Abstract
Boron neutron capture therapy (BNCT) was performed at the University of Missouri Research Reactor in mice bearing CT26 colon carcinoma flank tumors and the results were compared with previously performed studies with mice bearing EMT6 breast cancer flank tumors. Mice were implanted with CT26 tumors subcutaneously in the caudal flank and were given two separate tail vein injections of unilamellar liposomes composed of cholesterol, 1,2-distearoyl-sn-glycer-3-phosphocholine, and K[nido-7-CH3(CH2)15–7,8-C2B9H11] in the lipid bilayer and encapsulated Na3[1-(2`-B10H9)-2-NH3B10H8] within the liposomal core. Mice were irradiated 30 hours after the second injection in a thermal neutron beam for various lengths of time. The tumor size was monitored daily for 72 days. Despite relatively lower tumor boron concentrations, as compared to EMT6 tumors, a 45 minute neutron irradiation BNCT resulted in complete resolution of the tumors in 50% of treated mice, 50% of which never recurred. Median time to tumor volume tripling was 38 days in BNCT treated mice, 17 days in neutron-irradiated mice given no boron compounds, and 4 days in untreated controls. Tumor response in mice with CT26 colon carcinoma was markedly more pronounced than in previous reports of mice with EMT6 tumors, a difference which increased with dose. The slope of the dose response curve of CT26 colon carcinoma tumors is 1.05 times tumor growth delay per Gy compared to 0.09 times tumor growth delay per Gy for EMT6 tumors, indicating that inherent radiosensitivity of tumors plays a role in boron neutron capture therapy and should be considered in the development of clinical applications of BNCT in animals and man.
Introduction
Boron neutron capture therapy (BNCT) is a binary form of radiation therapy that involves the selective delivery of boron to target tissues, followed by irradiation with thermal neutrons. When irradiated with low-energy neutrons (<0.4 eV), 10B, a naturally occurring isotope of boron, captures a neutron and undergoes a nuclear reaction that results in the release of an alpha particle and a lithium nucleus [1], [2], [3]. These particles have a high Linear Energy Transfer (LET) and short track lengths (5–9 μm), resulting in significant radiation killing effect in an area roughly equivalent to one cell diameter [4], [5]. In general, it is intended that the boron delivery agents, as well as the low energy neutron beam, have very little biologic effect by themselves [6]. In this respect, cell killing selectivity is maximized in those tissues with high concentrations of 10B which are irradiated [7].
Similar to other forms of high LET radiation therapy, the radiobiology of BNCT is complex [8], [9]. While the maximum therapeutic effect of BNCT is realized from the boron neutron capture reaction products, there are several other dose sources which contribute to the total dose delivered during BNCT [10], [11]. Gamma rays are produced during the BNCT reaction and hydrogen neutron capture [12]. The thermal neutron beam at the MURR contains acceptable levels of fast neutrons and gamma rays remaining after the neutron filters [13]. Fast neutrons produce interact with hydrogen and produce high LET recoil protons through elastic scatter. High LET protons are also produced when 14N captures a thermal neutron. The total physical dose delivered in a BNCT treatment is the sum of all of the physical dose components [10]. Based on the beam characteristics as shown in Table 1, there are two variables that determine total dose delivered to a tissue: the tissue boron concentration and the time length of irradiation [14]. The total biologic effective dose is much more complex, and must be determined experimentally with very specific endpoints [15].
Table 1.
Dose Contributions of Various Sources for the MURR Thermal Neutron Beam Line
| Dose Component | Physical Dose Rate |
|---|---|
| Gamma dose from beam | 3.4 cGy/min |
| 1H capture photons | 1.4 cGy/min |
| 14N capture protons | 1.1 cGy/min |
| BNCT reaction (Li and He) | 0.43 cGy/min/ppm 10B |
BNCT research has been ongoing for over 50 years, including several direct clinical investigations in human patients with cancer [1], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]. While some efficacy has been shown in the human treatment of glioblastoma, head and neck cancer, and melanoma, the main shortcomings of BNCT have been in terms of neutron delivery, boron delivery, and prudent use of animal models of human disease [21], [24], [26], [27], [28]. While most BNCT facilities still use a nuclear reactor as a neutron source, filtering methods have improved since the initial investigations into this therapy. These advances have resulted in improved neutron spectra, allowing reduced gamma and fast neutron dose components, as well as the use of epithermal neutrons to allow for deeper penetration into tissues and skin sparing [14], [29], [30], [31]. Currently, there remains a need for new boron-delivery compounds and a critical assessment of clinical uses. Ideally, these new boron compounds should be non-toxic, bear a high boron payload, and feature selective delivery to malignant tissues [2], [32], [33].
The need for appropriate selection of tumor models for BNCT has been demonstrated by the limited success of early trials to treat glioblastoma multiforme [1], [16], [18], [19]. These results are not surprising, given the difficulty associated with treating this aggressive brain malignancy [34]. BNCT has already met with a measure of success when applied to different clinical scenarios including treatment of melanoma and recurrent tumors of the head and neck [21], [26], [28], [35], [36], [37]. In an effort to develop new, more cancer-specific boron delivery compounds and direct them to alternative tumor models, Kueffer et al. demonstrated BNCT in mice bearing EMT6 mammary tumors using boron-rich liposomes as a delivery system [38]. These liposomal nanoparticles deliver two boron clusters compounds, K[nido-7-CH3(CH2)15–7,8-C2B9H11] (MAC) and Na3[1-(2`-B10H9)-2-NH3B10H8] (TAC), which carry a high boron payload [24], [25]. These compounds are incorporated into liposomes, with MAC in the lipid bilayer membrane, and TAC in the hydrophilic liposome core. Liposomes containing both MAC and TAC are then administered intravenously and, by means of the enhanced permeability and retention (EPR) effect of neoplastic vasculature, accumulate preferentially in the tumor [39], [40], [41]. In mice with allograft EMT6 mammary carcinoma tumors, delivery of these compounds via a double-injection protocol resulted in tumor boron concentrations as high as 67.8 μg B per gram of tumor tissue, and a 30 minute irradiation nearly doubled the time required for EMT6 tumors to reach 500 mm3. [38] This study seeks to further validate these compounds in a second murine tumor model, namely CT26 colon carcinoma, and to compare BNCT efficacy with results from the previous EMT6 experiments.
Results
Biodistribution Studies
Studies were carried out to evaluate the boron concentrations in blood, tumor and normal organs in mice bearing CT26 tumors. These experiments were designed to mirror the studies done involving mice with EMT6 tumors [38]. Once tumors were approximately 100–150 mm3, mice were administered two separate MAC/TAC liposome injections of 200 μL via the lateral tail vein delivered 24 h apart, resulting in a total boron dose of 360–371 μg B per injection. Blood, liver, spleen, kidney, tumor, skin, and tail were collected at necropsy at multiple time points and were subsequently analyzed for their boron content using inductively coupled plasma atomic emission spectroscopy (ICP-OES). The biodistribution results are presented in Figure 1. An average peak tumor boron concentration of 36 ppm was observed 18 hours after the second injection (n = 9), though boron levels in the tumor remained relatively consistent from 18 to 72 hours. The maximum tumor to blood boron ratio of 3.1:1 occurred 72 hours after the second injection, at which time the average tumor boron concentration was 28 ppm (n = 6) (Supporting information Table 1).
Figure 1.
Mean boron concentration in tissue after a double injection of MAC/TAC liposomes in mice with CT26 tumors. Error bars indicate the standard deviation of the measurements.
Irradiation Studies
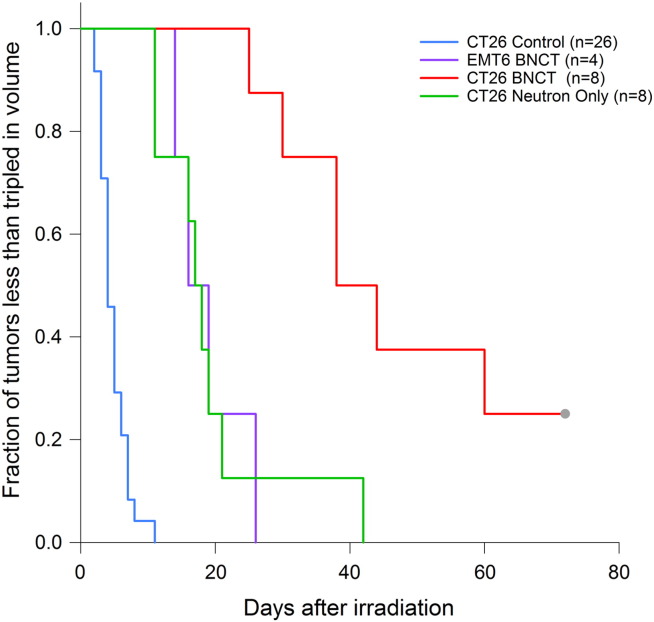
After biodistribution studies were completed, a series of neutron irradiation studies were performed to evaluate the therapeutic response of CT26 tumors to BNCT using these liposomes. The mice were irradiated for 30, 45, 60, or 90 minutes. The irradiation started 30 hours after the second liposome injection. Figure 2 illustrates the tumor growth delay after a 45 minute irradiation, comparing untreated controls (n = 29), mice receiving only a 45 minute neutron irradiation (n = 8), and mice receiving a 45 minute irradiation 30 hours after the liposome injection protocol (n = 8). The 45 minute neutron irradiation resulted in a delivered dose of 8.3 Gy to the BNCT-treated mice and 2.7 Gy to the neutron-only mice. The tumors of control mice reached 500 mm3 in 7 days, the tumors of neutron-only mice reached 500 mm3 in 21 days, and those of BNCT-treated mice required 41 days to reach the same volume. These values were all statistically different from one another (P = .00985). Additionally, 4 of the 8 mice in the BNCT-treated group experienced an at least temporary complete tumor regression, 2 of which remained in remission until the end of the study period (73 days after irradiation). No mice in the neutron-only group had resolution of their tumors after a 45 minute irradiation. A Kaplan–Meier curve demonstrating the time for tumors to triple in size is presented for different irradiation groups in Figure 3.
Figure 2.
Tumor growth delay after irradiation of multiple tumor models. This figure presents the mean tumor volume normalized to the tumor volume of that population at day 0. All non-control mice were irradiated for 45 minutes.
Figure 3.
Kaplan–Meier analysis depicting time required for tripling of tumor volume after irradiation.
Dose–Response Comparison
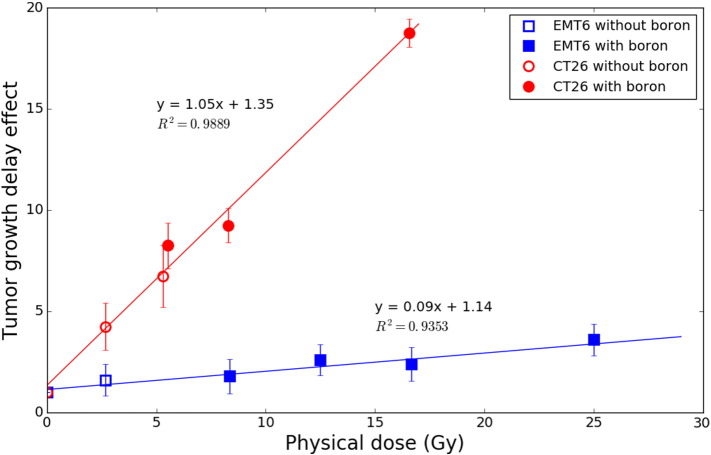
As already discussed, the total physical dose delivered in BNCT is the sum of each of the dose components. Since the constituent dose contributions are directly influenced by filtration and neutron profiles, the derivation of dose components is specific for a given neutron beam. A dose–response comparison was made without distinguishing these two variables and is presented as Figure 4. For EMT6 tumors, a delivered dose of ~8.30 Gy (30 min irradiation) results in a relative tumor growth delay of 1.8 (18 days vs. 10 days). This response increases in a near-linear fashion to 2.4 (24 days vs. 10 days) after a delivered dose of ~16.60 Gy (60 min irradiation), resulting in a slope of m = 0.09 times delayed tumor growth per Gy delivered (R2=0.9353). This effect is more pronounced in mice bearing CT26 tumors, where similar doses result in delays of 9.25 (37 days vs. 4 days) and 18.75 (75 days vs. 4 days), respectively, and an overall slope of m = 1.05 times delayed tumor growth per Gy (R2=0.9889).
Figure 4.
Comparison of growth delay effect for EMT6 and CT26 tumors after delivery of BNCT. Filled markers are indicative of a dose including a BNCT component, while open markers represent irradiations with no boron present.
Discussion
Boron Compounds
All animals in these studies received two separate injections of non-permeable liposome constructs containing MAC in the lipid bilayer, and TAC in the aqueous core. Liposome infusion was initially fractionated due to limitations on the injectable volume in mice, and the 24 hour time point was chosen based on pharmacokinetic properties. These compounds are not evaluable non-invasively as studied, though our laboratory is actively developing similar agents that function as CT or MRI contrast agents, which would allow for evaluation of boron content without need for a biopsy [42], [43], [44]. The compounds studied here are also only passively targeted to the tumor via the EPR effect. While we have seen improved tumor targeting using functionalized nanoparticles over non-functionalized nanoparticles, we have not actively targeted our liposome platform (unpublished data).
Biodistribution Studies
The peak tumor boron concentration of 36 ppm observed in CT26 mice is markedly less than the 68 ppm in EMT6 tumors as reported by Kueffer et al. [38] There were several notable differences in the development of the tumors identified during this study. The growth rate of CT26 tumors is markedly different from that of EMT6 tumors. While tumors would generally take longer to reach a 100 mm3 volume in the mouse flanks (14 days vs. 7 days for EMT6 tumors), once measureable, the tumors grew at a much more rapid rate, resulting in the inclusion of several larger tumors in the biodistribution analysis. As reported earlier, we also noted that tumor size is inversely proportional to tumor boron concentration (Supporting Materials). [38] The wider range of tumor sizes in the CT26 mice would allow the inclusion of mice with larger tumors in the data, resulting in an overall decrease in the average tumor boron content for these mice. Also noteworthy is that, as tumor grows larger, the EMT6 tumors tend to develop a necrotic central region, whereas the CT26 tumors do not. Other factors which may have altered boron delivery to tumors include variability in tumor necrosis, vasculogenesis and permeability [40].
Irradiation Studies
Even though the tumor to blood boron ratio was poor at the 30 hour time point, we carried out the irradiation experiment to directly compare results of this study with the previous EMT6 experiment. Despite lower tumor boron concentrations in CT26 tumors, we observed a very robust response to BNCT. Indeed, 50% of the tumors treated with 8.3 Gy completely resolved, and half of these tumors did not recur for the life of the mouse. While Heber et al. recently published data demonstrating the complete resolution of some tumors using these same liposome compounds for BNCT of squamous cell carcinoma of the hamster check pouch, complete resolution in mice receiving a single BNCT session had not been seen by our group until the current studies [45]. There are at least two contributing factors to the noted response, both relating to the differences in the biology of these tumors. The first factor is a difference in the inherent radiosensitivity of the two tumor types. A marked antitumor response was noted in the mice with CT26 tumors that received only neutron irradiation, whereas a much less robust response was noted in the neutron-only mice in the EMT6 studies. In fact, in the initial studies using CT26 tumors, mice received a 90 minute irradiation resulting in complete resolution of the tumors in all mice (both BNCT (16.6 Gy) and neutron only controls(5.3 Gy)). The observed difference in biologic response with no change in the physical delivery of the radiation suggests an inherent biologic difference in the sensitivity of these tumors to radiation.
The other factor likely contributing to this difference is a result of the different growth patterns of CT26 tumors compared to EMT6 tumors. As noted above, CT26 tumors develop more slowly, but, once measureable, grow more quickly than EMT6 tumors. This observation suggests that the fraction number of cells which are actively dividing (growth fraction) in CT26 tumors is larger than in EMT6 tumors. Additionally, even as CT26 tumors reach sizes up to 2 cm in diameter, they retain an ellipsoid to spherical shape, whereas EMT6 tumors become flattened and scabbed. These observations correlate with early data indicating a large central area of focal necrosis in EMT6 tumors. A large area of necrosis is likely to inhibit the uptake of boron compounds due to inadequate blood supply. As a result, the boron distribution in these EMT6 tumors is likely more heterogeneous than in CT26 tumors of equal size. This heterogeneity would prevent the sterilization of tumor cells, evidenced by the regrowth of all EMT6 tumors, whereas complete local control for more than 90 days was seen in 25% of the mice with CT26 tumors. The presence of necrotic regions, and likely hypoxic regions, could also directly contribute to differences in radiosensitivity of the two tumor lines.
Dose–Response Comparison
Only total dose was considered in the dose–response comparison, without regards specifically to the presence of boron. Consequently, the doses reported are in terms of physical dose, rather than biologic dose, which was not possible to determine due to the absence of an observable endpoint. Thus, while the particles of the BNCT reaction have a higher linear energy transfer than the other components of the beam, they were weighted the same as the doses delivered to the neutron-only mice. It is worth noting that no dramatic change of slope was noted in the dose response curves, which would have potentially indicated an increased biologic effect at the higher doses, which included dose from the BNCT reaction. It is also worth noting that this study was performed in in vivo models treated with BNCT, which is in contrast to the majority of the studies comparing different cell lines to treatment with high LET radiation.
At all doses, the radiation delivered included both high LET radiation (BNCT components, recoil and capture protons, etc.) and low LET radiation (gamma rays), though only the mice that were irradiated after having been given boron compounds had alpha particle and lithium nucleus high LET components. Response to radiation therapy was measured by means of tumor growth delay, which incorporates all forms of cell loss, including necrotic, apoptotic and mitotic cell death. The increased biologic effect from high LET radiation is usually attributed to increased complexity of DNA damage in the form of double strand breaks and local multiply damaged sites resulting from a denser ionization pattern [46], [47]. This model of biologic effectiveness is supported by the relative indifference of high LET radiation to hypoxia, phase in the cell cycle and fractionation, as DNA repair is minimal when complex DNA damage occurs [15]. This model, however, does not account for the differences seen between cell lines in the current study. While cell kinetics and level of hypoxia could vary between the two cell lines, the current understanding of high LET killing from the BNCT reaction suggests that cell kill should be similar. The difference is most likely due to a difference in inherent radiosensitivity [48]. One component of inherent radiosensitivity is radiation-induced apoptosis, which has been shown to be non-p53-mediated in high LET radiation, which is in contrast to the p53-dependent apoptosis associated with low LET radiation [49], [50], [51]. However, in this study, apoptosis is likely not the only component, as the minimum tumor size is achieved 15 days after treatment in mice with CT26 tumors treated with BNCT, and apoptosis is expected to be seen within 72 hours of irradiation.
Ideally, multiple tumor lines should be evaluated in an attempt to systematically identify potential targets for BNCT. The logistical limitations of the current study required transport of mice to the MU Research Reactor where neutron irradiation was conducted and back to a separate mouse facility for monitoring. Transport of mice in this manner is not feasible using immune-compromised mice, and thus we were limited to tumor cell lines syngenic to the mice used in the experiments. Nevertheless, this data demonstrates that inherent radiosensitivity of the target plays a role in the outcome of boron neutron capture therapy, and suggests that appropriate selection of tumor systems for the application of BNCT is imperative. This data also reinforces the concept that relative biologic effectiveness (RBE) is dependent on multiple factors, including endpoint and tumor model.
Materials and Methods
Compounds
Boron compounds were prepared using previously published methods and subsequently formulated into liposomal suspensions [38], [52], [53], [54]. The mv diameter (mean diameter of the volume distribution, representing the center of gravity of the distribution) of formulated liposomes ranged from 89.1 nm to 109.2 nm as determined by dynamic light scattering. The boron concentrations of the liposome formulations were 1800–1853 ppm for the irradiation studies, and 2384 ppm for the biodistribution studies.
Cell Culture and Tumor Induction
CT26.CL25 cells were purchased from American Type Culture Collection and cultured in Dulbecco's Modified Eagle Media supplemented with 10% FBS as recommended by American Type Culture Collection. Cells in log phase were dissociated by incubation with TrypLE buffer (Life Technologies) for 10 min, followed by addition of FBS-containing medium to terminate TrypLE digestion. Cells were then centrifuged at 323×g for 8 min at room temperature using Fisher Scientific Accuspin 3R centrifuge, and cell pellets were resuspended in PBS solution. Cells were counted using a Countess Automatic Cell Counter (Life Technologies). For tumor induction, CT26 cells (1×106 cells/mouse) were inoculated into the right flank of female BALB/c mice having an average body weight of 20 ± 2 g, following standard protocols [55]. Animals were purchased in groups of 12 mice (Harlan Laboratories) for tumor induction, and were assigned to either biodistribution or irradiation studies at induction.
Biodistribution Studies
CT26 tumors were developed in the right flank of mice as described above. After the tumors reached a reliably measureable size (approximately 75 mm3), mice were administered two separate injections of 200 μL of the MAC/TAC liposome compound into the lateral tail vein, 24 hours apart. Mice were euthanized 18, 24, 30, 48, and 72 hours after the second liposome injection. Multiple tissues, including blood, liver, spleen, kidney, skin, tumor and tail were collected at the time of necropsy. Average tumor mass at the time of necropsy was 294.8 mg (range 84–634.7). Samples were digested and analyzed using ICP-OES as described previously by Kueffer et al. [38].
Therapeutic Effect Studies
CT26 tumors were developed in either the left or right flank of mice as described above. Once tumors reached a reliably measureable size (approximately 75 mm3), mice were administered two separate injections of 200 μL of the MAC/TAC liposome compound into the lateral tail vein, 24 hours apart. Average tumor size at the time of irradiation was 122.6 mg (range 57.8–216.1) for CT26, and 60.1 mg (range 21.6–103.9) for EMT6. Mice were transported to the University of Missouri Research Reactor 30 hours after the second injection, where they were anesthetized and placed in the thermal neutron beam for 30, 45, 60, or 90 minutes. Prior to irradiation, mice were anesthetized with a combination of 10 mg/kg xylazine and 80 mg/kg ketamine administered i.p. Cu/Au flux wires were affixed to the right or left flank (over the tumor) using tape before it was placed in a positioning gantry as described previously by Kueffer et al. [38] The average neutron flux was previously determined to be 8.8 x 108 neutrons/cm2s (±7%) integrated over the (thermal) energy range of 0.0 to 0.414 eV. The neutron spectrum validation has previously been described by Brockman et al. [14] Irradiation times varied from 30 to 90 minutes, and doses were determined using the sum of dose components from Table 1. After the irradiation procedure, mice were monitored with daily tumor measurements until humane endpoints were met. All procedures involving live animals were done in accordance with the Institutional Animal Care and Use Committee at the University of Missouri.
Data Analysis
Tumor volumes in mm3 were calculated using the equation , where l is the length (greatest diameter), w is the width (perpendicular to greatest diameter) and d is the depth of the tumor, all in mm. Biodistribution plots and tumor growth curves were constructed using Microsoft Excel 2013. In the dose–response comparison, the unit Growth Delay Effect, which was the quotient of the time to 350 mm3 of the treatment group divided by the time 350 mm3 of the control group, was used to account for the difference in growth rates between CT26 and EMT6 tumors. Time-to-event analysis was performed using the Kaplan–Meier method, and outcomes among groups were compared using the log-rank test. Survival analysis was performed using SigmaPlot 12.0.
The following are the supplementary data related to this article.
Tissue boron concentrations in mice bearing CT26 tumors receiving a double injection of MAC/TAC liposomes
Variation of CT26 tumor boron concentration as a function of tumor mass. All mice received equal injections of MAC/TAC liposomes according to the double-injection protocol.
Contributor Information
Charles A Maitz, Email: maitzc@missouri.edu.
Aslam A Khan, Email: khanasl@missouri.edu.
Peter J Kueffer, Email: pjkueffer@gmail.com.
John D Brockman, Email: brockmanJD@missouri.edu.
Jonathan Dixson, Email: dixsonj@biotek.com.
Satish S Jalisatgi, Email: jalisatgis@missouri.edu.
David W Nigg, Email: David.Nigg@inl.gov.
Thomas A Everett, Email: everettt@missouri.edu.
M Frederick Hawthorne, Email: hawthornem@missouri.edu.
References
- 1.Sweet W. Early history of development of boron neutron capture therapy of tumors. J Neurooncol. 1997;33:19–26. doi: 10.1023/a:1005752827194. [DOI] [PubMed] [Google Scholar]
- 2.Hawthorne MF. Role of Chemistry in the Development of Boron Neutron Capture Therapy of Cancer. Angew Chem Int Ed Engl. 1993;32:950–984. [Google Scholar]
- 3.Baum EM, Ernesti MC, Knox HD, Miller TR, Watson AM. 17th Edition. 2009. Nuclides and Isotopes Chart of the Nuclides. [Google Scholar]
- 4.Claesson K. 2011. Radiobiological effects of alpha-particles from Astatine-211: From DNA damage to cell death. [Google Scholar]
- 5.Soloway AH, Tjarks W, Barnum BA, Rong FG, Barth RF, Codogni IM, Wilson JG. The Chemistry of Neutron Capture Therapy. (Chem. Rev. 1998, 98, 1515. Published on the Web May 20, 1998) Chem Rev. 1998;98:2389–2390. doi: 10.1021/cr980493e. [DOI] [PubMed] [Google Scholar]
- 6.Barth RF. A critical assessment of boron neutron capture therapy: an overview. J Neurooncol. 2003;62 doi: 10.1007/BF02699929. [DOI] [PubMed] [Google Scholar]
- 7.Dorn RV., III Boron neutron capture therapy (BNCT): a radiation oncology perspective. Int J Radiat Oncol Biol Phys. 1994;28:1189–1201. doi: 10.1016/0360-3016(94)90494-4. [DOI] [PubMed] [Google Scholar]
- 8.González S, Santa Cruz G. The photon-isoeffective dose in boron neutron capture therapy. Radiat Res. 2012;178:609–621. doi: 10.1667/RR2944.1. [DOI] [PubMed] [Google Scholar]
- 9.Coderre JA, Turcotte JC, Riley KJ, Binns PJ, Harling OK, Kiger WS. Boron neutron capture therapy: cellular targeting of high linear energy transfer radiation. Technol Cancer Res Treat. 2003;2:355–375. doi: 10.1177/153303460300200502. [DOI] [PubMed] [Google Scholar]
- 10.Coderre J, Morris G. The radiation biology of boron neutron capture therapy. Radiat Res. 1999;151:1–18. [PubMed] [Google Scholar]
- 11.Hopewell J, Morris G, Schwint A, Coderre J. The radiobiological principles of boron neutron capture therapy: a critical review. Appl Radiat Isot. 2011;69:1756–1759. doi: 10.1016/j.apradiso.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Mughabghab S, Divadeenam M, NE, H. Academic Press; 1981. Neutron Resonance Parameters and Thermal Cross Sections. [Google Scholar]
- 13.Brockman JD, Nigg DW, Hawthorne MF, Lee MW, McKibben C. Characterization of a boron neutron capture therapy beam line at the University of Missouri Research Reactor. J Radioanal Nucl Chem. 2009;282:157–160. [Google Scholar]
- 14.Brockman J, Nigg D, Hawthorne M, McKibben C. Spectral performance of a composite single-crystal filtered thermal neutron beam for BNCT research at the University of Missouri. Appl Radiat Isot. 2009;67:5. doi: 10.1016/j.apradiso.2009.03.108. [DOI] [PubMed] [Google Scholar]
- 15.Hall EJ, Giaccia AJ. Lippincott Williams & Wilkins; 2006. Radiobiology for the Radiologist. [Google Scholar]
- 16.Barth RF, Coderre JA, Vicente MGH, Blue TE. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 17.IAEA . International Atomic Energy Agency; 2001. Current status of neutron capture therapy. [Google Scholar]
- 18.Diaz A. Assessment of the results from the phase I/II boron neutron capture therapy trials at the Brookhaven National Laboratory from a clinician's point of view. J Neurooncol. 2003;62:101–109. doi: 10.1007/BF02699937. [DOI] [PubMed] [Google Scholar]
- 19.Joensuu H, Kankaanranta L, Seppala T, Auterinen I, Kallio M, Kulvik M, Laakso J, Vahatalo J, Kortesniemi M, Kotiluoto P. Boron neutron capture therapy of brain tumors: clinical trials at the finnish facility using boronophenylalanine. J Neurooncol. 2003;62:123–134. doi: 10.1007/BF02699939. [DOI] [PubMed] [Google Scholar]
- 20.Kankaanranta L, Seppala T, Koivunoro H, Saarilahti K, Atula T, Collan J, Salli E, Kortesniemi M, Uusi-Simola J, Makitie A. Boron neutron capture therapy in the treatment of locally recurred head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:475–482. doi: 10.1016/j.ijrobp.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Kankaanranta L, Seppala T, Koivunoro H, Saarilahti K, Atula T, Collan J, Salli E, Kortesniemi M, Uusi-Simola J, Valimaki P. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2012;82:75. doi: 10.1016/j.ijrobp.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 22.Kankaanranta L, Seppala T, Koivunoro H, Valimaki P, Beule A, Collan J, Kortesniemi M, Uusi-Simola J, Kotiluoto P, Auterinen I. L-boronophenylalanine-mediated boron neutron capture therapy for malignant glioma progressing after external beam radiation therapy: a Phase I study. Int J Radiat Oncol Biol Phys. 2011;80:369–376. doi: 10.1016/j.ijrobp.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Capala J, Stenstam HB, Skold K, Munck af Rosenschold P, Giusti V, Perrson C, Brun A, Franzen L, Carlsson J. Boron neutron capture therapy for glioblastoma multiforme: clinical studies in Sweden. J Neurooncol. 2003;62:135–144. doi: 10.1007/BF02699940. [DOI] [PubMed] [Google Scholar]
- 24.Henriksson R, Capala J, Michanek A, Lindahl SA, Salfod LG, Franzen L, Blomquist E, Westlin JE, Bergenheim AT. Boron neutron capture therapy (BNCT) for glioblastoma multiforme: A phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA) Radiother Oncol. 2008;88 doi: 10.1016/j.radonc.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto T, Matsumura A, Nakai K, Shibata Y, Endo K, Sakurai F, Kishi T, Kumada H, Yamamoto K, Torii Y. Current clinical results of the Tsukuba BNCT trial. Appl Radiat Isot. 2004;61:1089–1093. doi: 10.1016/j.apradiso.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Menendez PR, Roth BMC, Pereira MD, Casal MR, Gonzalez SJ, Feld DB, Santa Cruz GA, Kessler J, Longhino J, Blaumann H. BNCT for skin melanoma in extremities: Updated Argentine clinical results. Appl Radiat Isot. 2009;67:S50–S53. doi: 10.1016/j.apradiso.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Gavin PP, Leathers CC, DeHaan CC, Bauer WW. Biodistribution of boron in dogs with spontaneous intracranial tumors following borocaptate sodium administration. Cancer Res. 1994;54(5):1259–1263. [PubMed] [Google Scholar]
- 28.Mishima YY, Ichihashi MM, Honda CC, Shiono MM. Neutron Capture. 1992. Advances in the control of human cutaneous primary and metastatic melanoma by thermal neutron capture therapy. [Google Scholar]
- 29.Rogus RD, Harling OK, Yanch JC. Mixed field dosimetry of epithermal neutron beams for boron neutron capture therapy at the MITR-II research reactor. Med Phys. 1994 doi: 10.1118/1.597267. [DOI] [PubMed] [Google Scholar]
- 30.POzzi EC, Thorp S, Brockman J, Miller M, Nigg D, Hawthorne M. Intercalibration of physical neutron dosimetry for the RA-3 and MURR thermal neutron sources for BNCT small-animal research. Appl Radiat Isot. 2011;69:1921–1923. doi: 10.1016/j.apradiso.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Wheeler F, Nigg D, Capala J, Watkins P, Vroegindeweij C, Auterinen I, Seppala T, Bleuel D. Boron neutron capture therapy (BNCT): implications of neutron beam and boron compound characteristics. Med Phys. 1999;26:1237–1244. doi: 10.1118/1.598618. [DOI] [PubMed] [Google Scholar]
- 32.Hawthorne M, Lee M. A critical assessment of boron target compounds for boron neutron capture therapy. J Neurooncol. 2003;62:33–45. doi: 10.1007/BF02699932. [DOI] [PubMed] [Google Scholar]
- 33.Luderer MJ, de la Puente P, Azab AK. Advancements in Tumor Targeting Strategies for Boron Neutron Capture Therapy. Pharm Res. 2015;32:2824–2836. doi: 10.1007/s11095-015-1718-y. [DOI] [PubMed] [Google Scholar]
- 34.Lacroix M, Abi-Said D, Fourney D, Gokaslan Z, Shi W, DeMonte F, Lang F, McCutcheon I, Hassenbusch S, Holland E. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 35.Kato I, Ono K, Sakurai Y, Ohmae M, Murahashi A, Imahori Y, Kirihata M, Nakazawa M, Yura Y. Effectiveness of boron neutron capture therapy for recurrent head and neck malignancies. Appl Radiat Isot. 2009;67 doi: 10.1016/j.apradiso.2009.03.103. [DOI] [PubMed] [Google Scholar]
- 36.Minoru S, Ituro K, Teruhito A, Junichi H, Kenichi Y, Miyuki N, Yoshihiro K, Yasunori A, Shin-Ichi H, Yoshinori S. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J Radiat Res (Tokyo) 2014 doi: 10.1093/jrr/rrt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busse PM, Harling OK, Palmer MR, Kiger WS, Kaplan J, Kaplan I, Chuang CF, Goorley JT, Riley KJ, Newton TH. A critical examination of the results from the Harvard-MIT NCT program phase I clinical trial of neutron capture therapy for intracranial disease. J Neurooncol. 2003;62:111–121. doi: 10.1007/BF02699938. [DOI] [PubMed] [Google Scholar]
- 38.Kueffer PJ, Maitz CA, Khan AA, Schuster SA, Shlyakhtina NI, Jalisatgi SS, Brockman JD, Nigg DW, Hawthorne MF. Boron neutron capture therapy demonstrated in mice bearing EMT6 tumors following selective delivery of boron by rationally designed liposomes. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1303437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 40.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Hawthorne M, Shelly K. Liposomes as drug delivery vehicles for boron agents. J Neurooncol. 1997;33:53–58. doi: 10.1023/a:1005713113990. [DOI] [PubMed] [Google Scholar]
- 42.Goswami LN, Ma L, Chakravarty S, Cai Q, Jalisatgi SS, Hawthorne MF. Discrete Nanomolecular Polyhedral Borane Scaffold Supporting Multiple Gadolinium(III) Complexes as a High Performance MRI Contrast Agent. Inorg Chem. 2013;52:1694–1700. doi: 10.1021/ic3017613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goswami LN, Ma L, Cai Q, Sarma SJ, Jalisatgi SS, Hawthorne MF. cRGD Peptide-Conjugated Icosahedral closo-B122- Core Carrying Multiple Gd3+−DOTA Chelates for αvβ3 Integrin-Targeted Tumor Imaging (MRI) Inorg Chem. 2013;52:1701–1709. doi: 10.1021/ic302340c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarma SJ, Khan AA, Goswami LN, Jalisatgi SS, Hawthorne MF. A Trimodal Closomer Drug-Delivery System Tailored with Tracing and Targeting Capabilities. Chem A Eur J. 2016;22:12715–12723. doi: 10.1002/chem.201602413. [DOI] [PubMed] [Google Scholar]
- 45.Heber EM, Hawthorne MF, Kueffer PJ, Garabalino MA, Thorp SI, Pozzi EC, Monti Hughes A, Maitz CA, Jalisatgi SS, Nigg DW. Therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes for oral cancer in the hamster cheek pouch model. Proc Natl Acad Sci U S A. 2014;111:16077–16081. doi: 10.1073/pnas.1410865111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tannock I, Bristow R, Hill R, Harrington L. Fifth Edition. McGraw-Hill Education; 2013. Basic Science of Oncology. [Google Scholar]
- 47.Durante M. New challenges in high-energy particle radiobiology. Br J Radiol. 2014 doi: 10.1259/bjr.20130626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrington K, Jankowska P, Hingorani M. Molecular biology for the radiation oncologist: the 5Rs of radiobiology meet the hallmarks of cancer. Clin Oncol (R Coll Radiol) 2007;19:561–571. doi: 10.1016/j.clon.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Ghorai A, Bhattacharyya NP, Sarma A, Ghosh U. Radiosensitivity and Induction of Apoptosis by High LET Carbon Ion Beam and Low LET Gamma Radiation: A Comparative Study. Forensic Sci. 2014;2014:10. doi: 10.1155/2014/438030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holgersson A, Jernberg AR, Persson LM, Edgren MR, Lewensohn R, Nilsson A, Brahme A, Meijer AE. Low and high LET radiation-induced apoptosis in M059J and M059K cells. Int J Radiat Biol. 2003;79:611–621. doi: 10.1080/09553000310001596995. [DOI] [PubMed] [Google Scholar]
- 51.Mori E, Takahashi A, Yamakawa N, Kirita T, Ohnishi T. High LET heavy ion radiation induces p53-independent apoptosis. J Radiat Res. 2009;50:37–42. doi: 10.1269/jrr.08075. [DOI] [PubMed] [Google Scholar]
- 52.Feakes D, Shelly K, Knobler C, Hawthorne M. Na3[B20H17NH3]: synthesis and liposomal delivery to murine tumors. Proc Natl Acad Sci U S A. 1994;91:3029–3033. doi: 10.1073/pnas.91.8.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgiev E, Shelly K, Feakes B, Kuniyoshi J, Romano S, Hawthorne MF. Synthesis of Amine Derivatives of the Polyhedral Borane Anion [B(20)H(18)](4)(−) Inorg Chem. 1996;35:5412–5416. doi: 10.1021/ic960171y. [DOI] [PubMed] [Google Scholar]
- 54.Feakes D, Shelly K, Hawthorne M. Selective boron delivery to murine tumors by lipophilic species incorporated in the membranes of unilamellar liposomes. Proc Natl Acad Sci U S A. 1995;92:1367–1370. doi: 10.1073/pnas.92.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shelly K, Feakes D, Hawthorne MF, Schmidt P, Krisch T, Bauer W. Model studies directed toward the boron neutron-capture therapy of cancer: boron delivery to murine tumors with liposomes. Proc Natl Acad Sci U S A. 1992;89:9039–9043. doi: 10.1073/pnas.89.19.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tissue boron concentrations in mice bearing CT26 tumors receiving a double injection of MAC/TAC liposomes
Variation of CT26 tumor boron concentration as a function of tumor mass. All mice received equal injections of MAC/TAC liposomes according to the double-injection protocol.