Abstract
In this study, we report on the bacterial diversity and their functional properties prevalent in tea garden soils of Assam that have low pH (3.8–5.5). Culture-dependent studies and phospholipid fatty acid analysis revealed a high abundance of Gram-positive bacteria. Further, 70 acid-tolerant bacterial isolates characterized using a polyphasic taxonomy approach could be grouped to the genus Bacillus, Lysinibacillus, Staphylococcus, Brevundimonas, Alcaligenes, Enterobacter, Klebsiella, Escherichia, and Aeromonas. Among the 70 isolates, 47 most promising isolates were tested for their plant growth promoting activity based on the production of Indole Acetic Acid (IAA), siderophore, and HCN as well as solubilization of phosphate, zinc, and potassium. Out of the 47 isolates, 10 isolates tested positive for the entire aforesaid plant growth promoting tests and further tested for quantitative analyses for production of IAA, siderophore, and phosphate solubilization at the acidic and neutral condition. Results indicated that IAA and siderophore production, as well as phosphate solubilization efficiency of the isolates decreased significantly (P ≤ 0.05) in the acidic environment. This study revealed that low soil pH influences bacterial community structure and their functional properties.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0864-9) contains supplementary material, which is available to authorized users.
Keywords: Acid soil bacteria, Fatty acid methyl ester, Plant growth promoting bacteria, Phospholipid fatty acid analysis, Bacterial diversity, 16S rDNA
Introduction
Tea is one of the oldest, non-alcoholic, popular beverages of the world. Assam, a northeastern state of India plays a major role in the Indian tea industry by contributing about 53% of the country’s and around 17% of the world’s total annual tea productions (Dikshit and Dikshit 2014). The state enjoys a distinct recognition as the largest tea-growing region in the world with a record number of 68,465 small tea gardens (area of 3–15 acre) and 825 large tea gardens (>15 acres) (Economic survey, Govt. of Assam, 2013–2014).
Ideally, the tea bushes prefer acidic soils with a pH range of 4.5–6.0. However, continuous and exhaustive cultivation and adoption of traditional practices have led to the deterioration of soil health resulting in an increase in soil acidity and aluminum toxicity. A recent study by Bandyopadhyay et al. (2014) highlighted the alarming increase of soil acidity in the tea gardens, ranging from very strongly acidic (pH 4.9) to extremely acidic (pH 4.4) condition with low cation exchange capacity and low base saturation status (<35%). Acidic soils are generally poor in fertility and water holding capacity. The authors reported that a substantial area with pH value less than 5.5 showed severe potassium (P), calcium (Ca), magnesium (Mg), and molybdenum (Mo) deficiency with an increase in aluminum (Al) and iron (Fe) toxicities. Therefore, it can be assumed that poor nutrient cycling along with several major climatic variables such as average temperature, average precipitation, drought intensity, and precipitation variability (Duncan et al. 2016) could be the factors affecting the decadal plummet in Assam’s tea production.
Recent studies have reported that Assam tea garden soils have a rich reserve of microbial diversity and community (Baruah et al. 2013; Huidrom and Sharma 2014). It is well known that microbes play an important role in the tea garden soil ecosystem as they promote better plant growth (Phukan et al. 2012), inhibit plant pathogens and tea pests (Balamurugan et al. 2011; Barthakur et al. 2004), help in the acquisition of minerals, and maintain the biogeochemical cycles. Despite the importance of soil microbial community in regulating the structure and function of the tea garden soil-ecosystem, there is a dearth of knowledge about the impact of increasing soil acidity on resident bacterial communities and their functionality in the tea garden soils of Assam.
Identification of thriving bacterial isolates added with plant growth promotion features could be useful for improving soil health in the deteriorated tea garden acid soils of Assam. Such a study is expected not only to provide an insight into the bacterial diversity but also help in understanding the mechanism of pH homeostasis. In this paper, we report on the bacterial community structure prevalent in the tea garden soils using culture-dependent methods and phospholipid fatty acids (PLFAs) profiling. Further, a comparative functionality of these microbes in promoting plant growth was assessed through various biochemical tests under both acidic and neutral environments.
Materials and methods
Sample collection
The soil samples were collected in sterile containers from the surface layer (0–20 cm) of 14 different tea gardens belonging to small tea growers of Jorhat district of Assam. Soil samples were collected in December 2014. Three replicates of each sample were taken randomly from different sites of the 14 gardens, kept in ice, and transported to the laboratory. The three replicates of each sample were pooled and mixed properly. The samples were then sieved through a 2-mm mesh, to remove any debris and equally divided into three portions. The first portion was used for determination of soil physicochemical properties, the second was used for microbial analysis by culture-dependent method, and the third portion was used for PLFA analysis. A control soil sample (pH 6.8) was taken from non-agricultural land for comparative physicochemical and PLFA study.
Soil physicochemical characteristics
Physical parameter of the soil samples such as pH, bulk density, particle density, total porosity, and maximum water holding capacity were determined as described by Viji and Prasanna (2012). The available phosphate was determined following the method of Bray and Kurtz (1945). Further, available potassium and organic carbon were determined using the method as described by Patel et al. (2014) and Sato et al. (2014), respectively.
Isolation of acid-tolerant bacteria
The soil samples were serial diluted with sterile saline (0.85% w/v NaCl in water) and proper dilution was plated on nutrient agar (NA) (Merck, Germany) medium (pH 7.0) and incubated at 30 °C for 24–48 h. The Colony Forming Units (CFU) was enumerated and non-redundant colonies based on the colony morphology were tested for acid tolerance (pH 3.5, 4.0 and 4.5) by adjusting the pH of nutrient broth (Merck, Germany) with HCl and incubated at 30 °C for 24–48 h at 150 rpm. The acid tolerance of the isolates was also evaluated under buffered condition using acetate buffer (pH 4.5). Nutrient broth prepared in acetate buffer was inoculated with bacterial isolate and incubated under the same condition. Tolerance to organic acid of the isolates was checked by growing the isolates in nutrient broth (pH 4.5) adjusted with acetic acid and citric acid. The isolates which survived at pH 4.5 or below (acid tolerant) were selected for further studies. The pure cultures of the acid-tolerant isolates were preserved in slants as working culture and as glycerol stocks using 50% (v/v) glycerol.
Phenotypic and biochemical characterization
Colony morphology of the isolates was recorded after growing the isolates in NA for 24 h. Gram staining and endospore staining were performed as per standard protocol. Growth at different NaCl concentration and temperatures were also observed. Protease activity and lecithinase activity of the isolates were determined in skim milk agar and MYP agar (Himedia, India) or nutrient agar supplemented with egg yolk, respectively. Biochemical characterization and carbohydrate utilization profile of the isolates were performed on pure cultures using API 20 cassettes and API 50 CH cassettes, respectively (BioMérieux, France) following the manufacturer’s instruction.
Molecular characterization
Molecular characterization of the isolates was carried out by 16S rDNA sequence analyses. Genomic DNA was isolated using GeneElute genomic DNA extraction kit (Sigma-Aldrich, USA) as per the manufacturer’s instructions. Amplification of 16S rDNA was carried out using the primer set U16SF (5′-AGAGTTTGATCMTGGCTCAG-3′) and U16SR (5′-TACGGYTACCTTGTTACGACTT-3′). The amplified products were sequenced using BigDye Terminator reagent in an ABI 377 automated DNA Sequencer (Applied Biosystems, USA). The 16S rDNA sequence reads obtained after sequencing were assembled into contig using CodonCode Aligner (CodonCode Corporation, USA). Further, BLAST analysis was employed to find the similarity of the sequences with known 16S rDNA sequences present in public database. The sequences were deposited in GenBank of National Center for Biotechnology Information (NCBI) and the accession numbers obtained.
Cellular fatty acid analysis
Fatty acid methyl ester (FAME) profiles of the isolates were analyzed using Gas Chromatography (Agilent 7820 Series II) controlled by MIS Sherlock® (MIDI, Inc., Newark, DE, USA) and Agilent ChemStation software. Aerobic library RTBA6 was used to carry out FAME analysis. The isolates were grown on Tryptic soy agar for 24 h at 28 °C and harvested. Saponification, methylation, extraction, and washing steps were performed according to the protocol provided by MIDI, Inc. (DE, USA). Extracted FAME preparations were run in batches with a calibration control. FAME analysis was expressed both as a graph of peak activity against retention time and as a percentage of total FAME for each isolate.
Phylogenetic and cluster analysis
Phylogenetic analysis of the isolates was carried out based on results of biochemical, cellular fatty acids profile, and 16S rDNA sequences. The R software was used to construct the dendrogram from biochemical and cellular fatty acid profile. Further, principal component analysis of cellular fatty acid data was also conducted using the R package (Kassambara and Mundt 2016; Sebastien et al. 2008). The phylogenetic tree was constructed using 16S rDNA sequences of the isolates along with the sequences of the most similar strains retrieved from the NCBI. The sequences were aligned with Clustal W using default parameters and a phylogenetic tree was constructed using the neighbor-joining method in MEGA6 software (http://www.megasoftware.net/) with Kimura-2 parameter correction and 1000-step bootstrap (Tamura et al. 2013).
PLFA analysis of the soil samples
High throughput phospholipid fatty acid analysis of the soil samples was performed as described by Buyer and Sasser (2012). The PLFA calibration standard (PLFAD1) (Agilent Technologies, Wilmington, DE, USA) was prepared as per the manufacture’s instruction and stored at −20 °C. For lipid extraction, 2 g of each soil sample was dried overnight under vacuum at room temperature in the centrifugal evaporator and extracted with Bligh–Dyer extractant containing internal standard. The lipids were further separated by solid phase extraction and eluted with 0.5 ml of methanol:chloroform:H2O (5:5:1). Later, the solution was dried under vacuum, transesterified and finally, the lipids were dissolved in 80 µl of hexane and transferred to gas chromatography vials with glass inserts and stored at −20 °C until further analysis. Gas chromatography was performed in an Agilent 7820 gas chromatograph (GC) (Agilent Technologies, Wilmington, DE, USA) equipped with autosampler, Agilent Ultra 2 column (25 m long × 0.2 mm internal diameter × 0.33 m film thickness), split–splitless inlet, and flame ionization detector controlled with MIS Sherlock® (MIDI, Inc., Newark, DE, USA) and analyzed using Agilent ChemStation software. FAMEs were identified using the PLFAD1 calibration mix and naming table. The individual PLFA obtained were expressed as BACTYPE data using Sherlock Commander Tool as per manufacturer’s instructions.
Determination of plant growth promoting (PGP) characteristics
A total of 47 Gram-positive isolates were tested qualitatively for their plant growth promoting (PGP) activities such as the production of indole acetic acid (IAA), cyanogen (HCN) and siderophore, and solubilization of P, K, and Zn. The isolates showing positive results for all the qualitative tests were further selected for quantitative estimation of phosphate solubilization, and IAA and siderophore production.
The qualitative and quantitative tests for indole acetic acid production by bacterial isolates were carried out following the method of Ahmad et al. (2008). Cyanogen (HCN) production was determined as described by Bakker and Schippers (1987). Siderophore production was determined both qualitatively and quantitatively following the method of Schwyn and Neilands (1987) while potassium solubilization ability of bacterial isolates was analyzed following the method of Parmar and Sindhu (2013). Zinc solubilization ability of the isolates was detected as per the method of Fasim et al. (2002). Qualitative phosphate solubilization ability of the isolates was evaluated using tri-calcium phosphate (TCP), aluminum phosphate (ALP), zinc phosphate (ZP), and calcium phytate (CP) as the source of phosphate. Pikovskaya’s agar medium was modified by replacing the original phosphate source with each of the phosphate sources. The isolates were spot inoculated on agar media and incubated at 28 °C for 3 days and observed for the appearance of clearing zone around the colonies.
Quantitative estimation of water-extractable free inorganic phosphate (Pi) was carried out as described by Jackson (1973) at pH 7.0 and 4.0 using the same source of phosphate (P) as mentioned above excluding tri-calcium phosphate at pH 4.0. The solubilization of phosphate was determined by measuring the absorbance at 600 nm and the amount of solubilized P was extrapolated from the standard curve of KH2PO4. Phytase activity was measured in terms of inorganic orthophosphate released from the phytic acid (CP) by phytase, following the method described by Raghavendra and Halami (2009).
Statistical analysis
Complete randomized design (CRD) method was used to analyze the physicochemical characteristics of the soil samples (P ≤ 0.05). The PGP activities of the isolates at pH 7.0 and 4.0 were analyzed using Student’s t test (P ≤ 0.05). Further, principal component analysis of the FAME data was carried out using the R package.
Results and discussion
Soil physicochemical profile
Soil characteristic is an important factor contributing to the microbial diversity. The pH of the soil samples varied between 3.8 and 4.2. Previous studies reported that majority of the tea garden soils in Assam fall under medium acidic category (Deka 2016; Dutta et al. 2008). The bulk density of the soil samples ranged between 0.76 and 1.02 g cm−3, while the particle density varied between 0.9 and 1.32 g cm−3. The total porosity of the soil samples ranged between 11.69 and 26.89. The organic carbon content of the samples was below 6%, whereas available P and K content of the samples ranged from 29 to 50 kg ha−1 and 214 to 380 kg ha−1, respectively. The water holding capacity among the samples varied widely from 20 to 50%. The physicochemical properties of the 14 soil samples along with the control soil are presented in Table 1. The physicochemical characteristics of the soil samples from the present study were similar to the properties of other acidic soils analyzed previously (Baruah et al. 2013). In acidic soil, availability of N, P, K, Ca2+, Mg2+, Na+, and K+ decreases, while Fe, Ni, Cu, Zn, Mn, and Al become more available and soluble, often leading to toxicity (Nath 2014). The availability of these minerals at higher level of concentration in soil is detrimental to both plant and microbes (Prasanth et al. 2013). The soil samples in the present study had good availability of P and K in contrast to previous reports (Baruah et al. 2013; Gogoi et al. 2016).
Table 1.
Physicochemical characteristics of the soil samples
| Sample codea | pH | Organic carbon (%) | Available P (kg ha−1) | Available K (kg ha−1) | Bulk density (g cm−3) | Particle density (g cm−3) | Total porosity (%) | Max. WHC (%) |
|---|---|---|---|---|---|---|---|---|
| S1 | 4.2 | 2.43 | 33.67 | 381.00 | 1.05 | 1.053 | 14.56 | 38.24 |
| S2 | 4.1 | 2.83 | 46.33 | 388.67 | 0.90 | 1.143 | 20.90 | 28.35 |
| S3 | 4.2 | 5.73 | 31.00 | 254.33 | 0.75 | 0.94 | 18.32 | 27.62 |
| S4 | 3.8 | 5.23 | 28.00 | 268.66 | 0.83 | 0.80 | 11.76 | 31.47 |
| S5 | 3.8 | 2.70 | 36.67 | 297.66 | 0.95 | 0.92 | 23.55 | 32.60 |
| S6 | 4.2 | 2.79 | 30.33 | 366.66 | 0.85 | 1.08 | 16.21 | 18.77 |
| S7 | 4.3 | 4.39 | 54.00 | 358.33 | 0.87 | 1.38 | 26.65 | 29.58 |
| S8 | 4.1 | 4.77 | 32.00 | 354.00 | 0.82 | 1.07 | 19.84 | 31.51 |
| S9 | 4.0 | 2.69 | 33.00 | 363.00 | 0.97 | 1.25 | 19.31 | 20.08 |
| S10 | 3.9 | 2.86 | 43.00 | 308.00 | 0.96 | 1.37 | 13.81 | 40.23 |
| S11 | 3.8 | 2.37 | 34.00 | 244.00 | 1.027 | 1.24 | 18.08 | 18.66 |
| S12 | 3.7 | 2.84 | 51.33 | 226.33 | 0.91 | 1.19 | 11.73 | 31.78 |
| S13 | 4 | 2.54 | 33.00 | 244.00 | 1.17 | 1.56 | 26.88 | 27.50 |
| S14 | 4.2 | 3.2 | 38.33 | 215.00 | 0.88 | 1.04 | 17.89 | 22.84 |
| Control | 6.8 | 3.14 | 52.66 | 346.00 | 0.92 | 1.12 | 21.55 | 32.18 |
| Sed (±) | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 |
| Df | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| * | * | * | * | * | * | * | * |
aThe values are represented as mean ± SE (n = 3)
* Significant difference between the control and each sample (P ≤ 0.05%)
Isolation of acid-tolerant bacteria
A total of 110 non-redundant colonies were selected for their acid tolerance using acidified broth. Out of the 110 colonies (regarded as individual isolate), 70 isolates were able to grow at pH 4.5 (medium acidic), while 55 isolates showed the ability to tolerate pH 4.0. Therefore, the 70 isolates designated as G1 to G70 that grew at pH 4.5 were taken for further studies. The results of the acid tolerance of the isolates are shown in Table 2. Out of 70 isolates, 45 isolates showed tolerance to citric acid, 10 isolates showed tolerance to acetic acid, and 12 isolates showed partial tolerance to acetate buffer (pH 4.5) and the rest of the isolates failed to grow in these conditions indicating the higher toxicity of acetate compared to citrate and HCl. The reason is probably due to the limited ability of HCl and citrate to penetrate bacterial cell wall. Unlike inorganic acid such as HCl, organic acids diffuse freely across the lipid bilayers of the bacterial cell wall in undissociated forms and liberate protons in the cytoplasm and lower the cytoplasmic pH (Booth 1985). It has also been reported that the undissociated acid intercalates into the lipid bilayer at low external pH (Stratford and Anslow 1998) leading to anion accumulation (Russell and Diez-Gonzalez 1998; Roe et al. 1998). In view of our interest in bacteria that are able to tolerate low pH condition, we evaluated the acid tolerance ability of the isolate in different acidic pHs (pH 3.5, 4.0 and 4.5) which mimicked the pH of the tea garden soil. Tolerance of the isolates to such low pH may be due to the adoption of different mechanisms. The basic strategy of bacteria to survive against any stress depends on the integrity of the cell membrane and DNA, maintenance of protein folding as well as intracellular pH (Booth 2002). Apart from these mechanisms, production of biofilms and alkali (Chen et al. 1996, 1998), as well as changes in membrane lipids (Quivey et al. 2000) have also been implicated as other mechanisms in bacteria that aid to combat acid stress.
Table 2.
Phenotypic and acid tolerance characteristics of the isolates
| Soil sample code | Isolate code | Colony morphology | Growth at | Gram reactiona | Shape | Motility | Endospore | |||
|---|---|---|---|---|---|---|---|---|---|---|
| pH 4.5 | pH 4.0 | pH 3.5 | pH 4.5–acetate buffer | |||||||
| S1 | G1 | Light yellow, smooth, glistening, opaque, circular, convex with entire mergin | + | + | − | − | + | C | – | – |
| G2 | Cream white, opaque, and rhizoid | + | + | − | − | + | B | – | + | |
| G3 | White, circular, smooth, opaque | + | + | − | − | + | C | – | – | |
| G4 | Light orange, circular, slightly convex, smooth | + | + | − | − | + | C | – | – | |
| G5 | White, translucent, irregular with a spreading margin | + | + | − | − | − | B | + | – | |
| S2 | G6 | Orange, circular, slightly convex, smooth | + | + | − | − | + | C | – | – |
| G7 | Cream white, round, smooth, slightly mucoid with entire margins | + | + | − | + | + | B | + | + | |
| G8 | White, opaque, smooth, spreaded | + | + | − | + | + | B | + | + | |
| G9 | Dull white, rough, opaque, round | + | + | − | + | + | B | + | + | |
| G10 | Cream white, irregular, rough opaque | + | + | − | − | + | B | + | + | |
| S3 | G11 | Gray white, smooth opaque, irregular | + | − | − | − | + | B | + | + |
| G12 | Dull white, rough, opaque, irregular | + | + | − | − | + | B | + | + | |
| G13 | Yellowish, smooth opaque, irregular | + | + | − | − | + | B | + | + | |
| G14 | Yellowish, smooth opaque, irregular | + | + | − | − | + | B | + | + | |
| G15 | White, opaque, smooth, spreaded | + | + | − | − | + | B | + | + | |
| S4 | G16 | Orange, circular, slightly convex, smooth | + | + | − | − | + | C | – | – |
| G17 | Cream white, irregular, rough opaque | + | + | − | − | + | B | + | + | |
| G18 | Cream white, round, smooth, slightly mucoid with entire margins | + | + | − | + | + | B | + | + | |
| G19 | Cream, rough, opaque, irregular | + | + | − | − | + | B | + | + | |
| G20 | White, smooth, glistening, opaque, circular, convex with entire margin | + | − | − | − | − | B | + | – | |
| S5 | G21 | Cream, circular with undulate margin | + | + | − | + | + | B | + | + |
| G22 | White, rough, irregular, opaque | + | + | − | − | + | B | + | + | |
| G23 | Orange, circular, slightly convex, smooth | + | + | – | – | + | C | – | – | |
| G24 | Cream, irregular with undulate margin | + | + | – | – | + | B | + | + | |
| G25 | Light yellow, irregular with undulate margin | + | + | – | – | + | B | + | + | |
| S6 | G26 | White, circular with undulate margin | + | + | – | – | + | B | + | + |
| G27 | Dull white, rough, opaque, irregular | + | + | – | – | + | B | + | + | |
| G28 | White, smooth moist, irregular, opaque | + | + | – | + | + | B | + | + | |
| G29 | Cream white, opaque, and rhizoid | + | + | – | – | + | B | + | + | |
| G30 | Orange, circular, slightly convex, smooth | + | + | – | – | + | C | – | – | |
| S7 | G31 | White, smooth, translucent, flat round | + | + | – | – | + | B | + | + |
| G32 | White, rough, irregular, opaque | + | + | – | – | + | B | + | + | |
| G33 | Dull white, rough, opaque, irregular | + | + | – | – | + | B | + | + | |
| G34 | Light orange, rough, opaque, irregular | + | + | – | – | + | B | + | + | |
| G35 | White, round, opaque, smooth, spreaded | + | + | – | – | + | B | + | + | |
| S8 | G36 | White, round, flat with irregular edges | + | – | – | – | – | B | + | – |
| G37 | Cream white, irregular, rough opaque colonies | + | + | – | – | + | B | + | + | |
| G38 | Yellowish, smooth opaque, irregular, waxy | + | + | – | – | + | B | + | + | |
| G39 | White, opaque, smooth, spreaded | + | + | – | + | + | B | + | + | |
| G40 | White, translucent and irregular with a spreading margin | + | + | – | – | – | B | + | – | |
| S9 | G41 | White, opaque, smooth | + | + | – | – | + | B | + | + |
| G42 | White, round, flat with irregular edges | + | – | – | – | – | B | + | – | |
| G43 | White, undulate with irregular margins | + | + | – | + | + | B | + | + | |
| G44 | Yellowish, smooth opaque, irregular | + | + | – | + | + | B | + | + | |
| G45 | Dull white, rough, opaque, irregular | + | + | – | + | + | B | + | + | |
| S10 | G46 | Orange, circular, slightly convex, Smooth | + | + | – | + | + | C | – | – |
| G47 | White, sticky, Glistening moist colonies | + | – | – | – | – | B | – | – | |
| G48 | White, round, opaque, smooth | + | + | – | + | + | B | + | + | |
| G49 | Dull white, rough, opaque, irregular | + | + | – | – | + | B | + | + | |
| G50 | White, opaque, smooth, spreaded | + | + | – | – | + | B | + | + | |
| S11 | G51 | White, translucent and irregular with a spreading margin | + | – | – | – | – | B | + | – |
| G52 | White, smooth, opaque, circular, convex with entire edge | + | – | – | – | – | B | + | – | |
| G53 | Yellowish gray, circular, smooth | + | + | – | – | + | C | – | – | |
| G54 | Dull white, undulate, with irregular margins | + | + | – | – | + | B | + | + | |
| G55 | White, sticky, round with entire margin | + | + | – | – | – | B | + | – | |
| S12 | G56 | White, opaque, smooth, spreaded | + | + | – | – | + | B | + | + |
| G57 | Yellowish, rough, opaque, irregular | + | + | – | – | + | B | + | + | |
| G58 | Smooth, circular, convex, translucent | + | + | – | – | – | B | + | – | |
| G59 | Light yellow, circular, slightly convex, smooth | + | + | – | – | + | C | – | – | |
| G60 | Yellowish, smooth opaque, irregular | + | + | – | – | + | B | + | + | |
| S13 | G61 | White, smooth opaque, irregular | + | – | – | – | + | B | + | + |
| G62 | White, smooth opaque, irregular | + | – | – | – | + | B | + | + | |
| G63 | White, smooth opaque, round | + | – | – | – | + | B | + | + | |
| G64 | Yellowish, smooth opaque, irregular | + | – | – | – | + | B | + | + | |
| G65 | Yellowish, smooth mucoid opaque, round | + | – | – | – | + | B | + | + | |
| S14 | G66 | White, with a regular margin, convex | + | – | – | – | + | B | + | + |
| G67 | Yellowish, Smooth, glistening, opaque, circular with entire margin | + | – | – | – | – | B | + | – | |
| G68 | White, translucent, and irregular margin | + | + | – | – | – | B | + | – | |
| G69 | Smooth, circular, convex, translucent | + | + | – | – | – | B | + | – | |
| G70 | White, flat with a irregular margin | + | – | – | – | + | B | + | + | |
a B Bacillus/rod, C Coccus; + positive test, − negative test
Taxonomic characterization of the isolates
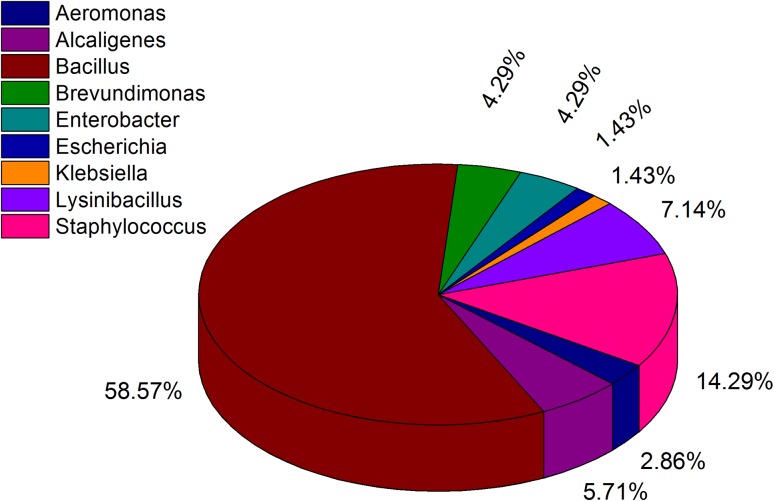
The colonies were white, orange or yellow in color with opaque, shiny, smooth or wrinkled, and mucoid or dry texture. The isolates could be divided into three groups: Gram-positive bacilli (66%), Gram-positive cocci (14%), and Gram-negative bacilli (16%) based on the Gram staining analysis. Most of the Gram-positive bacteria were able to grow at 40 °C and could tolerate up to 5% salt concentration, while the Gram-positive cocci were able to tolerate a higher salt concentration (10%). The Gram-positive bacilli were motile (except G2) and produced endospore. Motility was observed only in some Gram-negative isolates but did not form endospore. The Gram-positive cocci were non-motile and non-endospore forming. The results of biochemical and carbohydrate utilization tests (API 20E and API 50B) indicated that the isolates belong to the members of Bacillus, Lysinibacillus, Brevibacillus, Paenibacillus, Alkaligen, Aeromonas, Pseudomonas, Staphylococcus, Klebsiella, Escherichia, and Enterobacter. The results of phenotypic characterization are presented in Table 2 and the biochemical characteristics as well as carbohydrate utilization profiles are presented in Supplementary file (Table S1). The biochemical profiles of the isolates were similar to those mentioned in Bergey’s Manual of Systematic Bacteriology (Vos et al. 2009; Brenner et al. 2005). Biochemical and carbohydrate utilization tests are classical methods used for identification and differentiation of bacteria; however, these tests cannot distinguish among the closely related species. The 16S rDNA sequencing has been used as a reliable tool for identification and establishing phylogenetic relationships among bacteria (Borsodi et al. 2010). Several other studies also indicated 16S rDNA sequence analysis as an authenticated technique to study bacterial isolates at species level (Ludwig and Klenk 2001; Garrity and Holt 2001; Alam et al. 2011). The BLAST analysis of the 16S rDNA sequences of the 70 strains revealed that 42 isolates belonged to the genus Bacillus, 5 isolates to Lysinibacillus, 10 isolates to Staphylococcus, 2 isolates to Enterobacter, 3 isolates each to the genus Alcaligenes, Aeromonas, and Brevundimonas and 1 isolate each to the genus Escherichia and Klebsiella. Although, 16S rDNA sequences provide similarity between different orthologous in the range of 98–100%, it is unable to discern clearly among the closely related species such as the members of Bacillus genus (Fox et al. 1992). Therefore, FAME analysis of the isolates was further carried out to verify the identification obtained based on 16S rDNA sequences. As the types and relative abundances of fatty acids produced by a cell depends on the genotype of an organism, FAME analysis can be used for identification of different species and strains (Ehrhardt et al. 2010). The GenBank accession numbers, identity (%) and closest match along with the major fatty acid contents, FAME identification, and similarity index (%) of the isolates are presented in Supplementary file (Table S2). The whole cell fatty acid profile of the isolates indicated that branched chain (iso and anteiso) fatty acids were predominant in Gram-positive isolates, whereas straight chain and hydroxyl fatty acids were major fatty acids that occurred in Gram-negative bacteria. Microorganisms display species-specific fatty acid profiles (Suutari and Laakso 1994) and the membrane fatty acid composition of isolates in the present study is consistent with that of their corresponding species as described earlier (O’Leary and Wilkinson 1988). Both FAME and 16S rDNA sequencing data provided almost similar identification pattern. The FAME analysis aided in further identification of some isolates up to species level, which otherwise could not be differentiated on the basis of 16S rDNA sequencing alone. The results of both FAME and 16S rDNA sequencing analyses are also supported by the results of phenotypic and biochemical characteristics. The detailed result of the polyphasic approach of identification is depicted in Fig. 1. Our results revealed a narrow diversity of culturable bacteria in the tea plantation soils. The monoculture nature of the tea cultivation and soil acidity might have affected the microbial diversity in the tea garden soils. It has been reported that soil pH is one of the most dominant factors that affects the microbial community in soil (Fierer and Jackson, 2006; Rousk et al. 2009). Soil pH also regulates the carbon availability, nutrient availability, and the solubility of metals. In addition, soil pH may influence microbial biomass composition in soil (Rousk et al. 2009). Microbial diversity of soil depends on the types of plantation and the richness of rhizosphere in terms of nutrients such as sugars, amino acids, organic acids, hormones, and other small molecules derived from root exudates from which microorganisms obtain their energy (Badri et al. 2009). The physicochemical properties of soil including soil acidity and associated Al toxicity may also influence microbial selection process including the microbial abundance, its composition, and functional characteristics by affecting their metabolic activities (Rousk et al. 2010; Zhalnina et al. 2015; Rogelio Garcidueñas and Carlos 1996). The abundance of Bacillus, Lysinibacillus, and Staphylococcus in condition under study may be due to the nature of their cell wall structure that might aid in increasing their adaptability to low pH condition. Bacteria of the genus Bacillus and its derived genera are reported to exist in extreme environments including acidic soil (Yadav et al. 2015; Cihan et al. 2012).
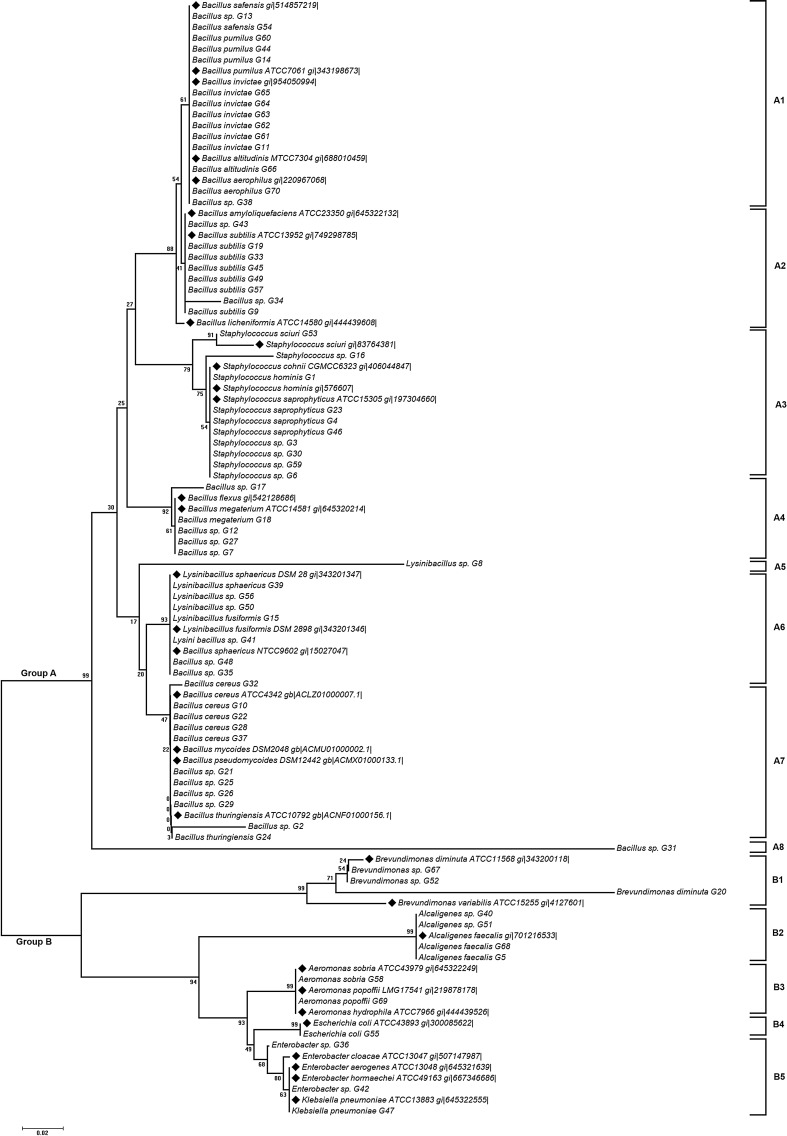
Fig. 1.
The bacterial diversity obtained from the polyphasic taxonomy
Phylogenetic and cluster analysis
The neighbor-joining tree based on 16S rDNA sequences of the 70 acid-tolerant isolates (Fig. 2) distributed them into 2 major clusters viz., Cluster 1 (Gram positive) and Cluster 2 (Gram negative). The tree was drawn to scale with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The phylogenetic tree indicated that each bacterial isolate was clustered to its corresponding strain from GenBank based on their sequence homology which was reflected by the bootstrap value in the node. Similar grouping patterns were also observed in the dendrogram prepared from biochemical and FAME data (Supplementary file, Fig. S1, S2). Principal component analysis of the FAME data grouped the isolates into four different clusters (Supplementary file, Fig. S3). Among the four clusters, cluster 2 comprised the highest numbers of isolates, while cluster 3 comprised of the least numbers of isolates. Cluster 1 and 2 contained the Gram-positive isolates, whereas Cluster 3 and 4 comprised of Gram-negative isolates.
Fig. 2.
Phylogenetic analysis of the isolates based on their 16SrRNA gene sequences. GenBank sequences are marked with the bold bullet point preceding the name of the strain
Microbial community structure
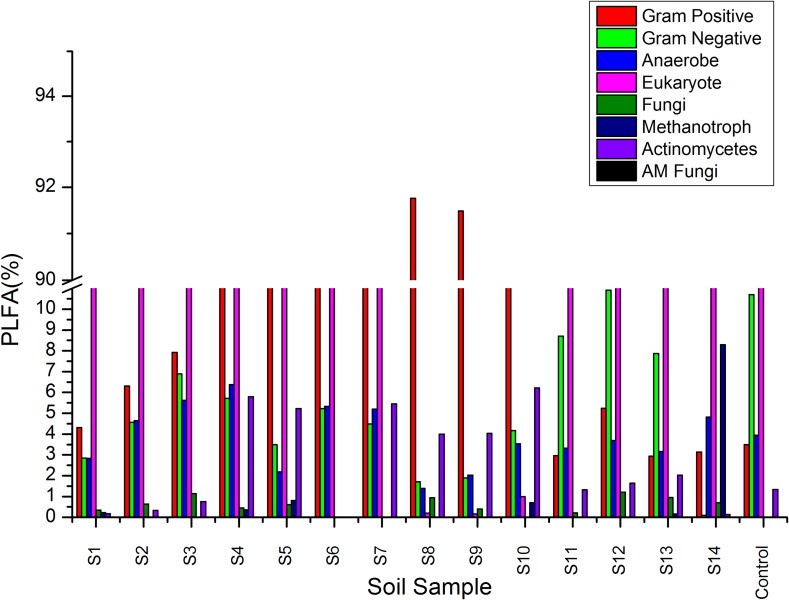
The BACTYPE analysis revealed an abundance of Gram-positive bacteria in the analyzed soil samples except for the samples S11, S12, and S13 (Fig. 3 and Supplementary file Table S3). The abundance of methanotrophs, arbuscular mycorrhiza (AM fungi), fungi, anaerobe, and actinomycetes varied among the soil samples. The eukaryote abundance in the samples ranged between 30 and 89% except for three samples (S8, S9, and S10) where it was less than 1%. The PLFA analysis revealed an abundance of Gram-positive bacteria indicating a correlation between soil acidity with microbial abundance. The PLFA analysis is an analytical procedure for the evaluation of biological communities in soil and provides better insights over plate counts, and regarded as an alternative method for assessing the difference in microbial community (Yao et al. 2000).
Fig. 3.
Graphical representation of BACTYPE analysis of PLFA data
Plant growth promoting (PGP) activities of the isolates
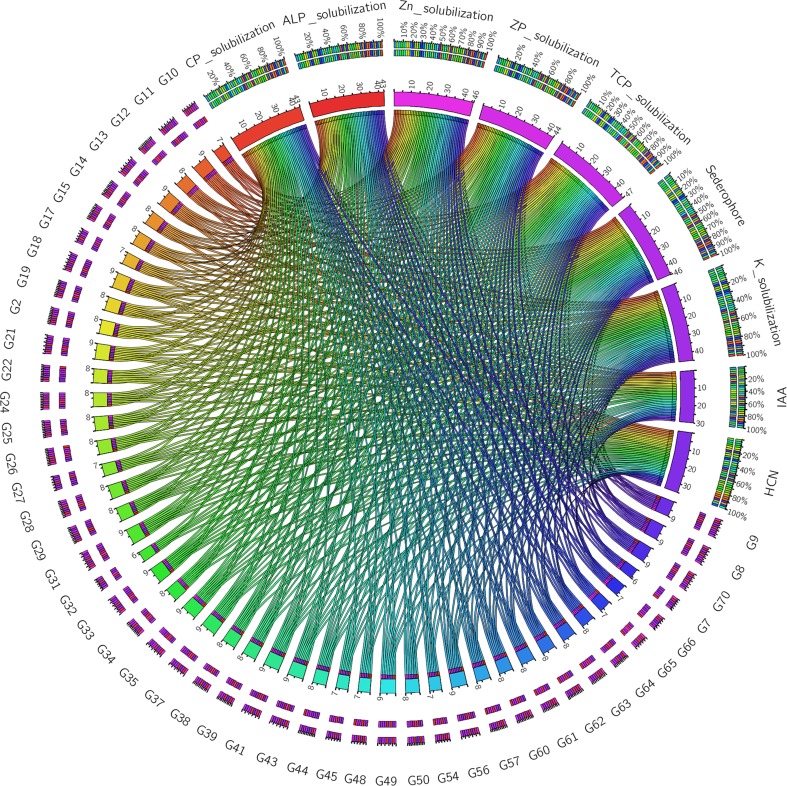
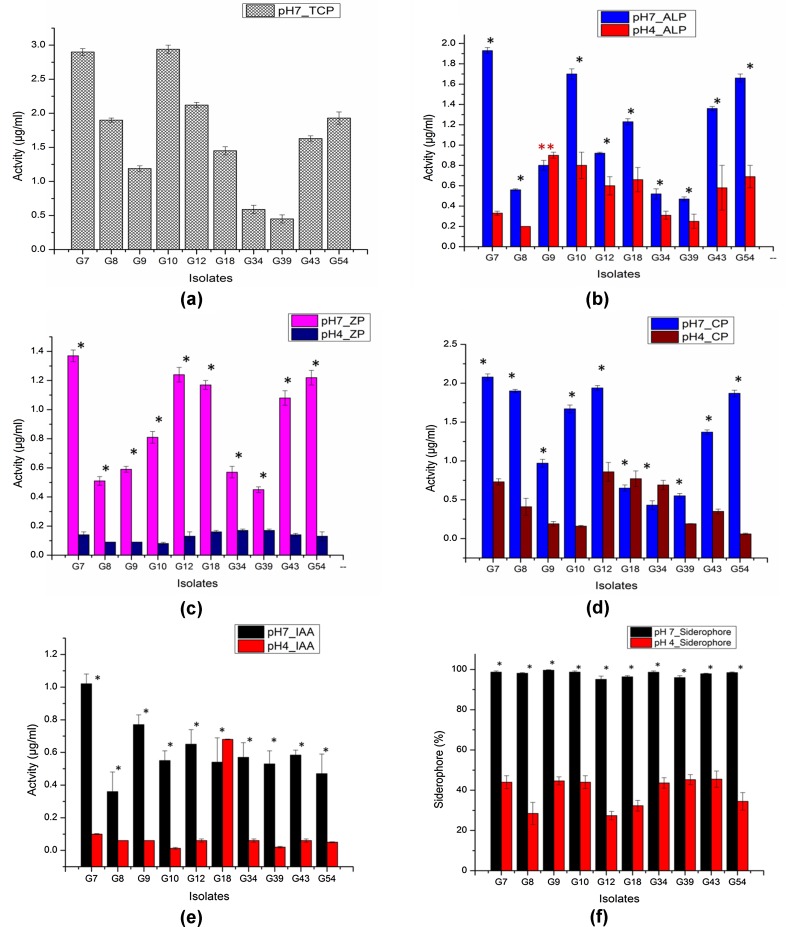
Plant growth promoting bacteria (PGPB) may stimulate plant growth, directly or indirectly and is considered as an attractive alternative to chemical fertilizers (Bano and Musarrat 2003; Vessey 2003; Pindi and Sultana 2014). A number of studies have reported the isolation of Gram-positive bacteria with PGP properties from different sources (Sharma et al. 2015, Kumar et al. 2012; Jha and Subramanian 2014). Due to greater abundance of Gram-positive bacteria found in the present study, only Gram-positive bacilli (47 isolates) were evaluated for their PGP activities. Among the 47 isolates, 46 isolates were able to solubilize TCP, 44 isolates were able to solubilize ALP and ZP and 42 isolates had the capacity to solubilize CP. Tri-calcium phosphate is generally used as a substrate to examine phosphate solubilization activity of microbes, however, solubility of TCP increases with increase in acidity (Bashan et al. 2013). Therefore, ALP, ZP, and CP were included as the source of P since these forms of P are known to exist in higher proportions in acidic soils. Thirty-four isolates showed siderophore production, while 29 isolates tested positive for HCN production. Out of the 47 isolates, 46 isolates secreted IAA and were able to solubilize both Zn and K. The production of HCN by the PGPB is beneficial for the plants since HCN has antifungal activity and may help in biological control of fungal pathogens (Haas and Defago 2005). The results of qualitative PGP activities are represented in Table 3 and Fig. 4. Among the 47 isolates, only 10 isolates tested positive for the entire range of PGP activities analyzed in the present study and hence these were further taken for quantitative estimation. Earlier studies focusing on bacterial PGP activities were conducted in neutral conditions (Giongo et al. 2010; Sang et al. 2014; Majeed et al. 2015; Islam et al. 2015); however, it is important to assess the PGP activity in acid stress condition if the isolates are to be assessed for their activity or used as bio-inoculum in acidic soil. Hence, quantitative PGP activities of the selected isolates were assayed in acidic (pH 4.0) condition taking neutral (pH 7.0) condition as control. The pH of the medium had influence on the functionality of the isolates. The PGP activities of the isolates in acidic pH reduced significantly (P ≤ 0.05) when compared to that of neutral pH. Phosphate solubilization activity of the isolates was tested quantitatively using four different substrates. The isolates displayed variations in their P solubilization activity depending on the substrate and pH. Bacillus cereus G10 showed the highest phosphate solubilization activity (2.94 ± 0.06 µg/ml) when TCP was used as substrate. However, when ALP was used as substrate, Bacillus sp. G7 showed the highest P solubilization activity (1.93 ± 0.03) at pH 7.0, but the activity was reduced to 0.33 ± 0.02 at pH 4.0. The highest ALP solubilizing activity (0.90 ± 0.03) at pH 4.0 was shown by Bacillus subtilis G9. The same isolate also showed the highest ZP and CP solubilizing activity (1.37 ± 0.04 and 2.08 ± 0.04, respectively) at pH 7.0. However, the same isolate showed low ZP and CP solubilizing activity at pH 4.0 (0.2 ± 0.04 and 0.9 ± 0.03, respectively). Maximum solubilization of ZP and CP at pH 4.0 was shown by isolate G18 and G12, respectively. Phosphorus is a key element in the nutrition of plants. Although P is abundant in soils in both inorganic and organic forms, it is a major limiting factor for plant growth as it is in an unavailable form for root uptake. Inorganic P mostly occurs in insoluble mineral complexes such as AlPO4 (Havlin et al. 1999) that cannot be absorbed by plants (Rengel and Marschner 2005). The ability of the isolates to solubilize AlPO4 and convert it to plant available form is an important characteristic under conditions where P is a limiting factor especially in acidic soils. Bacillus subtilis G9 produced the highest amount (1.02 ± 0.06) of IAA at pH 7.0, while isolate G18 produced maximum amount (0.68 ± 0.002) of IAA at pH 4.0. Phytohormone like IAA helps in development and distribution of plant roots, resulting in a better nutrient uptake from the soil (Li et al. 2008). The variation in the ability of PGPB to produce IAA has earlier been reported (Mansour et al. 1994; Zahir et al. 2000). This may be attributed to the various biosynthetic pathways, location of the genes involved, regulatory sequences, and the presence of enzymes to convert active-free IAA into conjugated forms (Patten and Glick 1996) and indeed the pH of the medium. This reason may also be applicable to other PGP activities. Bacillus subtilis G9 displayed the highest siderophore production (99.63 ± 0.22) at pH 7.0 and isolate G43 produced the highest siderophore at pH 4.0. The result of quantitative PGP tests is given in Fig. 5 and Table 4. Siderophores produced by bacteria may promote the plant growth either by providing iron to plant, or by decreasing the availability of iron to plant pathogens, resulting in weak growth of pathogens (Szilagyi-Zecchin et al. 2014). It is also reported that siderophores promote auxin synthesis by chelating metals such as Al, Cd, Ni, and Fe which otherwise inhibit auxin production, thereby enhancing plant growth (Dimkpa et al. 2008).
Table 3.
Plant growth promoting activities of the isolates (qualitative)
| Isolatea code | P solubilization | Siderophore | Zn solubilization | K solubilization | IAA | HCN | |||
|---|---|---|---|---|---|---|---|---|---|
| TCP | ALP | Ca-Phytate | ZP | ||||||
| G2 | + | + | + | + | + | + | – | + | + |
| G7 | + | + | – | + | + | + | + | – | – |
| G8 | + | + | + | + | + | + | + | + | + |
| G9 | + | + | + | + | + | + | + | + | + |
| G10 | + | + | + | + | – | + | + | + | – |
| G11 | + | + | + | + | + | + | + | + | + |
| G12 | + | + | + | + | + | + | + | + | – |
| G13 | + | + | + | + | + | + | + | + | + |
| G14 | + | + | + | + | + | + | – | + | + |
| G15 | + | + | + | + | + | + | + | + | – |
| G17 | + | – | + | + | – | + | + | + | + |
| G18 | + | + | + | + | + | + | + | + | – |
| G19 | + | + | + | – | + | + | + | + | + |
| G21 | + | + | + | + | + | + | + | + | + |
| G22 | + | + | – | + | + | + | + | + | + |
| G24 | + | + | + | + | + | + | + | + | – |
| G25 | + | + | + | + | + | + | + | + | – |
| G26 | + | + | + | + | – | + | + | + | + |
| G27 | + | – | + | – | + | + | + | + | + |
| G28 | + | + | + | + | + | + | + | + | – |
| G29 | + | + | + | + | + | – | + | + | + |
| G31 | + | + | + | + | + | + | + | + | + |
| G32 | + | – | + | – | + | + | + | + | – |
| G33 | + | + | + | + | + | + | + | + | + |
| G34 | + | + | + | + | + | + | + | + | – |
| G35 | + | + | + | + | + | + | + | + | + |
| G37 | + | + | + | + | + | + | + | + | + |
| G38 | + | + | + | + | + | + | + | + | – |
| G39 | + | + | + | + | + | + | + | + | – |
| G41 | + | + | + | + | + | + | + | + | + |
| G43 | + | + | + | + | + | + | + | + | + |
| G44 | + | + | – | + | + | + | + | + | + |
| G45 | + | – | + | + | – | + | + | + | + |
| G48 | + | + | + | + | – | + | + | + | – |
| G49 | + | + | + | + | + | + | + | + | + |
| G50 | + | + | + | + | + | + | + | + | – |
| G54 | + | + | + | + | – | + | + | + | – |
| G56 | + | + | + | + | + | + | + | + | + |
| G57 | + | + | + | + | – | + | + | + | + |
| G60 | + | + | + | + | – | + | + | + | + |
| G61 | + | + | + | + | – | + | + | + | + |
| G62 | + | + | + | + | – | + | + | + | + |
| G63 | + | + | + | – | – | + | + | + | + |
| G64 | + | + | + | + | + | + | + | + | – |
| G65 | + | + | + | + | – | + | + | + | – |
| G66 | + | + | – | + | + | + | + | + | – |
| G70 | + | + | + | + | + | + | + | + | + |
−: negative for the test
a+: positive for the test
Fig. 4.
Circos plot representing the qualitative PGP tests of the isolates
Fig. 5.
Graphical representation of quantitative PGP tests of the isolates. Asterisk indicates the significant differences between the two conditions and double asterisk indicates no significant difference between the two conditions
Table 4.
Quantitative plant growth promoting activities of the isolates
| Tests* | Condition | Activity/isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G7 | G8 | G9 | G10 | G12 | G18 | G34 | G39 | G43 | G54 | ||
| TCP (µg/ml) | pH 7 | 2.90 ± 0.05 | 1.90 ± 0.03 | 1.19 ± 0.04 | 2.94 ± 0.06 | 2.12 ± 0.04 | 1.45 ± 0.06 | 0.59 ± 0.06 | 0.45 ± 0.06 | 1.63 ± 0.04 | 1.93 ± 0.09 |
| ALP (µg/ml) | pH 7 | 1.93 ± 0.03 | 0.56 ± 0.01 | 0.80 ± 0.05a | 1.70 ± 0.05 | 0.92 ± 0.01 | 1.23 ± 0.03 | 0.52 ± 0.05 | 0.47 ± 0.02 | 1.36 ± 0.02 | 1.66 ± 0.04 |
| pH 4 | 0.33 ± 0.02 | 0.20 ± 0.00 | 0.90 ± 0.03a | 0.80 ± 0.13 | 0.60 ± 0.09 | 0.66 ± 0.12 | 0.31 ± 0.04 | 0.25 ± 0.07 | 0.58 ± 0.22 | 0.69 ± 0.11 | |
| ZP (µg/ml) | pH 7 | 1.37 ± 0.04 | 0.51 ± 0.03 | 0.59 ± 0.02 | 0.81 ± 0.04 | 1.24 ± 0.05 | 1.17 ± 0.03 | 0.57 ± 0.04 | 0.45 ± 0.02 | 1.08 ± 0.05 | 1.22 ± 0.05 |
| pH 4 | 0.14 ± 0.02 | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.08 ± 0.008 | 0.13 ± 0.03 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.03 | |
| CP (µg/ml) | pH 7 | 2.08 ± 0.04 | 1.90 ± 0.02 | 0.97 ± 0.05 | 1.67 ± 0.05 | 1.94 ± 0.03 | 0.65 ± 0.04 | 0.43 ± 0.057 | 0.55 ± 0.03 | 1.37 ± 0.03 | 1.87 ± 0.04 |
| pH 4 | 0.73 ± 0.04 | 0.41 ± 0.11 | 0.19 ± 0.03 | 0.16 ± 0.01 | 0.86 ± 0.12 | 0.77 ± 0.10 | 0.69 ± 0.06 | 0.19 ± 0.003 | 0.35 ± 0.03 | 0.06 ± 0.01 | |
| IAA (µg/ml) | pH 7 | 1.02 ± 0.06 | 0.36 ± 0.12 | 0.77 ± 0.06 | 0.55 ± 0.06 | 0.65 ± 0.09 | 0.54 ± 0.15 | 0.57 ± 0.09 | 0.53 ± 0.08 | 0.58 ± 0.03 | 0.47 ± 0.12 |
| pH 4 | 0.10 ± 0.003 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.013 ± 0.005 | 0.06 ± 0.01 | 0.68 ± 0.002 | 0.06 ± 0.01 | 0.02 ± 0.005 | 0.06 ± 0.01 | 0.05 ± 0.003 | |
| Siderophore (%) | pH 7 | 98.67 ± 0.54 | 98.12 ± 0.28 | 99.63 ± 0.22 | 98.67 ± 0.54 | 95.04 ± 1.68 | 96.32 ± 0.61 | 98.61 ± 0.59 | 95.93 ± 0.94 | 97.81 ± 0.31 | 98.43 ± 0.35 |
| pH 4 | 44.00 ± 3.21 | 28.45 ± 5.5 | 44.63 ± 2.0 | 44.004 ± 3.21 | 27.38 ± 2.08 | 32.32 ± 2.64 | 43.61 ± 2.64 | 45.26 ± 2.51 | 45.47 ± 4.04 | 34.43 ± 4.35 | |
* The values are represented as mean ± SD (n = 3); same superscript letter within a column indicates non-significant value, otherwise significant (p ≤ 0.05). Row 3 (TCP) was not considered for test of significance
Conclusion
The tea plantations sustain the ecosystem that supports various life forms including microbes. However, continuous and unsustainable monoculture practices have affected the fertility of the tea garden soils and also led to increase in soil acidity. The present study provides an insight into the bacterial community structure of tea garden soils of Assam as revealed by culture-dependent and PLFA analysis. This study indicated that soil acidity exerts selective pressure on microbial diversity. Gram-positive bacteria of the genus Bacillus were found in abundance in acidic tea garden soils of the present study. Many of the Bacillus isolates also displayed PGP activities which, however, decreased significantly in acidic conditions. Development of a bio-formulation with these isolates and testing them under field conditions, mimicking acid and neutral soil pH may provide greater evidence. Further study to understand the acid stress resistance mechanism of these isolates which is presently being investigated may lead to identification of gene(s) conferring acid-tolerant trait that might later help in manipulating beneficial microbes suitable for use in acidic soil.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are indebted to the Vice-Chancellor of Assam Agricultural University, Dr. K. M. Bujarbaruah for his idea and encouragement to take up the project. The authors thank Dr. B. K. Sarmah, Director, DBT-AAU Centre, AAU, Jorhat and Dr. M. K. Modi, Head, Department of Agricultural Biotechnology, AAU, Jorhat for providing the necessary facilities to carry out the research work. The help received from Mr. Debashis Panda, Research Scholar, Distributed Information Centre, AAU, in statistical analysis is also duly acknowledged. This project was supported by Grant received from DBT-AAU Centre, Assam Agricultural University.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interests.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0864-9) contains supplementary material, which is available to authorized users.
References
- Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizobacteria for their multiple plant growth promoting activities. Microbiol Res. 2008;163:173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Alam SI, Bansod S, Goel AK, Singh L. Characterization of an environmental strain of Bacillus thuringiensis from a hotspring in western Himalayas. Curr Microbiol. 2011;62:547–556. doi: 10.1007/s00284-010-9743-x. [DOI] [PubMed] [Google Scholar]
- Badri DV, Weir TL, van der Lelie D, Vivanco JM. Rhizosphere chemical dialogues: plant–microbe interactions. Curr Opin Biotechnol. 2009;20(6):642–650. doi: 10.1016/j.copbio.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Bakker AW, Schippers B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth-stimulation. Soil Biol Biochem. 1987;19:451–457. doi: 10.1016/0038-0717(87)90037-X. [DOI] [Google Scholar]
- Balamurugan A, Jayanthi R, Nepolean P, Pallavi V, Premkumar R. Studies on cellulose degrading bacteria in tea garden soils. Afr J Plant Sci. 2011;5(1):22–27. [Google Scholar]
- Bandyopadhyay S, Dutta D, Chattopadhyay T, Reza SK, Dutta DP, Baruah U, Sarkar D, Singh SK. Characterization and classification of some tea-growing soils of Jorhat district, Assam. Agropedology. 2014;24(02):138–145. [Google Scholar]
- Bano N, Musarrat J. Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr Microbiol. 2003;46:0324–0328. doi: 10.1007/s00284-002-3857-8. [DOI] [PubMed] [Google Scholar]
- Barthakur BK, Sarmah SR, Dutta P, Singh K. Effect of microbial bioagents in controlling certain pest and diseases of tea. J Mycopathol Res. 2004;42(1):83–88. [Google Scholar]
- Baruah BK, Das B, Medhi C, Misra AK. Fertility status of soil in the tea garden belts of Golaghat district, Assam, India. J Chem. 2013 [Google Scholar]
- Bashan Y, Kamnev AA, de-Bashan LE. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. BiolFertil Soils. 2013;49:465–479. doi: 10.1007/s00374-012-0737-7. [DOI] [Google Scholar]
- Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR. Stress and the single cell: Intra-population diversity is a mechanism to ensure survival upon exposure to stress. Int J Food Microbiol. 2002;178:19–30. doi: 10.1016/S0168-1605(02)00239-8. [DOI] [PubMed] [Google Scholar]
- Borsodi AK, Kiss RI, Cech G, Vajna B, Tóth EM, Márialigeti K. Diversity and activity of cultivable aerobic planktonic bacteria of a saline lake located in Sovata, Romania. Folia Microbiol. 2010;55(5):461–466. doi: 10.1007/s12223-010-0077-7. [DOI] [PubMed] [Google Scholar]
- Bray RH, Kurtz LT. Determination of total, organic and available forms of phosphorus in soils. Soil Sci. 1945;59(1):39–46. doi: 10.1097/00010694-194501000-00006. [DOI] [Google Scholar]
- Brenner DJ, Krieg NR, Staley JT, Garrity GM, editors. Bergey’s manual of systematic bacteriology. 2. New York: Springer-Verlag; 2005. [Google Scholar]
- Buyer JS, Sasser M. High throughput phospholipid fatty acid analysis of soils. Appl Soil Ecol. 2012;61:127–130. doi: 10.1016/j.apsoil.2012.06.005. [DOI] [Google Scholar]
- Chen YY, Clancy KA, Burne RA. Streptococcus salivarius urease: genetic and biochemical characterization and expression in a dental plaque streptococcus. Infect Immun. 1996;64:585–592. doi: 10.1128/iai.64.2.585-592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Weaver CA, Mendelsohn DR, Burne RA. Transcriptional regulation of the Streptococcus salivarius 57I urease operon. J Bacteriol. 1998;180:5769–5775. doi: 10.1128/jb.180.21.5769-5775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihan AC, Tekin N, Ozcan B, Cokmus C. The genetic diversity of genus Bacillus and the related genera revealed by 16S rRNA Gene sequences and ARDRA analyses isolated from geothermal regions of Turkey. Braz J Microbiol. 2012;43(1):309–324. doi: 10.1590/S1517-83822012000100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka A (2016) Aproteogenomic study to elucidate acid tolerance mechanism in soil bacteria. Ph. D thesis, Assam Agricultural University, Jorhat
- Dikshit KR, Dikshit JK. North-East India: land, people and economy. Dordrecht: Springer; 2014. [Google Scholar]
- Dimkpa CO, Svatos A, Dabrowska P, Schmidt A, Boland W, Kothe E. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere. 2008;74:19–25. doi: 10.1016/j.chemosphere.2008.09.079. [DOI] [PubMed] [Google Scholar]
- Duncan JMA, Saikia SD, Gupta N, Biggs M. Observing climate impacts on tea yield in Assam, India. Appl Geogr. 2016;77:64–71. doi: 10.1016/j.apgeog.2016.10.004. [DOI] [Google Scholar]
- Dutta J, Bhuyan B, Misra AK. Chemical estimation of soil fertility status in and around the tea gardens of Gohpur sub-division. Assam. Int J Chem Sci. 2008;6(2):1099–1105. [Google Scholar]
- Economic survey, Govt. of Assam (2013–2014) (http://www.planassam.info/economic_survey_assam_13-14/Economic_Survey_%202013-14.pdf (visited on 23 May 2017)
- Ehrhardt CJ, Chu V, Brown TC, Simmons TL, Swan BK, Bannan J, Robertson JM. Use of fatty acid methyl ester profiles for discrimination of Bacillus cereus T-Strain spores grown on different media. Appl Environ Microbiol. 2010;76:1902–1912. doi: 10.1128/AEM.02443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasim F, Ahmed N, Parsons N, Gadd GM. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol Lett. 2002;213:1–6. doi: 10.1111/j.1574-6968.2002.tb11277.x. [DOI] [PubMed] [Google Scholar]
- Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA. 2006;103(3):626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GE, Wisotzkey JD, Jurtshunk P. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:66–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- Garrity GM, Holt JG. The road map to the Manual. In: Boone DR, Castenholz RW, Garrity GM, editors. Bergey’s manual of systematic bacteriology. 2. New York: Springer; 2001. pp. 119–166. [Google Scholar]
- Giongo A, Beneduzi Anelise AA, Vargas LK, Stroschein MR, Eltz FL, Bodanese-Zanettini MH, Passaglia LMP. Isolation and characterization of two plant growth-promoting bacteria from the rhizoplane of a legume (Lupinus albescens) in sandy soil. Revista Brasileira de Ciência do Solo. 2010;34(2):361–369. doi: 10.1590/S0100-06832010000200009. [DOI] [Google Scholar]
- Gogoi S, Mishra G, Deka AK. Soil nutrient dynamics in tea agroforestry ecosystem of Golaghat district of Assam, India. Agric Sci Digest. 2016;36(3):185–190. [Google Scholar]
- Haas D, Defago G. Biological control of soil-borne pathogens by fluorescent Pseudomonas. Nat Rev Microbiol. 2005;3(4):307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- Havlin J, Beaton J, Tisdale SL, Nelson W. Soil fertility and fertilizers. An introduction to nutrient management. 6. New Jersey: Prentice Hall; 1999. [Google Scholar]
- Huidrom P, Sharma GD. Microbial bioremediation of pesticide residues in tea soil. Int Interdisc Res J. 2014;4:261–275. [Google Scholar]
- Islam S, Akanda AM, Prova A, Islam MT, Hossain MM. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol. 2015;6:1360. doi: 10.3389/fmicb.2015.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ML. Soil chemical analysis. Printice Hall of India: New Delhi; 1973. p. 392. [Google Scholar]
- Jha Y, Subramanian RB. Characterization of root-associated bacteria from paddy band its growth-promotion efficacy. 3 Biotech. 2014;4:325–330. doi: 10.1007/s13205-013-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A, Mundt F (2016) Factoextra: extract and visualize the results of multivariate data analyses, R package version 1.0.3. https://CRAN.R-project.org/package=factoextra
- Kumar P, Dubey RC, Maheshwari DK. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res. 2012;167(8):493–499. doi: 10.1016/j.micres.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Li JH, Wang ET, Chen WF, Chen WX. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol Biochem. 2008;40:238–246. doi: 10.1016/j.soilbio.2007.08.014. [DOI] [Google Scholar]
- Ludwig W, Klenk HP. Overview: a phylogenetic backbone and taxonomic framework for procaryotic systematics. In: Boone DR, Castenholz RW, Garrity GM, editors. Bergey’s manual of systematic bacteriology. 2. New York: Springer; 2001. pp. 49–65. [Google Scholar]
- Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol. 2015;6:198. doi: 10.3389/fmicb.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour FA, Ildesuguy HS, Hamedo HA. Studies on plant growth regulators and enzyme production by some bacteria. J Qatar Univ Sci. 1994;14:81–288. [Google Scholar]
- Nath TN. Status of macronutrients (N, P and K) in some selected tea growing soil of Sivasagar district of Assam, India. Int Res J Chem. 2014;7:12–29. [Google Scholar]
- O’Leary WM, Wilkinson SG. Gram-positive bacteria. In: Ratledge C, Wilkinson SG, editors. Microbial lipids. London: Academic Press; 1988. pp. 117–201. [Google Scholar]
- Parmar P, Sindhu SS. Potassium solubilization by rhizosphere bacteria: influence of nutritional and environmental conditions. J Microbiol Res. 2013;3(1):25–31. [Google Scholar]
- Patel BK, Jain SA, Jagtap MS, Patel KP, Patel DH. Study of presence of available potassium in soil of LunawadaTaluka territory. Arch Appl Sci Res. 2014;6(1):79–84. [Google Scholar]
- Patten CL, Glick BR. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol. 1996;42(3):207–220. doi: 10.1139/m96-032. [DOI] [PubMed] [Google Scholar]
- Phukan I, Madhab M, Sarmah SR, Bordoloi M, Nair SC, Dutta P, et al. Exploitation of PGP microbes of tea for improvement of plant growth and pest suppression: a novel approach. Two Bud. 2012;59(1):69–74. [Google Scholar]
- Pindi PKT, Sultana PKV. Plant growth regulation of Bt-cotton through Bacillus species. 3 Biotech. 2014;4:305–315. doi: 10.1007/s13205-013-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth KM, Sreekala PP, Sandip S, Kripa PK, Sreejesh KK. Heavy metals and its fraction as in soils of Koratty region, Kerala. Res J Recent Sci. 2013;2(ISC-2012):171–176. [Google Scholar]
- Quivey RG, Faustoferri R, Jr, Monahan K, Marquis R. Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol Lett. 2000;189:89–92. doi: 10.1111/j.1574-6968.2000.tb09211.x. [DOI] [PubMed] [Google Scholar]
- Raghavendra P, Halami PM. Screening, selection and characterization of phytic acid degrading lactic acid bacteria from chicken intestine. Int J Food Microbiol. 2009;133(1–2):129–134. doi: 10.1016/j.ijfoodmicro.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Rengel Z, Marschner P. Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol. 2005;168:305–312. doi: 10.1111/j.1469-8137.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- Roe AJ, McLaggan D, Davidson I, O’Byrne C, Booth IR. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol. 1998;180:767–772. doi: 10.1128/jb.180.4.767-772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelio Garcidueñas P, Carlos C. Microbial interactions with aluminium. Biometals. 1996;9(3):311–316. doi: 10.1007/BF00817932. [DOI] [PubMed] [Google Scholar]
- Rousk J, Brookes PC, Bååth E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol. 2009;75(6):1589–1596. doi: 10.1128/AEM.02775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010;4(10):1340–1351. doi: 10.1038/ismej.2010.58. [DOI] [PubMed] [Google Scholar]
- Russell JB, Diez-Gonzalez F. The effects of fermentation acids on bacterial growth. Adv Microb Physiol. 1998;39:205–234. doi: 10.1016/S0065-2911(08)60017-X. [DOI] [PubMed] [Google Scholar]
- Sang HJ, Mayank AG, Se-Chul C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res. 2014;169(1):83–98. doi: 10.1016/j.micres.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Sato JH, de Figueiredo CC, Marchão RL, Madari BE, Benedito LEC, Busato JG, de Mendes SD. Methods of soil organic carbon determination in Brazilian savannah soils. Sci Agric. 2014;71(4):302–308. doi: 10.1590/0103-9016-2013-0306. [DOI] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Sebastien L, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;25(1):1–18. [Google Scholar]
- Sharma A, Singh P, Kumar S, Kashyap PL, Srivastava AK, Chakdar H, Singh RN, Kaushik R, Saxena AK, Sharma AK. Deciphering diversity of salt-tolerant Bacilli from saline soils of eastern Indo-gangetic plains of India. Geomicrobiol J. 2015;32(2):170–180. doi: 10.1080/01490451.2014.938205. [DOI] [Google Scholar]
- Stratford M, Anslow PA. Evidence that sorbic acid does not inhibit yeast as a classic ‘weak acid preservative’. Lett Appl Microbiol. 1998;27:203–206. doi: 10.1046/j.1472-765X.1998.00424.x. [DOI] [PubMed] [Google Scholar]
- Suutari M, Laakso S. Microbial fatty acids and thermal adaptation. Crit Rev Microbiol. 1994;20:285–328. doi: 10.3109/10408419409113560. [DOI] [PubMed] [Google Scholar]
- Szilagyi-Zecchin VJ, Ikeda AC, Hungria M, Adamoski D, Kava-Cordeiro V, Glienke C, Galli-Terasawa LV. Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. AMB Express. 2014;4:26. doi: 10.1186/s13568-014-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- Viji R, Prasanna PR. Assessment of water holding capacity of major soil series of Lalgudi, Trichy, India. J Environ Res Dev. 2012;7(1A):393–398. [Google Scholar]
- Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer K-H, Whitman WB, editors. Bergey’s manual of systematic bacteriology. New York: Springer-Verlag; 2009. [Google Scholar]
- Yadav AN, Verma P, Kumar M, Pal KK, Dey R, Gupta A, Padaria JC, Gujar GT, Kumar S, Suman A, Prasanna R, Saxena AK. Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann Microbiol. 2015;65:611–629. doi: 10.1007/s13213-014-0897-9. [DOI] [Google Scholar]
- Yao H, He ZL, Wilson M, Campbell CD. Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb Ecol. 2000;40(3):223–237. doi: 10.1007/s002480000053. [DOI] [PubMed] [Google Scholar]
- Zahir A, Abbas SA, Khalid M, Arshad M. Structure dependent microbially derived plant hormones by improving growth of maize seedlings. Pak J Biol Sci. 2000;3:289–291. doi: 10.3923/pjbs.2000.289.291. [DOI] [Google Scholar]
- Zhalnina K, Dias R, de Quadros PD, Davis-Richardson A, Camargo FAO, Clark IM, McGrath SP, Hirsch PR, Triplett EW. Soil pH determines microbial diversity and composition in the park grass experiment. Microb Ecol. 2015;69(2):395–406. doi: 10.1007/s00248-014-0530-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.