Abstract
Background and objectives
There are inconsistent reports on the association of dietary protein intake with serum albumin and outcomes among patients on hemodialysis. Using a new normalized protein catabolic rate (nPCR) variable accounting for residual renal urea clearance, we hypothesized that higher baseline nPCR and rise in nPCR would be associated with higher serum albumin and better survival among incident hemodialysis patients.
Design, setting, participants, & measurements
Among 36,757 incident hemodialysis patients in a large United States dialysis organization, we examined baseline and change in renal urea clearance–corrected nPCR as a protein intake surrogate and modeled their associations with serum albumin and mortality over 5 years (1/2007–12/2011).
Results
Median nPCRs with and without accounting for renal urea clearance at baseline were 0.94 and 0.78 g/kg per day, respectively (median within-patient difference, 0.14 [interquartile range, 0.07–0.23] g/kg per day). During a median follow-up period of 1.4 years, 8481 deaths were observed. Baseline renal urea clearance–corrected nPCR was associated with higher serum albumin and lower mortality in the fully adjusted model (Ptrend<0.001). Among 13,895 patients with available data, greater rise in renal urea clearance–corrected nPCR during the first 6 months was also associated with attaining high serum albumin (≥3.8 g/dl) and lower mortality (Ptrend<0.001); compared with the reference group (a change of 0.1–0.2 g/kg per day), odds and hazard ratios were 0.53 (95% confidence interval, 0.44 to 0.63) and 1.32 (95% confidence interval, 1.14 to 1.54), respectively, among patients with a change of <−0.2 g/kg per day and 1.62 (95% confidence interval, 1.35 to 1.96) and 0.76 (95% confidence interval, 0.64 to 0.90), respectively, among those with a change of ≥0.5 g/kg per day. Within a given category of nPCR without accounting for renal urea clearance, higher levels of renal urea clearance–corrected nPCR consistently showed lower mortality risk.
Conclusions
Among incident hemodialysis patients, higher dietary protein intake represented by nPCR and its changes over time appear to be associated with increased serum albumin levels and greater survival. nPCR may be underestimated when not accounting for renal urea clearance. Compared with the conventional nPCR, renal urea clearance–corrected nPCR may be a better marker of mortality.
Keywords: Dietary protein intake (DPI), residual kidney function, mortality, hemodialysis, albumin, protein catabolic rate (PCR), Dietary Proteins, Fluid Therapy, Follow-Up Studies, Humans, Odds Ratio, Proportional Hazards Models, renal dialysis, Serum Albumin, urea, Urinary Tract Physiological Phenomena
Introduction
Protein-energy wasting (PEW), manifested by inadequate dietary protein intake or low serum albumin, is a common condition and a predictor of mortality in patients on dialysis (1–4). PEW is characterized by simultaneous loss of body protein and energy stores among patients with advanced CKD (5–7). Among patients on dialysis, the protein requirement is higher than in healthy adults because of the removal of amino acid by the dialysis treatment and the protein catabolic or antianabolic state caused by the uremia, inflammation, oxidative stress, and exposure to bioincompatible dialysis materials (8). The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines recommend a dietary protein intake of 1.2 g protein per 1 kg of ideal body wt per day for patients on maintenance hemodialysis (9). However, the role of dietary protein intake on serum albumin levels remains controversial on the basis of current published data mostly from cross-sectional studies (10,11).
The urea kinetic–based protein catabolic rate, which is usually normalized for body weight (normalized protein catabolic rate [nPCR]), is often interpreted as a measure of dietary protein intake. During steady-state conditions, protein intake is equal to or slightly greater than nPCR (9). Although serum albumin is a strong predictor of mortality among incident and prevalent hemodialysis patients (2,12,13), the mortality predictability of nPCR in patients on hemodialysis remains controversial. A previous study of patients on hemodialysis showed that there was no association between mortality and either baseline or 6-month follow-up measurements of nPCR (14), whereas other studies have shown an increased risk of death among patients with low levels or decreasing trend in nPCR (15–18).
These inconsistent results may be partly due to the fact that previous studies calculated nPCR without accounting for the contribution of residual renal urea clearance (rCLurea) (19,20). In addition, most studies evaluated the association between nPCR and serum albumin in a cross-sectional manner. We, therefore, hypothesized that nPCR accounting for rCLurea (i.e., rCLurea-corrected nPCR) provides better mortality risk stratification among incident hemodialysis patients while retaining its association with better survival and that a rise in nPCR, not baseline nPCR, is associated with attaining higher serum albumin.
Materials and Methods
Patients
We retrospectively analyzed clinical data from all incident in-center hemodialysis patients ages ≥18 years old treated in facilities operated by a large United States dialysis organization from January 1, 2007 to December 31, 2011 (21). Patient follow-up was divided into 20 consecutive patient-quarters representing 91-day intervals from the start of dialysis. Among 208,820 incident dialysis patients, we identified 133,134 patients who were treated only with conventional hemodialysis. We excluded patients with missing data on serum albumin, single-pool Kt/V (spKt/V), and rCLurea at baseline and those with multiple measurements of BUN on the same day, resulting in a cohort of 36,757 patients for evaluation of the association between baseline nPCR and mortality (Supplemental Figure 1). Differences in baseline characteristics among included versus excluded patients are shown in Supplemental Table 1. We further restricted to 13,895 patients with available data on serum albumin, spKt/V, and rCLurea at the third patient-quarter to evaluate the association of change in nPCR during the first 6 months of dialysis (i.e., from the first quarter to the third quarter) with attained serum albumin ≥3.8 g/dl and mortality risk. The study was approved by the Institutional Review Committees of the University of California, Irvine and Los Angeles Biomedical Research Institute at Harbor–University of California, Los Angeles, with the exemption of obtaining written consent given the large sample size, anonymity of the patients studied, and nonintrusive nature of the research.
Demographic, Clinical, and Laboratory Measures
Information on demographics, cause of ESRD, access type, presence of comorbidities, and death was obtained from the large dialysis organization’s database. Most blood samples were collected before dialysis. Dialysis dose was estimated by spKt/V using the urea kinetic model. Serum albumin was assayed using the bromocresol green method throughout the study period. The average serum urea concentrations during the urine collections were assumed to be 90% of the predialysis concentrations according to the approach by Daugirdas (22), and rCLurea was calculated as described with adjustment for body surface area (23,24) as presented in Supplemental Material. As an indicator of daily protein intake, nPCR was calculated accounting for rCLurea (i.e., rCLurea-corrected nPCR) and expressed as nPCRdial+renal. In contrast, nPCR calculated without accounting for rCLurea (i.e., non-rCLurea–corrected nPCR), as commonly used in current clinical practice, is expressed as nPCRdial. To account for varying frequencies of dialysis treatments per week (two versus three times), the correction component for the predialysis BUN (C0) for nPCRdial+renal and nPCRdial equations is used as shown in Supplemental Material. To minimize measurement variability, all repeated measures for each patient during any given patient-quarter were averaged. The averaged values during the first patient-quarter were used as baseline data.
Statistical Analyses
To examine differences between nPCRdial and nPCRdial+renal levels, we used the Bland–Altman plot and summarized the differences by rCLurea strata (<1.5, 1.5 to <3.0, 3.0 to <4.5, and ≥4.5 ml/min per 1.73 m2). Baseline characteristics between the high and low baseline nPCRdial+renal groups (<1.2 and ≥1.2 ml/min per 1.73 m2, respectively) were summarized as proportions, means (±SD), or medians (interquartile ranges [IQRs]). Given the relatively large sample size of this study, baseline characteristics were compared by standardized differences, of which 0.2, 0.5, and 0.8 were considered small, medium, and large differences, respectively, and ≥0.1 was defined as a meaningful imbalance (25). Ten categories of baseline nPCRdial+renal were created: <0.6 and ≥1.4 g/kg per day and eight incremental categories of 0.1 g/kg per day in between. Likewise, change in nPCRdial+renal from the first quarter to the third quarter of dialysis was calculated, and nine categories were created as follows: <−0.2 and ≥0.5 g/kg per day and seven incremental categories of 0.1 g/kg per day in between.
Logistic regression models were used to estimate associations between baseline nPCRdial+renal and having baseline serum albumin ≥3.8 g/dl and between change in nPCRdial+renal and attained serum albumin ≥3.8 g/dl at the third patient-quarter. The Cox proportional hazards model was used to estimate the associations between the exposure categories and all-cause mortality. The hazard proportionality was confirmed by the Schoenfeld residuals plot. Both baseline nPCRdial+renal and change in nPCRdial+renal were also modeled as continuous variables, and their relationship with outcomes (mortality or attained serum albumin level ≥3.8 g/dl at the third patient-quarter) was estimated using restricted cubic spline functions with four knots at the fifth, 35th, 65th, and 95th percentiles of each index and referent at the median of nPCRdial+renal. For each analysis, associations were adjusted by using three levels of hierarchical models: (1) unadjusted model; (2) case mix–adjusted models that included age, sex, race/ethnicity, diabetes mellitus, vascular access type, spKt/V, and rCLurea at baseline as well as the nine preexisting comorbidities listed in Table 1; and (3) case mix– and malnutrition-inflammation cachexia syndrome (MICS)–adjusted models that included all above-mentioned covariates plus surrogates of nutritional and inflammatory status, including body mass index and 11 laboratory variables listed in Table 1. For models evaluating changes in nPCR, adjustment for baseline nPCR was added to all models. Missing data in covariates (<1% for most laboratory variables and 4% for serum creatinine) were imputed by the mean or median of the existing values as appropriate. All analyses were carried out using STATA MP, version 13.1 (StataCorp, College Station, TX).
Table 1.
Baseline characteristics of 36,757 patients on incident hemodialysis according to renal CLurea-corrected nPCR level
| Variable | Total, n=36,757 | renal CLurea-corrected nPCR at baseline | Std Diff. | |
|---|---|---|---|---|
| <1.2, n=29,685 | ≥1.2, n=7072 | |||
| Baseline nPCR, g/kg per day | 0.94 (0.77–1.14) | 0.87 (0.73–1.01) | 1.35 (1.27–1.50) | 2.95 |
| Age, yr | 62±15 | 62±15 | 61±14 | 0.08 |
| Women, % | 37 | 39 | 29 | 0.21 |
| Race/ethnicity, % | ||||
| Non-Hispanic white | 54 | 54 | 54 | 0.01 |
| Black | 28 | 30 | 19 | 0.27 |
| Hispanic | 11 | 10 | 16 | 0.20 |
| Asian | 3 | 3 | 7 | 0.19 |
| Others | 3 | 3 | 5 | 0.07 |
| ESRD reason, % | ||||
| Diabetes | 47 | 46 | 48 | 0.04 |
| Hypertension | 28 | 29 | 26 | 0.05 |
| GN | 10 | 10 | 11 | 0.03 |
| Cystic kidney disease | 2 | 2 | 2 | 0.04 |
| Others | 13 | 13 | 13 | 0.02 |
| Access type, % | ||||
| Central venous catheter | 74 | 75 | 71 | 0.09 |
| AV fistula | 18 | 17 | 22 | 0.12 |
| AV graft | 4 | 4 | 3 | 0.04 |
| AV other | <0.1 | <0.1 | <0.1 | 0.00 |
| Unknown | 4 | 4 | 4 | 0.00 |
| Comorbidity, % | ||||
| Hypertension | 50 | 51 | 48 | 0.06 |
| Atherosclerotic heart disease | 14 | 14 | 14 | 0.01 |
| Congestive heart failure | 38 | 38 | 37 | 0.01 |
| Cerebrovascular disease | 2 | 2 | 1 | 0.04 |
| Other cardiovascular disease | 15 | 16 | 14 | 0.03 |
| History of cancer | 2 | 3 | 2 | 0.02 |
| HIV | 0.4 | 0.4 | 0.4 | 0.02 |
| COPD | 5 | 5 | 4 | 0.04 |
| Dyslipidemia | 26 | 26 | 27 | 0.02 |
| spKt/V | 1.55±0.36 | 1.53±0.35 | 1.66±0.39 | 0.37 |
| Body mass index, kg/m2 | 27.4 (23.6–32.7) | 27.5 (23.7–32.9) | 26.9 (23.4–31.9) | 0.08 |
| Renal CLurea, ml/min per 1.73 m2 | 2.92 (1.54–4.76) | 2.62 (1.36–4.30) | 4.38 (2.74–6.42) | 0.70 |
| Laboratories | ||||
| WBC, 1000/μl | 7.5 (6.1–9.0) | 7.5 (6.1–9.0) | 7.5 (6.2–9.0) | 0.03 |
| Lymphocyte, % | 21±7 | 21±7 | 20±7 | 0.09 |
| Hemoglobin, g/dl | 11.2±1.1 | 11.2±1.1 | 11.4±1.1 | 0.21 |
| Albumin, g/dl | 3.57±0.46 | 3.54±0.46 | 3.69±0.41 | 0.34 |
| Corrected calcium, mg/dl | 9.1±0.5 | 9.1±0.5 | 9.0±0.6 | 0.14 |
| Phosphorus, mg/dl | 5.0±1.1 | 4.9±1.1 | 5.3±1.2 | 0.34 |
| Intact PTH, pg/ml | 314 (202–479) | 310 (199–478) | 328 (216–482) | 0.07 |
| ALP, IU/L | 84 (67–110) | 85 (68–111) | 81 (64–105) | 0.15 |
| Creatinine, mg/dl | 5.9±2.4 | 5.8±2.3 | 6.1±2.4 | 0.14 |
| TIBC, mg/dl | 230 (201–260) | 227 (198–257) | 241 (214–271) | 0.35 |
| Transferrin saturation, % | 23±8 | 23±8 | 23±8 | 0.11 |
| Ferritin, ng/ml | 268 (157–451) | 269 (157–453) | 268 (156–445) | 0.01 |
| Bicarbonate, mEq/L | 23.5±2.6 | 23.7±2.6 | 22.7±2.6 | 0.37 |
Continuous values are expressed as mean±SD if normally distributed or median (interquartile range) if skewed. Differences in patient characteristics between two groups were compared by Std Diff., of which 0.8, 0.5, and 0.2 were considered large, medium, and small differences, respectively, and ≥0.1 was defined as meaningful imbalance. nPCRdial+renal, normalized protein catabolic ratedialysis+renal; Std Diff., standardized difference; nPCR, normalized protein catabolic rate; AV, arteriovenous; COPD, chronic obstructive pulmonary disease; spKt/V, single-pool Kt/V; CLurea, urea clearance; WBC, white blood cell; PTH, parathyroid hormone; ALP, alkaline phosphatase; TIBC, total iron binding capacity.
Results
Differences between Baseline nPCR Values with and without Accounting for rCLurea
Median values (IQR) of nPCRdial and nPCRdial+renal at baseline were 0.78 (0.65–0.94) and 0.94 (0.77–1.14) g/kg per day, respectively. The median (IQR) difference between these two indices was 0.14 (0.07–0.23) g/kg per day. There was a trend toward larger differences between these two indices across higher averaged values of nPCRdial and nPCRdial+renal (Figure 1A). Patients with greater rCLurea had higher nPCRdial+renal; among patients with rCLurea<1.5, 1.5 to <3.0, 3.0 to <4.5, and ≥4.5 ml/min per 1.73 m2, nPCRdial+renal showed consistently higher values with median (IQR) values of 0.81 (0.67–0.98), 0.91 (0.76–1.08), 0.98 (0.82–1.16), and 1.07 (0.89–1.28), respectively, while median (IQR) values of nPCRdial were 0.77 (0.64–0.93), 0.79 (0.66–0.94), 0.80 (0.67–0.94), and 0.78 (0.65–0.94) g/kg per day, respectively. Differences between nPCRdial and nPCRdial+renal were significantly larger across higher rCLurea categories: 0.04 (95% confidence interval [95% CI], 0.01 to 0.06), 0.11 (95% CI, 0.09 to 0.14), 0.18 (95% CI, 0.14 to 0.22), and 0.28 (95% CI, 0.21 to 0.36) g/kg per day, respectively (Pinteraction<0.001) (Figure 1B).
Figure 1.
Normalized protein catabolic rate (nPCR) was substantially underestimated among patients with residual kidney function when renal urea clearance (rCLurea) was not accounted for. Comparison between baseline rCLurea-corrected nPCR (nPCRdial) and baseline non-rCLurea–corrected nPCR (nPCRdial+renal) in (A) the Bland–Altman plot and (B) the box plot according to rCLurea categories.
Baseline Demographic, Clinical, and Laboratory Characteristics according to Baseline rCLurea-Corrected nPCR
Among 36,757 patients, the mean±SD age was 62±15 years old, 37% were women, 54% were non-Hispanic white, 28% were black, and 47% had diabetes as the cause of ESRD (Table 1). Patients were dichotomized on the basis of baseline nPCRdial+renal <1.2 g/kg per day (n=29,685; 81%) versus ≥1.2 g/kg per day (n=7072; 19%). The latter nPCR range is the recommended target range set forth by the KDOQI clinical practice guidelines (8). Compared with patients with nPCRdial+renal<1.2 g/kg per day at baseline, those who had greater nPCRdial+renal (≥1.2 g/kg per day) were more likely to be men and Hispanic or Asian and less likely to be black; were more likely to use arteriovenous fistula; had higher spKt/V, rCLurea, hemoglobin, albumin, phosphorus, creatinine, and total iron binding capacity; and had lower serum calcium, alkaline phosphatase, and bicarbonate.
Association of Baseline rCLurea-Corrected nPCR with Serum Albumin Levels and All-Cause Mortality
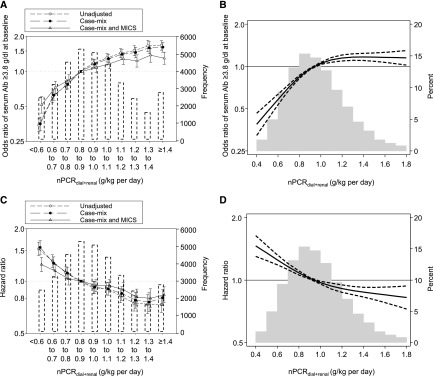
A total of 8481 deaths were observed during the follow-up period (up to 5 years; median [IQR] =1.4 [0.7–2.5] years), with a mortality rate of 13.5/100 patient-yr. There was an unadjusted trend toward higher baseline serum albumin across higher baseline nPCRdial+renal categories (Table 2), which were robust against adjustments (Ptrend<0.001 for all models) (Figure 2A, Supplemental Table 2). Additionally, there was a consistent trend toward lower mortality risk across higher nPCRdial+renal irrespective of adjustment model, albeit with an attenuation in the fully adjusted model (Ptrend<0.001 for all models) (Table 2, Figure 2C, Supplemental Table 3). Compared with the reference group (0.8–0.9 g/kg per day), baseline nPCRdial+renal <0.7 g/kg per day was significantly associated with higher mortality; fully adjusted hazard ratio (HRs) were 1.25 (95% CI, 1.14 to 1.37) and 1.14 (95% CI, 1.05 to 1.24) for nPCRdial+renal<0.6 and 0.6 to <0.7 g/kg per day, respectively. Patients with nPCRdial+renal≥1.1 g/kg per day had a lower mortality risk compared with the referent: fully adjusted HRs, 0.89 (95% CI, 0.81 to 0.98), 0.81 (95% CI, 0.73 to 0.90), 0.79 (95% CI, 0.69 to 0.89), and 0.83 (95% CI, 0.75 to 0.92) for nPCRdial+renal=1.1–1.2, 1.2–1.3, 1.3–1.4, and ≥1.4 g/kg per day, respectively. Consistent results were observed in the models using restricted cubic spline functions (Figure 2B and D).
Table 2.
Categories of baseline renal urea clearance-corrected normalized protein catabolic rate (nPCRdial+renal) in 36,757 incident patients
| Baseline nPCRdial+renal, g/kg per day | Group Size, n (% of Total) | Mean±SD Baseline Serum Albumin, g/dl | All-Cause Death per 100 patient-yr (n) |
|---|---|---|---|
| <0.6 | 2571 (7) | 3.24±0.54 | 20.8 (774) |
| 0.6 to <0.7 | 3384 (9) | 3.42±0.48 | 17.9 (959) |
| 0.7 to <0.8 | 4757 (13) | 3.50±0.45 | 15.4 (1198) |
| 0.8 to <0.9 | 5558 (15) | 3.56±0.44 | 13.8 (1336) |
| 0.9 to <1.0 | 5337 (15) | 3.61±0.42 | 12.7 (1191) |
| 1.0 to <1.1 | 4594 (13) | 3.64±0.42 | 12.6 (1011) |
| 1.1 to <1.2 | 3484 (9) | 3.65±0.42 | 11.4 (717) |
| 1.2 to <1.3 | 2521 (7) | 3.68±0.41 | 10.4 (479) |
| 1.3 to <1.4 | 1660 (5) | 3.69±0.41 | 10.2 (305) |
| ≥1.4 | 2891 (8) | 3.69±0.42 | 10.1 (511) |
| Total | 36,757 (100) | 3.57±0.46 | 13.5 (8481) |
Figure 2.
Higher baseline renal urea clearance-corrected normalized protein catabolic rate (nPCRdial+renal) levels were associated with higher likelihood of having baseline serum albumin (Alb) ≥3.8 g/dl and lower mortality among 36,757 incident hemodialysis patients. Likelihood of having baseline serum Alb ≥3.8 g/dl using (A) baseline nPCRdial+renal categories with three-level hierarchical adjustment models and (B) restricted cubic splines in the fully adjusted model. Association between baseline nPCRdial+renal and mortality using (C) baseline nPCRdial+renal categories with three-level hierarchical adjustment models, and (D) restricted cubic splines in the fully adjusted model. The histograms in (A) and (C) show the number of patients in each category. (B) and (D) were truncated at the first and 99th percentiles of data. MICS, malnutrition-inflammation cachexia syndrome.
Association of Change in rCLurea-Corrected nPCR with Serum Albumin Level and All-Cause Mortality
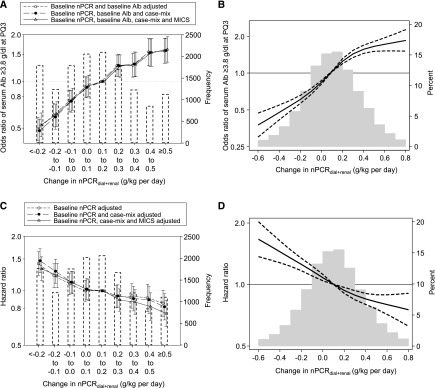
Among 13,895 patients, patients with a greater rise in nPCRdial+renal had lower baseline nPCRdial+renal and serum albumin (Table 3). There was a consistent trend toward higher likelihood of having serum albumin ≥3.8 g/dl at the third patient-quarter across greater rise in nPCRdial+renal categories, irrespective of adjustment model and without attenuation (Ptrend<0.001 for all models) (Figure 3A, Supplemental Table 4). Consistent results were also observed in models using restricted cubic spline functions (Figure 3B). Similarly, greater rise in nPCRdial+renal was also associated with higher likelihood of an increase in serum albumin ≥0.2 g/dl per 6 months (Supplemental Figure 2).
Table 3.
Categories of change in renal urea clearance-corrected normalized protein catabolic rate (nPCRdial+renal) during the first 6 months in 13,895 incident hemodialysis patients
| Change in nPCRdial+renal, g/kg per day | Group Size (% of Total) | Mean±SD Baseline nPCRdial+renal, g/kg per day | Mean±SD Baseline Serum Albumin, g/dl | All-Cause Death, per 100 patient-yr (n) |
|---|---|---|---|---|
| <−0.2 | 1802 (13) | 1.32±0.31 | 3.67±0.42 | 12.8 (369) |
| −0.2 to <−0.1 | 1235 (9) | 1.10±0.25 | 3.65±0.44 | 13.1 (277) |
| −0.1 to <0.0 | 1793 (13) | 1.02±0.24 | 3.65±0.43 | 12.6 (391) |
| 0.0 to <0.1 | 2061 (15) | 0.97±0.24 | 3.62±0.44 | 12.0 (422) |
| 0.1 to <0.2 | 2102 (15) | 0.93±0.24 | 3.63±0.43 | 12.3 (434) |
| 0.2 to <0.3 | 1704 (12) | 0.90±0.22 | 3.58±0.44 | 11.9 (344) |
| 0.3 to <0.4 | 1227 (9) | 0.89±0.23 | 3.58±0.45 | 12.1 (251) |
| 0.4 to <0.5 | 851 (6) | 0.87±0.24 | 3.53±0.44 | 12.1 (181) |
| ≥0.5 | 1120 (8) | 0.85±0.24 | 3.51±0.45 | 11.4 (208) |
| Total | 13,895 (100) | 1.00±0.29 | 3.61±0.44 | 12.3 (2877) |
Figure 3.
Greater increase in renal urea clearance-corrected normalized protein catabolic rate (nPCRdial+renal) between the first and the third patient-quarter was associated with higher likelihood of attaining serum albumin (Alb) ≥3.8 g/dl at the third patient-quarter and lower mortality among 13,895 incident hemodialysis patients. Likelihood of attaining serum Alb ≥3.8 g/dl at 6 months after dialysis initiation using (A) change in nPCRdial+renal categories with three-level adjustment models and (B) restricted cubic splines in the fully-adjusted model. Association between change in nPCRdial+renal using (C) change in nPCRdial+renal categories with three-level adjustment models and (D) restricted cubic splines in the fully adjusted model. The histograms in (A) and (C) show the number of patients in each category. (B) and (D) were truncated at the first and 99th percentiles of data. MICS, malnutrition-inflammation cachexia syndrome; PQ3, third patient-quarter.
Among 13,895 patients with available data on change in nPCRdial+renal, 2877 patients died during the follow-up period (up to 4.5 years; median [IQR] =1.5 [0.8–2.4] years). Greater rise in nPCRdial+renal categories had lower overall crude rates of all-cause death, despite lower baseline nPCRdial+renal and serum albumin (Ptrend<0.001) (Table 3). There was an inverse linear relationship between change in nPCRdial+renal and mortality risk (Ptrend<0.001 for all models) (Figure 3C, Supplemental Table 5). Compared with the reference group (a rise of 0.1–0.2 g/kg per day between the first and third patient-quarters), a decline in nPCRdial+renal≥0.1 g/kg per day was significantly associated with higher mortality risk: HR, 1.32 (95% CI, 1.14 to 1.54) and HR, 1.22 (95% CI, 1.04 to 1.42) for decrease in nPCRdial+renal>0.2 and >0.1–0.2 g/kg per day, respectively. However, a rise in nPCRdial+renal≥0.4 g/kg per day showed a lower mortality risk in fully adjusted models: HR, 0.82 (95% CI, 0.69 to 0.97) and HR, 0.76 (95% CI, 0.64 to 0.90) for rise in nPCRdial+renal=0.4–0.5 and >0.5 g/kg per day, respectively. Consistent results were also observed in models using restricted cubic splines (Figure 3D).
Mortality Risk Associated with Discordance between nPCR Values with versus without Accounting for rCLurea
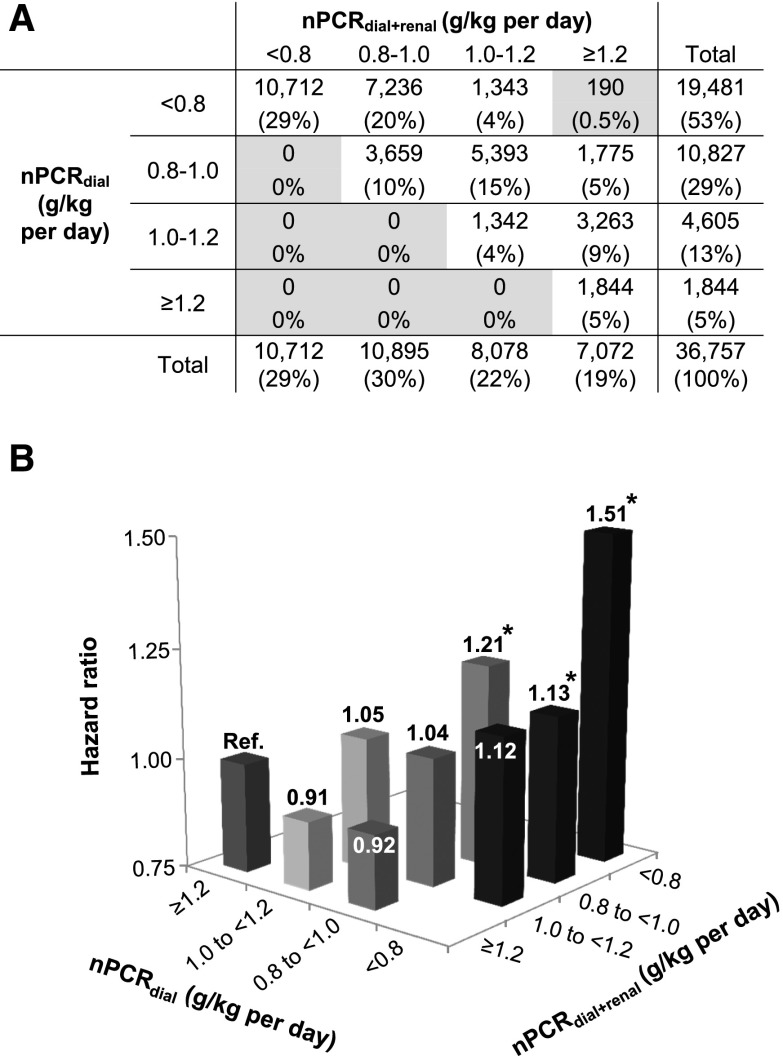
To examine the mortality risk associated with discordance between nPCR values with versus without accounting for rCLurea, we conducted a Cox regression analysis with case-mix adjustment after categorizing patients according to nPCRdial+renal and nPCRdial values (i.e., <0.8, 0.8 to <1.0, 1.0 to <1.2, and ≥1.2 g/kg per day). For example, among 10,827 patients who were categorized as having nPCRdial levels of 0.8 to <1.0 g/kg per day, there were 3659 (34%) who were concordantly categorized as having nPCRdial+renal levels of 0.8 to <1.0 g/kg per day (Figure 4A). There was an incrementally lower risk of all-cause death across increasing nPCRdial+renal categories within each category of nPCRdial (Ptrend<0.001) (Figure 4B). However, this relationship did not persist on examination from the opposite axis. Among patients who were categorized as having nPCRdial+renal levels of 0.8 to <1.0 g/kg per day, the adjusted mortality risk was paradoxically higher in greater nPCRdial values (i.e., HR, 1.13 [95% CI, 1.00 to 1.27] in <0.8 g/kg per day versus HR, 1.21 [95% CI, 1.07 to 1.37] in 0.8 to <1.0 g/kg per day; P=0.04). Furthermore, there were no significant trends observed across categories of nPCRdial within the other nPCRdial+renal categories (Ptrend=0.73 in 1.0 to <1.2 g/kg per day and Ptrend=0.46 in ≥1.2 g/kg per day).
Figure 4.
Normalized protein catabolic rate (nPCR) was underestimated according to renal urea clearance (rCLurea) levels among 36,757 incident hemodialysis patients, leading to an overestimation of all-cause mortality risk associated with low nPCR. (A) Concordance and discordance of rCLurea-corrected nPCR (nPCRdial+renal) and non-rCLurea-corrected nPCR (nPCRdial) and (B) mortality risk associated with categories defined by the combination of nPCRdial and nPCRdial+renal among 36,757 incident hemodialysis patients in a case mix–adjusted model. Gray cells in A indicate groups in which hazard ratios were not reported due to the limited number of patients. *P<0.05.
Discussion
In this longitudinal and national cohort of over 36,000 incident hemodialysis patients with documented data on residual kidney function, we found that nPCR without accounting for rCLurea tended to underestimate nPCR values, especially among patients with greater rCLurea. We also showed that baseline nPCRdial+renal (i.e., rCLurea-corrected nPCR) was inversely associated with mortality. Additionally, change in nPCRdial+renal during the first 6 months of hemodialysis was a significant correlate of attaining high serum albumin and mortality risk independent of baseline nPCRdial+renal and other MICS markers. To our knowledge, this is the first study to examine the association of nPCR with serum albumin levels and mortality accounting for the contribution of rCLurea.
In current clinical practice, nPCR is calculated without accounting for rCLurea, resulting in the underestimation of total urea excretion from the body among patients with residual kidney function. One previous study showed that there was no association of nPCR at either baseline or 6 months follow-up with mortality outcomes (14). However, it was acknowledged in this study that there may be bias toward the null due to lack of uniformity of the postdialysis blood flow for BUN measurement, which may have affected the precision of nPCR values (14). Given the large error in calculation of nPCR and the strong association with patient health-related quality of life and clinical outcomes, we believe that rCLurea should be periodically monitored and accounted for when evaluating protein intake using nPCR among patients with residual kidney function.
Our study showed a clear inverse linear trend toward lower mortality across higher nPCRdial+renal, whereas previous studies have shown a reverse J-shaped association, where patients with nPCRdial≥1.4 g/kg per day had a higher mortality risk (16,17). The association between nPCRdial+renal and mortality in our study was robust, even after rigorous adjustment for case mix variables and MICS markers. The difference in results between our study and the previous studies is likely attributed to the difference between nPCRdial and nPCRdial+renal; the differences between these two variables are especially greater among patients with higher nPCR levels (Figure 1). In our study, there also seemed to be an incrementally higher mortality risk associated with lower nPCRdial+renal levels within the same nPCRdial category, suggesting that a better risk stratification of all-cause mortality attributed to nPCR accounting for rCLurea. The paradoxically lower mortality risk associated with lower nPCRdial among patients who were categorized as having low nPCRdial+renal (i.e., 0.8–<1.0 g/kg per day) may be due to residual kidney function, a strong predictor of better survival among patients on hemodialysis (19,20).
Higher rise in nPCRdial+renal was a correlate of attaining higher serum albumin at the third patient-quarter, despite lower baseline nPCRdial+renal and serum albumin. Inflammation decreases serum albumin levels by reducing the rate of synthesis via downregulation of albumin gene transcription (26–29). Malnutrition and inflammation are closely related to each other and orchestrate the development of PEW (3,5), and recent randomized clinical trials showed that protein supplementation reduced inflammation and increased serum albumin levels (30,31). Moreover, higher rise in nPCRdial+renal predicted lower mortality. These associations between baseline or change in nPCRdial+renal and mortality seemed to be attenuated in the fully adjusted model, but it is likely due to overadjustment for MICS markers, which also included serum albumin and other potential intermediate factors in the causal pathway between dietary protein intake and survival. Rise in nPCR over the first 6 months of dialysis may be one of the representations of increased appetite and intake in relation to the resolution of uremic symptoms (32). Nevertheless, baseline or change in nPCRdial+renal was independently associated with all-cause death even after adjustment for MICS-related variables. Therefore, nPCR may be a useful predictor of mortality in patients on hemodialysis among others (3,33) as included in composite nutritional scores (34,35).
Several limitations of our study should be noted. First, there are day-to-day fluctuations in nPCR caused by changes in daily protein intake (36). However, the use of nPCR data that were averaged over a treatment quarter might mitigate the effect of these fluctuations on our examined associations. Second, the use of nPCR as a reflection of daily protein intake assumes that protein metabolism is in equilibrium at the time of measurement (37). This is not always the case with patients on hemodialysis, because they are susceptible to various comorbidities, which may induce protein catabolism. Additionally, information about rCLurea was not available for all patients. Therefore, our results may not be representative of the entire cohort and may be subject to selection biases. As shown in Supplemental Table 1, patients with available rCLurea were more likely to be non-Hispanic white and had higher spKt/V, albumin, and body mass index. However, adding rCLurea data to the calculation of nPCR may have provided a more accurate approach in evaluating nPCR. Although we adjusted for a number of inflammatory markers, such as serum ferritin, total iron binding capacity, blood white blood cells, and lymphocyte percentage (38–40), other inflammatory markers, such as C-reactive protein, were not available.
In conclusion, our study shows that both higher baseline values and greater increase in dietary protein intake, represented by rCLurea-corrected nPCR, are independently associated with attaining higher serum albumin levels and lower all-cause mortality among incident hemodialysis patients. Compared with conventional nPCR, rCLurea-corrected nPCR may be a better marker of mortality among patients on hemodialysis with substantial residual kidney function. Future studies should investigate effective nutrition treatments in patients on hemodialysis and their effects on clinical outcomes.
Disclosures
C.P.K. has received honoraria from Abbott Nutrition (Abbott Park, IL), Relypsa (Redwood City, CA), Sanofi-Aventis (Bridgewater, NJ), and ZS Pharma (Coppell, TX) and grant support from Shire (Lexington, MA). K.K.-Z. has received commercial honoraria and/or support from Abbott (Abbott Park, IL), Abbvie (North Chicago, IL), Alexion (New Haven, CT), Amgen (Thousand Oaks, CA), Astra-Zeneca (Wilmington, DE), Aveo (Cambridge, MA), Chugai (Berkeley Heights, NJ), DaVita (Denver, CO), Fresenius (Waltham, MA), Genentech (South San Francisco, CA), Haymarket Media (London, England), Hospira (Lake Forest, IL), Kabi (Lake Zurich, IL), Keryx (Boston, MA), Novartis (New York, NY), Pfizer (New York, NY), Relypsa, Resverlogix (Calgary, Alberta, Canada), Sandoz (Princeton, NJ), Sanofi (Bridgewater, NJ), Shire, Vifor (Zurich, Switzerland), UpToDate (Waltham, MA), and ZS Pharma.
Supplementary Material
Acknowledgments
We thank DaVita Clinical Research for providing the clinical data for this research.
The study was supported by National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) of the National Institutes of Health (NIH) research grants K24-DK091419 (to K.K.-Z.), R01-DK078106 (to K.K.-Z.), and R01-DK095668 (to K.K.-Z.) and philanthropic grants from Mr. Harold Simmons (to K.K.-Z.), Mr. Louis Chang (to K.K.-Z.), Dr. Joseph Lee (to K.K.-Z.), and AVEO, Inc. (to K.K.-Z.). Y.O. is supported by the Shinya Foundation for International Exchange of Osaka University Graduate School of Medicine Grant. Y.O. is also supported by the Uehara Memorial Foundation Research Fellowship. E.S. is supported by Office of Research and Development of the Department of Veterans Affairs career development award IK2-CX001266-01. C.M.R. is supported by NIH (NIDDK) grant K23-DK102903, and C.P.K. is supported by NIH (NIDDK) grants R01-DK096920 and U01-DK102163.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13141216/-/DCSupplemental.
References
- 1.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H Jr., Kopple JD, Greenland S: Revisiting mortality predictability of serum albumin in the dialysis population: Time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 20: 1880–1888, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K: Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care 18: 254–262, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko GJ, Obi Y, Tortorici AR, Kalantar-Zadeh K: Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care 20: 77–85, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Kopple JD: Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr 129[1S Suppl]: 247S–251S, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Mehrotra R, Kopple JD: Nutritional management of maintenance dialysis patients: Why aren’t we doing better? Annu Rev Nutr 21: 343–379, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Kopple JD; National Kidney Foundation K/DOQI Work Group : The National Kidney Foundation K/DOQI clinical practice guidelines for dietary protein intake for chronic dialysis patients. Am J Kidney Dis 38[Suppl 1]: S68–S73, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Foundation NK: Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 35[Suppl 2]: S1–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Friedman AN, Fadem SZ: Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol 21: 223–230, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Kaysen GA, Greene T, Daugirdas JT, Kimmel PL, Schulman GW, Toto RD, Levin NW, Yan G; HEMO Study Group : Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am J Kidney Dis 42: 1200–1211, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Kovesdy CP, Kalantar-Zadeh K: Review article: Biomarkers of clinical outcomes in advanced chronic kidney disease. Nephrology (Carlton) 14: 408–415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergström J: Nutrition and mortality in hemodialysis. J Am Soc Nephrol 6: 1329–1341, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Pifer TB, McCullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, Young EW: Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int 62: 2238–2245, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Supasyndh O, Lehn RS, McAllister CJ, Kopple JD: Normalized protein nitrogen appearance is correlated with hospitalization and mortality in hemodialysis patients with Kt/V greater than 1.20. J Ren Nutr 13: 15–25, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Shinaberger CS, Kilpatrick RD, Regidor DL, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K: Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis 48: 37–49, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Ravel VA, Molnar MZ, Streja E, Kim JC, Victoroff A, Jing J, Benner D, Norris KC, Kovesdy CP, Kopple JD, Kalantar-Zadeh K: Low protein nitrogen appearance as a surrogate of low dietary protein intake is associated with higher all-cause mortality in maintenance hemodialysis patients. J Nutr 143: 1084–1092, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K: Nutritional predictors of early mortality in incident hemodialysis patients. Int Urol Nephrol 46: 129–140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K: Preservation of residual kidney function in hemodialysis patients: Reviving an old concept. Kidney Int 90: 262–271, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obi Y, Rhee CM, Mathew AT, Shah G, Streja E, Brunelli SM, Kovesdy CP, Mehrotra R, Kalantar-Zadeh K: Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol 27: 3758–3768, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, Katz R, Molnar MZ, Nissenson A, Ravel V, Streja E, Himmelfarb J, Mehrotra R: Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 30: 1208–1217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daugirdas JT: Physiologic principles and urea kinetic modeling. In: Handbook of Dialysis, 5th Ed., edited by Daugirdas JT, Blake PG, Ing TS, Philadelphia, Lippincott Williams & Wilkins, 2014, pp 59–60 [Google Scholar]
- 23.Hemodialysis Adequacy 2006 Work Group : Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Mosteller RD: Simplified calculation of body-surface area. N Engl J Med 317: 1098, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28: 3083–3107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaysen GA, Dubin JA, Müller HG, Mitch WE, Rosales LM, Levin NW: Relationships among inflammation nutrition and physiologic mechanisms establishing albumin levels in hemodialysis patients. Kidney Int 61: 2240–2249, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Kaysen GA, Dubin JA, Müller HG, Rosales L, Levin NW, Mitch WE; HEMO Study Group NIDDK : Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int 65: 1408–1415, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Birch HE, Schreiber G: Transcriptional regulation of plasma protein synthesis during inflammation. J Biol Chem 261: 8077–8080, 1986 [PubMed] [Google Scholar]
- 29.Princen JM, Nieuwenhuizen W, Mol-Backx GP, Yap SH: Direct evidence of transcriptional control of fibrinogen and albumin synthesis in rat liver during the acute phase response. Biochem Biophys Res Commun 102: 717–723, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Tomayko EJ, Kistler BM, Fitschen PJ, Wilund KR: Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. J Ren Nutr 25: 276–283, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Rhee CM, You AS, Koontz Parsons T, Tortorici AR, Bross R, St-Jules DE, Jing J, Lee ML, Benner D, Kovesdy CP, Mehrotra R, Kopple JD, Kalantar-Zadeh K: Effect of high-protein meals during hemodialysis combined with lanthanum carbonate in hypoalbuminemic dialysis patients: Findings from the FrEDI randomized controlled trial [published online ahead of print September 22, 2016]. Nephrol Dial Transplant doi:10.1093/ndt/gfw323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehrotra R, Berman N, Alistwani A, Kopple JD: Improvement of nutritional status after initiation of maintenance hemodialysis. Am J Kidney Dis 40: 133–142, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Panichi V, Cupisti A, Rosati A, Di Giorgio A, Scatena A, Menconi O, Bozzoli L, Bottai A: Geriatric nutritional risk index is a strong predictor of mortality in hemodialysis patients: Data from the Riscavid cohort. J Nephrol 27: 193–201, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Thijssen S, Wong MM, Usvyat LA, Xiao Q, Kotanko P, Maddux FW: Nutritional competence and resilience among hemodialysis patients in the setting of dialysis initiation and hospitalization. Clin J Am Soc Nephrol 10: 1593–1601, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazairac AH, de Wit GA, Grooteman MP, Penne EL, van der Weerd NC, van den Dorpel MA, Nubé MJ, Lévesque R, Ter Wee PM, Bots ML, Blankestijn PJ; CONTRAST investigators : A composite score of protein-energy nutritional status predicts mortality in haemodialysis patients no better than its individual components. Nephrol Dial Transplant 26: 1962–1967, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Kloppenburg WD, Stegeman CA, de Jong PE, Huisman RM: Relating protein intake to nutritional status in haemodialysis patients: How to normalize the protein equivalent of total nitrogen appearance (PNA)? Nephrol Dial Transplant 14: 2165–2172, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Panzetta G, Tessitore N, Faccini G, Maschio G: The protein catabolic rate as a measure of protein intake in dialysis patients: Usefulness and limits. Nephrol Dial Transplant 5[Suppl 1]: 125–127, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH: Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant 19: 141–149, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kalantar-Zadeh K, Kleiner M, Dunne E, Ahern K, Nelson M, Koslowe R, Luft FC: Total iron-binding capacity-estimated transferrin correlates with the nutritional subjective global assessment in hemodialysis patients. Am J Kidney Dis 31: 263–272, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Reddan DN, Klassen PS, Szczech LA, Coladonato JA, O’Shea S, Owen WF Jr., Lowrie EG: White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant 18: 1167–1173, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.