Abstract
Background
TKA commonly involves substantial blood loss and tranexamic acid has been used to reduce blood loss after TKA. Numerous clinical trials have documented the efficacy and safety of intravenous (IV) or intraarticular (IA) use of tranexamic acid. Combined administration of tranexamic acid also has been suggested; however, there is no consensus regarding the ideal route of tranexamic acid administration.
Questions/Purposes
(1) To compare the efficacy of tranexamic acid in terms of total blood loss and the allogeneic transfusion rate among three routes of administration: IV alone, IA alone, and combined IV and IA. (2) To compare these regimens in terms of venous thromboembolism (VTE) and the frequency of wound complications.
Methods
In total, 376 patients undergoing TKA between March 2014 and March 2015 were randomized to four groups by the route of tranexamic acid administration: IV only, IA only, low-dose combined (IV + IA injection of 1 g), and high-dose combined (IV + IA injection of 2 g). The calculated total blood loss, allogeneic transfusion rate, decrease in hemoglobin, the frequency of symptomatic deep vein thrombosis and pulmonary embolism, wound complications, and periprosthetic joint infection were compared among the groups. Total blood loss was calculated using estimated total body blood volume and hemoglobin loss. The decision regarding when to transfuse was determined based on preset criteria.
Results
The high- and low-dose combined groups and the IA-only group had lower total blood loss (564 ± 242 mL, 642 ± 242 mL, and 633 ± 205 mL, respectively) than the IV-only group (764 ± 217 mL; mean differences = 199 mL [95% CI, 116–283 mL], p < 0.001; 121 mL [95% CI, 38–205 mL], p = 0.001; 131 mL [95% CI, 47–214 mL], p < 0.001); no differences were found among the other three groups. No patients in any study group received an allogeneic transfusion. One patient in the IV-only group had a symptomatic pulmonary embolism develop, but no other symptomatic VTE events occurred in any group. In addition, no differences were observed in wound complications, such as superficial wound necrosis (one patient in the IV-only and the high-dose combined group, respectively) and oozing (IV-only, IA-only, low-dose combined, high-dose combined = 3%, 4%, 4%, and 7%; p = 0.572) between the groups. No patients had a periprosthetic joint infection.
Conclusion
IA tranexamic acid administration further reduces blood loss after TKA in comparison to IV use alone; no additional effect in further reducing blood loss was found in combination with IV tranexamic acid. Appropriately powered studies are needed to confirm the safety of this route of administration as the preferred route of administration in TKA.
Level of Evidence
Level I, therapeutic study.
Keywords: Tranexamic Acid, Wound Complication, Periprosthetic Joint Infection, Reduce Blood Loss, Total Blood Loss
Introduction
A TKA is a surgery that alleviates pain and improves function but involves substantial blood loss that might result in allogeneic blood transfusion [11, 32, 43]. Allogeneic blood transfusion exposes patients to risks like immunologic reactions and transmission of diseases [15], and is associated with substantial cost to the healthcare system. Numerous strategies have been suggested to address this issue [32], with the use of antifibrinolytics to control perioperative blood loss a popular method [36]. Tranexamic acid, a synthetic analog of the amino acid lysine that inhibits fibrinolysis by competitively blocking the lysine binding sites of plasminogen [12, 42], has found widespread use. Tranexamic acid has been found to reduce blood loss in TKA and THA without an increase in thromboembolic complications [23]. While there is consensus regarding the efficacy of tranexamic acid in reducing blood loss, numerous issues persist regarding the best regimen and route of administration [30].
Tranexamic acid can be administered during the perioperative period either intravenously (IV), intraarticularly (IA), or by a combination of these measures. IV administration of tranexamic acid is a common practice with evidence favoring its effectiveness in reducing blood loss and the rate of allogeneic transfusion use in patients undergoing TKA [7, 22, 49]. However, concerns regarding complications like thromboembolic events after systemic administration [40] and the need for a prolonged effect to reduce postoperative blood loss [8] have led to interest in the topical administration of tranexamic acid. In addition, several studies [24, 26, 33, 38] have explored the possibility of using combined administration of tranexamic acid considering the advantages of both methods, but to our knowledge, only one published study compared all three methods of tranexamic acid administration as a strategy for reducing blood loss after TKA [48].
The purpose of our study was to compare the efficacy of tranexamic acid in patients undergoing TKA in terms of total blood loss and the allogeneic transfusion rate among the three routes of administration: IV, IA, and combined IV and IA. In addition, we wanted to compare these regimens in terms of venous thromboembolism (VTE) and the frequency of wound complications.
Patients and Methods
This study was a randomized controlled study. After obtaining institutional review board approval, a total of 444 patients scheduled for elective unilateral primary TKA between March 2014 and March 2015 were assessed for eligibility (Fig. 1). Exclusion criteria were: a diagnosis other than primary osteoarthritis, an acquired or congenital coagulopathy, patients receiving current anticoagulation therapy, preoperative hepatic or renal dysfunction or severe ischemic heart disease, and a history of thromboembolic disease. A total of 396 patients were enrolled and randomized to one of the four tranexamic acid groups (IV-only, IA-only, low-dose combined, and high-dose combined groups) using a computer-generated randomization table with a permutation block of six (99 in each group). All the enrolled patients provided informed consent for this study.
Fig. 1.
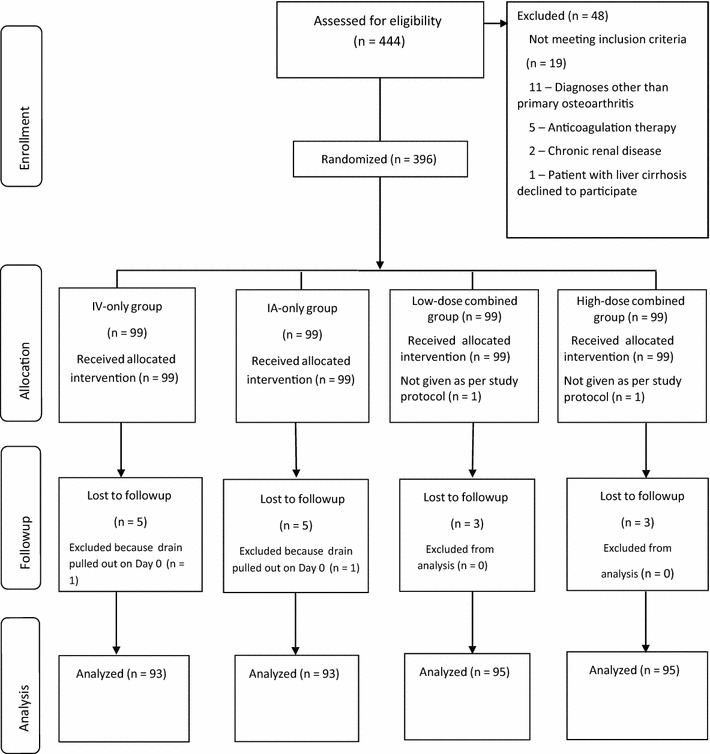
The flow diagram shows the number of patients assessed and included at each stage of the trial. IV = intravenous; IA = intraarticular.
Administration of Tranexamic Acid
Patients assigned to the IV-only group were given intraoperative tranexamic acid with a weight-adjusted dosage (10 mg/kg) 30 minutes before tourniquet deflation; the same dose was repeated 3 hours after surgery. The calculated dose of tranexamic acid was mixed in 100 mL of normal saline and given as a slow IV injection. For patients assigned to the IA-only group, 2 g of tranexamic acid in 30 mL of normal saline was injected in the joint after closure of the retinaculum and quadriceps tendon but before subcutaneous closure. Patients in the low-dose combined group were given an IA injection of 1 g tranexamic acid in 30 mL of normal saline in addition to weight-adjusted intraoperative and postoperative IV administrations. Similarly, patients in the high-dose combined group were given an IA injection of 2 g tranexamic acid in 30 mL of normal saline in addition to intraoperative and postoperative IV administration of tranexamic acid.
Perioperative Management and Surgical Procedures
All patients were managed using the same perioperative protocols. Patients underwent a systematic medical clearance protocol that identifies patients with anemia, which is defined as hemoglobin less than 12 g/dL for females and less than 13 g/dL for males. Patients with a diagnosis of anemia were prescribed oral iron unless other underlying diseases were suspected. Patients who were taking acetylsalicylic acid, antiplatelet agents, or nonselective cyclooxygenase inhibitors were asked to discontinue the medications 7 to 10 days before surgery.
All surgical procedures were performed by one surgeon (TKK) using the standard medial parapatellar approach with application of the aforementioned blood conservation protocol. All patients were given spinal anesthesia. A pneumatic tourniquet was used on all patients and was inflated with a safe margin of approximately 150 mm Hg above preinflation systolic blood pressure. Before implant fixation, the femoral canal was plugged with a bone block and all prostheses were fixed with cement. The patella was resurfaced in all patients. After completing prosthesis fixation, the tourniquet was deflated and arterial bleeding was cauterized. A closed suction drain was placed subcutaneously with the intention to reduce blood loss and wound complication risk [44]. A compressive dressing then was applied with a CryoCuff (Howmedicare, Seoul, Korea) and knee immobilizer during the first 24 hours. The drain was removed approximately 24 hours after surgery. Allogeneic blood transfusion was given if the hemoglobin level fell below 7.0 mg/dL or if anemic symptoms such as dyspnea or tachycardia persisted even after volume replacement in patients with hemoglobin levels between 7.0 and 8.0 mg/dL. This transfusion trigger was chosen based on several previous studies that reported that the use of a restrictive hematologic algorithm with the hemoglobin level of 7.0 g/dL as the trigger does not increase adverse events related to postoperative anemia [14, 20, 39]. When transfusion was indicated based on those criteria, 1 unit of packed red blood cells was transfused at a time to increase the hemoglobin level to 8.0 g/dL. IV iron and erythropoietin were administered to patients without anemic symptoms but with a hemoglobin level between 7.0 and 8.0 mg/dL. Preoperative autologous blood donation was not performed in any patient during the study period. Our routine protocol of discharge was the fifth day after unilateral TKA during the study period.
All patients were given thromboprophylaxis according to our individualized approach protocol. Patients were assessed preoperatively for the risk of pulmonary embolism or bleeding and classified in four risk-stratified categories. A designated postoperative thromboprophylactic regimen was administered for each of the four categories: (1) standard risk for pulmonary embolism and bleeding: intermittent pneumatic compression during admission and aspirin 100 mg once a day for 5 weeks; (2) elevated risk for pulmonary embolism and standard risk for bleeding: intermittent pneumatic compression during admission and 10 mg rivaroxaban once a day for 10 days followed by 100 mg aspirin once a day for 25 days; (3) standard risk for pulmonary embolism and elevated risk for bleeding: intermittent pneumatic compression only during admission; and (4) elevated risk for pulmonary embolism and bleeding: intermittent pneumatic compression during admission and 100 mg aspirin once a day for 5 weeks. The risk-stratified categories were not considered in the randomization process to designate the four study groups.
Outcome Measures
The primary outcome measures were total blood loss, allogeneic transfusion rate, and incidence of VTE events. The total blood loss was calculated using a formula described in previous studies, which is based on patient blood volume and decrease in hemoglobin [18, 31, 37]. Blood volume was estimated according to the formula of Nadler et al. [37], which took body mass, height, and sex into account. Decrease in hemoglobin was defined as the difference between the preoperative hemoglobin level and the postoperative hemoglobin level on the fifth postoperative day [18], when the hemoglobin level was typically the lowest in our patient population or when the hemoglobin level was lowest before blood transfusion [29]. Patients who were given an allogeneic transfusion, which had been decided with strict transfusion criteria applied, were noted. All symptomatic VTE events were meticulously noted. Routine screening for deep vein thrombosis was not performed. CT angiography was done for patients with suspicious symptoms including pain, swelling, and tenderness in the thigh or calf and consultations with hematologists were sought to confirm the diagnosis and to plan treatment for diagnosed VTE events.
The secondary outcomes were postoperative decrease in hemoglobin, the proportions of patients with hemoglobin lower than 7.0, 8.0, and 9.0 g/dL, and the proportions of patients with wound complications, including deep periprosthetic joint infection.
To calculate the postoperative decrease in hemoglobin, we subtracted the hemoglobin level on postoperative Day 5 from the preoperative hemoglobin level unless patients were given an allogeneic transfusion. If patients received a transfusion, the lowest hemoglobin level before transfusion was used for the calculation. As certain cutoff values of the hemoglobin level are frequently used as transfusion triggers, the proportions of patients with hemoglobin levels less than 7.0, 8.0, and 9.0 g/dL were calculated. Wound complications, namely, oozing, superficial wound necrosis, and periprosthetic joint infection, were noted.
Statistical Analysis
The four groups were compared based on the primary and secondary outcome variables. ANOVA with post hoc Tukey’s honestly significant difference test was used for continuous variables (total blood loss, decrease in hemoglobin,), and the chi-square test was used for categorical variables (allogeneic transfusion incidence, proportions of patients with hemoglobin levels lower than 7.0, 8.0, and 9.0 g/dL, and the incidences of wound complications and VTE events). Statistical analyses were performed using SPSS Version 20.0 for Windows (IBM Corp, Armonk, NY, USA), and p values less than 0.05 were considered significant. The statistical power of the study was calculated by G-Power software (Version 3.1.9.2; Heinrich-Heine-Universität Düsseldorf, Dusseldorf, Germany) and found to be 0.98. The sample size was determined based on the difference in the primary outcome, which was calculated total blood loss. Based on preliminary data, the difference of calculated total blood loss between groups was 101 mL with a SD of 227 mL. Sample size estimation at an alpha of 0.05 and power of 80% indicated that 79 patients would be required for each arm. To compensate for the expected dropouts (15%), we needed 99 patients per group. The patients and a clinical investigator (SC) who prospectively collected all of the clinical information were unaware of the group identities until the final data analysis.
There were no differences across the groups in terms of demographic data or preoperative hematologic values (Table 1). Of the 444 patients assessed, 48 were excluded for the following reasons: 11 for diagnoses other than primary osteoarthritis, five owing to use of anticoagulation therapy, two with chronic renal disease, one with liver cirrhosis, and 29 who declined to participate (Fig. 1). A total of 396 patients (knees) undergoing unilateral TKA were enrolled. Twenty patients were excluded from final analysis owing to various reasons (eg, two patients because the dose was not given per protocol; 16 were lost to followup, and two pulled out the suction drain) leaving a total of 376 patients (knees) included in the final analysis: IV only (IV-only group, n = 93), IA only (IA-only group, n = 93), IV and IA injection of 1 g (low-dose combined group, n = 95), and IV and IA injection of 2 g (high-dose combined group, n = 95) tranexamic acid in 30 mL of normal saline.
Table 1.
Demographic variables and preoperative hematologic values*
| Variable | IV-only (n = 93) | IA-only (n = 93) | Low-dose combined (n = 95) | High-dose combined (n = 95) | p Value# |
|---|---|---|---|---|---|
| Sex (female) | 87 (94%) | 88 (95%) | 83 (87%) | 88 (93%) | 0.261 |
| Age (years) | 73.4 ± 6.2 | 72.3 ±7.4 | 72.6 ± 6.3 | 72.1 ± 6.9 | 0.511 |
| Height (cm) | 152.3 ± 6.1 | 152.6 ±6.5 | 153.7 ±7.3 | 152.7 ± 6.2 | 0.525 |
| Weight (kg) | 62.4 ± 8.6 | 62.3 ±9.6 | 63.1 ± 9.4 | 64.6 ± 10.7 | 0.346 |
| BMI (kg/m2) | 26.9 ± 3.6 | 26.71 ±3.3 | 26.7 ± 3.6 | 27.7 ± 4.2 | 0.194 |
| Preoperative Hb (g/dL) | 13.2 ± 1.2 | 12.9 ± 1.3 | 13.2 ± 0.9 | 12.8 ± 1.3 | 0.124 |
*Data are presented as means ± SD or as number of patients (%); #p values were calculated using ANOVA for continuous variables (age, height, weight, BMI, and Hb) and chi-square test for the categorical variable (gender); IV = intravenous, IA = intraarticular, Hb = hemoglobin.
Results
The combined use of IV and IA administration of tranexamic acid reduced blood loss more than IV use alone. IA use alone also resulted in improved blood loss reduction compared with IV use alone; these results were similar to those of the combined use groups (Table 2). The high-dose and low-dose combined groups and the IA-only group had less total blood loss (564 ± 242 mL, 642 ± 242 mL, and 633 ± 205 mL, respectively) than the IV-only group (764 ± 217 mL; mean differences = 199 mL, [95% CI, 116–283 mL], p < 0.001; 121 mL, [95% CI, 38–205 mL], p = 0.001]; 131 mL, [95% CI, 47–214 mL], p < 0.001), and no differences were found among the three groups that received IA tranexamic acid (p > 0.05) (Table 3). The high-dose and the low-dose combined groups and the IA-only group had smaller decreases in hemoglobin (2.0 ± 0.8 g/dL, 2.3 ± 0.8 g/dL, 2.4 ± 0.8 g/dL, respectively) than the IV-only group (2.9 ± 0.9 g/dL; mean differences = 0.9 g/dL, [95% CI, 0.5–1.2 g/dL], p < 0.001; 0.5 g/dL, [95% CI, 0.2–0.9 g/dL], p = 0.001; 0.5 g/dL, [95% CI, 0.2–0.8 g/dL], p < 0.001), and the high-dose combined group had a smaller decrease in hemoglobin than the low-dose combined group or the IA-alone group (mean differences = 0.3 g/dL, [95% CI, 0–0.6 g/dL], p = 0.047; 0.3 g/dL, [95% CI, 0–0.6 g/dL], p = 0.033) (Table 4). No patients in any of the four groups received an allogeneic transfusion and no differences were found among the groups in the proportions of patients with hemoglobin less than 7.0 g/dL, 8.0 g/dL, and 9.0 g/dL (Table 2).
Table 2.
Efficacy of outcome variables*
| Variable | IV-only group (n = 93) | IA-only group (n = 93) | Low-dose combined group (n = 95) | High-dose combined group (n = 95) | p Value |
|---|---|---|---|---|---|
| Total blood loss (mL) | 764 ± 217 | 633 ± 205 | 642 ± 242 | 564 ± 242 | < 0.001 |
| Hb drop (g/dL) | 2.9 ± 0.9 | 2.4 ± 0.8 | 2.3 ± 0.8 | 2.0 ± 0.8 | < 0.001 |
| Allogeneic transfusion | 0 | 0 | 0 | 0 | NA |
| Patients with Hb < 7.0 g/dL | 0 | 0 | 0 | 0 | NA |
| Patients with Hb < 8.0 g/dL | 1 (1%) | 2 (2%) | 0 | 0 | 0.288 |
| Patients with Hb < 9.0 g/dL | 8 (9%) | 6 (7%) | 3 (3%) | 5 (5%) | 0.449 |
*Data presented as means ± SD or number of patients (%); #calculated using ANOVA for continuous variables (total blood loss, blood loss via drain, Hb drop) and chi-square test for categorical variables (patients with Hb < 8.0 g/dL or < 9.0 g/dL); IV = intravenous, IA = intraarticular, Hb = hemoglobin, NA = not applicable.
Table 3.
Total blood loss comparisons
| Variable | IV-only group | IA-only group | Low-dose combined group | High-dose combined group | p Value* |
|---|---|---|---|---|---|
| Total blood loss (mL)† | 764 ± 217 | 633 ± 205 | 642 ± 242 | 564 ± 242 | < 0.001 |
| Post hoc test‡ | |||||
| IV only | −131 (47–214; p < 0.001) | −121 (−205 to −38; p = 0.001) | −199 (−283 to −116; p < 0.001) | ||
| IA only | 131 (47–214; p < 0.001) | 9 (−74 to 93; p = 0.992) | −69 (−152 to 15; p = 0.146) | ||
| Low-dose combined | 121 (38–205; p = 0.001) | −9 (−92 to 74; p = 0.992) | −78 (−161 to 5; p = 0.073) | ||
| High-dose combined | 199 (116–283; p < 0.001) | 69 (−15 to 152; p = 0.146) | 78 (−5 to 161; p = 0.073) |
*Using ANOVA for comparison among groups; †values are expressed as mean ± SD; ‡values are expressed as mean difference (95% CI; p value) using Tukey’s Honestly Significant Difference test; IV = intravenous, IA = intraarticular.
Table 4.
Hemoglobin drop comparisons
| Variable | IV-only group | IA-only group | Low-dose combined group | High-dose combined group | p Value* |
|---|---|---|---|---|---|
| Hb drop (g/dL)† | 2.9 ± 0.9 | 2.4 ± 0.8 | 2.3 ± 0.8 | 2.0 ± 0.8 | < 0.001 |
| Post hoc test‡ | |||||
| IV-only | −0.5 (−0.8 to −0.2; p < 0.001) | −0.5 (−0.9 to −0.2; p < 0.001) | −0.9 (−1.2 to −0.5; p < 0.001) | ||
| IA-only | 0.5 (0.2–0.8; p < 0.001) | 0 (−0.3 to 0.3; p = 0.999) | −0.3 (−0.6 to 0; p = 0.033) | ||
| Low-dose combined | 0.5 (0.2–0.9; p < 0.001) | 0 (−0.3 to 0.3; p = 0.999) | −0.3 (−0.6 to 0; p = 0.047) | ||
| High-dose combined | 0.9 (0.5–1.2; p < 0.001 | 0.3 (0–0.6; p = 0.033) | 0.3 (0–0.6; p = 0.047) | ||
*Using ANOVA for comparison among groups; †values are expressed as mean ± SD; ‡values are expressed as mean difference (95% CI; p value) using Tukey’s Honestly Significant Difference test; IV = intravenous, IA = intraarticular.
The proportions of patients with symptomatic deep vein thrombosis, pulmonary embolism, and wound complication were extremely low in all study groups and no notable group differences were observed (Table 5). Only one patient in the IV-only group had a symptomatic pulmonary embolism, which was treated with low molecular weight heparin followed by warfarin without any residual sequelae. No other patients had symptomatic deep vein thrombosis or other thromboembolic events. Only minor wound complications (superficial wound necrosis and oozing) occurred, and these were uncommon in all groups (range, 3%–7%), with no between-group differences. No patients in any group had a deep periprosthetic joint infection develop during the surveillance period.
Table 5.
Venous thromboembolism, wound complications, and deep infection*
| Variable | IV-only group (n = 93) | IA-only group (n = 93) | Low-dose combined group (n = 95) | High-dose combined group (n = 95) | p Value |
|---|---|---|---|---|---|
| Symptomatic deep vein thrombosis | 0 | 0 | 0 | 0 | NA |
| Symptomatic pulmonary embolism | 1 (1%) | 0 | 0 | 0 | NA |
| Thromboembolic event | 1 (1%) | 0 | 0 | 0 | NA |
| Superficial wound necrosis | 1 (1%) | 0 | 0 | 1 (1%) | NA |
| Oozing | 3 (3%) | 4 (4%) | 4 (4%) | 7 (7%) | 0.572 |
| Deep infection | 0 | 0 | 0 | 0 | NA |
*Data are presented as numbers of patients; #p values were calculated using chi-square test for categorical variables; IV = intravenous; IA = intraarticular; NA = not applicable.
Discussion
The efficacy of tranexamic acid in reducing blood loss and allogeneic transfusion rate has been reported in numerous studies but there has been debate regarding the ideal route of administration [26, 30, 47, 48, 53]. Our study was conducted to compare the effects of tranexamic acid, in terms of total blood loss and allogeneic blood transfusion rate, when administered by IV, IA, or combined IV and IA routes. Several studies have compared the effects of topical and IV administration of tranexamic acid [8, 19, 35, 50], while others have compared the effects of combined versus IV or IA administration of tranexamic acid in TKA [24, 26, 33]. Our study is unique as we attempted to compare three different modalities of tranexamic acid administration (ie, IV alone, IA alone, combined IV and IA) at the same time. Our major finding is that IA tranexamic acid administration further reduces blood loss after TKA compared with IV use alone, and a combination of IV and IA administration will not provide additional advantages in reducing blood loss.
Our findings need to be interpreted in light of the following limitations. First, our study population had a high female predominance (92%), which might be a confounding factor. If there is sex-dependent difference in bleeding risk, it may restrict our findings from being extrapolated to a population with a different sex composition. However, only 11% of eligible patients were excluded from this study during the study period, and there was no difference in the sex ratio between the four groups. The gender composition of all patients in our TKA database is similar to the gender composition of the study cohort and also is similar to national results in South Korean patients [10, 28]. Second, we did not have a placebo group, as there is already ample published evidence in favor of tranexamic acid in this setting [1, 52, 54], and because to do so would be depriving the patients of its beneficial effects and subjecting them to the potential risks of increased blood loss and transfusion. Third, we did not include patients who underwent simultaneous or staged bilateral TKAs, and excluded patients presently taking anticoagulants as they might be expected to have greater blood loss; therefore, our findings may not to be applicable to these patients. Fourth, the vacuum drain was placed in the subcutaneous space, which is different from the typical intraarticular placement. This factor needs to be taken into account when comparing the amount of blood loss in our patients with those reported in other studies. Our practice is supported by a previous study that found that the use of a subcutaneous vacuum drain reduces blood loss without increasing wound complications [44]. Fifth, no blood analyses were performed to estimate serum tranexamic acid levels and no ultrasonography was done to assess asymptomatic deep vein thrombosis and pulmonary embolism as there is substantial evidence supporting the safety of tranexamic acid administration and a low incidence of complications [52]. Sixth, because we excluded a small number of patients who were receiving anticoagulants, we cannot rule out the possibility that combined administration is more effective in those patients, compared with IA or IV administration. However, as only five patients receiving anticoagulants were excluded from this study, the effect of this exclusion would not be substantial.
The major finding of our study was that the IA group and the high- and low-dose combined groups had less total blood loss than IV-only group, and there was no difference between the three groups that included IA administration in the regimen. Our findings suggest that an IA injection of tranexamic acid could be viewed as a more effective mode of administration of tranexamic acid, compared with IV administration. This finding is supported by a systematic review which found better efficacy of topical administration of tranexamic acid than IV use in an indirect comparison of the two methods [2]. However, several recent meta-analyses showed that IA and IV administration are comparable in effectiveness in decreasing blood loss and blood transfusion rate during TKA [9, 16, 17, 35]. We found less blood loss in the IA-only group than the IV-only group, but the difference was not sufficiently great to be clinically meaningful and the transfusion rate was not different. Therefore the efficacy of the two methods may be equivalent from this clinical standpoint. However, IA administration is a simple, surgeon-directed method, and it may have other benefits as well; a previous study showed that IA administration of tranexamic acid also reduced knee swelling in addition to reducing blood loss after TKA [25]. Another study showed that IA administration of tranexamic acid may improve early functional outcomes after TKA [46]. Theoretically, IA administration may lessen potential systemic adverse risk of tranexamic acid, as the topical application leads to 70% lower systemic absorption [51]. Considering the comparable (or even better) efficacy of IA administration, along with the potential benefits, we believe IA administration of tranexamic acid could be the preferred route in patients undergoing primary unilateral TKA.
Our findings also suggest that there is no compelling reason to combine IV administration with IA use, in terms of the blood loss and transfusion rate. Although the decrease in hemoglobin was lower in the high-dose combined group than the IA-only group, the difference was only 0.4 g/dL. This difference did not result in a lower transfusion rate or total blood loss. Enhanced efficacy of combined administration was reported in several studies [24, 26, 38]. However, there seems to be little published evidence comparing IA with combined administration of tranexamic acid [33, 48]. A recent study in which 120 patients were randomized to a topical, combined, or control group showed that the regimen used in the combined group was more effective than that used in the topical group in lowering the postoperative decrease in hemoglobin (1.9 g/dL vs 2.4 g/dL) [33]. However, there were no differences in the mean calculated blood loss and transfusion rate, which agrees with our results. In another study which compared topical, IV, combined, and placebo groups, no additional advantage of combined administration over topical or IV administration was found [48]. Our findings are consistent with the studies that question the necessity of combination approaches to administration [33, 48]. Again, combined administration did not show any remarkable advantage over IA administration of tranexamic acid alone in patients undergoing unilateral TKA.
Although our findings suggest that combined administration of tranexamic acid is not more beneficial than IA use alone in patients undergoing primary unilateral TKA, it may be considered in patients undergoing simultaneous bilateral TKAs. Kim et al. [29] reported that the decrease in hemoglobin in patients having simultaneous TKAs (5.1 g/dL) was much larger than that in patients having a unilateral TKA (3.8 g/dL) without tranexamic acid administration. Therefore the combined use of tranexamic acid may be helpful in reducing the decrease in hemoglobin and transfusion rate in this clinical scenario, although further studies will be required.
Tranexamic acid, as an antifibrinolytic agent, causes concern in terms of a possible increase in VTE complications after TKA. Although it is established that tranexamic acid does not inhibit fibrinolytic activity in the vein wall [4], its effect on a disrupted endothelium is unknown. We found no symptomatic deep vein thrombosis and only one pulmonary embolism among all of our study patients. Our findings are consistent with those of a systematic review [30] which found that no previous studies have reported an increased incidence of symptomatic deep vein thrombosis or pulmonary embolism with the use of tranexamic acid in TKA at any dose, timing, or route of administration [3, 5, 6, 13, 18, 21, 25, 27, 34, 41, 45, 51, 55]. The safety of this drug also is supported by several meta-analyses [1, 52, 54]. However, we did not routinely screen patients for deep vein thrombosis or pulmonary embolism unless the patient presented with clinical symptoms, and we also did not perform any specific tests looking for adverse effects related to the use of tranexamic acid. Therefore, we cannot exclude the possibility that the use of tranexamic acid increases the incidence of subclinical adverse events. Although we did not observe a difference in symptomatic thromboembolic events, our study was far too small to make safety claims on this point; future meta-analyses will need to aggregate the results of many randomized trials to evaluate the safety of these regimens.
IA tranexamic acid administration further reduces blood loss after unilateral TKA compared with IV use alone, regardless of its combination with IV administration. In this study, no additional effect of IV combination was found in further reducing blood loss compared with IA alone. IA administration is a more-effective mode of administration of tranexamic acid than systemic use for this purpose, and combined IV administration need not be considered additionally for primary unilateral TKA.
Acknowledgment
We thank Nimesh Prakash Jain MS (Orth) (Bombay Hospital and Research Centre, Marine Lines, Mumbai, Maharashtra, India) for contributions in writing the manuscript.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
This work was performed at the Joint Reconstruction Center, Seoul National University Bundang Hospital.
A comment to this article is available at http://dx.doi.org/10.1007/s11999-017-5322-9.
References
- 1.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93:1577–1585. doi: 10.1302/0301-620X.93B12.26989. [DOI] [PubMed] [Google Scholar]
- 2.Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96:1005–1015. doi: 10.1302/0301-620X.96B8.33745. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48:519–525. doi: 10.1111/j.1537-2995.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 4.Astedt B, Liedholm P, Wingerup L. The effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann Chir Gynaecol. 1978;67:203–205. [PubMed] [Google Scholar]
- 5.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–440. [PubMed] [Google Scholar]
- 6.Camarasa Godoy MA, Serra-Prat M, Palomera Fanegas E. Effectiveness of tranexamic acid in routine performance of total knee replacement surgery. Rev Esp Anestesiol Reanim. 2008;55:75–80. doi: 10.1016/S0034-9356(08)70513-9. [DOI] [PubMed] [Google Scholar]
- 7.Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res. 2011;469:2874–2880. doi: 10.1007/s11999-011-1874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JY, Chin PL, Moo IH, Pang HN, Tay DK, Chia SL, Lo NN, Yeo SJ. Intravenous versus intra-articular tranexamic acid in total knee arthroplasty: a double-blinded randomised controlled noninferiority trial. Knee. 2016;23:152–156. doi: 10.1016/j.knee.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Chen Z, Cui S, Li Z, Yuan Z. Topical versus systemic tranexamic acid after total knee and hip arthroplasty: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95:e4656. doi: 10.1097/MD.0000000000004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho HJ, Chang CB, Kim KW, Park JH, Yoo JH, Koh IJ, Kim TK. Gender and prevalence of knee osteoarthritis types in elderly Koreans. J Arthroplasty. 2011;26:994–999. doi: 10.1016/j.arth.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Cushner FD, Friedman RJ. Blood loss in total knee arthroplasty. Clin Orthop Relat Res. 1991;269:98–101. [PubMed] [Google Scholar]
- 12.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 13.Engel JM, Hohaus T, Ruwoldt R, Menges T, Jurgensen I, Hempelmann G. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg. 2001;92:775–780. doi: 10.1213/00000539-200103000-00041. [DOI] [PubMed] [Google Scholar]
- 14.Fakhry SM, Fata P. How low is too low? Cardiac risks with anemia. Crit Care. 2004;8(suppl 2):S11–14. [DOI] [PMC free article] [PubMed]
- 15.Fiebig E. Safety of the blood supply. Clin Orthop Relat Res. 1998;357:6–18. doi: 10.1097/00003086-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Shi Z, Han B, Ye Y, You T, Jing J, Li J. Comparing efficacy and safety of 2 methods of tranexamic acid administration in reducing blood loss following total knee arthroplasty: a meta-analysis. Medicine (Baltimore). 2016;95:e5583. doi: 10.1097/MD.0000000000005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96:1937–1944. doi: 10.2106/JBJS.N.00060. [DOI] [PubMed] [Google Scholar]
- 18.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–599. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 19.Hamlin BR, DiGioia AM, Plakseychuk AY, Levison TJ. Topical versus intravenous tranexamic acid in total knee arthroplasty. J Arthroplasty. 2015;30:384–386. doi: 10.1016/j.arth.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. [DOI] [PubMed]
- 21.Hiippala S, Strid L, Wennerstrand M, Arvela V, Mantyla S, Ylinen J, Niemela H. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth. 1995;74:534–537. doi: 10.1093/bja/74.5.534. [DOI] [PubMed] [Google Scholar]
- 22.Hiippala ST, Strid LJ, Wennerstrand MI, Arvela JV, Niemela HM, Mantyla SK, Kuisma RP, Ylinen JE. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84:839–844. doi: 10.1213/00000539-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31:529–537. doi: 10.1177/0310057X0303100507. [DOI] [PubMed] [Google Scholar]
- 24.Huang Z, Ma J, Shen B, Pei F. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29:2342–2346. doi: 10.1016/j.arth.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, Kubo S, Matsumoto T, Matsushita T, Chin T, Iguchi T, Kurosaka M, Kuroda R. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35:1639–1645. doi: 10.1007/s00264-010-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain NP, Nisthane PP, Shah NA. Combined administration of systemic and topical tranexamic acid for total knee arthroplasty: can it be a better regimen and yet safe? A randomized controlled trial. J Arthroplasty. 2016;31:542–547. doi: 10.1016/j.arth.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83:596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 28.Kim HA, Kim S, Seo YI, Choi HJ, Seong SC, Song YW, Hunter D, Zhang Y. The epidemiology of total knee replacement in South Korea: national registry data. Rheumatology (Oxford). 2008;47:88–91. doi: 10.1093/rheumatology/kem308. [DOI] [PubMed] [Google Scholar]
- 29.Kim TK, Chang CB, Kang YG, Seo ES, Lee JH, Yun JH, Lee SH. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22:1870–1878. doi: 10.1007/s00167-013-2492-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim TK, Chang CB, Koh IJ. Practical issues for the use of tranexamic acid in total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22:1849–1858. doi: 10.1007/s00167-013-2487-y. [DOI] [PubMed] [Google Scholar]
- 31.Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28:1473–1476. doi: 10.1016/j.arth.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine BR, Haughom B, Strong B, Hellman M, Frank RM. Blood management strategies for total knee arthroplasty. J Am Acad Orthop Surg. 2014;22:361–371. doi: 10.5435/JAAOS-22-06-361. [DOI] [PubMed] [Google Scholar]
- 33.Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30:776–780. doi: 10.1016/j.arth.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012;470:2605–2612. doi: 10.1007/s11999-012-2310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May JH, Rieser GR, Williams CG, Markert RJ, Bauman RD, Lawless MW. The assessment of blood loss during total knee arthroplasty when comparing intravenous vs intracapsular administration of tranexamic acid. J Arthroplasty. 2016;31:2452–2457. doi: 10.1016/j.arth.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Melvin JS, Stryker LS, Sierra RJ. Tranexamic acid in hip and knee arthroplasty. J Am Acad Orthop Surg. 2015;23:732–740. doi: 10.5435/JAAOS-D-14-00223. [DOI] [PubMed] [Google Scholar]
- 37.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 38.Nielsen CS, Jans O, Orsnes T, Foss NB, Troelsen A, Husted H. Combined intra-articular and intravenous tranexamic acid reduces blood loss in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2016;98:835–841. doi: 10.2106/JBJS.15.00810. [DOI] [PubMed] [Google Scholar]
- 39.Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86:1512–1518. doi: 10.2106/00004623-200407000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Raveendran R, Wong J. Tranexamic acid reduces blood transfusion in surgical patients while its effects on thromboembolic events and mortality are uncertain. Evid Based Med. 2013;18:65–66. doi: 10.1136/eb-2012-100872. [DOI] [PubMed] [Google Scholar]
- 41.Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20:2494–2501. doi: 10.1007/s00167-012-1942-5. [DOI] [PubMed] [Google Scholar]
- 42.Royston D. Blood-sparing drugs: aprotinin, tranexamic acid, and epsilon-aminocaproic acid. Int Anesthesiol Clin. 1995;33:155–179. doi: 10.1097/00004311-199500000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee. 2000;7:151–155. doi: 10.1016/S0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 44.Seo ES, Yoon SW, Koh IJ, Chang CB, Kim TK. Subcutaneous versus intraarticular indwelling closed suction drainage after TKA: a randomized controlled trial. Clin Orthop Relat Res. 2010;468:2168–2176. doi: 10.1007/s11999-010-1243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:1869–1874. doi: 10.1007/s00167-012-2079-2. [DOI] [PubMed] [Google Scholar]
- 46.Serrano Mateo L, Goudarz Mehdikhani K, Cáceres L, Lee YY, Gonzalez Della Valle A. Topical tranexamic acid may improve early functional outcomes of primary total knee arthroplasty. J Arthroplasty. 2016;31:1449–1452. doi: 10.1016/j.arth.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Shin YS, Yoon JR, Lee HN, Park SH, Lee DH. Intravenous versus topical tranexamic acid administration in primary total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016 July 14. [Epub ahead of print]. [DOI] [PubMed]
- 48.Song EK, Seon JK, Prakash J, Seol YJ, Park YJ, Jin C. Combined administration of IV and topical tranexamic acid is not superior to either individually in primary navigated TKA. J Arthroplasty. 2017;32:37–42. doi: 10.1016/j.arth.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 49.Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46:1206–1211. doi: 10.1034/j.1399-6576.2002.461007.x. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee. 2014;21:987–993. doi: 10.1016/j.knee.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, Syed KA, Muhammad Ovais Hasan S, De Silva Y, Chung F. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92:2503–2513. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 52.Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94:1153–1159. doi: 10.2106/JBJS.K.00873. [DOI] [PubMed] [Google Scholar]
- 53.Yuan ZF, Yin H, Ma WP, Xing DL. The combined effect of administration of intravenous and topical tranexamic acid on blood loss and transfusion rate in total knee arthroplasty: combined tranexamic acid for TKA. Bone Joint Res. 2016;5:353–361. doi: 10.1302/2046-3758.58.BJR-2016-0001.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:1742–1752. doi: 10.1007/s00167-011-1754-z. [DOI] [PubMed] [Google Scholar]
- 55.Zohar E, Fredman B, Ellis M, Luban I, Stern A, Jedeikin R. A comparative study of the postoperative allogeneic blood-sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesth Analg. 1999;89:1382–1387. doi: 10.1097/00000539-199912000-00010. [DOI] [PubMed] [Google Scholar]


