Abstract
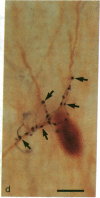
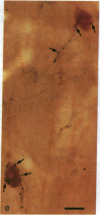
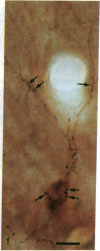
Information flow and processing in hippocampal neuronal networks is determined by a wide range of inhibitory mechanisms [e.g., feedforward or feedback, gamma-aminobutyrate (GABA) A or B receptor-mediated, perisomatic shunting, or distal dendritic inhibition], each subserving specialized functions. These forms of local inhibition are mediated by morphologically and neurochemically well-defined, mostly GABA-containing, interneurons, which control large populations of principal cells through their extensive axonal arborizations. These neurons can serve as ideal targets for subcortical pathways, such as those originating in the septum or raphe, which exercise a global control over hippocampal activity. This intriguing possibility prompted us to study whether the profound effect of the serotonergic raphe-hippocampal pathway is mediated by inhibitory interneurons or whether a direct diffuse action on the principal cells is dominant. We demonstrate that axons of this pathway form multiple synaptic contacts with hippocampal GABAergic interneurons. Interestingly, the serotonergic afferents selectively innervate the somata and dendritic trees of GABAergic neurons that contain the 28-kDa calcium-binding protein calbindin D28K, but never those that contain another calcium-binding protein, parvalbumin. These results show that the mechanism by which the serotonergic pathway may exert a powerful influence on hippocampal function involves the modulation of local inhibitory circuits. Furthermore, the selectivity in the choice of target GABAergic interneurons suggests a strong functional specialization among inhibitory circuits, as well as among the subcortical input pathways originating in the septum and raphe.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf S. Y., Miller J. J. The role of a raphe serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience. 1978;3(6):539–550. doi: 10.1016/0306-4522(78)90018-0. [DOI] [PubMed] [Google Scholar]
- Baimbridge K. G., Miller J. J. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982 Aug 12;245(2):223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- Blaxter T. J., Carlen P. L., Davies M. F., Kujtan P. W. gamma-Aminobutyric acid hyperpolarizes rat hippocampal pyramidal cells through a calcium-dependent potassium conductance. J Physiol. 1986 Apr;373:181–194. doi: 10.1113/jphysiol.1986.sp016041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Goddard G. V., Riives M. Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J Physiol. 1983 Jan;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Leung L. W., Vanderwolf C. H. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983 Oct;287(2):139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Celio M. R. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986 Feb 28;231(4741):995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- Colino A., Halliwell J. V. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987 Jul 2;328(6125):73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988 Nov 10;336(6195):170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Gorcs T. J., Liposits Z., Palay S. L., Chan-Palay V. Serotonin neurons on the ventral brain surface. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7449–7452. doi: 10.1073/pnas.82.21.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson A. J., Penke B., Erdei A., Chubb I. W., Somogyi P. Antisera to gamma-aminobutyric acid. I. Production and characterization using a new model system. J Histochem Cytochem. 1985 Mar;33(3):229–239. doi: 10.1177/33.3.3973378. [DOI] [PubMed] [Google Scholar]
- Kalén Peter, Rosegren Evald, Lindvall Olle, Björklund Anders. Hippocampal Noradrenaline and Serotonin Release over 24 Hours as Measured by the Dialysis Technique in Freely Moving Rats: Correlation to Behavioural Activity State, Effect of Handling and Tail-Pinch. Eur J Neurosci. 1989 May;1(3):181–188. doi: 10.1111/j.1460-9568.1989.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Katsumaru H., Kosaka T., Heizmann C. W., Hama K. Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res. 1988;72(2):347–362. doi: 10.1007/BF00250256. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Hama K. Two subtypes of non-pyramidal cells in rat hippocampal formation identified by intracellular recording and HRP injection. Brain Res. 1987 May 12;411(1):190–195. doi: 10.1016/0006-8993(87)90700-1. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Katsumaru H., Hama K., Wu J. Y., Heizmann C. W. GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987 Sep 1;419(1-2):119–130. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- Köhler C., Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982 Apr;7(4):951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988 Apr;8(4):1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov H. G., Grzanna R., Molliver M. E. The serotonin innervation of the cerebral cortex in the rat--an immunohistochemical analysis. Neuroscience. 1980;5(2):207–227. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Malouf A. T., Robbins C. A., Schwartzkroin P. A. Phaclofen inhibition of the slow inhibitory postsynaptic potential in hippocampal slice cultures: a possible role for the GABAB-mediated inhibitory postsynaptic potential. Neuroscience. 1990;35(1):53–61. doi: 10.1016/0306-4522(90)90119-o. [DOI] [PubMed] [Google Scholar]
- Mulligan K. A., Törk I. Serotoninergic innervation of the cat cerebral cortex. J Comp Neurol. 1988 Apr 1;270(1):86–110. doi: 10.1002/cne.902700108. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O. G., Strecker R. E., Daszuta A., Björklund A. Combined cholinergic and serotonergic denervation of the forebrain produces severe deficits in a spatial learning task in the rat. Brain Res. 1988 Jun 21;453(1-2):235–246. doi: 10.1016/0006-8993(88)90163-1. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. 5-Hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci. 1988 Nov;11(11):496–500. doi: 10.1016/0166-2236(88)90011-2. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G., Segal M. Raphe cells grafted into the hippocampus can ameliorate spatial memory deficits in rats with combined serotonergic/cholinergic deficiencies. Brain Res. 1989 Jan 23;478(1):184–186. doi: 10.1016/0006-8993(89)91495-9. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G., Segal M. Serotonin releasers modulate reactivity of the rat hippocampus to afferent stimulation. Neurosci Lett. 1988 Nov 22;94(1-2):173–176. doi: 10.1016/0304-3940(88)90290-x. [DOI] [PubMed] [Google Scholar]
- Ropert N. Inhibitory action of serotonin in CA1 hippocampal neurons in vitro. Neuroscience. 1988 Jul;26(1):69–81. doi: 10.1016/0306-4522(88)90128-5. [DOI] [PubMed] [Google Scholar]
- Segal M. Serotonin attenuates a slow inhibitory postsynaptic potential in rat hippocampal neurons. Neuroscience. 1990;36(3):631–641. doi: 10.1016/0306-4522(90)90006-p. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Haby M., Leresche N., Crunelli V. The GABAB antagonist phaclofen inhibits the late K+-dependent IPSP in cat and rat thalamic and hippocampal neurones. Brain Res. 1988 May 17;448(2):351–354. doi: 10.1016/0006-8993(88)91275-9. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J. Antisera to gamma-aminobutyric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections of cat striate cortex. J Histochem Cytochem. 1985 Mar;33(3):249–257. doi: 10.1177/33.3.2579124. [DOI] [PubMed] [Google Scholar]
- Vanderwolf C. H. A general role for serotonin in the control of behavior: studies with intracerebral 5,7-dihydroxytryptamine. Brain Res. 1989 Dec 18;504(2):192–198. doi: 10.1016/0006-8993(89)91356-5. [DOI] [PubMed] [Google Scholar]
- Wouterlood F. G. Anterograde neuroanatomical tracing with Phaseolus vulgaris-leucoagglutinin combined with immunocytochemistry of gamma-amino butyric acid, choline acetyltransferase or serotonin. Histochemistry. 1988;89(5):421–428. doi: 10.1007/BF00492597. [DOI] [PubMed] [Google Scholar]