Abstract
Background and objectives
Technique failure is a major limitation of peritoneal dialysis. Our study aimed to identify center- and patient-level predictors of peritoneal dialysis technique failure.
Design, setting, participants, & measurements
All patients on incident peritoneal dialysis in Australia from 2004 to 2014 were included in the study using data from the Australia and New Zealand Dialysis and Transplant Registry. Center- and patient-level characteristics associated with technique failure were evaluated using Cox shared frailty models. Death-censored technique failure and cause-specific technique failure were analyzed as secondary outcomes.
Results
The study included 9362 patients from 51 centers in Australia. The technique failure rate was 0.35 (95% confidence interval, 0.34 to 0.36) episodes per patient-year, with a sevenfold variation across centers that was mainly associated with center-level characteristics. Technique failure was significantly less likely in centers with larger proportions of patients treated with peritoneal dialysis (>29%; adjusted hazard ratio, 0.83; 95% confidence interval, 0.73 to 0.94) and more likely in smaller centers (<16 new patients per year; adjusted hazard ratio, 1.10; 95% confidence interval, 1.00 to 1.21) and centers with lower proportions of patients achieving target baseline serum phosphate levels (<40%; adjusted hazard ratio, 1.15; 95% confidence interval, 1.03 to 1.29). Similar results were observed for death-censored technique failure, except that center target phosphate achievement was not significantly associated. Technique failure due to infection, social reasons, mechanical causes, or death was variably associated with center size, proportion of patients on peritoneal dialysis, and/or target phosphate achievement, automated peritoneal dialysis exposure, icodextrin use, and antifungal use. The variation of hazards of technique failure across centers was reduced by 28% after adjusting for patient-specific factors and an additional 53% after adding center-specific factors.
Conclusions
Technique failure varies widely across centers in Australia. A significant proportion of this variation is related to potentially modifiable center characteristics, including peritoneal dialysis center size, proportion of patients on peritoneal dialysis, and proportion of patients on peritoneal dialysis achieving target phosphate level.
Keywords: Anti-Bacterial Agents, Australia, Glucans, Glucose, Hemoglobins, hospitalization, Humans, New Zealand, peritoneal dialysis, Peritonitis, Phosphates, Registries, renal dialysis, icodextrin
Introduction
One of the major reasons for low peritoneal dialysis (PD) prevalence is a high attrition rate from PD programs due to technique failure (1–3). Although there are both patient- and center-level characteristics that contribute to technique failure, very few studies have specifically investigated the association between center-level characteristics and technique failure (center effects).
Huisman et al. (4) observed that there was great variability in technique survival across centers, with smaller centers and centers with lower proportions of patients on PD experiencing higher rates of death-censored technique failure. Similarly, Schaubel et al. (5) reported a significant association between center characteristics, including cumulative number of patients treated with PD and the percentage of patients started on PD, and technique survival using data from the Canadian Organ Replacement Registry. In another study, Afolalu et al. (6) identified that death-censored technique failure was higher in units with smaller numbers of patients on PD (<25 patients). More recently, an analysis of the French Language Peritoneal Dialysis Registry found that center characteristics accounted for 52% of the disparities in early (within 6 months) PD failure across centers and that center size was significantly associated with early PD failure (7). The findings of a systematic review of observational studies examining the effect of center volume on outcomes of patients on dialysis further support an association between center size and technique survival, with larger centers experiencing better technique survival (8).
Most studies that have examined the relationship between center characteristics and technique survival have focused primarily on center size and have not examined other potentially important characteristics, such as PD prescription practices, infection-related characteristics, attainment of guideline-recommended biochemical and hematologic targets, and transplanting center status. This study aimed to more comprehensively examine the relationship between a diverse range of center characteristics and overall, death-censored, and cause-specific technique failure.
Materials and Methods
Study Population
The study included all patients on incident PD in Australia from the period of January of 2004 to December of 2014. Patients with missing data and those from centers with <5 patient-years of total follow-up were excluded. Deidentified data were obtained from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry (9). Permission to analyze the data was also granted by an ANZDATA Registry executive.
Patient-Level Characteristics
The patient-level characteristics examined in this study were age at initiation of dialysis, sex, race, body mass index (BMI), smoking status (current, former, or never), presence of cardiovascular disease (defined as a composite of ischemic heart disease, cerebrovascular disease, and peripheral vascular disease), presence of diabetes mellitus, chronic lung disease, primary renal disease, later referral to nephrologist (defined as <3 months before initiation of RRT), initial RRT modality, initial PD modality, and socioeconomic position (reported as Index of Relative Socioeconomic Advantage and Disadvantage [IRSAD] scores) (10). IRSAD scores were divided into quartiles, with the lowest quartile used as the reference group in analyses. BMI was divided into four groups: underweight (BMI<18.5 kg/m2), normal range (BMI=18.5–24.9 kg/m2), overweight (BMI=25–29.9 kg/m2), and obese (BMI≥30 kg/m2) on the basis of the World Health Organization classification of BMI. The normal-range BMI group was used as the reference group in analyses.
Center-Level Characteristics
The center-level characteristics examined in this study were transplant center status (defined as whether at least one kidney transplant was performed in the same hospital as the PD center during the study period [2004–2014]), center size (calculated as the mean annual number of patients on incident PD at the center), PD proportion (estimated from the proportion of all center patients on dialysis treated with PD), automated peritoneal dialysis (APD) exposure (defined as the proportion of center patients on PD exposed to APD at least once), peritoneal equilibration test performance (defined as the proportion of center patients who had a peritoneal equilibration test performed within the first 6 months of PD commencement), icodextrin exposure (defined as the proportion of center patients treated with icodextrin), target serum phosphate performance (defined as the proportion of center patients on PD with baseline phosphate level of <6.4 mg/dl), and target hemoglobin (defined as the proportion of center patients on PD with baseline hemoglobin levels within the national guideline-recommended targets of 10–12 g/dl [2004–2010] or 10–11.5 g/dl [2011–2014]). The peritonitis-related center characteristics examined in this study were the proportion of culture-negative peritonitis related to all episodes of peritonitis in center, the proportion of peritonitis episodes hospitalized for treatment, the proportion of peritonitis episodes receiving complete empirical antibiotic coverage (defined as episodes treated with antibiotics covering both Gram-positive and -negative organisms at presentation), and the proportion of peritonitis episodes treated with antifungal prophylaxis in center. The dialysis center for each patient was defined as the center where PD was commenced. All center-level characteristics, except transplanting center status, were divided into quartiles on the basis of the total number of study participants. The second and third quartiles were combined and served as the reference group in analyses. According to this method, all of the center-level characteristics, except transplanting center status, were categorized into three groups (first quartile, second and third quartiles combined, and fourth quartile). For example, center size was categorized as <16, 16–48, and >48 patients on incident PD per year, whereas percentages of patients treated with PD were categorized as <18%, 18%–29%, and >29%. The era of PD commencement was divided into two periods, 2004–2009 and 2010–2014, with the earlier period as the reference group in analyses.
Outcomes of the Study
The primary outcome was technique failure defined as transfer to hemodialysis (HD) for ≥30 days or death (including death within 30 days of transferring to HD) as per the previously published standardized definition (11). The secondary outcomes were death-censored technique failure and cause-specific death-censored technique failure. The specific causes of death-censored technique failure were infection, inadequate dialysis, and social and mechanical causes as per the previously published standardized definition (11). As a secondary outcome analysis, technique failure was also analyzed using the 180-day criterion defined as transfer to HD for ≥180 days or death (including death within 180 days of transferring to HD) (11).
Statistical Analyses
Patient- and center-level characteristics are presented as frequencies (percentages) for categorical variables, mean±SD for continuous normally distributed variables, and median (interquartile range) for continuous non-normally distributed variables.
For the primary outcome, all patient-level characteristics with P values <0.20 in univariable Cox proportional hazards regression models with shared frailty were included as fixed effects in a patient-level multivariable model. Cox proportional hazard with shared frailty model was used to account for the fact that patients were clustered within the center. A final multivariable model included all covariates from the patient-level model and center-level covariates with P values <0.20 in univariable Cox regression models. The era of PD commencement was also fitted as a fixed effect covariate in the final model to adjust for era effect. Proportional hazards assumptions for Cox regression were assessed using Schoenfeld residuals. Because the proportional hazards assumption was found to be violated for first RRT modality, a time-dependent effect for first RRT was included in the Cox shared frailty model used to analyze the primary outcome, in which data were censored at the time of transplantation or the end of the study. There was no biologically meaningful interaction between covariates. Because evidence of collinearity was identified between center size and proportion of patients on PD with respect to the primary outcome, a separate analysis was performed for center size and proportion of patients on PD. The likelihood ratio test was used to compare the patient-level with the final model. The percentage reduction in the random effect of technique failure across the centers was calculated as the ratio of the difference in SDs between the two models divided by the SD of the smaller model.
All covariates in the final model for the primary outcome were included in models for the secondary outcomes. The cause-specific hazard model was used to analyze death-censored technique failure, with death and transplantation as competing events. A similar method was used to analyze the individual cause of technique failure with censoring of the other competing events (12). All data were analyzed using Stata (version 14.0; StataCorp LP, College Station, TX). P values <0.05 were considered statistically significant.
Results
A total of 9642 patients commenced PD during the study period (2004–2014). Of these, 46 (0.4%) patients from 12 dialysis centers with <5 patient-years of total follow-up period were excluded along with 234 (2.5%) patients with missing demographic data. Consequently, 9362 patients on incident PD from 51 centers were included in the study. Of these 9362 patients, 3691 (39%) transferred to HD, 1244 (13%) received a kidney transplant, and 2122 (23%) died during the study period. A total of 28 centers were categorized as small centers, 19 centers were categorized as average-sized centers, and four centers were categorized as large centers. The baseline patient- and center-level characteristics are presented in Table 1.
Table 1.
Patient- and center-level characteristics
| Characteristics | Descriptive Statistics |
|---|---|
| Patient characteristics, n=9362 | |
| Men | 5555 (59) |
| Age, yr | 59.4±15.4 |
| Race | |
| White | 6885 (74) |
| Asian | 1095 (12) |
| ATSI | 697 (7) |
| Maori–Pacific Islanders | 358 (4) |
| Others | 327 (3) |
| Body mass index, kg/m2 | 27.0±5.8 |
| <18.5 | 319 (3) |
| 18.5–24.9 | 3369 (36) |
| 25–29.9 | 3229 (35) |
| ≥30 | 2445 (26) |
| Primary renal disease | |
| GN | 2406 (26) |
| Diabetes mellitus | 3143 (34) |
| Renovascular disease | 1353 (14) |
| Polycystic kidney disease | 553 (6) |
| Others | 1907 (20) |
| Comorbidities | |
| Diabetes mellitus | 4056 (43) |
| Cardiovascular disease | 4346 (46) |
| Chronic lung disease | 1370 (15) |
| Smoking status | |
| Nonsmoker | 4454 (48) |
| Current smoker | 1167 (12) |
| Former smoker | 3741 (40) |
| Late nephrology referral | 1878 (20) |
| Modality of PD (CAPD) | 6913 (74) |
| PD as first RRT modality | 6889 (65) |
| IRSAD scores | 978±79 |
| <934 | 2341 (25) |
| 934–983 | 2349 (25) |
| >983–1032 | 2335 (25) |
| >1032 | 2337 (25) |
| Center characteristics, n=51 | |
| Transplant center, n (%) | 18 (35) |
| Center size (no. of incident patients per year) | 14 (8–25) |
| Patients on PD (over total patients on dialysis), % | 20 (15–25) |
| Exposure to APDa | 63 (40–80) |
| Exposure to icodextrina | 49 (35–70) |
| PET performed at PD initiationa | 72 (55–80) |
| Culture-negative peritonitisb | 16 (10–20) |
| Antifungal prophylaxis useb | 71 (30–85) |
| Patients empirically received both antibioticsb | 85 (77–90) |
| Hospitalization for peritonitisb | 74 (60–85) |
| Hemoglobin in targeta | 42 (37–45) |
| Phosphate in targeta | 45 (40–51) |
Data are presented as number (%), mean±SD, or median (interquartile range). ATSI, Aboriginal and Torres Strait Islander; PD, peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; IRSAD, Index of Relative Socioeconomic Advantage and Disadvantage; APD, automated peritoneal dialysis; PET, peritoneal equilibration test.
Percentage of all participating patients on PD in a center.
Percentage of all peritonitis in a center.
Technique Failure
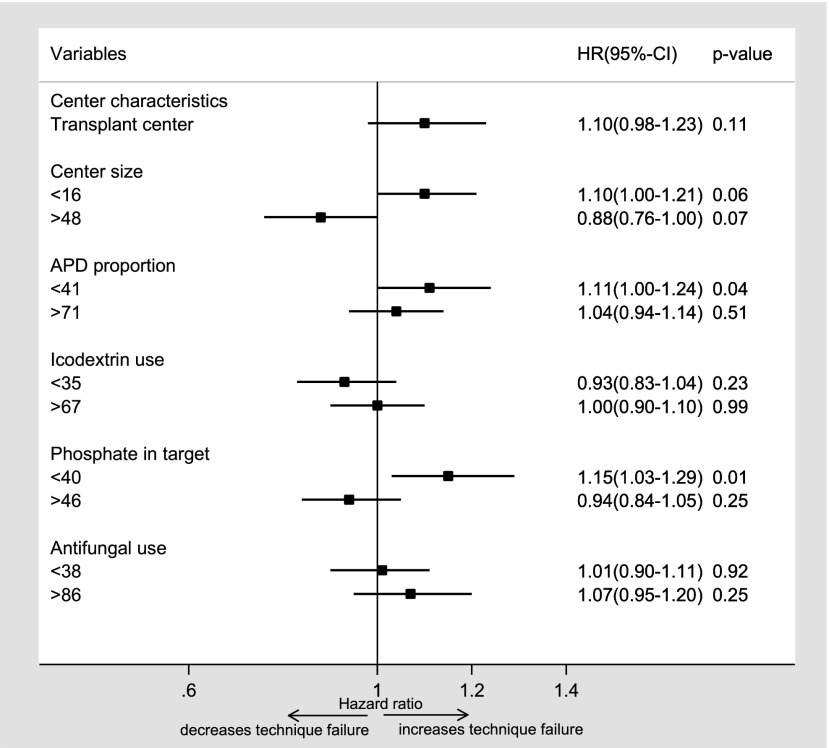
There were 5813 episodes of technique failure over 16,504 patient-years of follow-up. The overall technique failure rate was 0.35 (95% confidence interval [95% CI], 0.34 to 0.36) episodes per patient-year, with a sevenfold variation in technique failure rates among centers. Technique failure was significantly less likely in centers with larger proportions of patients treated with PD (>29%; adjusted hazard ratio [AHR], 0.83; 95% CI, 0.73 to 0.94) (Supplemental Table 1) and more likely in smaller centers (<16 new patients per year; AHR, 1.10; 95% CI, 1.00 to 1.21) and centers with lower proportions of patients achieving baseline serum phosphate levels in target (<40%; AHR, 1.15; 95% CI, 1.03 to 1.29) (Figure 1, Table 2). The other center characteristics were not associated with technique failure.
Figure 1.
Forest plot showing the association between center-level characteristics and technique failure after adjusting for age, sex, race, body mass index, smoking status, primary renal disease, diabetes mellitus, cardiovascular disease, chronic lung disease, late nephrology referral, initial modality of peritoneal dialysis, initial modality of RRT, socioeconomic position, and era of peritoneal dialysis commencement. For each variable, the reference group is the middle category (combined second and third quartiles). APD, automated peritoneal dialysis; 95% CI, 95% confidence interval; HR, hazard ratio.
Table 2.
Multivariable Cox shared frailty models for technique failure defined as 30 and 180 days
| Covariates | Technique Failure 30 d | Technique Failure 180 d | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Era (2004–2009) | 1.0 | Reference | 1.0 | Reference | ||
| Era (2010–2014) | 0.87 | 0.82 to 0.93 | <0.001 | 0.80 | 0.75 to 0.85 | <0.001 |
| Patient-level characteristics | ||||||
| Age (decade) | 1.08 | 1.05 to 1.10 | <0.001 | 1.12 | 1.09 to 1.14 | <0.001 |
| Men | 1.00 | 0.94 to 1.06 | 0.92 | 0.99 | 0.93 to 1.05 | 0.76 |
| Race | <0.001 | <0.001 | ||||
| White | 1.00 | Reference | 1.00 | Reference | ||
| Asian | 0.72 | 0.65 to 0.80 | <0.001 | 0.71 | 0.64 to 0.79 | <0.001 |
| ATSI | 1.06 | 0.94 to 1.19 | 0.35 | 1.10 | 0.97 to 1.24 | 0.15 |
| MP | 0.75 | 0.63 to 0.89 | 0.001 | 0.73 | 0.61 to 0.88 | 0.001 |
| Other | 0.60 | 0.48 to 0.75 | <0.001 | 0.61 | 0.48 to 0.77 | <0.001 |
| BMI, kg/m2 | <0.001 | <0.001 | ||||
| <18.5 | 1.11 | 0.96 to 1.30 | 0.15 | 1.09 | 0.93 to 1.28 | 0.27 |
| 18.5–24.9 | 1.00 | Reference | 1.00 | Reference | ||
| 25–29.9 | 0.98 | 0.92 to 1.05 | 0.55 | 0.95 | 0.89 to 1.01 | 0.13 |
| ≥30 | 1.12 | 1.04 to 1.20 | 0.001 | 1.10 | 1.02 to 1.18 | 0.01 |
| Smoking status | 0.01 | 0.01 | ||||
| Nonsmoker | 1.00 | Reference | 1.00 | Reference | ||
| Current smoker | 1.10 | 1.01 to 1.20 | 0.02 | 1.11 | 1.02 to 1.22 | 0.01 |
| Former smoker | 1.08 | 1.02 to 1.15 | <0.01 | 1.08 | 1.02 to 1.15 | 0.01 |
| Diabetes mellitus | 1.05 | 0.96 to 1.15 | 0.29 | 1.12 | 1.02 to 1.22 | 0.02 |
| Cardiovascular disease | 1.30 | 1.23 to 1.38 | <0.001 | 1.35 | 1.27 to 1.44 | <0.001 |
| Chronic lung disease | 1.10 | 1.03 to 1.19 | <0.01 | 1.09 | 1.02 to 1.18 | 0.02 |
| Primary renal disease | 0.06 | 0.06 | ||||
| GN | 1.00 | Reference | 1.00 | Reference | ||
| Diabetes nephropathy | 1.12 | 1.01 to 1.24 | 0.03 | 1.15 | 1.03 to 1.28 | 0.01 |
| Hypertension | 0.97 | 0.88 to 1.05 | 0.48 | 1.02 | 0.93 to 1.12 | 0.70 |
| Polycystic kidney disease | 1.06 | 0.93 to 1.20 | 0.42 | 0.94 | 0.81 to 1.08 | 0.36 |
| Other/unknown | 0.98 | 0.91 to 1.06 | 0.66 | 1.03 | 0.95 to 1.13 | 0.46 |
| Late referral | 1.10 | 1.03 to 1.18 | <0.01 | 1.07 | 1.00 to 1.15 | 0.05 |
| Initial modality of RRT (PD)a | ||||||
| Overall | 0.64 | 0.59 to 0.70 | <0.001 | 0.62 | 0.57 to 0.68 | <0.001 |
| At 6 mo | 0.67 | 0.63 to 0.72 | <0.001 | 0.65 | 0.61 to 0.70 | <0.001 |
| At 1 yr | 0.71 | 0.66 to 0.75 | <0.001 | 0.68 | 0.64 to 0.73 | <0.001 |
| At 2 yr | 0.78 | 0.73 to 0.82 | <0.001 | 0.75 | 0.70 to 0.79 | <0.001 |
| Initial PD modality (CAPD) | 0.87 | 0.81 to 0.93 | <0.001 | 0.86 | 0.80 to 0.93 | <0.001 |
| IRSAD scoresb | 0.53 | 0.52 | ||||
| <934 | 1.00 | Reference | 1.00 | Reference | ||
| 934–983 | 0.99 | 0.92 to 1.06 | 0.70 | 0.99 | 0.92 to 1.07 | 0.86 |
| >983–1032 | 0.98 | 0.91 to 1.06 | 0.65 | 0.99 | 0.91 to 1.07 | 0.72 |
| >1032 | 0.94 | 0.87 to 1.02 | 0.16 | 0.94 | 0.87 to 1.03 | 0.17 |
| Center-level characteristics | ||||||
| Transplant center | 1.10 | 0.98 to 1.23 | 0.11 | 1.09 | 0.98 to 1.23 | 0.11 |
| Center size (incident patients per 1 yr) | 0.02 | 0.02 | ||||
| <16 | 1.10 | 1.00 to 1.21 | 0.06 | 1.10 | 1.00 to 1.20 | 0.04 |
| 16–48 | 1.00 | Reference | 1.00 | Reference | ||
| >48 | 0.88 | 0.76 to 1.00 | 0.07 | 0.90 | 0.79 to 1.03 | 0.12 |
| APD exposure,c % | 0.13 | 0.18 | ||||
| <41 | 1.11 | 1.00 to 1.24 | 0.04 | 1.10 | 0.99 to 1.22 | 0.08 |
| 41–71 | 1.00 | Reference | 1.00 | Reference | ||
| >71 | 1.04 | 0.94 to 1.14 | 0.51 | 1.06 | 0.96 to 1.17 | 0.25 |
| Icodextrin use,c % | 0.46 | 0.55 | ||||
| <35 | 0.93 | 0.83 to 1.04 | 0.23 | 0.94 | 0.84 to 1.05 | 0.28 |
| 35–67 | 1.00 | Reference | 1.00 | Reference | ||
| >67 | 1.00 | 0.90 to 1.10 | 0.99 | 1.0 | 0.90 to 1.10 | 0.94 |
| Phosphate in target,c % | 0.004 | <0.01 | ||||
| <40 | 1.15 | 1.03 to 1.29 | 0.01 | 1.14 | 1.02 to 1.27 | 0.02 |
| 40–46 | 1.00 | Reference | 1.00 | Reference | ||
| >46 | 0.94 | 0.84 to 1.05 | 0.25 | 0.94 | 0.84 to 1.05 | 0.27 |
| Antifungal use,d % | 0.50 | 0.79 | ||||
| <38 | 1.01 | 0.90 to 1.11 | 0.92 | 0.99 | 0.89 to 1.09 | 0.78 |
| 38–86 | 1.00 | Reference | 1.00 | Reference | ||
| >86 | 1.07 | 0.95 to 1.20 | 0.25 | 1.03 | 0.92 to 1.15 | 0.61 |
HR, hazard ratio; 95% CI, 95% confidence interval; ATSI, Aboriginal and Torres Strait Islander; MP, Maori–Pacific Islanders; BMI, body mass index; PD, peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; IRSAD, Index of Relative Socioeconomic Advantage and Disadvantage; APD, automated peritoneal dialysis.
There was a significant interaction between initial modality of RRT and time (P<0.001). The overall HR and HR at three time points are given.
Socioeconomic position reported as IRSAD scores, with higher scores reflecting higher socioeconomic position.
Percentage of all participating patients in the center.
Percentage of peritonitis in center.
The patient-level predictors of technique failure are presented in Table 2. There was a significant interaction between initial modality of RRT and time (P<0.001), whereby the adjusted hazard of technique failure for patients commenced on PD as the initial modality of RRT increased over time (hazard ratio [HR], 0.67; 95% CI, 0.63 to 0.72 at 6 months; HR, 0.71; 95% CI, 0.66 to 0.75 at 1 year; HR, 0.78; 95% CI, 0.73 to 0.82 at 2 years).
Similar results were observed when technique failure was analyzed using the 180-day definition, (Table 2).
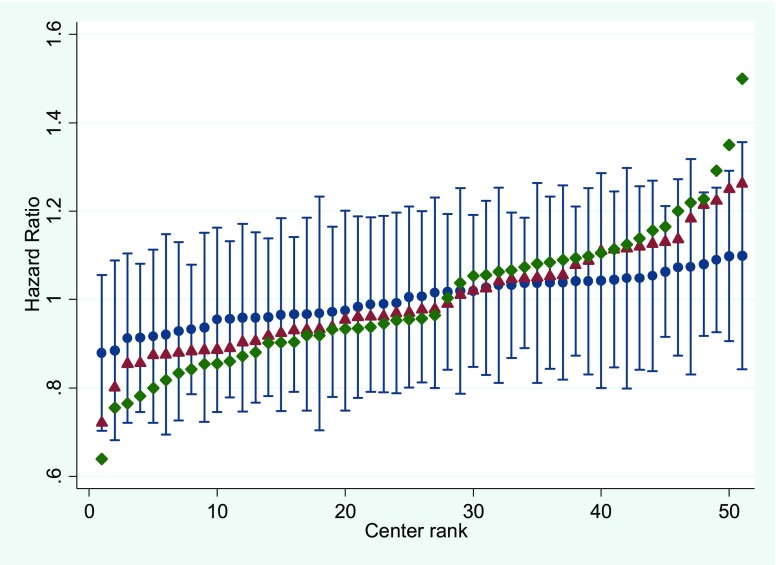
The variation in hazards of technique failure across centers was reduced by 28% after adjusting for patient-level characteristics and an additional 53% after adjusting for center-level characteristics (Figure 2).
Figure 2.
Variation in hazard of technique failure across 51 Australian peritoneal dialysis centers during the period of 2004–2014 in unadjusted (green diamonds), patient-level adjusted (red triangles), and patient- and center-level adjusted (blue circles) models with SEMs. Dialysis centers are ranked by hazard of technique failure.
Death-Censored Technique Failure
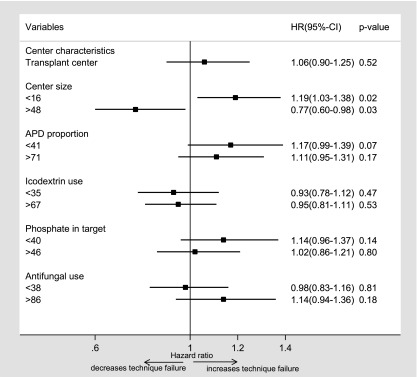
The median death-censored technique failure rate was 0.22 (95% CI, 0.21 to 0.23) episodes per patient-year. Death-censored technique failure was significantly more likely in smaller centers (<16; AHR, 1.19; 95% CI, 1.03 to 1.38) (Figure 3, Table 3) and less likely in centers with larger proportions of patients treated with PD (>29%; AHR, 0.83; 95% CI, 0.73 to 0.94) (Supplemental Table 1). None of the other center-level characteristics were significantly associated with death-censored technique failure.
Figure 3.
Forest plot showing the association between center-level characteristics and death-censored technique failure after adjusting for age, sex, race, body mass index, smoking status, primary renal disease, diabetes mellitus, cardiovascular disease, chronic lung disease, late nephrology referral, initial modality of peritoneal dialysis, initial modality of RRT, socioeconomic position, and era of peritoneal dialysis commencement. For each variable, the reference group is the middle category (combined second and third quartiles). APD, automated peritoneal dialysis; 95% CI, 95% confidence interval; HR, hazard ratio.
Table 3.
Multivariable Cox shared frailty models for death-censored technique failure defined as 30 and 180 days
| Covariates | Technique Failure 30 d | Technique Failure 180 d | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Era (2004–2009) | 1.0 | Reference | 1.0 | Reference | ||
| Era (2010–2014) | 0.93 | 0.86 to 0.99 | 0.04 | 0.82 | 0.75 to 0.89 | <0.001 |
| Patient-level characteristics | ||||||
| Age (decade) | 0.93 | 0.91 to 0.96 | <0.001 | 0.93 | 0.90 to 0.96 | <0.001 |
| Men | 1.07 | 0.98 to 1.14 | 0.06 | 1.12 | 1.04 to 1.22 | <0.01 |
| Race | <0.001 | <0.001 | ||||
| White | 1.00 | Reference | 1.00 | Reference | ||
| Asian | 0.79 | 0.70 to 0.89 | <0.001 | 0.80 | 0.70 to 0.92 | 0.002 |
| ATSI | 1.12 | 0.97 to 1.30 | 0.12 | 1.19 | 1.01 to 1.40 | 0.04 |
| MP | 0.85 | 0.69 to 1.03 | 0.10 | 0.86 | 0.68 to 1.08 | 0.19 |
| Other | 0.67 | 0.52 to 0.87 | 0.003 | 0.67 | 0.50 to 0.90 | <0.01 |
| BMI, kg/m2 | <0.001 | <0.001 | ||||
| <18.5 | 1.06 | 0.88 to 1.30 | 0.53 | 1.0 | 0.79 to 1.25 | 0.99 |
| 18.5–24.9 | 1.00 | Reference | 1.00 | Reference | ||
| 25–29.9 | 1.08 | 0.99 to 1.17 | 0.07 | 1.04 | 0.95 to 1.14 | 0.41 |
| ≥30 | 1.27 | 1.17 to 1.39 | <0.001 | 1.32 | 1.20 to 1.46 | <0.001 |
| Smoking status | 0.03 | 0.08 | ||||
| Nonsmoker | 1.00 | Reference | 1.00 | Reference | ||
| Current smoker | 1.09 | 0.98 to 1.21 | 0.10 | 1.08 | 0.96 to 1.22 | 0.19 |
| Former smoker | 1.10 | 1.02 to 1.19 | 0.01 | 1.10 | 1.00 to 1.20 | 0.03 |
| Diabetes mellitus | 0.98 | 0.87 to 1.10 | 0.73 | 1.01 | 0.89 to 1.16 | 0.87 |
| Cardiovascular disease | 1.12 | 1.04 to 1.21 | 0.003 | 1.08 | 0.99 to 1.18 | 0.07 |
| Chronic lung disease | 1.05 | 0.96 to 1.16 | 0.29 | 0.98 | 0.88 to 1.10 | 0.75 |
| Primary renal disease | <0.001 | 0.004 | ||||
| GN | 1.00 | Reference | 1.00 | Reference | ||
| Diabetes nephropathy | 0.98 | 0.86 to 1.11 | 0.73 | 0.96 | 0.82 to 1.11 | 0.54 |
| Hypertension | 0.83 | 0.74 to 0.94 | 0.002 | 0.86 | 0.75 to 0.98 | 0.02 |
| Polycystic kidney disease | 1.20 | 1.04 to 1.38 | 0.01 | 1.11 | 0.94 to 1.31 | 0.22 |
| Other/unknown | 0.86 | 0.78 to 0.95 | 0.003 | 0.85 | 0.76 to 0.95 | <0.01 |
| Late referral | 1.06 | 0.98 to 1.16 | 0.15 | 1.02 | 0.92 to 1.23 | 0.71 |
| Initial modality of RRT (PD)a | ||||||
| Overall | 0.69 | 0.63 to 0.76 | <0.001 | 0.70 | 0.62 to 0.79 | <0.001 |
| At 6 mo | 0.73 | 0.67 to 0.79 | <0.001 | 0.73 | 0.66 to 0.80 | <0.001 |
| At 1 yr | 0.76 | 0.70 to 0.82 | <0.001 | 0.76 | 0.70 to 0.83 | <0.001 |
| At 2 yr | 0.84 | 0.78 to 0.91 | <0.001 | 0.82 | 0.75 to 0.90 | <0.001 |
| Initial PD modality (CAPD) | 0.98 | 0.90 to 1.07 | 0.70 | 0.86 | 0.80 to 0.93 | <0.001 |
| IRSAD scoresb | 0.91 | 0.71 | ||||
| <934 | 1.00 | Reference | 1.00 | Reference | ||
| 934–983 | 1.02 | 0.93 to 1.12 | 0.66 | 1.03 | 0.93 to 1.15 | 0.52 |
| >983–1032 | 1.02 | 0.93 to 1.13 | 0.64 | 1.05 | 0.94 to 1.17 | 0.39 |
| >1032 | 0.99 | 0.90 to 1.09 | 0.90 | 0.99 | 0.88 to 1.11 | 0.91 |
| Center-level characteristics | ||||||
| Transplant center | 1.06 | 0.90 to 1.25 | 0.52 | 1.05 | 0.89 to 1.25 | 0.54 |
| Center size (incident patients per 1 yr) | 0.004 | <0.001 | ||||
| <16 | 1.19 | 1.03 to 1.38 | 0.02 | 1.23 | 1.07 to 1.43 | <0.01 |
| 16–48 | 1.00 | Reference | 1.00 | Reference | ||
| >48 | 0.77 | 0.60 to 0.98 | 0.03 | 0.79 | 0.63 to 0.99 | 0.04 |
| APD exposure,c % | 0.15 | 0.04 | ||||
| <41 | 1.17 | 0.99 to 1.39 | 0.07 | 1.14 | 0.97 to 1.35 | 0.11 |
| 41–71 | 1.00 | Reference | 1.00 | Reference | ||
| >71 | 1.11 | 0.95 to 1.31 | 0.17 | 1.22 | 1.04 to 1.42 | 0.01 |
| Icodextrin use,c % | 0.69 | 0.68 | ||||
| <35 | 0.93 | 0.78 to 1.12 | 0.47 | 0.94 | 0.79 to 1.22 | 0.50 |
| 35–67 | 1.00 | Reference | 1.00 | Reference | ||
| >67 | 0.95 | 0.81 to 1.11 | 0.53 | 0.94 | 0.81 to 1.10 | 0.46 |
| Phosphate in target,c % | 0.32 | 0.66 | ||||
| <40 | 1.14 | 0.96 to 1.37 | 0.14 | 1.08 | 0.91 to 1.29 | 0.37 |
| 40–46 | 1.00 | Reference | 1.00 | Reference | ||
| >46 | 1.02 | 0.86 to 1.21 | 0.80 | 1.05 | 0.89 to 1.24 | 0.60 |
| Antifungal use,d % | 0.32 | 0.47 | ||||
| <38 | 0.98 | 0.83 to 1.16 | 0.81 | 0.95 | 0.80 to 1.11 | 0.50 |
| 38–86 | 1.00 | Reference | 1.00 | Reference | ||
| >86 | 1.14 | 0.94 to 1.36 | 0.18 | 1.07 | 0.90 to 1.29 | 0.44 |
HR, hazard ratio; 95% CI, 95% confidence interval; ATSI, Aboriginal and Torres Strait Islander; MP, Maori–Pacific Islanders; BMI, body mass index; PD, peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; IRSAD, Index of Relative Socioeconomic Advantage and Disadvantage; APD, automated peritoneal dialysis.
There was a significant interaction between initial modality of RRT and time (P<0.001). The overall HR and HR at three time points are given.
Socioeconomic position reported as IRSAD scores, with higher scores reflecting higher socioeconomic position.
Percentage of all participating patients in the center.
Percentage of peritonitis in center.
When death-censored technique failure was analyzed using the 180-day definition, similar results were observed, except that centers with higher APD use were associated with a higher hazard of technique failure (Table 3).
The variation in hazards of death-censored technique failure across centers was reduced by 15% after adjusting for patient-level characteristics and an additional 37% after adjusting for center-level characteristics (Supplemental Figure 1).
Cause-Specific Death-Censored Technique Failure
The causes of technique failure were infection (n=1577; 27%), social (n=502; 9%), inadequate dialysis (n=709; 12%), mechanical (n=748; 13%), death (n=1972; 34%), and other reasons (n=305; 5%).
Center size and proportion of patients on PD were significantly associated with technique failure due to social and mechanical causes (Supplemental Table 2). Poorer center achievement of target phosphate was associated with technique failure due to infection, social reasons (Supplemental Table 2), and death (Supplemental Table 3). Lower center APD exposure was associated with technique failure due to infection, whereas higher exposure was associated with technique failure due to social reasons. Higher center icodextrin use was associated with lower technique failure due to social reasons. Centers with higher or lower antifungal use were associated with higher hazards of infection-related technique failure compared with centers with average antifungal use (Supplemental Table 2).
Technique Failure over Time
There was a significant improvement in technique failure (AHR, 0.87; 95% CI, 0.82 to 0.93) (Table 2) and death-censored technique failure (AHR, 0.93; 95% CI, 0.86 to 0.99) (Table 3) between the periods 2004–2009 and 2010–2014. Similar findings were observed using the 180-day definition for technique failure (AHR, 0.80; 95% CI, 0.75 to 0.85) and death-censored technique failure (AHR, 0.82; 95% CI, 0.75 to 0.89). From 2010, the hazards of technique failure due to infection (AHR, 0.64; 95% CI, 0.57 to 0.72) and death (AHR, 0.78; 95% CI, 0.70 to 0.87) decreased, whereas the hazards of technique failure due to social reasons (AHR, 1.22; 95% CI, 1.00 to 1.47) increased.
Discussion
This study showed that technique failure varied considerably across PD centers, and although some of this variation was related to patient-level factors, a considerable proportion was related to modifiable center-level factors, particularly center size and the center’s proportion of patients treated with PD, and proportion of patients with achieved baseline phosphate within target level. Similar results were observed for death-censored technique failure, except that the factor of centers with proportion of patients with achieved target phosphate level was no longer significant. Technique failure due to infective, social, or mechanical causes or death was variably associated with center size, proportion of patients on PD, proportion of patients achieving target phosphate level, APD use, icodextrin use, and antifungal use.
These findings are in keeping with those of previous studies that have reported smaller center size as a risk factor for death-censored technique failure in patients on PD (4–6,8,13).Using registry data from The Netherlands, Huisman et al. (4) reported that smaller centers (defined as centers with <20 patients on PD) were associated with higher rates of death-censored technique failure. Similarly, using data from 17,900 patients on PD recorded in the Canadian Organ Replacement Register between 1981 and 1997, Schaubel et al. (5) reported that higher cumulative numbers of patients on PD in centers were associated with better technique survival. Afolalu et al. (6) observed that death-censored technique failure rate was higher in United States centers with ≤25 patients on PD than those with >25 patients on PD. Recently, Guillouet et al. (7) also observed that greater center experience with PD, defined as more than ten new patients per year, was associated with a lower risk of early PD failure. In a systematic review and meta-analysis of ten nonexperimental studies evaluating the association between center volume and patient outcomes, Pieper et al. (8) reported that higher center volume was associated with a lower risk of technique failure, with a median relative effect measure of 0.73 (0.25–0.94).
In addition to the observation that smaller centers (and hence, smaller patient with PD exposure) were associated with technique failure, this study also found that centers that treated proportionally more of their patients on dialysis with PD as opposed to HD were likely to have lower technique failure rates. A similar finding was reported in Canadian patients (5).
Taken together, these results suggest that less cumulative PD experience and a lesser degree of PD specialization adversely affect PD technique survival. In contrast to previous investigations, this study took another step by analyzing the relationship between these center characteristics and cause-specific technique failure. In particular, smaller center size was associated with higher hazards of technique failure related to social and mechanical causes. This finding may reflect center inexperience with managing PD-related complications, such as poor catheter flow or catheter malfunction, leading to a higher rate of transferring patients on PD to HD after such complications are encountered due to suboptimal management of the complications and/or a tendency to give up more quickly on PD when problems arise. Conversely, centers with higher (>29%) proportions of their patients on dialysis treated with PD were found to be less likely to experience technique failure due to social and mechanical causes.
Another interesting finding was the association between higher center icodextrin use and lower social technique failure. This may have been related to the fact that icodextrin use has been associated with improved quality of life (14) and fluid balance (15) compared with conventional dialysis solution, thereby potentially facilitating patient motivation to persist with PD.
Poorer center achievement of serum phosphate targets was also associated with technique failure due to social reasons, infection, and death. This finding may be explained by complications of hyperphosphatemia (e.g., pruritus, vascular calcification, and cardiovascular disease) (16,17) or a type 1 statistical error (chance finding). Alternatively, poor attainment of serum phosphate targets may reflect less rigorous overall center care or adherence to guidelines.
The study also showed that infection-related technique failure was associated with high or low center antifungal prophylaxis use and low APD use. These center characteristics have been reported to be associated with higher risks of peritonitis in a previous ANZDATA Registry study (18). Low antifungal use could be a surrogate marker of poorer general compliance with peritonitis prevention guideline recommendations, whereas high antifungal use might reflect indication bias (i.e., centers with an infection problem may be more inclined to prescribe antifungal prophylaxis). The fewer connections with APD compared with continuous ambulatory PD may have reduced opportunity for contamination and mitigated infection risk, although the literature is conflicted regarding APD and infection risk (19).
Perhaps the most important finding of this study was that it provided important evidence that modifiable center practices (center effects) seemed to account for an appreciable portion of observed center variation in technique failure rates beyond that conferred by patient-level characteristics. Significantly, the variation in hazard of technique failure across centers was reduced by 28% after adjusting for patient-level factors and an additional 53% after adding center-level factors.
It is also important to note that the overall risk of technique failure in Australia was significantly reduced after 2009 (20–22), which was mainly related to a decrease in technique failure due to infection and death. This improvement probably reflected the effectiveness of systematic, coordinated, national peritonitis prevention programs instituted since 2009.
This is one of the largest and most comprehensive studies to date examining the relationship between center effects and overall, death-censored, and cause-specific technique failure in patients on incident PD. It included all PD centers in Australia, thereby mitigating ascertainment bias. It also used robust statistical methodologies, allowing multivariable adjustment of both patient- and center-level characteristics and thereby, limiting residual confounding on a center-level basis.
The strengths of this study should be balanced against its limitations, which include the possibility of residual confounding as well as selection, reporting, and coding biases. The limited data collected by the ANZDATA Registry prevented inclusion and analysis of important center-level information (e.g., nurse and physician staffing levels, patient and staff training protocols, clinical pathways, exit site care, infection control processes, frequency of PD multidisciplinary governance meetings, etc.) as well as patient-level information (compliance, level of education, employment status, severity of comorbidities, distance from PD center, assisted PD, climatic factors, residual renal function, etc.). Any changes in dialysis center by patients on PD were not considered. Finally, the results of this Australian study may not be generalizable to other countries.
In conclusion, this study showed important variation in technique failure rates between centers in Australia and evidence that an appreciable proportion of this variation may have been related to modifiable center effects. Specifically, smaller center size, a lower proportion of patients treated with PD, and a lower proportion of patients achieving target serum phosphate levels were independently associated with technique failure and cause-specific technique failure (particularly related to infection, social and mechanical causes, and death). It is possible that addressing these center effects (e.g., by amalgamating and/or centralizing smaller PD units, directing more patients on incident RRT to PD, or optimizing infection control practices within centers in line with International Society for Peritoneal Dialysis (ISPD) recommendations) may extend the length of time that patients spend on PD. Future prospective studies collecting more detailed center- and patient-level data, such as the international Peritoneal Dialysis Outcomes and Practice Patterns Study, should endeavor to identify which modifiable PD center characteristics (e.g., center size, PD proportion, nurse and physician staffing levels, PD training and retraining protocols, infection control protocols and practices, quality improvement activities, etc.) are most strongly associated with superior technique survival.
Disclosures
Y.C. has received research grants from Fresenius Medical Care. C.H. has previously received research grants and travel sponsorships from Baxter Healthcare and Fresenius Medical Care. K.S. has received speaker’s honoraria from Baxter Healthcare, Roche, Amgen, and Boehringer Ingelheim and conference or meeting sponsorships from Shire, Roche, Boehringer Ingelheim, Amgen, Sanofi, and Novartis. D.W.J. has received consultancy fees, research grants, speaker’s honoraria, and travel sponsorships for Baxter Healthcare and Fresenius Medical Care. All other authors have no conflict of interest to declare.
Supplementary Material
Acknowledgments
The authors acknowledge the substantial contributions of the entire Australia and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients) in providing information for and maintaining the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry database. The data reported here have been supplied by the ANZDATA Registry.
Y.C. is supported by a National Health and Medical Research Council Early Career Fellowship. D.W.J. is supported by a National Health and Medical Research Council Practitioner Fellowship.
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the ANZDATA Registry.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Are Peritoneal Dialysis Center Characteristics a Modifiable Risk Factor to Improve Peritoneal Dialysis Outcomes?,” on pages 1032–1034.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12321216/-/DCSupplemental.
References
- 1.Chaudhary K: Peritoneal dialysis drop-out: Causes and prevention strategies. Int J Nephrol 2011: 434608, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perl J, Davies SJ, Lambie M, Pisoni RL, McCullough K, Johnson DW, Sloand JA, Prichard S, Kawanishi H, Tentori F, Robinson BM: The peritoneal dialysis outcomes and practice patterns study (PDOPPS): Unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int 36: 297–307, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrotra R, Devuyst O, Davies SJ, Johnson DW: The current state of peritoneal dialysis. J Am Soc Nephrol 27: 3238–3252, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huisman RM, Nieuwenhuizen MG, Th de Charro F: Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in The Netherlands. Nephrol Dial Transplant 17: 1655–1660, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Schaubel DE, Blake PG, Fenton SS: Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 60: 1517–1524, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO: Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 29: 292–296, 2009 [PubMed] [Google Scholar]
- 7.Guillouët S, Veniez G, Verger C, Béchade C, Ficheux M, Uteza J, Lobbedez T: Estimation of the center effect on early peritoneal dialysis failure: A multilevel modelling approach. Perit Dial Int 36: 519–525, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieper D, Mathes T, Marshall MR: A systematic review of the impact of center volume in dialysis. BMC Res Notes 8: 812, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald SP: Australia and New Zealand dialysis and transplant registry. Kidney Int Suppl (2011) 5: 39–44, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socio-Economic Indexes for Areas (SEIFA), 2011: Available at: http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa. Accessed October 16, 2016
- 11.Lan PG, Clayton PA, Johnson DW, McDonald SP, Borlace M, Sud K, Boudville N: Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: Proposal for a standardized definition of technique failure. Perit Dial Int 36: 623–630, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC, Lee DS, Fine JP: Introduction to the analysis of survival data in the presence of competing Risks. Circulation 133: 601–609, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans D, Lobbedez T, Verger C, Flahault A: Would increasing centre volumes improve patient outcomes in peritoneal dialysis? A registry-based cohort and Monte Carlo simulation study. BMJ Open 3: e003092, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo A, Wolfson M, Holt R: Early quality of life benefits of icodextrin in peritoneal dialysis. Kidney Int Suppl 62: S72–S79, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimbürger O, Simonsen O, Davenport A, Tranaeus A, Divino Filho JC: Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338–2344, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr ., Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM: Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ Res 109: 697–711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadeau-Fredette A-C, Johnson DW, Hawley CM, Pascoe EM, Cho Y, Clayton PA, Borlace M, Badve SV, Sud K, Boudville N, McDonald SP: Center-specific factors associated with peritonitis risk-A multi-center registry analysis. Perit Dial Int 36: 509–518, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, Struijk DG, Teitelbaum I, Johnson DW: ISPD peritonitis recommendations: 2016 Update on prevention and treatment. Perit Dial Int 36: 481–508, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jose MD, Johnson DW, Mudge DW, Tranaeus A, Voss D, Walker R, Bannister KM: Peritoneal dialysis practice in Australia and New Zealand: A call to action. Nephrology (Carlton) 16: 19–29, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Mudge DW, Boudville N, Brown F, Clayton P, Duddington M, Holt S, Johnson DW, Jose M, Saweirs W, Sud K, Voss D, Walker R: Peritoneal dialysis practice in Australia and New Zealand: A call to sustain the action. Nephrology (Carlton) 21: 535–546, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Cho Y, Johnson DW: Peritoneal dialysis-related peritonitis: Towards improving evidence, practices, and outcomes. Am J Kidney Dis 64: 278–289, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.