Abstract
Background
Prosthetic implant infections caused by Staphylococcus aureus and epidermidis are major challenges for early diagnosis and treatment owing to biofilm formation on the implant surface. Extracellular DNA (eDNA) is actively excreted from bacterial cells in biofilms, contributing to biofilm stability, and may offer promise in the detection or treatment of such infections.
Questions/purposes
(1) Does DNA structure change during biofilm formation? (2) Are there time-dependent differences in eDNA production during biofilm formation? (3) Is there differential eDNA production between clinical and control Staphylococcal isolates? (4) Is eDNA production correlated to biofilm thickness?
Methods
We investigated eDNA presence during biofilm formation in 60 clinical and 30 control isolates of S aureus and S epidermidis. The clinical isolates were isolated from patients with infections of orthopaedic prostheses and implants: 30 from infected hip prostheses and 30 from infected knee prostheses. The control isolates were taken from healthy volunteers who had not been exposed to antibiotics and a hospital environment during the previous 3 and 12 months, respectively. Control S epidermidis was isolated from the skin of the antecubital fossa, and control S aureus was isolated from the nares. For the biofilm experiments the following methods were used to detect eDNA: (1) fluorescent staining with 4′,6-diamidino-2-phenylindole (DAPI), (2) eDNA extraction using a commercial kit, and (3) confocal laser scanning microscopy for 24-hour biofilm observation using propidium iodide and concanavalin-A staining; TOTO®-1 and SYTO® 60 staining were used for observation and quantification of eDNA after 6 and 24 hours of biofilm formation. Additionally antibiotic resistance was described.
Results
eDNA production as observed by confocal laser scanning microscopy was greater in clinical isolates than controls (clinical isolates mean ± SD: 1.84% ± 1.31%; control mean ± SD: 1.17% ± 1.37%; p < 0.005) after 6 hours of biofilm formation. After 24 hours, the amount of eDNA was greater in biofilms of S epidermidis than in biofilms of S aureus (S aureus mean ± SD: 1.35% ± 2.0%; S epidermidis mean ± SD: 6.42% ± 10.6%; p < 0.05). Clinical isolates of S aureus and S epidermidis produced more eDNA than control isolates at 6 hours of biofilm formation. The extraction method also showed that clinical isolates produced substantially greater amounts of eDNA than controls.
Conclusions
S aureus and S epidermidis exhibit a differential production of DNA with time. Clinical isolates associated with implant infections produce greater amounts of eDNA than controls. Future research might focus on the diagnostic value of eDNA as a surrogate laboratory marker for biofilm formation in implant infections.
Clinical relevance
eDNA should be considered as a potential future diagnostic tool or even a possible target to modify biofilms for successful treatment of biofilm-associated infections.
Introduction
Microbial biofilms are an important factor in the pathophysiology of prosthetic joint infections. Data show that prosthetic joint infections account for 25% of failed knee arthoplasties and 15% of failed hip arthroplasties [1, 2, 5]. Patients may be faced with prolonged and often futile antimicrobial treatment where implant removal is ultimately inevitable [8, 32]. Biofilms are a natural niche in which bacteria may survive in an adverse environment [17]. After initial bacterial adhesion on the implant surface, colonization and proliferation occurs. Producing extracellular matrix consisting of extrapolymeric substances, for example, exopolysaccharides, proteins, and nucleic acids, bacteria exhibit several mechanisms to survive: (1) mechanical persistence; (2) metabolic changes such as changes from an aerobic to an anaerobic state; (3) exchanging genetic information, for example, resistance genes; and (4) hiding from the immune system.
Multiple properties of Staphylococcus epidermidis and Staphylococcus aureus are involved in biofilm formation [9, 19]. The presence of extracellular DNA (eDNA), which is actively released through designated cells, has generated increased interest [25, 26]. The production of eDNA regulates the properties of extrapolymeric substances in response to environmental influences. Biofilm formation and production of extrapolymeric substances is related to an active release of eDNA, originating from bacteria that survived in an unfavorable environment. S aureus and S epidermidis rely on different autolytic mechanisms in which they use eDNA: eDNA produced by S epidermidis plays an important role during the attachment phase [21, 22]. This eDNA production is mediated by the autolysin protein AtIE that induces lysis of a small bacterial fraction enabling eDNA to be set free by S epidermidis during the surface attachment [4, 13]. In contrast, eDNA of S aureus originates from altruistic cell lysis and seems to be responsible for biofilm maturation, which is controlled by the cid operon [31]. Moreover, S aureus eDNA seems to establish a functional net structure in the biofilm matrix to tether cells together [6, 28]. Although the role of eDNA has been investigated in basic biofilm research [18, 22, 25], unresolved issues remain regarding eDNA in clinical biofilms: a remarkable variability of eDNA was reported in a study on clinical isolates of S epidermidis from patients with orthopaedic wound and implant infections [28].
This topic might be important to clinicians because prosthetic joint infection attributable to S aureus and S epidermidis are associated with frequent failure of long-term antibiotic treatment, revision surgery, and a debilitating course for patients. Determination of eDNA of the causative pathogen may be a new tool to identify bacterial strains with high eDNA content in biofilms as a potential therapeutic target or to adjust the treatment decisions, for example, early surgical intervention, to fight these infections more efficiently.
In the current study, we investigated the presence of eDNA in S aureus and S epidermidis, isolated either from patients with prosthetic joint infections or from control subjects without active infections. Using separate methods to observe DNA and eDNA and quantify eDNA and confocal laser scanning microscopy for observation, we asked: (1) Does DNA structure change during biofilm formation? (2) Are there time-dependent differences in eDNA production during biofilm formation? (3) Is there differential eDNA production between clinical and control Staphylococcal isolates? (4) Is eDNA production correlated to biofilm thickness?
Materials and Methods
Isolates and Strains Used in the Experiments
During 2000 to 2015, isolates from patients with infections of orthopaedic prostheses and implants were collected and stored at −80 °C. The explanted prostheses had to be transported from the operating theater to the microbiology laboratory under standardized conditions within no more than 2 hours to be included in our study. The explanted prostheses were placed in sterile boxes filled with phosphate buffered saline (PBS) and vortexed first for 30 seconds. Thereafter, boxes with implants were sonicated for 5 minutes and again vortexed for 30 seconds. The sonicated fluid was distributed on blood agar plates and cultured. Growing bacteria were counted and identified using routine microbiologic laboratory methods. Additionally the results obtained after implant sonication were confirmed with microbiologic cultures of intraoperative fluid samples to exclude possible contamination [14].
The pathogens of 60 patients (27 male, 33 female) were included in the study. The median age of the patients was 71 years (17–89 years). Thirty-one patients (51.5%) had infection of a primary prosthesis and 29 patients (48.5%) had infection of a secondary prosthesis.
Thus, overall 30 clinical isolates from infected hip prostheses (hip) (15 S aureus and 15 S epidermidis) and 30 isolates from infected knee prostheses (knee) (15 S aureus and 15 S epidermidis) were investigated. Control S aureus (n =15) and S epidermidis (n =15) isolates were collected from 30 volunteers without active infections who had not been exposed to antibiotics for 3 months and to a hospital environment during the previous 12 months. All S epidermidis isolates were collected from skin of the antecubital fossa. Control S aureus was isolated from the nares. All isolates were identified using standard microbiologic methods. S epidermidis DSM 3269 and S aureus American Type Culture Collection (ATCC®) 25921 (ATCC, Manassas, VA, USA; www.atcc.org) were used as reference strains.
Characteristics of Clinical and Control Isolates
Susceptibility Testing
All bacteria were tested for susceptibility to oxacillin, rifampicin, ciprofloxacin, and vancomycin (disc diffusions test), using standard laboratory methods according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria (http://www.eucast.org/clinical_breakpoints/).
Biofilm Experiments
Biofilm Formation Testing
All experiments leading to biofilm formation were done on overnight cultures growing at 37 °C on Columbia agar + 5% sheep blood plates (bioMerieux SA, Marcy l’Etoile, France). After incubation the isolates and the reference strains were used for biofilm preparation using a modified protocol described by Christensen et al. [3]. Other than in the above-mentioned protocol, Brain Heart Infusion Broth (Sigma-Aldrich, St Louis, USA) was used for all experiments, as established in previous biofilm experiments [11, 12]. The 96-well flat bottom plates (Corning® Costar®; Corning Life Sciences, Tewksbury, MA, USA) with bacterial suspensions were incubated for 24 hours at 37 °C. Biofilm quantification was determined by measuring the optical density after staining with crystal violet and counting the viable bacteria (viable bacteria count) in the biofilms.
For crystal violet staining, biofilms were fixed using 150 µL 2% glutaraldehyde/PBS. This fixing solution was chosen for its superior preserving qualities. The extinction of retained crystal violet was measured at 620 nm wavelengths using the FLUOstar® Omega microtiter-plate reader (BMG LABTECH, Ortenberg, Germany). All biofilm experiments were done five times for each isolate to minimize variability. Additionally, confocal laser scanning microscopy was used to confirm biofilm formation.
To measure viable bacteria in biofilms, the supernatant containing planktonic cells was aspirated. The quantification of viable bacteria counts of S epidermidis and S aureus was assessed by serial dilutions; 100 µL of each dilution was plated onto blood agar plates. After 48 hours incubation at 37 °C, growth of colony forming units was counted.
Detection of eDNA
Measurements of eDNA were performed on 24-hour biofilms using two methods: (1) fluorescence using 4′, 6-diamidino-2-phenylindole (DAPI), and (2) extraction of eDNA using the polymer mediated enrichment kit.
DAPI
DAPI is a fluorescence stain method to observe the DNA in cells. This staining method does not penetrate living bacterial cells such as staphylococci and was chosen to establish the amount of eDNA in living biofilms [7]. This procedure was performed overnight in black-bottom flat-well plates (Corning® Costar®; Corning Life Sciences). Biofilms grown for 24 hours were washed twice with PBS, air dried, and then stained with DAPI dissolved in PBS: 2 drops DAPI in 1 mL PBS (NucBlue® Fixed Cell Ready Probes® Reagent; Thermo Fisher Scientific, Waltham, MA, USA). To avoid cell penetration and thus staining intracellular DNA, staining was applied for 5 minutes only. The fluorescence of free eDNA in biofilms was measured immediately using the FLUOstar® Omega microplate reader at 355/460 nm.
Extraction Using the Polymer Mediated Enrichment Kit
In this method 24-hour biofilms of the same isolate were scraped and pooled in 200 µL PBS. After vortexing for 20 seconds to break up the biofilms, the suspension was filtered using 0.22 Millex®-GS (Merck Millipore Ltd, Tullagreen, Ireland) filters in a glass tube. eDNA was extracted using the commercially available Polymer Mediated Enrichment Free-Circulating DNA Extraction Kit (Analytik Jena AG, Jena, Germany) according to the manufacturer’s instructions. The amount of extracted eDNA was measured using the NanoDropTM 2000c Spectrophotometer (Thermo Fisher Scientific).
Confocal Laser Scanning Microscopy
Observation of 24-hour Biofilm Development in µ-Dishes in Dead Bacteria (reference strains)
To assess the role of eDNA during 24-hour biofilm development, 1 mL of each reference (diluted in Brain Heart Infusion Medium 1.5 × 107), was cultivated in single 24-well Ibidi µ-Dishes (Ibidi Treat 1, 5 polymer coverslip, tissue culture treated; Ibidi GmbH, Planegg/Martinsried, Germany). Biofilms were grown at 37 °C for 24 hours on an orbital shaker. Every hour one well was taken off for further investigation. Biofilms were washed two times in PBS and fixed with 4% glutaraldehyde. First we observed general DNA production and its structure at different times of biofilm formation. Therefore, propidium iodide was used to observe the dense DNA of the dead bacteria (Molecular Probes®; Thermo Fisher Scientific). Polysaccharides, representing the most characteristic fraction of the extrapolymeric substances, were stained using concanavalin-A (ConA) (Sigma-Aldrich Corp, St Louis, MO, USA).
eDNA Observation on 6- and 24-hour Biofilms
To observe eDNA expression and its distribution in live biofilms, TOTO®-1 and SYTO® 60 (Molecular Probes®) were used as recommended [24]. The working concentration of TOTO®-1 was 2 µmol/L and the counterstain SYTO® 60 was used at a concentration of 10 µmol/L. TOTO®-1 stains the free eDNA surrounding living and dead cells, whereas SYTO® 60 is able to penetrate only the cell wall of dead cells and stains the contained DNA red. Biofilms were grown in Ibidi 96-well µ-plates for optical microscopy. Confocal laser scanning microscopy was done after 6 and 24 hours. We used the same settings and region of interest for every investigation. The images were made using the LSM 780 confocal microscope system (Zeiss, Oberkochen, Germany). ImageJ (https://imagej.nih.gov/ij/) was used to score the images of stained biofilms. Every measurement was made in triplicate.
Statistical Methods
Descriptive statistics, IBM SPSS® Version 23.0 (IBM Corp, Armonk, NY, USA) was used to analyze the data. The unpaired two-sided Student’s t-test was used to compare eDNA production of the two staphylococcal species depending on the antibiotic exposure (control and clinical). Spearman’s rank correlation was used to assess eDNA production and biofilm thickness. Probability values less than 0.05 were considered statistically significant.
Results
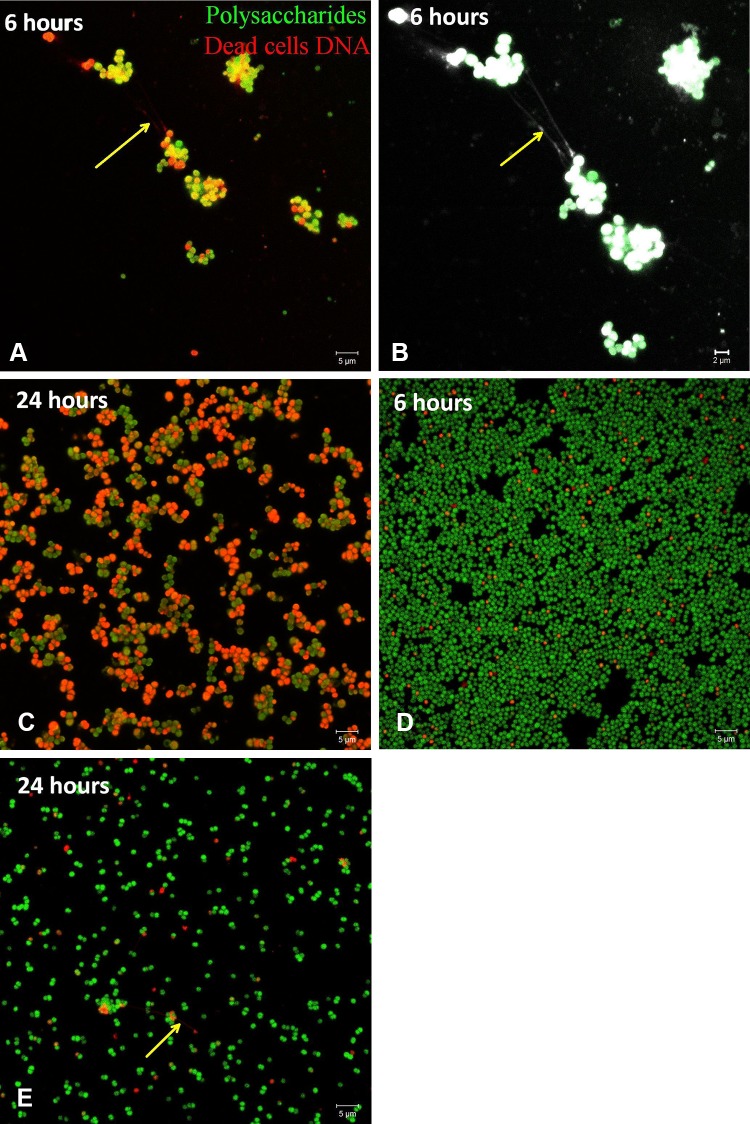
During the 24-hour observation of the reference strains we observed differences in the pattern of biofilm formation: S aureus ATCC25923 aggregated and formed various grape-like aggregations of bacterial cells coated by single polysaccharides before spreading on the surface and forming a biofilm layer (Fig. 1A–B). In contrast, S epidermidis biofilms started with scattered cells spreading over the surface until reaching confluence and their maximum thickness at 24 hours without forming grapes or clusters (Fig. 1C–D). Most interestingly, we observed tiny filamentous bond-like structures between dead cells at different stages depending on the staphylococcal species. In S aureus biofilms the filaments were detected mainly after 5 to 6 hours of biofilm formation (Fig. 1A–B, arrow), whereas the filaments in S epidermidis biofilms were detected after 24 hours of biofilm formation (Fig. 1E, arrow).
Fig. 1A–E.
(A) The S aureus biofilm after 6 hours of incubation shows grape-like groups and suspected eDNA filaments (arrow) before spreading. (B) A magnification is shown of Illustration A, using a filter to better observe the filamentous bonds (arrow). (C) After 24 hours of incubation, a biofilm layer is forming. (D) The S epidermidis biofilm after 6 hours of incubation shows scattered cells over the surface. (E) After 24 hours of incubation, suspected eDNA filaments (arrow) are produced. (Original magnification, ×1000; scale bar: 5 µm).
eDNA staining of 24-hour biofilms with TOTO®-1 revealed less eDNA in biofilms of all S aureus isolates than eDNA in biofilms of all S epidermidis isolates (S aureus mean ± SD: 1.35% ± 2.0%; S epidermidis mean ± SD: 6.42% ± 10.6%; p < 0.05). The same staining showed greater production of eDNA in all clinical isolates at 6 hours regardless of their species (clinical isolates mean ± SD: 1.84% ± 1.31%; control mean ± SD: 1.17% ± 1.37%; p < 0.005). However, only biofilms of S aureus isolates showed differences in eDNA production at 6 and 24 hours (6-hour biofilms: clinical isolates mean ± SD: 1. 97% ± 1.51%; control mean ± SD: 0.88% ± 0.72%; p < 0.005; 24-hour biofilms: clinical isolates mean ± SD: 1.83% ± 2.30%; control mean ± SD: 0.38% ± 0.19%; p < 0.05). The amount of eDNA decreased in clinical and control biofilms of S aureus at 24 hours.
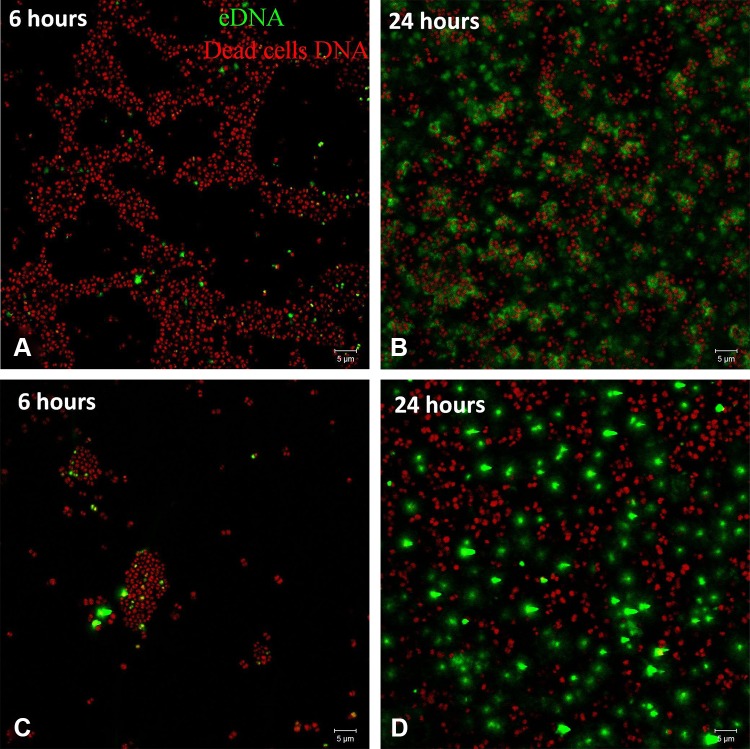
For S epidermidis there was no difference of eDNA between clinical isolates and controls (6-hour clinical isolates mean ± SD: 1.71% ± 1.07%; control mean ± SD: 1.45% ± 1.78%; 24-hour clinical isolates mean ± SD: 6.98% ± 12.62%; control mean ± SD: 5.3% ± 4.57%; (Fig. 2). The amount of eDNA at 24-hours was increased in clinical and control biofilms compared with 6-hour biofilms. A dense net of filamentous structures stained green with TOTO®-1 was seen on the confocal laser scanning microscopy image of S epidermidis on the 24-hour biofilms.
Fig. 2A–D.
Clinical S epidermidis biofilms after (A) 6 hours and (B) 24 hours, and control isolates of S epidermidis biofilms after (C) 6 hours (D) and 24 hours are shown. The amount of dead cells DNA (red) measured after 6 hours was higher (p < 0.005) on biofilms of clinical isolates of S epidermidis than for control isolates. Generally eDNA (green) production was higher (p < 0.05) on all biofilms of clinical isolates than on control isolates. (Original magnification, ×1000; scale bar: 5 µm).
Regarding polymer mediated enrichment extraction on biofilms, all clinical isolates showed greater amounts of eDNA than biofilms of control isolates (clinical isolates mean ± SD: 88.6 ± 14.8 ng/µL versus control isolates mean ± SD: 69.6 ± 20.6 ng/µL; p < 0.005). In contrast, there was no difference in eDNA production between clinical and control biofilms using the fluorescence DAPI stain (clinical isolates mean ± SD: 2551 ± 909 fluorescence units; control mean ± SD: 2500 ± 1419 fluorescence units) (Table 1). Comparing clinical and control isolates of S aureus and S epidermidis, both methods showed concordant results: the amount of eDNA in biofilms of S aureus clinical isolates was greater than on biofilms of S aureus control isolates (p < 0.001) (Table 1). However, DAPI but not polymer mediated enrichment showed that eDNA in biofilms of S epidermidis clinical isolates was lower than on biofilms of S epidermidis control isolates (mean ± SD, 3292 ± 1520 fluorescence units; p < 0.05) (Table 1).
Table 1.
Amounts of eDNA measured for clinical and control S aureus and S epidermidis
| Type of isolate | DAPI (fluorescence units) | PME (ng/µL) | Confocal laser scanning microscopy mean % area eDNA (TOTO®-1; green)* | |
|---|---|---|---|---|
| 6-hour | 24-hour | |||
| S aureus (n = 45) | 2446 ± 2577 | 74.9 ± 21.6 | 1.61 ± 1.39 | 1.35 ± 2.00 |
| S epidermidis (n = 45) | 2622 ± 1113 | 89.6 ± 12.7 | 1.62 ± 1.33 | 6.42 ± 10.60 |
| No difference | No difference | No difference | p = 0.01 | |
| S aureus | ||||
| Clinical isolates (n = 30) | 2814 ± 1058 | 84 ± 18.12 | 1.97 ± 1.51 | 1.83 ± 2.30 |
| Control isolates (n = 15) | 1707 ± 720 | 57 ± 16.2 | 0.88 ± 0.72 | 0.38 ± 0.19 |
| p = 0.001 | p = 0.001 | p = 0.002 | p = 0.01 | |
| S epidermidis | ||||
| Clinical isolates (n = 30) | 2287 ± 647 | 93 ± 8.5 | 1.71 ± 1.07 | 6.98 ± 12.62 |
| Control isolates (n = 15) | 3292 ± 1520 | 83 ± 16.4 | 1.45 ± 1.78 | 5.3 ± 4.57 |
| p = 0.005 | p = 0.005 | No difference | No difference | |
| Clinical isolates (n = 60) | 2551 ± 909 | 88.6 ± 14.8 | 1.84 ± 1.31 | 4.41 ± 9.36 |
| Control isolates (n = 30) | 2500 ± 1419 | 69.6 ± 20.6 | 1.17 ± 1.37 | 2.84 ± 4.04 |
| No difference | p = 0.001 | p = 0.03 | No difference | |
*Cyanine dimer TOTO®-1 to stain extracellular DNA (green); DAPI = 4’,6-diamidino-2-phenylindole; PME = polymer mediated enrichment.
Biofilm thickness determined using optical density depended mainly on the species, and whether the isolate was a clinical isolate or a control isolate from the skin of healthy control subjects. There were no differences between optical densities of biofilms of S epidermidis (0.42 ± 0.29) and S aureus (0.30 ± 0.19). Furthermore no differences between optical densities of biofilms of all clinical (0.40 ± 0.28) compared with control isolates (0.29 ± 0.18) were observed. The biofilm optical densities of S aureus clinical isolates (0.35 ± 0.2) were greater than those of S aureus control isolates (0.2 ± 0.13; p < 0.005). The biofilm optical densities of S epidermidis control isolates (0.37 ± 0.17) were greater than those of S aureus control isolates (0.2 ± 0.13; p < 0.005). Spearman’s rank correlation showed no relationship between production of eDNA by S aureus (clinical and control isolates) and biofilm production (rho = 0.29; p = 0.05). Similarly, no relationship was observed using Spearman’s rho between production of eDNA by S epidermidis (clinical and control isolates) and biofilm (rho = 0.12; p = 0.42).
Antibiotic susceptibility testing revealed less susceptibility to antimicrobials among the clinical isolates compared with controls. The clinical S epidermidis isolates were resistant to oxacillin (15/30), rifampicin (nine of 30), and ciprofloxacin (14/30). Control S epidermidis isolates were resistant to oxacillin and ciprofloxacin (three of 15). In comparison, clinical S aureus was resistant to oxacillin (eight of 30), ciprofloxacin (seven of 30), and rifampicin (one of 30). All tested isolates were sensitive to vancomycin (Table 2).
Table 2.
Antimicrobial characteristics of the clinical and control isolates
| Antibiotic resistance | Knee prosthesis n = 15 |
Hip prosthesis n = 15 |
Controls (nose) n = 15 |
Knee prosthesis n = 15 |
Hip prosthesis n = 15 |
Controls (cubital fossa) n =15 |
|---|---|---|---|---|---|---|
| Oxacillin resistant | 4 (27%) | 4 (27%) | 0 | 7 (47%) | 8 (53%) | 3 (20%) |
| Rifampicin resistant | 0 | 1 (7%) | 0 | 5 (33%) | 4 (27%) | 0 |
| Ciprofloxacin resistant | 2 (13%) | 5 (33%) | 0 | 7 (47%) | 7 (47%) | 3 (20%) |
| Vancomycin resistant | 0 | 0 | 0 | 0 | 0 | 0 |
Discussion
eDNA in biofilms plays an important role in biofilm formation and maturation [4, 18]. It is actively produced by a small bacterial population, and is essential for biofilm structure and stability [27]. In addition to its role in biofilm structure and stability, eDNA serves an additional role in antimicrobial resistance [7]. As such, eDNA may play a potential role in the diagnosis and treatment of biofilm-related infections. Using confocal laser scanning microscopy for observation, and separate methods to observe and quantify DNA and eDNA we asked (1) whether DNA structure changes during biofilm formation, (2) are there time-dependent differences in eDNA production during biofilm formation, (3) is there differential eDNA production between clinical and control Staphylococcal isolates, and (4) is eDNA production correlated to biofilm thickness?
One limitation of our study is that the observation method using confocal microscopy and the extraction method cannot be directly compared although both methods were used for different purposes. The extraction method is robust and may be used for experiments in future clinical studies. Additionally, the fluorescent stain SYTO® 60 has a very short half-life making quantification problematic. The DAPI stain, which we intentionally used to selectively stain eDNA proved to be quite nonspecific in contrast to previous reports where DAPI penetrated the cells only after longer staining times [7, 16]. Finally, the use of brain heart infusion broth as the growth medium could be seen as a limitation since, in general, tryptic soy broth medium is used to enhance biofilm formation of S epidermidis producing polysaccharide intercellular adhesion [30]. Knowing that alternative pathways of biofilm formation based on the extracellular matrix binding protein (Embp) or through the release of eDNA are present, we opted for brain heart infusion broth based on our previous experiences with this medium [11, 12].
Propidium iodide stains DNA either in dead cells or dense eDNA structures [24]. Overall, eDNA is pivotal for the formation of biofilms in clinical isolates of S epidermidis and S aureus [22]. We observed filamentous structures stained by propidium iodide in S aureus (Fig. 1A, arrow) and S epidermidis, (Fig. 1D, arrow). Similar structures were observed in live biofilms of S epidermidis stained with TOTO®-1. TOTO®-1 stain is not cell permeable and exhibits a high DNA binding affinity owing to its four positive charges [24]. Furthermore, TOTO®-1 stain has no time constraints and it shows high sensitivity for observation of tiny and delicate eDNA structures. It may be possible that these filaments represent eDNA forming a network, as described by Dengler et al. [6].
TOTO®-1 staining to observe and quantify eDNA using confocal microscopy showed greater eDNA production in S epidermidis than in S aureus at 24 hours. An explanation for this finding may be that S aureus, being mostly a transient part of the human microbiota, starts biofilm formation only under certain conditions [23], whereas S epidermidis as a constant part of the human skin microbiota, relies on the constant production of eDNA to stabilize biofilms and to persist on different surfaces such as the human skin [10]. At 6 hours we detected substantially higher amounts of eDNA in biofilms of clinical isolates of S aureus and S epidermidis compared with control isolates, but not at 24 hours owing to the higher variance. The high amount of eDNA at 6 hours confirms previous studies in which S aureus used eDNA in the initial attachment and accumulation phase [15, 20, 29] . Qin et al. [27] indirectly supported this finding, stating that DNase, as DNA cleaving enzyme, was able to disturb or negatively influence biofilms during the first 6 hours of biofilm formation.
In the current study, biofilm thickness of all isolates was assessed using optical density measurements after crystal violet staining. According to our results, biofilm thickness did not correlate with eDNA production, as reported by Doroshenko et al. [7]. Biofilms of clinical isolates showed no differences in optical densities compared with biofilms of control isolates. Confocal laser scanning microscopy was most useful showing the growth pattern during biofilm formation.
Information regarding eDNA production of clinical isolates is scarce and our results indicate that clinical isolates of S aureus und S epidermidis produced substantially more eDNA than control isolates. Staining with TOTO®-1 and SYTO® 60 allowed observation and quantification of eDNA using confocal laser scanning microscopy. Further research is needed to determine whether the amount of eDNA produced by clinical isolates might be considered as an additional diagnostic tool in staphylococcal biofilm infections. eDNA also might be a future target to modify or disrupt biofilms. Finally, characterizing pathogens by their eDNA production may serve as an outcome predictor of prosthetic joint infections and thus play a role in treatment decisions.
Acknowledgments
Open access funding provided by Medical University of Vienna. We thank Hirschl Alexander MD, PhD (Department Clinical Microbiology, Medical University of Vienna, Vienna, Austria), for providing the clinical staphylococcal isolates.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
A comment to this article is available at http://dx.doi.org/10.1007/s11999-017-5360-3.
References
- 1.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 3.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das T, Sharma PK, Busscher HJ, van der Mei HC, Krom BP. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl Environ Microbiol. 2010;76:3405–3408. doi: 10.1128/AEM.03119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254–3262. doi: 10.1007/s11999-014-3543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dengler V, Foulston L, DeFrancesco AS, Losick R. An electrostatic net model for the role of extracellular DNA in biofilm formation by Staphylococcus aureus. J Bacteriol. 2015;197:3779–3787. doi: 10.1128/JB.00726-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doroshenko N, Tseng BS, Howlin RP, Deacon J, Wharton JA, Thurner PJ, Gilmore BF, Parsek MR, Stoodley P. Extracellular DNA impedes the transport of vancomycin in Staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob Agents Chemother. 2014;58:7273–7282. doi: 10.1128/AAC.03132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fey PD. Modality of bacterial growth presents unique targets: how do we treat biofilm-mediated infections? Curr Opin Microbiol. 2010;13:610–615. doi: 10.1016/j.mib.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furustrand Tafin U, Betrisey B, Bohner M, Ilchmann T, Trampuz A, Clauss M. Staphylococcal biofilm formation on the surface of three different calcium phosphate bone grafts: a qualitative and quantitative in vivo analysis. J Mater Sci Mater Med. 2015;26:130. doi: 10.1007/s10856-015-5467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galdbart JO, Allignet J, Tung HS, Ryden C, El Solh N. Screening for Staphylococcus epidermidis markers discriminating between skin-flora strains and those responsible for infections of joint prostheses. J Infect Dis. 2000;182:351–355. doi: 10.1086/315660. [DOI] [PubMed] [Google Scholar]
- 11.Gattringer KB, Suchomel M, Eder M, Lassnigg AM, Graninger W, Presterl E. Time-dependent effects of rifampicin on staphylococcal biofilms. Int J Artif Organs. 2010;33:621–626. doi: 10.1177/039139881003300907. [DOI] [PubMed] [Google Scholar]
- 12.Hajdu S, Holinka J, Reichmann S, Hirschl AM, Graninger W, Presterl E. Increased temperature enhances the antimicrobial effects of daptomycin, vancomycin, tigecycline, fosfomycin, and cefamandole on staphylococcal biofilms. Antimicrob Agents Chemother. 2010;54:4078–4084. doi: 10.1128/AAC.00275-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 14.Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res. 2011;29:617–622. doi: 10.1002/jor.21286. [DOI] [PubMed] [Google Scholar]
- 15.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74:470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MB, Criss AK. Fluorescence microscopy methods for determining the viability of bacteria in association with mammalian cells. J Vis Exp. 2013;79:e50729. doi: 10.3791/50729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19:1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan JB, Jabbouri S, Sadovskaya I. Extracellular DNA-dependent biofilm formation by Staphylococcus epidermidis RP62A in response to subminimal inhibitory concentrations of antibiotics. Res Microbiol. 2011;162:535–541. doi: 10.1016/j.resmic.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauderdale KJ, Malone CL, Boles BR, Morcuende J, Horswill AR. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J Orthop Res. 2010;28:55–61. doi: 10.1002/jor.20943. [DOI] [PubMed] [Google Scholar]
- 20.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PloS One. 2009;4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer C, Moritz R, Kirschner C, Borchard W, Maibaum R, Wingender J, Flemming HC. The role of intermolecular interactions: studies on model systems for bacterial biofilms. Int J Biol Macromol. 1999;26:3–16. doi: 10.1016/S0141-8130(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 22.Montanaro L, Poggi A, Visai L, Ravaioli S, Campoccia D, Speziale P, Arciola CR. Extracellular DNA in biofilms. Int J Artif Organs. 2011;34:824–831. doi: 10.5301/ijao.5000051. [DOI] [PubMed] [Google Scholar]
- 23.Moormeier DE, Bose JL, Horswill AR, Bayles KW. Temporal and stochastic control of Staphylococcus aureus biofilm development. MBio. 2014;5:e01341–14. [DOI] [PMC free article] [PubMed]
- 24.Okshevsky M, Meyer RL. Evaluation of fluorescent stains for visualizing extracellular DNA in biofilms. J Microbiol Methods. 2014;105:102–104. doi: 10.1016/j.mimet.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Okshevsky M, Meyer RL. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol. 2015;41:341–352. doi: 10.3109/1040841X.2013.841639. [DOI] [PubMed] [Google Scholar]
- 26.Okshevsky M, Regina VR, Meyer RL. Extracellular DNA as a target for biofilm control. Curr Opin Biotechnol. 2015;33:73–80. doi: 10.1016/j.copbio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, Qu D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007;153:2083–2092. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- 28.Ravaioli S, Campoccia D, Visai L, Pirini V, Cangini I, Corazzari T, Maso A, Poggio C, Pegreffi F, Montanaro L, Arciola CR. Biofilm extracellular-DNA in 55 Staphylococcus epidermidis clinical isolates from implant infections. Int J Artif Organs. 2011;34:840–846. doi: 10.5301/ijao.5000057. [DOI] [PubMed] [Google Scholar]
- 29.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2007;104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohde H, Frankenberger S, Zahringer U, Mack D. Structure, function and contribution of polysaccharide intercellular adhesin (PIA) to Staphylococcus epidermidis biofilm formation and pathogenesis of biomaterial-associated infections. Eur J Cell Biol. 2010;89:103–111. doi: 10.1016/j.ejcb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Sharma-Kuinkel BK, Mann EE, Ahn JS, Kuechenmeister LJ, Dunman PM, Bayles KW. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J Bacteriol. 2009;191:4767–4775. doi: 10.1128/JB.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly. 2005;135:243–251. doi: 10.4414/smw.2005.10934. [DOI] [PubMed] [Google Scholar]