Abstract
Background and objectives
Frailty is common among patients on hemodialysis and associated with adverse outcomes. However, little is known about changes in frailty over time and the factors associated with those changes.
Design, setting, participants, & measurements
To address these questions, we examined 762 participants in the A Cohort to Investigate the Value of Exercise/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD cohort study, among whom frailty was assessed at baseline and 12 and 24 months. We used ordinal generalized estimating equations analyses and modeled frailty (on a scale from zero to five possible components) and death during follow-up.
Results
The mean frailty score at baseline was 1.9, and the distribution of frailty scores was similar at each evaluation. However, most participants’ scores changed, with patients improving almost as often as worsening (overall change, 0.2 points per year; 95% confidence interval, 0.1 to 0.3). Hispanic ethnicity (0.6 points per year; 95% confidence interval, 0.0 to 1.1) and diabetes (0.7 points per year; 95% confidence interval, 0.3 to 1.0) were associated with higher frailty scores and higher serum albumin concentration with lower frailty scores (−1.1 points per g/dl; 95% confidence interval, −1.5 to −0.7). In addition, patients whose serum albumin increased over time were less likely to become frail, with each 1-g/dl increase in albumin associated with a 0.4-point reduction in frailty score (95% confidence interval, −0.80 to −0.05). To examine the underpinnings of the association between serum albumin and frailty, we included serum IL-6, normalized protein catabolic rate, and patient self-report of hospitalization within the last year in a second model. Higher IL-6 and hospitalization were statistically significantly associated with worse frailty at any point and worsening frailty over time, whereas normalized protein catabolic rate was not independently associated with frailty.
Conclusions
There was substantial year to year variability in frailty scores, with approximately equal numbers of patients improving and worsening. Markers of inflammation and hospitalization were independently associated with worsening frailty. Studies should examine whether interventions to address inflammation or posthospitalization rehabilitation can improve the trajectory of frailty.
Keywords: hemodialysis; frailty; end-stage renal disease; physical function; functional status; chronic kidney disease; Adiposity; diabetes mellitus; Follow-Up Studies; Hispanic Americans; hospitalization; Humans; Inflammation; Interleukin-6; obesity; renal dialysis; Serum Albumin; IL6 protein, human
Introduction
Frailty is a medical syndrome that is characterized by diminished strength, endurance, and reduced physiologic function, and it increases an individual’s vulnerability for loss of independence and death (1). Fried et al. (2) proposed a clinical phenotype of frailty that consists of at least three of weak grip strength, exhaustion, slow walking speed, low physical activity, and unintentional weight loss. This frailty construct has been associated with higher mortality among community-dwelling elders and become a de facto gold standard (3).
The prevalence of frailty is considerably higher among patients on hemodialysis than among community-dwelling elders: at 30%–42% (4,5), and frail patients are at higher risk of hospitalization and mortality than nonfrail patients (5,6). Cross-sectional studies suggest that the prevalence of frailty increases as kidney disease progresses (7). These data support the concept of a spiral of deconditioning, in which chronic disease can lead to inactivity and deconditioning, and deconditioning can lead to further inactivity and disability (8). However, although chronic disease and aging contribute to frailty, there may also be potentially reversible contributors, such as acute illnesses, inflammation, and poor nutritional status, and frailty may not always be a permanent or progressive condition. Little is known about the evolution or trajectory of frailty among patients with ESRD. Therefore, we aimed to ascertain the extent to which frailty changes over a 2-year period in patients on hemodialysis and the clinical factors associated with development, worsening, or improvement in frailty in this population.
Materials and Methods
Study Participants
A Cohort to Investigate the Value of Exercise/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD (ACTIVE/ADIPOSE) enrolled 771 patients from 14 dialysis facilities in the San Francisco Bay area and the Atlanta, Georgia metropolitan area during 2009–2011 and followed them with annual assessments for up to 2 years through 2013 (9). Participants were over 18 years of age, on dialysis for at least 3 months, English or Spanish speaking, and able to provide informed consent. The study was approved by the University of California, San Francisco and the Emory University Institutional Review Boards, and patients provided written informed consent for study participation.
Clinical and Laboratory Evaluation
Study coordinators interviewed participants, measured physical performance (gait speed and grip strength), and administered study questionnaires immediately before or during a dialysis session. Spanish-speaking participants received standardized instructions and questionnaires in Spanish from Spanish-speaking study coordinators. Coordinators reviewed patients’ medical records to ascertain the presence of comorbid conditions and dialysis adequacy. Patients’ data were linked to data from the ESRD Medical Evidence Report (Centers for Medicare and Medicaid Services Form 2728) and the patients file available in the US Renal Data System.
Blood was drawn immediately before a dialysis session within 1 month of the testing sessions. Samples were centrifuged, aliquoted, stored at −80°C, and transported to the core laboratory, where they were stored over liquid nitrogen at −196°C until the time of assay. We measured serum albumin in duplicate using a Polychem Autoanalyzer 180 (Polymedco, Cortland Manor, NY), and mean values were used in analyses. The intra-assay coefficient of variation (CV) was 2.5%, and the interassay CV was 3.2%. IL-6 was measured by ELISA (EMD Millipore Corp., St. Charles, MO). The intra-assay CV was 4.5%, and the interassay CV was 2.6%.
Frailty
We assessed frailty using the components of the Fried frailty phenotype (2,4) at study enrollment and 12 and 24 months (4). Weight loss was determined by asking participants whether they had lost >10 lb in the last year unintentionally. Exhaustion was on the basis of responses to questions about endurance and energy from the Center for Epidemiologic Studies depression scale (10). Low physical activity was ascertained from the short version of the Minnesota Leisure Time Physical Activity questionnaire (11). Grip strength was measured using a handheld dynamometer (Jamar; Lafayette Instrument, Lafayette, IN). Participants performed three tests with each hand, and the mean of the strongest hand was used to determine frailty using cutoffs on the basis of sex and body mass index (2). For gait speed, participants walked at their usual pace over a 15-ft course, and the faster of two trials was recorded. Patients received a total of zero to five points according to the number of these potential criteria that they met (2).
Statistical Analyses
Participant characteristics were described using mean (SD) or median (interquartile range) and proportions for categorical variables. We compared participant characteristics according to baseline frailty score using ANOVA, Wilcoxon rank sum, or chi-squared tests as appropriate.
To determine factors associated with frailty and change in frailty over time, we used ordinal generalized estimating equations analysis, which can accommodate within-patient dependencies arising from serial measurement. For these analyses, frailty was considered on an ordered scale of zero to five at each time point (baseline and 12 and 24 months) on the basis of the number of frailty components present, and death was considered worse than maximal frailty. This analysis included all participants with information about frailty status at baseline (n=762); 641 had information about frailty status or death at 12 months (84%), and 536 had information at 24 months (70%). Patients missing data for any reason other than death were censored (Figure 1). We first examined the associations of the following potential predictors with frailty and change in frailty: age, sex, race (white versus nonwhite), ethnicity (Hispanic versus non-Hispanic), body mass index as a categorical variable (<20, 20 to <25, 25 to <30, and ≥30 kg/m2), diabetes, atherosclerotic heart disease, heart failure, use of a tunneled catheter for dialysis, and serum albumin concentration. Comorbidities were considered to be present if they were noted on the Medical Evidence Report or in the patients’ medical records at study entry. Catheter use and serum albumin concentration were updated at each time point. For variables assessed at baseline only, we used models that included time, the variable of interest, and a multiplicative interaction term between time and the potential predictor variable. For variables that were measured or assessed at each time point, we included time, baseline value (continuous for albumin concentration and dichotomous for catheter use), and change since baseline in the models.
Figure 1.
Study flow diagram. Patients with a frailty score or death at 12 or 24 months contributed outcome information during follow-up. HD, hemodialysis.
In a second model, because serum albumin may be a marker of nutritional status, inflammation, or general health status, we added additional variables to explore these possible mechanisms explaining the association between serum albumin and frailty, including normalized protein catabolic rate (nPCR), IL-6, and hospitalization within the prior year, all of which were also updated at each time point. nPCR was calculated as an estimate of dietary protein intake on the basis of urea kinetic modeling (12) during the same month that frailty was assessed. Hospitalization information was obtained by patient report. We validated self-reported hospitalization against Medicare claims for hospitalization in the subset of the cohort with Medicare as the primary payer during each of the three time periods (−12 months to baseline, baseline to 12 months, and 12–24 months), and we found good agreement (Supplemental Table 1).
In sensitivity analyses, we repeated these analyses (1) using the original designations of the Fried frailty phenotype (nonfrail [zero points], prefrail [one to two points], and frail [three to five points]) (2) and (2) without time updating the covariates. We used SAS, version 9.4 (SAS Institute, Inc.) for all statistical analyses.
Results
Participants and Baseline Frailty Status
ACTIVE/ADIPOSE enrolled 771 patients, of whom 762 (99%) completed baseline frailty assessments and were included in this study. Participant mean age was 57.2±14.2 years old, 40.7% were women, 61.5% were black, and 53.4% had diabetes (Table 1). The mean number of frailty components present at baseline was 1.9±1.2 (median =2; 25th–75th percentile =0–4). Table 1 shows participants’ characteristics according to the number of frailty components present at baseline. Patients with higher frailty scores were older and more likely to be women and have diabetes, atherosclerotic heart disease, and heart failure. Patients who were frailer also had lower serum albumin concentration.
Table 1.
Participant characteristics according to baseline frailty status
| Characteristic | Entire Cohort, n=762 | Frailty Score | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0, n=95 | 1, n=211 | 2, n=216 | 3, n=154 | 4, n=73 | 5, n=13a | |||
| Age, yr | 57.2±14.2 | 52.1±12.6 | 54.2±13.8 | 56.9±14.3 | 60.9±14.4 | 63.7±13.5 | 64.2±8.3 | <0.001 |
| Sex, % women | 40.7 | 32.6 | 37.9 | 41.2 | 37.7 | 60.3 | 61.5 | 0.003 |
| Race, % | 0.23 | |||||||
| Black | 61.5 | 69.5 | 67.3 | 57.9 | 52.6 | 64.4 | — | |
| White | 23.9 | 18.9 | 20.9 | 25.9 | 29.2 | 23.3 | — | |
| Asian | 11.0 | 9.5 | 10.9 | 11.6 | 12.3 | 8.2 | — | |
| Other | 3.5 | 2.1 | 0.9 | 4.6 | 5.8 | 4.1 | — | |
| Ethnicity, % Hispanic | 12.7 | 10.5 | 9.0 | 15.3 | 16.2 | 12.3 | — | 0.28 |
| BMI, kg/m2a | 28.2±6.9 | 26.5±5.9 | 28.1±6.7 | 28.3±6.9 | 28.8±7.3 | 29.5±8.1 | 26.5±5.9 | 0.11 |
| Diabetes, % | 53.4 | 31.6 | 46.4 | 56.5 | 61.7 | 69.9 | — | <0.001 |
| ASHD, % | 33.1 | 24.2 | 26.5 | 30.6 | 43.5 | 46.6 | — | <0.001 |
| HF, % | 34.5 | 29.5 | 26.1 | 33.8 | 41.6 | 45.2 | — | <0.001 |
| Dialyzing via catheter, % | 20.7 | 15.8 | 20.4 | 21.8 | 20.1 | 28.8 | — | 0.34 |
| Albumin, g/dl | 4.0±0.4 | 4.1±0.3 | 4.0±0.3 | 4.0±0.3 | 3.9±0.4 | 3.8±0.4 | 3.7±0.4 | <0.001 |
—, National Institutes of Health policy prohibits providing specific count information when n<10. Although not all comorbid conditions are present in fewer than ten individuals in this group, for all conditions, either the number of individuals with or without the condition is less than ten.; BMI, body mass index; ASHD, atherosclerotic heart disease; HF, heart failure.
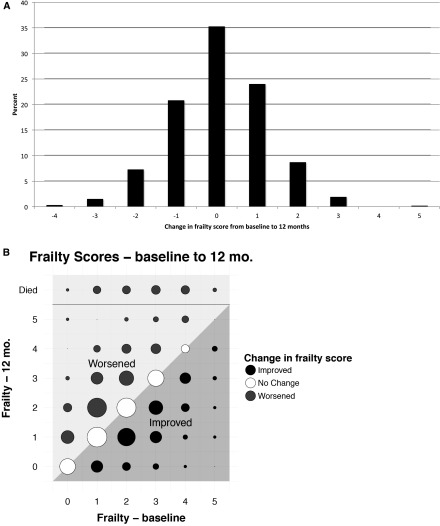
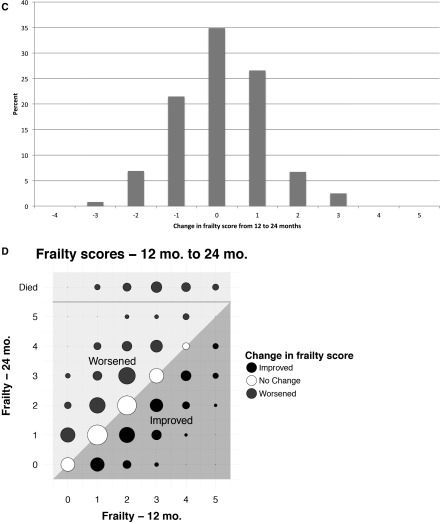
During follow-up, 104 patients died, 76 moved to a different facility, 19 changed to home dialysis (peritoneal dialysis or home hemodialysis), 37 received a kidney transplant, and a small number withdrew from the study or dialysis. In addition, some patients were not available for measurement at 12 (n=19) or 24 months (n=13) due to hospitalization, being too sick, vacation, or other reasons (Figure 1). Overall, 587 patients were assessed for frailty at 12 months, and 54 died, so that 641 patients contributed information at the 12-month follow-up. At 24 months, 486 patients were assessed for frailty (536 contributed to analyses, including the 50 who had died). The distribution of frailty scores at 12 and 24 months was similar to the distribution at baseline, although there was a slight but statistically significant increase in the unadjusted frailty score on average over time (0.2 points per year; 95% confidence interval [95% CI], 0.1 to 0.3). However, the similar overall distributions and small increase over time masked considerable underlying change (Figure 2). Only 35% of patients did not have at least a one-point change in score between 0 and 12 months. Of note, improvement in frailty was as common as worsening, and change occurred throughout the spectrum of baseline frailty scores (Figure 2, A and B). Similarly, 65% of patients had a change of at least one point between 12 and 24 months, and the pattern of changes was remarkably similar over the second year of observation (Figure 2, B and C).
Figure 2.
Changes in frailty scores between baseline and 12 months and between 12 and 24 months. A shows a histogram of the change in scores from baseline to 12 months. The distribution of change was fairly symmetrical around zero. However, only 35% of patients did not change; more patients changed by one point than stayed the same. B shows frailty score at baseline on the x axis and 12 months on the y axis. The area of the circles is proportional to the number of patients in each point. The diagonal line indicates no change in frailty score, and the shading of the dots and the areas of the graph indicate improving or worsening of frailty. This illustration highlights that change occurred across the spectrum of baseline frailty score. C and D show similar data for changes between 12 and 24 months, and the changes were remarkably similar over the second year of observation.
We examined predictors of frailty and change in frailty score using multivariable ordinal generalized estimating equations including demographic and clinical variables as covariates (Table 2, model 1). In this model, Hispanic ethnicity, diabetes, and serum albumin concentration were the strongest correlates of frailty. Hispanic patients had a 0.6-point higher frailty score on average (95% CI, 0 to 1.1) at any given time, and patients with diabetes had a 0.7-point higher score (95% CI, 0.3 to 1.0). However, the interaction terms of time with Hispanic ethnicity and diabetes were not statistically significant, indicating that the change in frailty score over time (i.e., the trajectory of frailty) did not differ significantly between Hispanic and non-Hispanic patients or between patients with and without diabetes. Higher serum albumin concentration was associated with lower frailty score (−1.1 points per 1 g/dl; 95% CI, −1.5 to −0.7), and serum albumin was the only variable significantly related to change in frailty, with each 1-g/dl increase in albumin associated with a 0.4-point reduction in frailty score (95% CI, −0.8 to −0.1).
Table 2.
Predictors of frailty and change in frailty score over time
| Variable | Model 1a | Model 2 | ||
|---|---|---|---|---|
| Difference in Frailty Score, Points | Change in Frailty, Points per Yearb | Difference in Frailty Score, Points | Change in Frailty, Points per Yearb | |
| Age, per 10 yr | 0.2 (−0.1 to 0.5) | 0.0 (−0.1 to 0.1) | 0.2 (0.1 to 0.3) | 0.0 (−0.1 to 0.1) |
| Women | 0.3 (−0.0 to 0.6) | 0.1 (−0.1 to 0.3) | 0.4 (0.0 to 0.7) | 0.1 (−0.1 to 0.2) |
| Race, nonwhite | 0.1 (−0.3 to 0.6) | −0.0 (−0.3 to 0.3) | 0.0 (−0.4 to 0.5) | 0.0 (−0.2 to 0.3) |
| Ethnicity, Hispanic | 0.6 (0.0 to 1.1) | −0.1 (−0.5 to 0.2) | 0.6 (0.1 to 1.2) | −0.1 (−0.4 to 0.2) |
| BMI, kg/m2 | ||||
| <20 | −0.1 (−0.3 to 0.5) | 0.3 (−0.2 to 0.6) | 0.0 (−0.7 to 0.6) | 0.3 (−0.2 to 0.7) |
| 20 to <25 | Reference | Reference | Reference | Reference |
| 25 to <30 | 0.0 (−0.4 to 0.4) | 0.2 (0.0 to 0.4) | 0.0 (−0.4 to 0.4) | 0.2 (0.0 to 0.5) |
| ≥30 | 0.1 (−0.3 to 0.5) | 0.2 (−0.1 to 0.4) | 0.1 (−0.3 to 0.4) | 0.1 (−0.1 to 0.4) |
| Diabetes | 0.7 (0.3 to 1.0) | −0.1 (−0.3 to 0.1) | 0.6 (0.3 to 0.9) | −0.1 (−0.3 to 0.1) |
| ASHD | 0.2 (−0.2 to 0.5) | 0.1 (−0.2 to 0.2) | 0.0 (−0.2 to 0.2) | 0.0 (−0.2 to 0.2) |
| Heart failure | 0.3 (0.0 to 0.6) | 0.0 (−0.2 to 0.2) | 0.3 (−0.1 to 0.6) | 0.0 (−0.2 to 0.2) |
| Dialysis via catheter | 0.3 (−0.1 to 0.7) | 0.3 (−0.2 to 0.7) | 0.1 (−0.3 to 0.5) | 0.1 (−0.3 to 0.5) |
| Serum albumin, g/dl | −1.1 (−1.5 to −0.7) | −0.4 (−0.8 to −0.1) | −0.6 (−1.1 to −0.2) | −0.1 (−0.5 to 0.3) |
| Hospitalization in last year | — | — | 0.6 (0.3 to 1.0) | 0.6 (0.3 to 0.8) |
| IL-6, pg/ml | — | — | 0.3 (0.2 to 0.5) | 0.3 (0.1 to 0.4) |
| nPCR, g/kg | — | — | −0.4 (−0.9 to 0.1) | −0.4 (−0.9 to 0.1) |
There were n=732 patients with complete data for all covariates. BMI, body mass index; ASHD, atherosclerotic heart disease; nPCR, normalized protein catabolic rate.
-, these variables were not included in model 1.
For nonvarying predictors, change in frailty represents the interaction term between time and each variable. For time-updated variables, this column shows the association with the change in that variable over time.
When we added IL-6 as an inflammatory marker, nPCR as an indicator of protein intake, and self-reported hospitalization within the last year as a general health indicator, each of which was associated with frailty and change in frailty in univariate analysis, to a second multivariable model (Table 2, model 2), IL-6 was directly correlated with frailty and change in frailty (0.3 points per 1 pg/ml; 95% CI, 0.2 to 0.5 and 0.3 points per 1 pg/ml; 95% CI, 0.1 to 0.4, respectively). Similarly, hospitalization within the last year was also associated with frailty and change in frailty (0.6 points higher among patients hospitalized in the year before assessment; 95% CI, 0.3 to 1.0 and 0.6 points for new hospitalization in follow-up; 95% CI, 0.3 to 0.8). nPCR was not significantly associated with frailty (−0.4 per 1 g/kg; 95% CI, −0.9 to 0.1). Serum albumin concentration remained associated with frailty, but the association was attenuated somewhat after adjustment for these additional covariates (−0.6 points per 1 g/dl; 95% CI, −1.1 to −0.2), and the association between serum albumin and change in frailty was no longer statistically significant. Hispanic ethnicity and diabetes remained associated with higher frailty, and older age and women were also associated with frailty in this model.
Results of sensitivity analysis using a three-level frailty outcome were similar to those of the primary analysis, although 95% CIs were wider (Supplemental Table 2). All of the same variables were associated with frailty when covariates were not updated (Supplemental Table 3).
Discussion
In this analysis of changes in frailty status, we found that most patients’ frailty score did not remain static from year to year. Although we had hypothesized that frailty would change in some patients and that improvement might be observed in some patients, we had not anticipated that there would be almost as much improvement as decline. We found that serum albumin concentration was an important predictor of frailty and its evolution and that the observed association was attenuated when IL-6, nPCR, and hospitalization in the prior year were accounted for. In a previous study using a modified definition of frailty, we found that a one-point higher frailty score was associated with an 87% higher risk of mortality (95% CI, 1.59 to 2.20) (13). Thus, the magnitudes of the associations that we observed in this study are likely to be clinically significant.
In addition to examination of traditional correlates of frailty, such as older age, women, and diabetes mellitus, which were associated with frailty as expected, we examined several patient characteristics that have received less emphasis, such as Hispanic ethnicity, dialysis via a catheter, and hospitalization within the prior year. Surprisingly, Hispanic ethnicity was associated with a bigger difference in frailty score (0.6 points; 95% CI, 0.1 to 1.2) than 10 years of age (0.2; 95% CI, 0.1 to 0.3) and comparable with diabetes in the magnitude of association (0.6 points; 95% CI, 0.3 to 0.9). However, the 95% CIs were wider for Hispanic ethnicity than for age and diabetes. We also found it remarkable that dialysis via a catheter was not independently associated with frailty in this cohort. It is possible that frail patients dialyzing with catheters were less likely to survive to enter our cohort given that the median vintage of the cohort was 2.7 years (25th–75th percentile, 1.2–5.4) (6). Furthermore, the rate of catheter use at the beginning of the study was relatively low at 21% and may reflect patient preference or access difficulties not related to frailty.
Sex, ethnicity, and diabetes were associated with frailty cross-sectionally but not longitudinally. It is possible that the 2-year time course of our study was too short to detect subtle differences in the trajectory of frailty among women, Hispanic patients, and those with diabetes that occur over longer periods of exposure or that these subgroups are more likely to experience acute events that affect the development of frailty but do not affect the trajectory. Only serum albumin was associated with change in frailty in our first model. On further exploration, inflammation and hospitalization seemed to at least partially explain the relation between albumin and frailty, but nPCR was not associated with frailty, which might indicate that low protein intake is a less important contributor to frailty or that its contribution is not independent of other factors, such as inflammation and comorbidity. This information might be useful in considering interventions to prevent or improve frailty given that inflammation, nutrition, and hospitalization are at least potentially modifiable factors.
Although the association between hospitalization in the intervening period and frailty is not an unexpected finding, it is worthy of some consideration. Hospitalization is a frequent occurrence among patients on hemodialysis. Recent recognition that readmission within 30 days of hospital discharge is particularly high in the dialysis population (14,15) has prompted discussion of strategies to prevent readmission (16). However, there has been less consideration of declines in functioning during hospitalization (17). Our findings suggest that hospitalization could be a major driver of frailty, and attention to nutrition and rehabilitation needs after hospitalization might be beneficial.
It may be possible to address inflammation either directly through administration of IL-1 inhibitors (18), IL-1 receptor antagonists (19,20), or TNF-a inhibitors (21) or indirectly by administering omega fatty acids (22,23) or nutritional supplements (23). Nutritional supplementation and anti-inflammatory interventions have also been shown to improve nutritional status (21,24). However, for the most part, these interventions have been tested in small numbers of patients, and potential effects on physical functioning and frailty have not been assessed. In light of our results, studies of this type of intervention should include assessments of physical function or frailty. Such studies are needed to ascertain whether addressing inflammation can improve frailty, because although our results could indicate that inflammation leads to frailty, they would also be consistent with other conditions (e.g., infection) leading to both worsening inflammation and worsening frailty.
Our study has several strengths, in particular the size of the cohort, the use of the Fried frailty phenotype, which allows for direct comparison with other populations, and the repeated measures of frailty. Frailty has generally been considered as a static entity, but our results challenge this notion and suggest possible strategies to mitigate frailty in the dialysis population.
Some weaknesses should also be acknowledged. Perhaps the most important is participant dropout. Thirty percent of participants left the cohort before the end of the study. The most common reasons for this were moving to another dialysis facility, changing to home dialysis therapy, receiving a transplant, and not being available for assessment because of hospitalization, vacation, or missed treatments. It is difficult to predict the extent to which participant dropout might have affected our results. Moving to another dialysis facility, the most common reason for loss to follow-up, is likely to introduce noninformative censoring, but other reasons for being unavailable for follow-up assessments might not have been independent of frailty (e.g., transplant recipients are likely to be less frail, and hospitalized patients are frailer). To minimize informative censoring, we included patients who died in our analysis as the highest category of frailty rather than censoring at death. Because we do not have claims data for the whole cohort, hospitalization was ascertained by patient self-report rather than from hospital claims. We found good but not perfect agreement in the subset with Medicare as the primary payer (Supplemental Table 1). Misclassification would be expected to bias estimates of the association with frailty toward the null. We also note that the racial, ethnic, and age distribution of our cohort does not match that of the United States dialysis population, because we included more black participants and our population was somewhat younger. However, we did not find evidence that race was associated with frailty or change in frailty.
In conclusion, frailty is present in a much higher percentage of patients receiving dialysis—even those who are nonelderly—than populations of community-dwelling elders (2), but the overall prevalence in our cohort changed little over time. However, there was substantial year to year change in frailty scores, with approximately equal numbers of patients improving and declining. Although older patients, women, Hispanics, and those with diabetes were more likely to be frail, only inflammation and hospitalization were independently associated with change in frailty. Future studies should examine whether interventions to address inflammation can improve frailty, whether assessment of frailty immediately after hospitalization could be helpful in predicting readmission and mortality, and whether rehabilitation in the setting of posthospitalization decline in functioning can improve outcomes.
Disclosures
None of the authors have any financial conflict of interest relevant to the content presented in this manuscript. L.S.D. is currently employed by Fresenius Medical Care North America.
Supplementary Material
Acknowledgments
Because K.L.J. was a Deputy Editor of the Clinical Journal of the American Society of Nephrology at the time of peer review, she was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK107269 and K24DK085153. In addition, C.D. was supported by Career Development Award 1K2CX000527 from the US Department of Veterans Affairs, Clinical Research and Development Program. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health through University of California, San Francisco - Clinical & Translational Science Institute grant UL1 TR000004.
The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12131116/-/DCSupplemental.
References
- 1.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J: Frailty consensus: A call to action. J Am Med Dir Assoc 14: 392–397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Xue QL: The frailty syndrome: Definition and natural history. Clin Geriatr Med 27: 1–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen KL, Dalrymple LS, Delgado C, Kaysen GA, Kornak J, Grimes B, Chertow GM: Association between body composition and frailty among prevalent hemodialysis patients: A US Renal Data System special study. J Am Soc Nephrol 25: 381–389, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, Walston JD, Segev DL: Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 61: 896–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen KL, Dalrymple LS, Glidden D, Delgado C, Kaysen GA, Grimes B, Chertow GM: Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol 11: 626–632, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalrymple LS, Katz R, Rifkin DE, Siscovick D, Newman AB, Fried LF, Sarnak MJ, Odden MC, Shlipak MG: Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol 8: 2091–2099, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Painter P, Johansen K: Physical functioning in end-stage renal disease. Introduction: A call to activity. Adv Ren Replace Ther 6: 107–109, 1999 [DOI] [PubMed] [Google Scholar]
- 9.US Renal Data System : USRDS 2011 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 10.Orme JG, Reis J, Herz EJ: Factorial and discriminant validity of the center for epidemiological studies depression (CES-D) scale. J Clin Psychol 42: 28–33, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Taylor HL, Jacobs DR Jr., Schucker B, Knudsen J, Leon AS, Debacker G: A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31: 741–755, 1978 [DOI] [PubMed] [Google Scholar]
- 12.Garred LJ, Barichello DL, Canaud BC, McCready WG: Simple equations for protein catabolic rate determination from pre dialysis and post dialysis blood urea nitrogen. ASAIO J 41: 889–895, 1995 [PubMed] [Google Scholar]
- 13.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 14.US Renal Data System : Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 15.Flythe JE, Katsanos SL, Hu Y, Kshirsagar AV, Falk RJ, Moore CR: Predictors of 30-day hospital readmission among maintenance hemodialysis patients: A hospital’s perspective. Clin J Am Soc Nephrol 11: 1005–1014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathew AT, Strippoli GF, Ruospo M, Fishbane S: Reducing hospital readmissions in patients with end-stage kidney disease. Kidney Int 88: 1250–1260, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Lo D, Chiu E, Jassal SV: A prospective pilot study to measure changes in functional status associated with hospitalization in elderly dialysis-dependent patients. Am J Kidney Dis 52: 956–961, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, Wang W, Smits G, Tengesdal I, Dinarello CA, Hung AM: IL-1 inhibition and vascular function in CKD. J Am Soc Nephrol 28: 971–980, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung AM, Ellis CD, Shintani A, Booker C, Ikizler TA: IL-1β receptor antagonist reduces inflammation in hemodialysis patients. J Am Soc Nephrol 22: 437–442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung AM, Limkunakul C, Placido JS, Siew ED, Ellis CD, Shintani A, Ikizler TA: Administration of IL-1ra improves adiponectin levels in chronic hemodialysis patients. J Nephrol 27: 681–688, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Don BR, Kim K, Li J, Dwyer T, Alexander F, Kaysen GA: The effect of etanercept on suppression of the systemic inflammatory response in chronic hemodialysis patients. Clin Nephrol 73: 431–438, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Asemi Z, Soleimani A, Shakeri H, Mazroii N, Esmaillzadeh A: Effects of omega-3 fatty acid plus alpha-tocopherol supplementation on malnutrition-inflammation score, biomarkers of inflammation and oxidative stress in chronic hemodialysis patients. Int Urol Nephrol 48: 1887–1895, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Daud ZA, Tubie B, Adams J, Quainton T, Osia R, Tubie S, Kaur D, Khosla P, Sheyman M: Effects of protein and omega-3 supplementation, provided during regular dialysis sessions, on nutritional and inflammatory indices in hemodialysis patients. Vasc Health Risk Manag 8: 187–195, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouque D, McKenzie J, de Mutsert R, Azar R, Teta D, Plauth M, Cano N; Renilon Multicentre Trial Study Group : Use of a renal-specific oral supplement by haemodialysis patients with low protein intake does not increase the need for phosphate binders and may prevent a decline in nutritional status and quality of life. Nephrol Dial Transplant 23: 2902–2910, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.