Abstract
Long-term potentiation (LTP) of synaptic transmission in the central nervous system is a key form of cortical plasticity. The insular cortex (IC) is known to play important roles in pain perception, aversive memory and mood disorders. LTP has been recently reported in the IC, however, the signaling pathway for IC LTP remains unknown. Here, we investigated the synaptic mechanism of IC LTP. We found that IC LTP induced by the pairing protocol was N-methyl-D-aspartate receptors (NMDARs) dependent, and expressed postsynaptically, since paired-pulse ratio (PPR) was not affected. Postsynaptic calcium is important for the induction of post-LTP, since the postsynaptic application of BAPTA completely blocked the induction of LTP. Calcium-activated adenylyl cyclase subtype 1 (AC1) is required for potentiation. By contrast, AC8 is not required. Inhibition of Ca2+ permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (CP-AMPARs) or protein kinase M zeta (PKMζ) reduced the expression of LTP. Our results suggest that calcium-stimulated AC1, but not AC8, can be a trigger of the induction and maintenance of LTP in the IC.
Keywords: Neuroscience
1. Introduction
Long-term potentiation (LTP) of synaptic transmission is a key synaptic model for investigating learning and memory in the central nervous system (CNS) (Bliss and Collingridge, 2013). In addition to the hippocampus, LTP has also been reported in pain-related brain regions such as anterior cingulate cortex (ACC) and spinal dorsal horn (Sandkuhler, 2007; Zhuo, 2008). Recent studies suggest that LTP in these areas play important roles in behavioral sensitization and emotional anxiety in chronic pain states (Koga et al., 2015; Bliss et al., 2016).
ACC and insular cortex (IC) are two main cortical regions for pain perception, taste aversive memory and emotional disorders (Etkin and Wager, 2007; Harris et al., 2009; Shema et al., 2011; Zhuo, 2016). Human imaging studies show that the IC is activated by noxious stimulation and electrical stimulation of the IC induces painful and somatic sensations (Ostrowsky et al., 2002). Moreover, inhibition or lesions in the IC can produces less pain suffering or smaller levels of empathic pain in patients (Greenspan et al., 1999; Bowsher, 2006; Gu et al., 2012). In addition, the acquisition and storage of different learning and memory, such as conditioned taste aversion (CTA), novel taste learning, avoidance and object recognition memory require the involvement of the IC (Berman and Dudai, 2001; Bermudez-Rattoni et al., 2005; Yefet et al., 2006). In addition, there are reports of the IC being involved in fear and fear related emotional disorders (Damasio et al., 2000; Ostrowsky et al., 2002). At the synaptic level, we have previously reported that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) mediate most of the basal excitatory synaptic transmission in the insular cortex (Koga et al., 2012). Both in vivo and in vitro brain slice works show that excitatory synapses in the IC can undergo LTP (Jones et al., 1999; Wei et al., 2002; Liu et al., 2013), and peripheral injury or aversive stimulation causes LTP in the IC (Rodriguez-Duran et al., 2011; Qiu et al., 2013).
Adenylyl cyclases (ACs) are enzymes for the key second messenger cyclic adenosine monophosphate (cAMP). AC subtype 1 (AC1) is mainly expressed in CNS and regulated by a calcium-calmodulin (CaM) signaling pathway (Wang et al., 2011; Zhuo, 2012). AC1 plays a critical role downstream of glutamate receptors and contributes to chronic pain-related neuronal plasticity in the ACC (Chen et al., 2014a; Li et al., 2014; Qiu et al., 2014). Furthermore, a recent study showed that AC1 activity is required for the increases of synaptic GluA1 (also known as GluR1) in the IC after nerve injury (Qiu et al., 2014). However, whether AC1 is required for LTP in the IC has not been investigated.
In the present study, we employed integrative methods including whole-cell patch clamp recording, pharmacology and gene knockout mice to investigate LTP in the IC. We showed that LTP in the IC was required for the calcium-stimulate signaling pathway via activation of AC1, but not AC8, by using genetically modified mice with deletions of AC1 (AC1-/-) or AC8 (AC8-/-), and a selective inhibitor for AC1, NB001. Furthermore, we demonstrated that the maintenance of post-LTP in the IC required activation of GluA1 subunits and protein kinase M zeta (PKMζ). These observations are pertinent for the understanding of pain processing, aversive memory and fear-related mood disorders in the IC.
2. Materials and methods
2.1. Animals and slice preparation
Adult C57BL/6 mice were purchased from Charles River (6–14 week old). AC1-/- and AC8-/- mice were a gift from Dr. Daniel R. Storm (University of Washington, Seattle, WA) and were maintained on a C57BL/6 background. All mice were maintained on a 12-h light/dark cycle with food and water provided ad libitum. All experiments related to mutant mice were performed blind to the genotype. Age-matched male C57BL/6 mice were used as controls for AC1-/-, AC8-/-. Mice were anesthetized with isoflurane, and coronal brain slices (300 μm) containing the IC were prepared using our previous methods (Wei et al., 2001; Li et al., 2010; Koga et al., 2012). Brain slices were transferred and kept at a chamber with oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) at a room temperature for at least 1 hr. ACSF solution contains (in mM) 124 NaCl, 2.5 KCl, 2CaCl2, 1 MgSO4, 25 NaHCO3, 1 NaH2PO4 and 10 glucose.
2.2. In vitro whole-cell patch-clamp recording
Experiments including whole-cell patch clamp recording were performed in a recording chamber on the stage of an Axioskop 2FS microscope (Zeiss) with infrared DIC optics for visualization of cells. In the present study, evoked EPSCs (eEPSCs) were recorded from the layer II/III neurons with an Axopatch 200B amplifier (Molecular Devices, CA) and the stimulations were delivered by a bipolar tungsten stimulating electrode placed in the layer V/VI of the IC slices. Control test pulses were given every 30 s. The recording pipettes (3–5 MΩ) were filled with solution containing (mM) 145 K-gluconate, 5 NaCl, 1 MgCl2, 0.2 EGTA, 10HEPES, 2 Mg-ATP, and 0.1 Na3-GTP (adjusted to pH7.2 with KOH). Picrotoxin (100 μM) was always used to block the γ-aminobutyric acid (A) (GABAA) receptor-mediated inhibitory currents in all experiments. The amplitudes of eEPSCs were adjusted to between 50–100 pA to obtain a baseline. Paired pulse stimulations with a 50 ms interpulse interval were given during recordings. As previously described, we isolated the NMDAR-mediated component of EPSCs pharmacologically in Mg2+-free ACSF ((in μM) 20CNQX, 1 glycine, and 100 picrotoxin). The recording pipettes were filled with solution containing (mM) 102 cesium gluconate, 5 TEA-chloride, 3.7 NaCl, 10 BAPTA, 0.2 EGTA, 20HEPES, 2 Mg-ATP, 0.3 Na-GTP, and 5 QX-314 chloride [N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium chloride] (adjusted to pH 7.2 with CsOH). NMDAR-mediated EPSCs were induced at 0.05 Hz. Access resistance was 15–30 MΩ and monitored throughout the experiment. Data were discarded if the access resistance changed >15% during the experiment. Data were filtered at 1 kHz and digitized at 10 kHz. During the experiments, the membrane potential was held at −60 mV.
To induce LTP in the IC, we used a protocol that is previously reported (Zhao et al., 2005). The LTP induction protocol contains 80 pulses stimulation of 2 Hz while the postsynaptic cell was held at +30 mV (or called the pairing protocol) (see Fig. 1d). In general, stable baseline EPSCs were collected before the induction of LTP. LTP was induced within 12 minutes after establishing the whole-cell configuration to avoid washout of intracellular contents that are critical for the establishment of synaptic plasticity (Zhao et al., 2005).
Fig. 1.
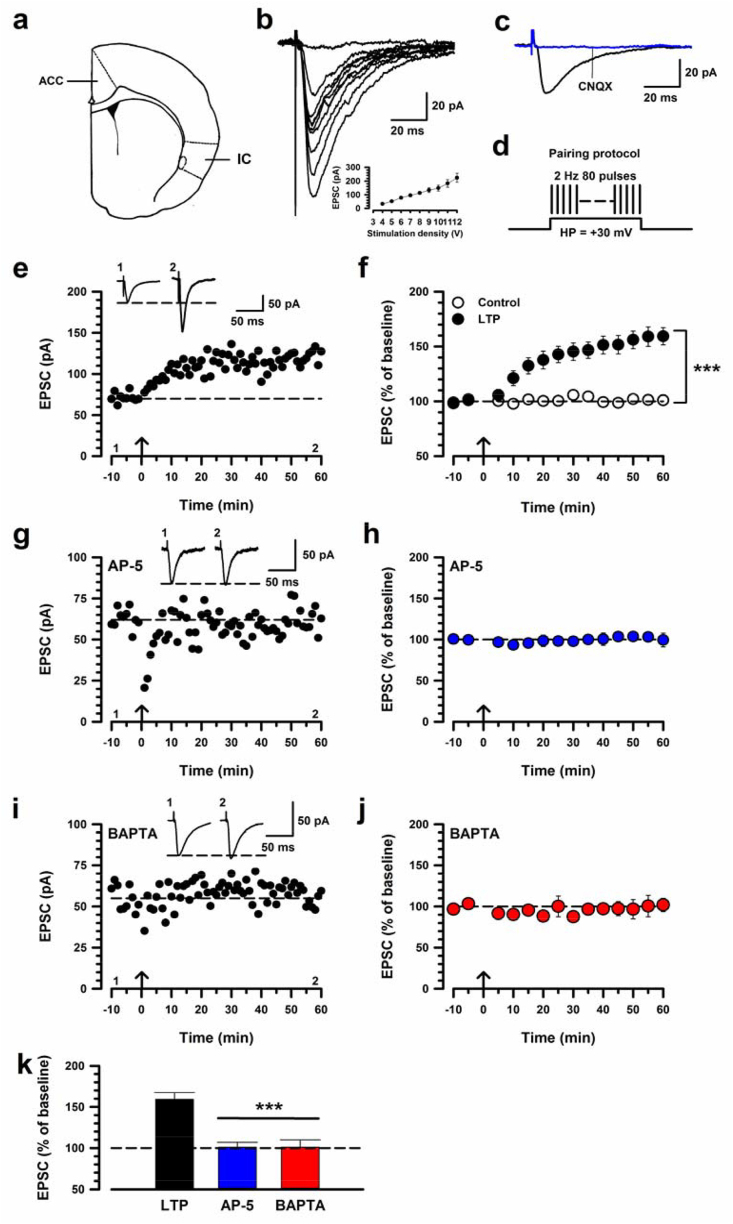
Postsynaptic calcium triggers IC LTP. (a) Brain diagram of adult mouse IC. (b) Top: sample traces show input-output relationship of AMPA receptor-mediated EPSCs in the IC. Bottom: Plots of input-output curve in WT mice IC (n = 8 neurons/5 mice). (c) The evoked EPSC was completely blocked by CNQX (20 μM). (d) A scheme illustrating the pairing protocol consisting of pairing 80 presynaptic pulses at 2 Hz with postsynaptic depolarization (holding at + 30 mV). (e) Sample traces at the indicated time points are shown above the plot. Top: sample traces of EPSCs with single-pulse stimulation during baseline (1) and 60 min after pairing protocol (2) at a holding membrane potential of −60 mV. (f) Pooled data to illustrate the time course of LTP (black, n = 14/12) and control (white, n = 11/11). There is significant difference between LTP and control (two-way ANOVA, F1,46 = 70.52, ***p < 0.001). (g) Bath application of AP-5 (50 μM) completely blocked the induction of LTP in one neuron. (h) Pooled data of AP-5 (n = 5 neurons/4 mice). (i) Postsynaptic application of BAPTA (20 mM in the recording pipette) completely blocked the induction of LTP in one neuron. (j) Pooled data of BAPTA (n = 5/4). (k) Summary of AP-5 and BAPTA on the induction of LTP. The amplitudes of EPSCs in AP-5 or BAPTA were significantly decreased compared with LTP (one-way ANOVA, F2,21 = 14.17, ***p < 0.001). Calibration, 50 pA, 50 ms. The mean amplitudes of EPSCs were determined at 50–60 min after pairing protocol. The arrow donates the time of pairing protocol. Error bars represent SEM; ***p < 0.001.
2.3. Pharmacological inhibition
D (−)-2-amino-5-phosphonopentanoic acid (AP5), KT5720, z-Pseudosubstrate inhibitory peptide (ZIP) and NASPM were obtained from Tocris Cookson (Bristol, UK). NB001 was provided by NeoBrain Pharmac Inc (Canada). AP5, NB001, ZIP and NASPM were dissolved in distilled water and KT5720 was dissolved in dimethyl sulfoxide (DMSO). Drugs were instantly diluted from the stock solutions to the final desired concentration in the ACSF. We found that the same amount of dimethyl sulfoxide diluted in ACSF had no effect on basal synaptic transmission and plasticity.
2.4. Data analysis
Data were collected and analyzed with Clampex 10.2 and Clampfit 10.2 software (Molecular Devices). For comparison between two groups, we used unpaired t-test or paired t-test. For comparison among three groups, we used one-way analysis of variance (ANOVA) or two-way ANOVA. Significance between groups was tested with a Holm-Sidak or Tukey tests to adjust for multiple comparisons. All data are presented as the means ± standard error of the mean (SEM). In all cases, p < 0.05 was considered statistically significant.
3. Results
3.1. Pairing protocol induces IC LTP
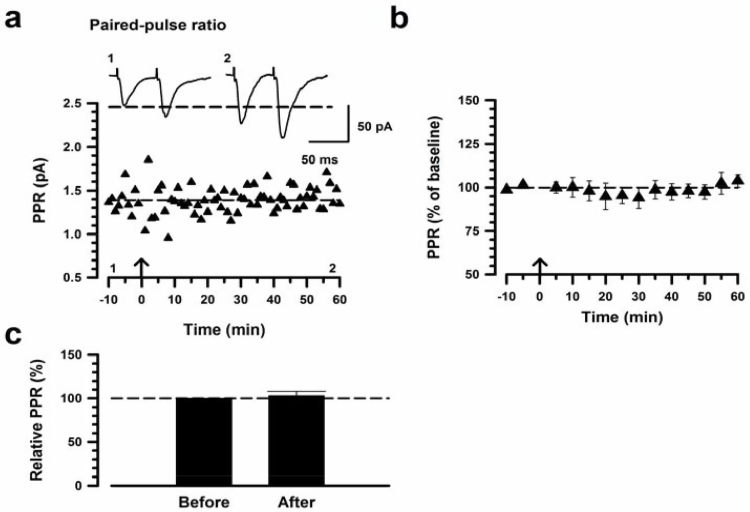
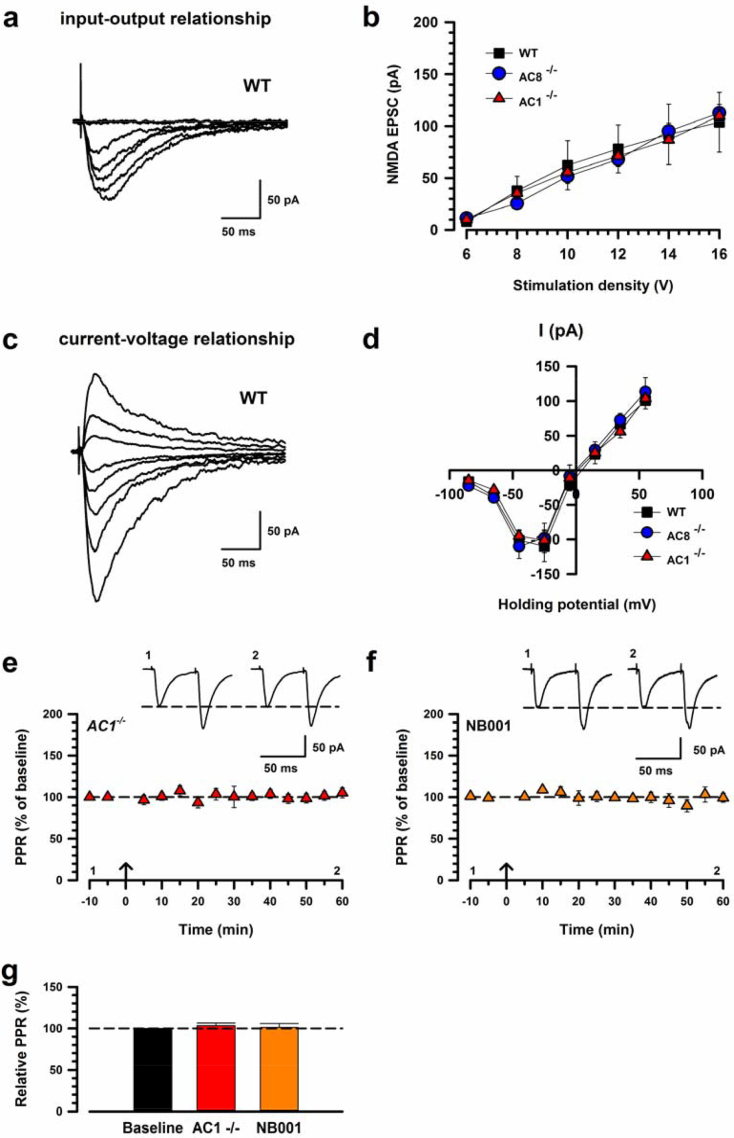
We performed whole-cell patch clamp recordings from visually identified neurons in layers II/III of the IC slices. A bipolar stimulation electrode was placed in deep layers to induce synaptic responses (n = 49 mice; Fig. 1a). Using repetitive stimulation at higher frequencies, we confirmed that these responses are monosynaptic in nature. We first recorded the input-output relationship of eEPSCs to examine whether excitatory synaptic transmission was altered in the IC neurons. Amplitudes of these eEPSCs increased with increases of stimulation density (Fig. 1b). Next, to test whether these EPSCs are mediated by glutamate, we bath applied an AMPA/Kinate receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM). The eEPSCs were completely blocked (Fig. 1c). These results indicate that glutamate is the major excitatory transmission in the IC as well as ACC. Then, we assessed that the pairing protocol induced the possible presynaptic facilitation. To investigate the possibility, we recorded paired-pulse ratio (PPR), using paired-pulse stimulation (interpulse interval of 50 ms). PPR was not affected for at least 1 h after the pairing protocol (Fig. 2). We next confirmed whether the pairing protocol can induce LTP at the IC synapses. We found that this pairing protocol robustly increased the amplitude of eEPSCs in IC neurons and that the LTP lasted for at least 1hr (159.2% ± 8.2%; Fig. 1e,f). In contrast, control neurons, which did not receive the pairing protocol, showed no change in the amplitude of eEPSCs (101.1% ± 1.7%; Fig. 1f). The pairing protocol and control groups were significantly different (unpaired t-test, t23 = -6.16, p < 0.001, LTP versus control; Fig. 1k). These results suggest that IC LTP by pairing protocol is unlikely purely expressed presynaptically for at least 1 h.
Fig. 2.
Pairing protocol purely induce post-LTP. (a) Top: sample traces of EPSCs with paired-pulse stimulation during baseline (1) and 60 min after the pairing protocol (2) at a holding membrane potential of −60 mV. Bottom: a time course plot of a representative single example. (b) Pooled data to illustrate the time course of PPR (n = 7/5). (c) Summary of PPR data before and after the pairing protocol. There is no significant difference between before and after (paired t-test, t6 = -0.64, p > 0.05). Calibration, 100 pA, 50 ms. The arrow denotes the time of pairing protocol.
3.2. NMDARs and postsynaptic calcium is involved in IC LTP
The activation of NMDARs is important for most forms of LTP. We previously reported that NMDA GluN2A (or called NR2A) and GluN2B (or called NR2B) receptors are required for the induction of LTP in the IC (Liu et al., 2013; Qiu et al., 2013). To confirm whether IC LTP induced by pairing protocol is NMDARs dependent, we applied an NMDAR antagonist, AP-5 (50 μM), in the bath solution (Fig. 1g). LTP was completely blocked under the presence of AP-5 (101.2% ± 6.0%, p < 0.001; Fig. 1h,k), indicating that LTP in the IC is NMDAR dependent. To further address the possible involvement of postsynaptic signaling pathways in the IC, we investigated the roles of postsynaptic calcium (Ca2+). We inhibited postsynaptic Ca2+ signaling by adding an internal Ca2+ chelator (BAPTA, 20 mM) in the recording pipette. We found that BAPTA completely blocked the induction of LTP (101.2% ± 8.8%, p < 0.001; Fig. 1i-k). Therefore, these results suggest that LTP can be induced in the IC through NMDARs.
3.3. LTP is mediated by AC1 activation
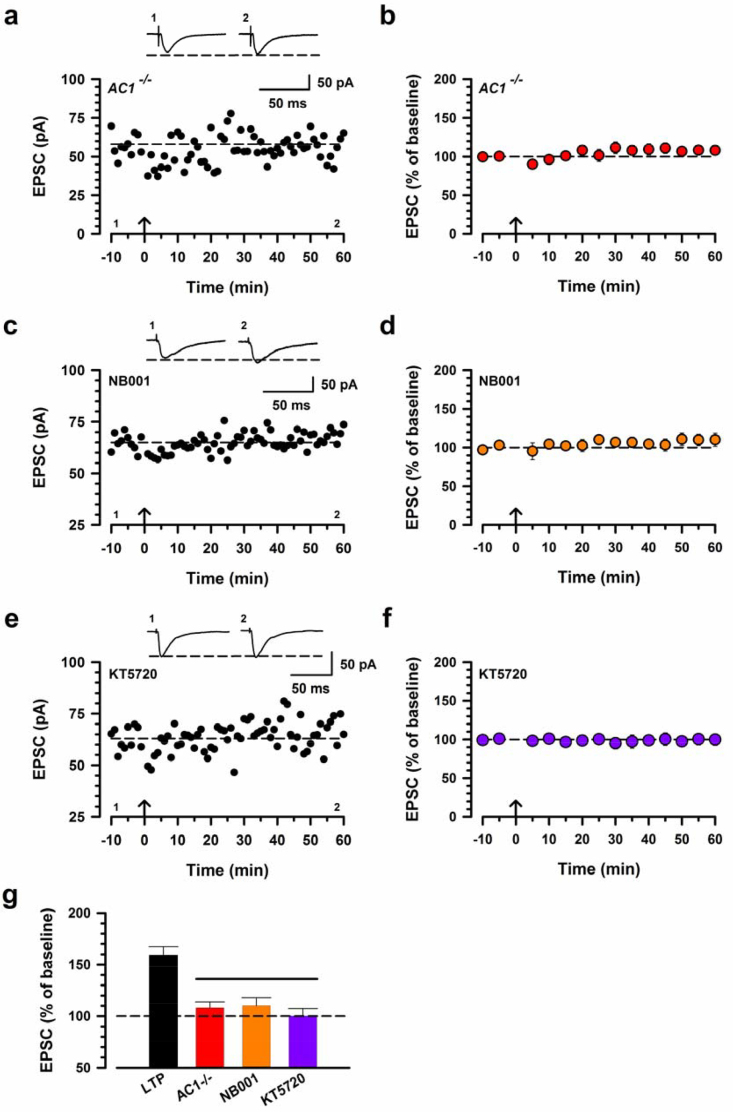
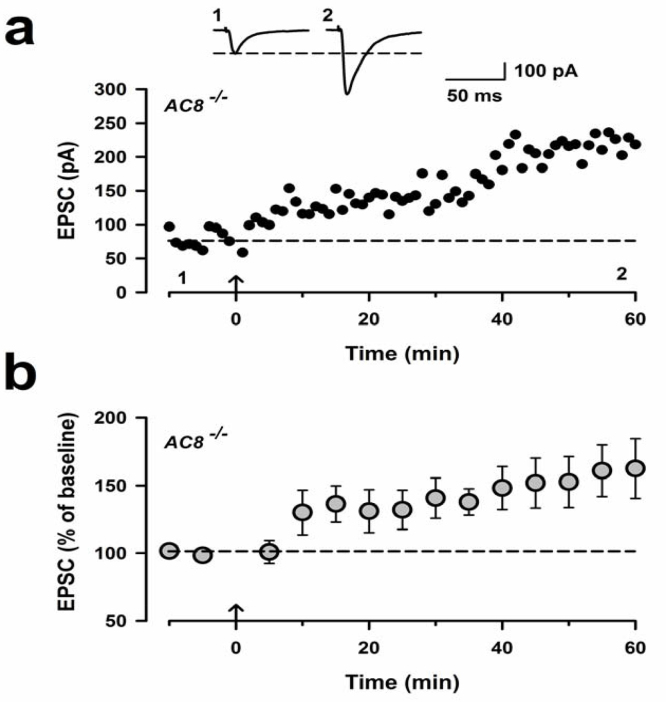
We next investigated that intracellular signaling pathways are required for LTP. ACs are the major Ca2+/calmodulin-stimulated adenylyl cyclase isoforms among the cAMP signaling pathway (Wang and Storm, 2003; Cooper and Crossthwaite, 2006). AC1 and AC8 were both expressed at high levels in two pain-related forebrain areas, the ACC and the IC, and contribute to activity-dependent gene activation. We studied the possible involvement of ACs by using AC1-/- or AC8-/- mice. AC1-/- mice failed to exhibit LTP (108.1% ± 5.6%, p < 0.001, AC1-/- versus control LTP; Fig. 3a,b and g). However, AC8-/- mice showed normal LTP (162% ± 20%; Fig. 4). Our recent studies have identified a selective inhibitor for AC1, NB001 (Wang et al., 2011). Therefore, to confirm the requirement of AC1 activity for LTP in the IC, we applied NB001 (20 μM) into the bath solution (Fig. 3c). We found that while baseline responses were not significantly affected, LTP was completely blocked by NB001 (110.1% ± 7.9%; Fig. 3d,g). These results indicate that AC1, but not AC8, is essential for the induction of LTP in the IC.
Fig. 3.
Loss of LTP in the IC of AC1-/- mice. (a) The pairing stimulation failed to induce LTP in the IC in one neuron of AC1-/- mouse. (b) Pooled data of AC1-/- mouse (n = 7 neurons/5 mice). (c) NB001 (20 μM) blocked the induction of LTP in one neuron. (d) Pooled data of NB001 (n = 7/5). (e) KT5720 (1 μM) blocked the induction of LTP. (f) Pooled data of KT5720 (n = 7/5). (g) Summary of the effect of AC1-/-, NB001 and KT5720 on the induction of LTP. The amplitudes of EPSCs in AC1-/-, NB001 or KT5720 were significantly decreased compared with control LTP (one-way ANOVA, F3,37 = 13.82, ***p < 0.001). Insets in a,b and e are example EPSC traces at time points indicated by the numbers in the graph. Calibration, 50 pA, 50 ms. The arrow denotes the time of pairing protocol. Error bars represent SEM; ***p < 0.001.
Fig. 4.
AC8−/− mice show normal LTP in the IC. (a) LTP in the IC was induced in one neuron of AC8−/− mouse. Sample traces at the indicated time points are shown above the plot. Calibration, 100 pA, 50 ms. (b) Pooled data to illustrate the induction of LTP in AC8-/- mice (n = 5 neurons/4 mice). The arrow denotes the time of pairing protocol. Error bars represent SEM.
3.4. PKA is involved in expression of LTP in the IC
PKA is important for cingulate LTP. AC1 generates cAMP, which activates PKA, which is required for LTP transduction in the hippocampus. We thus examined if PKA activity is required for the LTP induction in the IC. We applied a PKA inhibitor, KT5720 (1 μM), in the bath solution (Fig. 3e). We observed that induction of LTP in the IC was completely blocked by KT5720 (100.0% ± 7.4%, p < 0.001; Fig. 3f,g). Taken together, these results indicate that PKA is involved in a calcium-stimulated AC1 signaling cascade for the induction of LTP in the IC.
3.5. AC1 shows normal NMDARs −mediated EPSCs and does not affect glutamate release
One possible mechanism of reduced LTP is inhibition of NMDAR mediated responses. To examine if NMDARs mediated responses are affected in knockout mice, we examined NMDAR-mediated EPSCs in the IC of WT, AC1-/- or AC8-/- mice (Fig. 5a). There were no differences in total NMDAR-mediated input-output curves in the IC among WT, AC1-/- and AC8-/- (Fig. 5b). Next, to examine the voltage dependence of NMDAR-mediated currents, we recorded synaptic responses in voltage-clamp mode over a range of membrane potentials from −85 mV to +55 mV (Fig. 5c). The peak I–V curve for the NMDAR-mediated currents was outwardly rectifying. There is no difference among the linear parts of the I–V curve, which had a reversal potential of 4.3 ± 2.0 mV, −0.6 ± 2.0 mV, 1.1 ± 2.3 mV in WT, AC1-/- and AC8-/-, respectively (Fig. 5d) These results suggest that there are no differences in NMDAR-mediated synaptic transmission in WT, AC1-/- and AC8-/- mice in the IC.
Fig. 5.
NMDAR-mediated EPSCs and effect of PPR in AC1−/− mice. (a) Sample trace showing the input-output relationship of NMDAR-mediated EPSCs in a WT mice IC neuron. (b) There was no difference in the input-output curve of NMDAR-mediated EPSCs in the IC between WT mice (square, n = 7 neurons/5 mice), AC1-/- (triangle, n = 5/5) or AC8-/- (circle, n = 12/6) (two-way ANOVA, F2,147 = 0.1, p > 0.05). (c) Sample trace showing NMDAR-mediated EPSCs evoked at holding potentials of −85 mV to approximately +55 mV in a WT IC neuron. (d) Current-voltage plots for NMDAR-mediated EPSCs between WT mice (n = 7/6), AC1-/- mice (n = 5/4) and AC8−/− (n = 10/7) in the IC (two-way ANOVA, F2,152 = 0.23, p > 0.05). (e) Pooled data of PPR in AC1-/- mice (n = 5/3). (f) Pooled data of NB001 (n = 5/3). (g) Summary of PPR data before and after the pairing protocol. There is no significant difference among baseline, AC1-/- and NB001 (one-way ANOVA, F2,12 = 0.28, p > 0.05). Calibration, 100 pA, 50 ms. The arrow denotes the time of pairing protocol. Error bars represent SEM.
We recorded PPR of AC1-/- mice to investigate whether LTP affect presynaptic plasticity in AC1-/- mice (Fig. 5e). AC1-/- mice did not show a significant change in PPR after LTP as well as WT (Fig. 5g). We also pharmacologically applied NB001 but there was no difference in PPR between baseline and NB001 (Fig. 5f,g). These results indicate that IC LTP requires for postsynaptic AC1 activity.
3.6. GluA1 and PKMζ are important for the maintenance of post-LTP
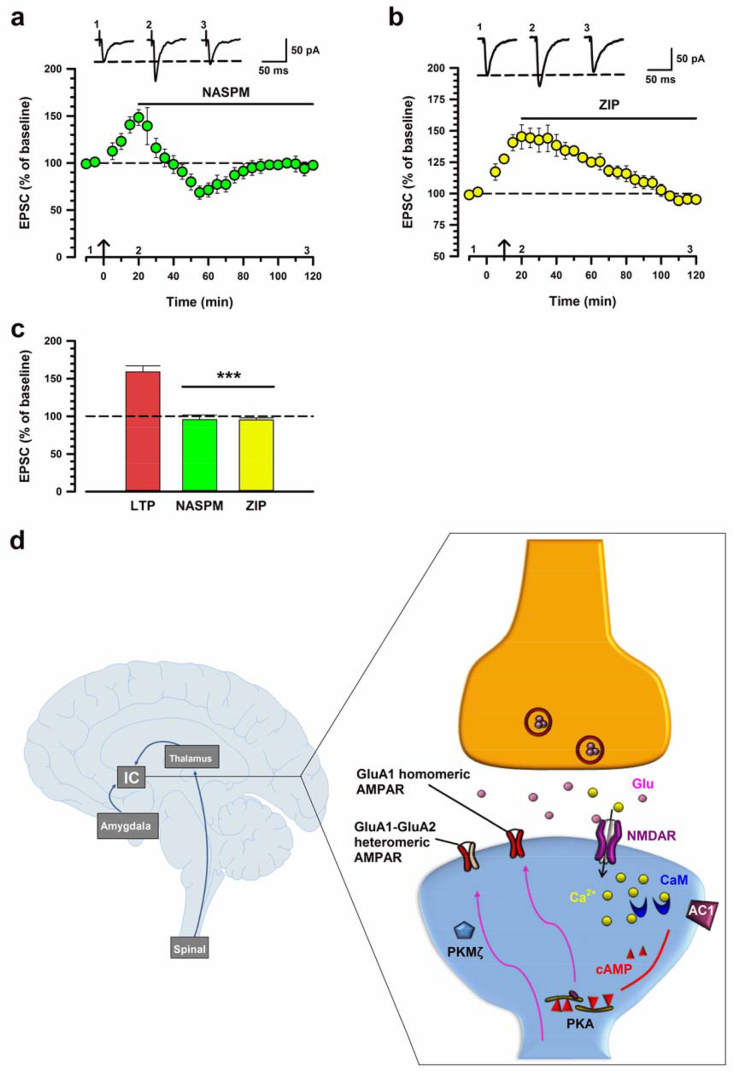
Our previous study of LTP in the ACC showed that the maintenance was mediated via postsynaptic AMPAR GluA1 subunits (Chen et al., 2014b). However, it is unknown if the maintenance of IC LTP can be mediated by GluA1 subunits. To test the possibility in the IC, we pharmacologically applied NASPM, a CP-AMPAR agonist, on IC LTP (Fig. 6a). We found that bath application of NASPM (50 μM) applied 20 min after the induction of LTP, was able to fully reverse LTP back to baseline values (95.7% ± 6.1% at 120 min, p < 0.001; Fig. 6c).
Fig. 6.
GluA1 and PKMζ are involved in the maintenance of IC LTP. (a) Pooled data of NASPM (50 μM) on the maintenance of LTP (n = 5 neurons/3 mice), applied 20 min after the LTP induction. (b) Pooled data of ZIP (5 μM) on the maintenance of LTP in the IC, applied 20 min after the LTP induction (n = 5/5). (c) Summary of effect for the maintenance of LTP. There are significant differences comparing LTP with NASPM and ZIP (one-way ANOVA, F2,21 = 18.94, p < 0.001). (d) Left: A simplified diagram shows that insular cortex (IC) receives projections from the spinal cord and amygdala. Right: A model for IC LTP. Activation of glutamate NMDARs triggers an increase in postsynaptic Ca2+ in dendritic spines. Ca2+ is an important intracellular signal for triggering a series of biochemical events that contribute to the induction and expression of LTP. After the activation of NMDARs, Ca2+ binds to calmodulin (CaM) and leads to activation of Ca2+-stimulated AC1 as well as Ca2+/CaM-dependent protein kinases. Subsequently, postsynaptic GluA1-containing AMPA receptors may be transferred into synaptic sites and contribute to enhanced synaptic responses. In addition, activation of AC1 leads to activation of PKA. Activation of cAMP-PKA drives the insertion of GluA1 homomers (CP‐AMPARs). PKMζ may maintain LTP by upregulating GluA1–GluA2 heteromers. Insets in a and b are example eEPSC traces at time points indicated by the numbers in the graph. The arrow denotes the time of pairing protocol. Calibration, 50 pA, 50 ms. Error bars represent SEM; ***p < 0.001.
We next tested whether PKMζ was involved in the maintenance of LTP in the IC because PKMζ is also thought to contribute to the maintenance of postsynaptic potentiation in different parts of the brain, including ACC (Li et al., 2010) and hippocampus (Sacktor, 2012). Bath application of ZIP (5 μM), which is an inhibitor of atypical PKC isoforms including PKMζ, applied 20 min after induction of LTP, completely blocked the maintenance of LTP (95.2% ± 2.6% at 120 min, Fig. 6b,c). These results suggest that both GluA1 subunits and PKMζ are critical for the maintenance of LTP in the IC.
4. Discussion
In this study, we provide a synaptic mechanism of the induction and maintenance of LTP in the IC. We demonstrated that LTP could be induced in the IC of adult mouse. This form of LTP was induced postsynaptically, and required calcium-dependent signaling pathways, including NMDARs, AC1 and PKA. AC8, another isoform of calcium-stimulated AC, was not required for the induction of LTP in the IC. The expression of IC LTP is likely postsynaptic. Bath application of NASPM or ZIP reversed LTP. GluA1 subunits and PKMζ may contribute to the maintenance of LTP in the IC (Fig. 6d). We cannot rule out other forms of LTP in the IC. Our preliminary data found that a presynaptic form of LTP reported in the ACC (Koga et al., 2015) can be also found in insular synapses (unpublished data).
4.1. Induction of LTP in the IC
There are several protocols that have been used to induce LTP in the IC. Jones et., al. reported tetanic stimulation (6 trains of 100 Hz for 1 s, 20 s interval) induces LTP (Jones et al., 1999), and Theta-bust stimulation (TBS) induced a prolonged potentiation that lasted for at least 3 h (Liu et al., 2013). In the present study, we found pairing protocol could induce LTP at IC synapses postsynaptically, since PPR was not affected for at least 1 h after the induction. NMDARs are known for the induction of LTP. Here, we found that LTP is completely blocked by NMDAR antagonist AP-5. This is similar to the mechanism of LTP induction in the ACC.
At ACC synapses, the activation of NMDARs leads to an increase in postsynaptic Ca2+ levels, and this postsynaptic Ca2+ signal is an essential component for the induction of LTP (Bliss et al., 2016). Here, we showed that BAPTA also completely blocked LTP, providing strong evidence that postsynaptic Ca2+ influx can be essential for LTP. The activation of CaM-dependent signaling pathways by Ca2+ binding activates AC (Wang et al., 2011; Zhuo, 2012). Similar to other cortical regions such as ACC (Liauw et al., 2005), AC1 but not AC8 is important for the induction of IC LTP. To exclude the possible side effects caused by gene deletion, we further confirm inhibiting AC1 activity by NB001 also blocked LTP. This is consistent with the biochemical observation that AC1 is much more sensitive than AC8. In the present, we cannot completely rule out that AC8 KO may be compensated. Consistent with ACC, our results suggest that AC1 contributes to cortical LTP in a way that differs from hippocampal CA1. Activated AC1 elevates the endogenous intracellular cAMP. As the downstream target of AC1, cAMP-dependent PKA has been documented, which activates MAPK that is required for LTP in the ACC. Therefore, PKA can be also critical for induction of LTP in the IC. In this study, we found an involvement of PKA in IC LTP, since KT5720, a PKA inhibitor, significantly blocked it. As with LTP in the ACC, it seems likely that AC1 generates cAMP, which activates PKA, and that this is required for LTP transduction in the IC.
4.2. Maintenance of post-LTP in the IC
We previously reported that CP-APMAR antagonist NASPM reduced synaptic potentiation in the ACC. Phosphorylation of GluA1 is important for GluA1 trafficking and synaptic plasticity (Lee et al., 2003). However, less is known if GluA1 is required for expression of LTP in the IC. In the present study, we found that NASPM reduced LTP in the IC. Our previous study reported that peripheral nerve injury caused an increase in the expression of AMPARs, and this increase require AC1 dependent signaling pathway including AKAP79/150, and PKA (Qiu et al., 2014). Thus, we suggest that LTP in the IC can be related to neuropathic pain.
PKMζ activity has also been known to be important for maintenance of LTP in hippocampus and ACC (Li et al., 2010; Sacktor, 2012). In this study, we showed that ZIP reversed LTP at IC synapses for the first time. This result suggests that PKMζ is specifically involved in the expression of LTP in the IC. In behavioral studies of rat IC, the critical role of PKMζ has been reported (Shema et al., 2007; Shema et al., 2011). Microinjection of ZIP into the IC produced analgesic effects in rats with nerve injury (Han et al., 2015). In the conditioned taste aversion test, inhibiting PKMζ produced erasure of long-term memory, and overexpression of PKMζ in the IC can enhance consolidated long-term memory (Shema et al., 2007; Shema et al., 2011). However, recent studies have questioned the selectivity of this peptide for PKMζ (Volk et al., 2013). Indeed, ZIP may also inhibit the other atypical protein kinase C (PKC) isoform, PKCι/λ (Sacktor, 2012). Therefore, additional studies are clearly needed to establish the precise mechanism underlying the inhibition of post-LTP in the IC by administration of this peptide.
4.3. Functional and pathological implications of IC
IC plays important roles in pain perception, emotion, mood disorders as well as taste learning and memory (Table 1). IC is activated by somatic and/or muscular pain (Craig et al., 2000; Henderson et al., 2007), and direct stimulation of IC induces pain and somatic sensation (Ostrowsky et al., 2002). We recently report that neuropathic pain induced by nerve injury could fully occlude the subsequent induction of LTP in the IC (Qiu et al., 2013), and that excitatory transmission in the IC underwent plastic changes after peripheral nerve injury (Qiu et al., 2014). Therefore, analgesic effects produced by NB001 may also be due to inhibition of pain-related plasticity in the IC when drugs are applied systemically (Wang et al., 2011; Zhang et al., 2014). Inhibiting pain-related plasticity in the IC may give us better ways to control chronic pain. IC also relates to fear, sadness, Posttraumatic stress disorder (PTSD), social anxiety disorders and phobias (Damasio et al., 2000; Etkin and Wager, 2007; Yoshihara et al., 2016). We have previously reported that there is a presynaptic form of LTP (pre-LTP) in the ACC and it contributes to pain-related anxiety (Koga et al., 2015). Actually, we could induce pre-LTP in the IC (unpublished data), thus, future studies are needed to investigate whether pre-LTP in the IC can relate to mood disorders, and to investigate the possible interaction between ACC and IC areas at molecular and behavioral levels. In addition, IC is an important brain area for the acquisition and storage of learning and memory such as CTA and novel taste learning (Bermudez-Rattoni et al., 2005; Yefet et al., 2006). It is known that IC LTP contributes to CTA and taste learning (Jones et al., 1999; Rodriguez-Duran et al., 2011). Previous studies have reported that NMDAR is involved in the process of taste learning (Rosenblum et al., 1997; Adaikkan and Rosenblum, 2015), and that an inhibition of PKMζ inhibits long-term CTA memory (Shema et al., 2007). Therefore, the present study may help us to understand the synaptic mechanism of these functions in the IC.
Table 1.
Studies performed in the insular cortex.
| Function | Study | Method | Result | Reference |
|---|---|---|---|---|
| pain | human | stereotactic implantation technique | posterior IC is involved in the processing of both painful and innocuous somaesthetic inputs | (Ostrowsky et al., 2002) |
| mice | electrophysiology | NMDARs contributes to neuropathic pain | (Qiu et al., 2013) | |
| mice | electrophysiology | AMPARs is enhanced after nerve injury | (Qiu et al., 2014) | |
| Emotion | human | fMRI | functional connectivity between the amygdala and anterior IC relates to fear | (Yoshihara et al., 2016) |
| human | PET | relation to sadness | (Damasio et al., 2000) | |
| human | fMRI, PET | PTSD, social anxiety disorder and specific phobia show great activities | (Etkin and Wager, 2007) | |
| Memory | mice | electrophysiology, CTA | LTD attenuates taste aversive memory | (Li et al., 2016) |
| rats | electrophysiology (in vivo), CTA | CTA induces LTP | (Rodriguez-Duran et al., 2011) | |
| rats | CTA | long-term memory is inhibited by an inhibition of PKMζ | (Shema et al., 2007) | |
| rats | electrophysiology (in vivo), CTA | NMDAR dependent LTP is required for taste learning | (Escobar et al., 1998) | |
| rats | CTA | PSD-5 induction is necessary for learning novel tastes | (Elkobi et al., 2008) | |
| rats | CTA | only the early robust trace is maintained by a NMDA-dependent CaMKII-AMPAR pathway | (Adaikkan and Rosenblum, 2015) | |
| rats | electrophysiology (in vivo) | LTP contributes to taste learning | (Jones et al., 1999) | |
| rats | CTA | NMDARs is involved in taste learning | (Rosenblum et al., 1997) |
Declarations
Author contribution statement
Manabu Yamanaka: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Takanori Matsuura: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Haili Pan: Performed the experiments; Analyzed and interpreted the data.
Min Zhuo: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Canadian Institute for Health Research (CIHR) Michael Smith Chair in Neurosciences and Mental Health, Canada Research Chair, CIHR operating grant (MOP-124807) and project grant (PJT-148648), Azrieli Neurodevelopmental Research Program and Brain Canada.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adaikkan C., Rosenblum K. A molecular mechanism underlying gustatory memory trace for an association in the insular cortex. eLife. 2015;4 doi: 10.7554/eLife.07582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D.E., Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F., Okuda S., Roozendaal B., McGaugh J.L. Insular cortex is involved in consolidation of object recognition memory. Learn. Mem. 2005;12:447–449. doi: 10.1101/lm.97605. [DOI] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol. Brain. 2013;6:5. doi: 10.1186/1756-6606-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L., Kaang B.K., Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 2016;17:485–496. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- Bowsher D. Somatic sensation and the insular-opercular cortex: relationship to central pain. Eur. Neurol. 2006;55:160–165. doi: 10.1159/000093575. [DOI] [PubMed] [Google Scholar]
- Chen T., O'Den G., Song Q., Koga K., Zhang M.M., Zhuo M. Adenylyl cyclase subtype 1 is essential for late-phase long term potentiation and spatial propagation of synaptic responses in the anterior cingulate cortex of adult mice. Mol. Pain. 2014;10:65. doi: 10.1186/1744-8069-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wang W., Dong Y.L., Zhang M.M., Wang J., Koga K., Liao Y.H., Li J.L., Budisantoso T., Shigemoto R., Itakura M., Huganir R.L., Li Y.Q., Zhuo M. Postsynaptic insertion of AMPA receptor onto cortical pyramidal neurons in the anterior cingulate cortex after peripheral nerve injury. Mol. Brain. 2014;7:76. doi: 10.1186/s13041-014-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D.M., Crossthwaite A.J. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol. Sci. 2006;27:426–431. doi: 10.1016/j.tips.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Craig A.D., Chen K., Bandy D., Reiman E.M. Thermosensory activation of insular cortex. Nat. Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L., Parvizi J., Hichwa R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Elkobi A., Ehrlich I., Belelovsky K., Barki-Harrington L., Rosenblum K. ERK-dependent PSD-95 induction in the gustatory cortex is necessary for taste learning, but not retrieval. Nat. Neurosci. 2008;11:1149–1151. doi: 10.1038/nn.2190. [DOI] [PubMed] [Google Scholar]
- Escobar M.L., Alcocer I., Chao V. The NMDA receptor antagonist CPP impairs conditioned taste aversion and insular cortex long-term potentiation in vivo. Brain Res. 1998;812:246–251. doi: 10.1016/s0006-8993(98)00931-7. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan J.D., Lee R.R., Lenz F.A. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain. 1999;81:273–282. doi: 10.1016/S0304-3959(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Gu X., Gao Z., Wang X., Liu X., Knight R.T., Hof P.R., Fan J. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Kwon M., Cha M., Tanioka M., Hong S.K., Bai S.J., Lee B.H. Plasticity-Related PKMzeta Signaling in the Insular Cortex Is Involved in the Modulation of Neuropathic Pain after Nerve Injury. Neural Plast. 2015:601767. doi: 10.1155/2015/601767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.E., Sundgren P.C., Craig A.D., Kirshenbaum E., Sen A., Napadow V., Clauw D.J. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.A., Gandevia S.C., Macefield V.G. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain. 2007;128:20–30. doi: 10.1016/j.pain.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Jones M.W., French P.J., Bliss T.V., Rosenblum K. Molecular mechanisms of long-term potentiation in the insular cortex in vivo. J. Neurosci. 1999;19:RC36. doi: 10.1523/JNEUROSCI.19-21-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K., Sim S.E., Chen T., Wu L.J., Kaang B.K., Zhuo M. Kainate receptor-mediated synaptic transmissions in the adult rodent insular cortex. J. Neurophysiol. 2012;108:1988–1998. doi: 10.1152/jn.00453.2012. [DOI] [PubMed] [Google Scholar]
- Koga K., Descalzi G., Chen T., Ko H.G., Lu J., Li S., Son J., Kim T., Kwak C., Huganir R.L., Zhao M.G., Kaang B.K., Collingridge G.L., Zhuo M. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron. 2015;85:377–389. doi: 10.1016/j.neuron.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.K., Takamiya K., Han J.S., Man H., Kim C.H., Rumbaugh G., Yu S., Ding L., He C., Petralia R.S., Wenthold R.J., Gallagher M., Huganir R.L. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Li W.G., Liu M.G., Deng S., Liu Y.M., Shang L., Ding J., Hsu T.T., Jiang Q., Li Y., Li F., Zhu M.X., Xu T.L. ASIC1a regulates insular long-term depression and is required for the extinction of conditioned taste aversion. Nat. Commun. 2016;7:13770. doi: 10.1038/ncomms13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.Y., Wang N., Wang Y.J., Zuo Z.X., Koga K., Luo F., Zhuo M. Long-term temporal imprecision of information coding in the anterior cingulate cortex of mice with peripheral inflammation or nerve injury. J. Neurosci. 2014;34:10675–10687. doi: 10.1523/JNEUROSCI.5166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.Y., Ko H.G., Chen T., Descalzi G., Koga K., Wang H., Kim S.S., Shang Y., Kwak C., Park S.W., Shim J., Lee K., Collingridge G.L., Kaang B.K., Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–1404. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- Liauw J., Wu L.J., Zhuo M. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J. Neurophysiol. 2005;94:878–882. doi: 10.1152/jn.01205.2004. [DOI] [PubMed] [Google Scholar]
- Liu M.G., Kang S.J., Shi T.Y., Koga K., Zhang M.M., Collingridge G.L., Kaang B.K., Zhuo M. Long-term potentiation of synaptic transmission in the adult mouse insular cortex: multielectrode array recordings. J. Neurophysiol. 2013;110:505–521. doi: 10.1152/jn.01104.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowsky K., Magnin M., Ryvlin P., Isnard J., Guenot M., Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb. Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Qiu S., Zhang M., Liu Y., Guo Y., Zhao H., Song Q., Zhao M., Huganir R.L., Luo J., Xu H., Zhuo M. GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J. Neurosci. 2014;34:13505–13515. doi: 10.1523/JNEUROSCI.1431-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S., Chen T., Koga K., Guo Y.Y., Xu H., Song Q., Wang J.J., Descalzi G., Kaang B.K., Luo J.H., Zhuo M., Zhao M.G. An increase in synaptic NMDA receptors in the insular cortex contributes to neuropathic pain. Sci. Signal. 2013;6:ra34. doi: 10.1126/scisignal.2003778. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Duran L.F., Castillo D.V., Moguel-Gonzalez M., Escobar M.L. Conditioned taste aversion modifies persistently the subsequent induction of neocortical long-term potentiation in vivo. Neurobiol. Learn. Mem. 2011;95:519–526. doi: 10.1016/j.nlm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Rosenblum K., Berman D.E., Hazvi S., Lamprecht R., Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J. Neurosci. 1997;17:5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor T.C. Memory maintenance by PKMzeta–an evolutionary perspective. Mol. Brain. 2012;5:31. doi: 10.1186/1756-6606-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Understanding LTP in pain pathways. Mol. Pain. 2007;3:9. doi: 10.1186/1744-8069-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R., Sacktor T.C., Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Shema R., Haramati S., Ron S., Hazvi S., Chen A., Sacktor T.C., Dudai Y. Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. [DOI] [PubMed] [Google Scholar]
- Volk L.J., Bachman J.L., Johnson R., Yu Y., Huganir R.L. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493:420–423. doi: 10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Storm D.R. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol. Pharmacol. 2003;63:463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- Wang H.S., Xu H., Wu L.J., Kim S.S., Chen T., Koga K., Descalzi G., Gong B., Vadakkan K.I., Zhang X.H., Kaang B.K., Zhuo M. Identification of an Adenylyl Cyclase Inhibitor for Treating Neuropathic and Inflammatory Pain. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001269. [DOI] [PubMed] [Google Scholar]
- Wei F., Wang G.D., Kerchner G.A., Kim S.J., Xu H.M., Chen Z.F., Zhuo M. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat. Neurosci. 2001;4:164–169. doi: 10.1038/83993. [DOI] [PubMed] [Google Scholar]
- Wei F., Qiu C.S., Liauw J., Robinson D.A., Ho N., Chatila T., Zhuo M. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat. Neurosci. 2002;5:573–579. doi: 10.1038/nn0602-855. [DOI] [PubMed] [Google Scholar]
- Yefet K., Merhav M., Kuulmann-Vander S., Elkobi A., Belelovsky K., Jacobson-Pick S., Meiri N., Rosenblum K. Different signal transduction cascades are activated simultaneously in the rat insular cortex and hippocampus following novel taste learning. Eur. J. Neurosci. 2006;24:1434–1442. doi: 10.1111/j.1460-9568.2006.05009.x. [DOI] [PubMed] [Google Scholar]
- Yoshihara K., Tanabe H.C., Kawamichi H., Koike T., Yamazaki M., Sudo N., Sadato N. Neural correlates of fear-induced sympathetic response associated with the peripheral temperature change rate. NeuroImage. 2016;134:522–531. doi: 10.1016/j.neuroimage.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Zhang M.M., Liu S.B., Chen T., Koga K., Zhang T., Li Y.Q., Zhuo M. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol. Brain. 2014;7 doi: 10.1186/1756-6606-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M.G., Toyoda H., Lee Y.S., Wu L.J., Ko S.W., Zhang X.H., Jia Y., Shum F., Xu H., Li B.M., Kaang B.K., Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31:199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zhuo M. Targeting neuronal adenylyl cyclase for the treatment of chronic pain. Drug Discov. Today. 2012;17:573–582. doi: 10.1016/j.drudis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Zhuo M. Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience. 2016;338:220–229. doi: 10.1016/j.neuroscience.2016.08.014. [DOI] [PubMed] [Google Scholar]