Abstract
The pathogenic bacterium Listeria monocytogenes can persist in food processing plants for many years, even when appropriate hygienic measures are in place, with potential for contaminating ready-to-eat products and, its ability to form biofilms on abiotic surfaces certainly contributes for the environmental persistence. In this research, L. monocytogenes was grown in biofilms up 8 days attached to stainless steel and glass surfaces, contributing for advancing the knowledge on architecture of mature biofilms, since many literature studies carried out on this topic considered only early stages of cell adhesion. In this study, biofilm populations of two strains of L. monocytogenes (serotypes 1/2a and 4b) on stainless steel coupons and glass were examined using regular fluorescence microscopy, confocal laser scanning microscopy and classic culture method. The biofilms formed were not very dense and microscopic observations revealed uneven biofilm structures, with presence of exopolymeric matrix surrounding single cells, small aggregates and microcolonies, in a honeycomb-like arrangement. Moreover, planktonic population of L. monocytogenes (present in broth media covering the abiotic surface) remained stable throughout the incubation time, which indicates an efficient dispersal mechanism, since the culture medium was replaced daily. In conclusion, even if these strains of L. monocytogenes were not able to form thick multilayer biofilms, it was noticeable their high persistence on abiotic surfaces, reinforcing the need to focus on measures to avoid biofilm formation, instead of trying to eradicate mature biofilms.
Keywords: Listeria monocytogenes, Abiotic surfaces, Persistence, Biofilms
Introduction
The ability to form biofilms is a key factor for the persistence of Listeria monocytogenes in processing plants and they represent a potential source of contamination of food products, especially ready-to-eat items.1, 2 Biofilm architectural characteristics, such as thickness, density and spatial arrangement are important to define the functional properties of these biological structures, including resistance and persistence.1, 3
Many studies indicated L. monocytogenes present weak to moderate ability to form biofilms, although it has been shown some isolates can adhere strongly to abiotic surfaces, depending also on the material they are made of.1, 2, 4 Sessile L. monocytogenes populations can reach 4–6 log CFU cm−2 but it not always forms thick multilayer biofilms (9–12 log CFU cm−2) as many other bacteria do.5, 6
In the present study, biofilms formed by two strains of L. monocytogenes on abiotic surfaces during prolonged incubation times (up to 8 days – 192 h) are described, using culture method and differential staining with viability dyes, combined with observations under Confocal Laser Scanning Microscopy (CLSM) and regular fluorescence microscopy.
Materials and methods
Bacterial strains and culture conditions
Two strains of L. monocytogenes were used for biofilm experiments: serotype 1/2a represents the main one recovered from food and from environmental areas; serotype 4b strains are the major cause of human listeriosis outbreaks.7 L. monocytogenes ATCC 19115 (serotype 4b) and L. monocytogenes IAL 633 (serotype 1/2a) were acquired from Adolfo Lutz Institute (São Paulo, Brazil) and stored at −80 °C in Brain Heart Infusion broth (BHI, Oxoid, UK) supplemented with 20% (v/v) glycerol (Synth, Brazil). Working cultures were made in BHI broth incubated at 37 °C for 24 h.
Biofilm formation by L. monocytogenes on stainless steel and glass coupons
Biofilms of L. monocytogenes were grown on stainless steel (AISI 304, 2.45 cm2) and glass coupons (2.65 cm2). Prior to inoculation, the coupons were cleaned and prepared according to Winkelströter et al.,8 placed in 24-wells polystyrene microtiter plates (TPP, Switzerland) and the wells were filled with 2 ml of an overnight culture of L. monocytogenes in Brain Heart Infusion (BHI) broth (Oxoid). The load of initial inoculum (ca. 108 CFU/ml) was checked at every experiment by plate counting on BHI agar plates (37 °C/24 h). A static pre-incubation of biofilms was done at 25 °C for 3 h,to favor bacterial adhesion9 and, incubation proceeded under orbital shaking (120 rpm) up to 8 days (192 h).
After 3, 24 and 96 h of incubation, to remove non-adherent bacterial cells, coupons were double rinsed with 1 ml of phosphate buffered saline (PBS) pH 7.0, transferred to clean wells and further incubated up to 192 h in 2 ml of fresh BHI broth, to promote bacterial growth.
At the selected times, planktonic cells of L. monocytogenes in the remnant BHI broth were plated onto BHI agar (BHI broth plus 1.5% bacteriological agar, Oxoid) and incubated at 37 °C for 24 h. To enumerate L. monocytogenes cells attached to the coupons, each coupon was rinsed three times with PBS to eliminate non-adhered cells, transferred to test tubes containing 10 ml of PBS, treated for 2 min in ultrasound bath (50–60 kHz) and vortexed for 1 min according to Leriche and Carpentier,10 with modification – it was necessary to use a shorter time of sonication to maintain cell viability, since in the original publication a less potent equipment was used (4 min in a 28 kHz). Tenfold dilutions of the biofilm suspensions were done in PBS, drop plated (10 μl) on BHI agar and incubated at 37 °C for 24 h, according to Herigstad et al.,11 with modifications.
The assays were performed as biological triplicates and the results were expressed as colony forming units (CFU) per cm2 or per ml, respectively, for sessile and planktonic cells.
Microscopic evaluation of biofilms formed by L. monocytogenes on stainless steel coupons and glass
Architectural features of L. monocytogenes biofilms, formed on stainless steel were observed by regular fluorescence microscopy. Additionally, biofilms of L. monocytogenes were grown in a special glass chamber (Nunc™ Lab-Tek™ II 8 Chambered Coverglass Nagle Nunc Int., USA) with optical characteristics adequate for studies with confocal laser scanning microscopy (CLSM). For that, 0.5 ml of overnight cultures of L. monocytogenes on BHI broth (Oxoid) were placed in the wells of the glass chamber, followed by incubation at 25 °C (room temperature) up to 192 h. Supernatants were replaced by fresh BHI broth (Oxoid) at times 3, 24 and 96 h.
After 24, 96 and 192 h of incubation, stainless steel coupons and the glass chamber wells were rinsed three times with PBS to remove planktonic cells and stained with Live/Dead BacLight® kit (Molecular Probes, USA). This kit contains two fluorescent dyes: Syto 9, that stains in green live cells with intact membranes and propidium iodide (PI), which marks in red cells with a compromised membrane, that are considered to be dead or dying.12
Biofilms on stainless steel coupons were observed using an epifluorescence microscope (Nikon 80i, Japan) equipped with filters to detect Syto 9 (Nikon B-2A, 450–490 nm) and PI (Nikon G-2A, 510–560 nm), combined with the software NIS-Elements AR 3.2 (Nikon).
Biofilms formed on the glass chamber were observed by CLSM (Leica TCS SP5-AOBS, Germany) equipped with argon laser (488 nm) for detection of cells stained with Syto 9 and helium laser (543 nm) for detection of PI marked cells. Analyses of images were performed using the software LAS AF version: 2.6.0 build 7266 (Leica), adjusted to capture images with a z-series of scans (xyz) of 0.55 μm thickness and standardized stacks of 0.13 μm. The software ImageJ MacBiophotonic was used to analyze the data (available <http://www.macbiophotonics.ca/imagej/installing_imagej.htm>). The results were expressed as fluorescence units per volume (FU/μm3), and the total volume of the biofilm was calculated by multiplying the value of the area observed by the depth value.
The images of L. monocytogenes biofilms grown on stainless steel coupons and on glass chamber were acquired as independent duplicate experiments, and ca. 3–5 images were captured each time, for both systems and strains.
Statistical analysis
Experimental data were analyzed by two-way ANOVA test, followed by Bonferroni and, P < 0.05 was considered for significant difference (GraphPadPrism, version 5.0, USA). Bonferroni test is adequate when few comparisons are done at once, being simple to understand and versatile. It is suitable to compare two values and to compute confidence intervals for each comparison (https://www.graphpad.com/guides/prism/6/statistics/index.htm?stat_the_bonferroni_method.htm).
Results
Both strains of L. monocytogenes adhered to stainless steel and glass coupons since 3 h of contact (105–106 CFU/cm2), and reached 106–108 CFU/cm2 after 24 h, with no further increase of sessile populations, despite incubation for up to 192 h. Planktonic cells of L. monocytogenes were abundant (107–1010 CFU/ml) over the course of experiments.
For both strains, under fluorescence microscopy (data not shown), green stained (viable) single cells or clusters of L. monocytogenes cells were seen in biofilms formed on stainless steel coupons and they were surrounded by a yellowish background. Only sparse non-viable/dying (red cells) were seen in all assays, except for 96 h. The yellowish color may result from overlapping of Syto 9 and PI dyes, as observed for L. monocytogenes ATCC 19115 and, it indicate the presence of EPS, extracellular DNA (e-DNA) or cell injury.
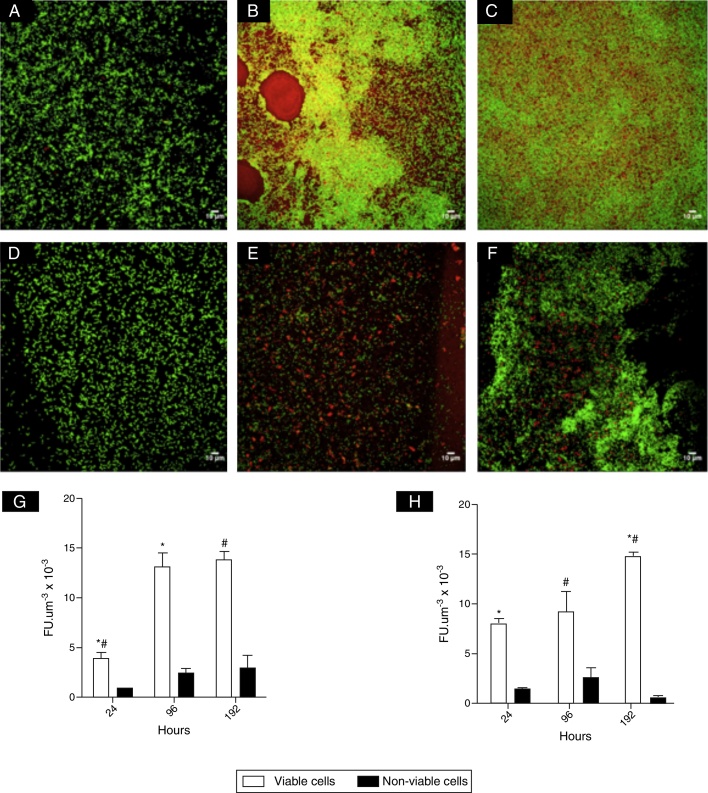
CLSM images (Fig. 1) revealed clusters and microcolonies of L. monocytogenes at 24 h of incubation, for both strains (Fig. 1A, D and E). However, honeycomb-like structures with channels predominated with advanced incubation (Fig. 1B, C and F). Fig. 1G and H shows the estimates of the number of viable cells of L. monocytogenes calculated from CLSM data and it was shown at 96 and 192 h there were significantly higher populations compared to 24 h. It was also noted that the biomass of non-viable cells did not increase significantly in mature biofilms.
Fig. 1.
Photomicrographs of biofilms of L. monocytogenes IAL 633 serotype 1/2a (A–C) and L. monocytogenes ATCC 19115 serotype 4b (D–F) acquired with CLSM (Leica SP5), ocular 10× and objective 63×, using immersion oil. Cells stained with SYTO 9 were green and cells stained with PI were red: 24 h (A and D); 96 h (B and E); 192 h (C and F). Estimates of viable and non-viable cells (G and H) respectively for L. monocytogenes ATCC 19115 and L. monocytogenes IAL 633 are also shown, expressed as fluorescence units per volume (FU μm−3). *#Denote significant statistical difference in viable cells.
Discussion
Biofilm formation by L. monocytogenes is influenced not only by lineage and origin of the strain, but also by intrinsic and extrinsic food factors. Under laboratorial conditions, Kadam et al.13 observed that nutrient availability affected biofilm formation by L. monocytogenes strains in polystyrene surfaces, highlighting that nutritionally poor media favored the growth in biofilm state. On the other hand, Zeraik and Nitschke14 demonstrated that L. monocytogenes produced more biofilm in nutrient rich medium. In the present study, BHI broth was used because it has been reported as an effective medium to promote biofilm formation by L. monocytogenes isolates from different origins, at various surfaces.2, 15 It was observed 106–108 CFU of L. monocytogenes/cm2 were present in biofilms formed on stainless steel and glass coupons, despite incubation times evaluated. Previous studies on L. monocytogenes biofilms also demonstrated that, after a quick initial adhesion to surfaces, populations did not increase largely.5, 6 The present results also indicated after initial colonization of abiotic surfaces, the number of planktonic L. monocytogenes cells remained constant due to dispersal from biofilms, which agrees with the report by Gram et al.5. Oliveira et al.,6 also reported the biotransfer potential of L. monocytogenes from biofilms to the surrounding medium, highlighting the importance of this as a source of contamination in food contact surfaces.
The microscopic observations done for L. monocytogenes mature biofilms showed a honey comb-like structure, that is known to offer fitness advantages due to improvement of mechanical stability of biofilm and absorption of nutrients by bacterial cells.16 Borucki et al.4 reported L. monocytogenes produced a dense 3-D biofilm structure, while Pilchová et al.17 demonstrated also a honeycomb-like, as the present study. The uneven colonization of abiotic surfaces is not uncommon for L. monocytogenes in biofilms,6, 18, 19 although there is also a literature report on a thin biofilm structure covering most of the abiotic surface.3 In this study, CLSM revealed in biofilms up 192 h for the two strains of L. monocytogenes there were hollows of different sizes, suggestive of cell death or dispersal (Fig. 1B). Another structural aspect that should be emphasized is the presence of a “red carpet” surrounding viable cells at later incubation times, which may indicate of the presence of extracellular DNA. Moreover, the overlap of the dyes Syto 9 and PI (Fig. 1B, yellow color) also suggests cell lysis and release of e-DNA.20
In conclusion, L. monocytogenes did not form thick biofilms but they presented a firm and highly organized structure, with cells under different physiological states, which can offer selective advantage for bacterial survival under suboptimal conditions.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors are grateful to Multiuser Laboratory of Confocal Microscopy – FMRP-USP (São Paulo Research Foundation – FAPESP process# 2004/08868-0) for the CLSM analysis, and to the Biochemistry Laboratory, Department of Physics and Chemistry – FCFRP-USP for the use of the Microplate Reader. This research was funded by São Paulo Research Foundation – FAPESP (Processes #2010/10051-3 and 2011/07062-6).
Associate Editor: Susana Saad
References
- 1.Mosquerá-Fernandez M., Rodríguez-López P., Cabo M.L., Balsa-Canto E. Numerical spatio-temporal characterization of Listeria monocytogenes biofilms. Int J Food Microbiol. 2014;18:2–183. doi: 10.1016/j.ijfoodmicro.2014.05.005. 26-36. [DOI] [PubMed] [Google Scholar]
- 2.Doijad S.P., Barbuddhe S.B., Garg S. Biofilm-forming abilities of Listeria monocytogenes serotypes isolated from different sources. PLoS ONE. 2015;10(9):e0137046. doi: 10.1371/journal.pone.0137046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridier A., Dubois-Brissonet F., Boubetra A., Thomas V., Briandet R. The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J Microbiol Methods. 2010;82:64–70. doi: 10.1016/j.mimet.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Borucki M.K., Peppin J.D., White D., Loge F., Call D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol. 2003;69:7336–7342. doi: 10.1128/AEM.69.12.7336-7342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gram L., Bagge-Ravn D., Ng Y.Y., Gymoese P., Vogel B.F. Influence of food soiling matrix on cleaning and disinfection efficiency on surface attached Listeria monocytogenes. Food Control. 2007;8:1165–1171. [Google Scholar]
- 6.Oliveira M.M.M., Brugnera D.F., Alves E., Piccoli R.H. Biofilm formation by Listeria monocytogenes on stainless steel surface and biotransfer potential. Braz J Microbiol. 2010;41:97–106. doi: 10.1590/S1517-838220100001000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y., Breidt J.R.F., Kathariou S. Competition of Listeria monocytogenes serotype 1/2a and 4b strains in mixed-culture biofilms. Appl Environ Microbiol. 2009:5846–5852. doi: 10.1128/AEM.00816-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkelströter L.K., Gomes B.C., Thomaz M.R.S., Souza V.M., De Martinis E.C.P. Lactobacillus sakei 1 and its bacteriocin influence adhesion of Listeria monocytogenes on stainless steel surface. Food Control. 2011;22:1404–1407. [Google Scholar]
- 9.Chae M.S., Schraft H. Comparative evaluation of adhesion and biofilm formation of different Listeria monocytogenes strains. Int J Food Microbiol. 2010;62:103–111. doi: 10.1016/s0168-1605(00)00406-2. [DOI] [PubMed] [Google Scholar]
- 10.Leriche V., Carpentier B. Viable but nonculturable Salmonella typhimurium in single- and binary-species biofilms in response to chlorine treatment. J Food Prot. 1995;58:1186–1191. doi: 10.4315/0362-028X-58.11.1186. [DOI] [PubMed] [Google Scholar]
- 11.Herigstad B., Hamilton M., Heersink J. How to optimize the drop plate method for enumerating bacteria. J Microbiol Methods. 2001;44:121–129. doi: 10.1016/s0167-7012(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 12.Joshi S.G., Paff M., Friedman G. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am J Infect Control. 2010;38:293–301. doi: 10.1016/j.ajic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Kadam S.R., den Besten H.M.W., van der Veen S., Zwietering M.H., Moezelaar R., Abee T. Diversity assessment of Listeria monocytogenes biofilm formation: impact of growth condition, serotype and strain origin. Int J Food Microbiol. 2013;165:259–264. doi: 10.1016/j.ijfoodmicro.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Zeraik A.E., Nitschke M. Influence of growth media and temperature on bacterial adhesion to polystyrene surfaces. Braz Arch Biol Technol. 2012;55(4):569–576. [Google Scholar]
- 15.Tomiĉić R.M., Ĉabarkapa I.S., Vukmirović D.M., Lević J.D., Tomiĉić Z.M. Influence of growth conditions on biofilm formation of Listeria monocytogenes. Food Feed Res. 2016;43(1):19–24. [Google Scholar]
- 16.Schaudinn C., Stoodley P., Kainović A. Bacterial biofilms, other structures seen as mainstream concepts. Microbe. 2001;2:231–237. [Google Scholar]
- 17.Pilchová T., Hernould M., Prévost H., Demnerová K., Pazlarová J., Tresse O. Influence of food processing environments on structure initiation of static biofilm of Listeria monocytogenes. Food Control. 2014;35:366–372. [Google Scholar]
- 18.Kalmokoff M.L., Austin J.W., Wan X.D., Sanders G., Banerjee S., Farber J.M. Adsorption, attachment and biofilm formation among isolates of Listeria monocytogenes using model conditions. J Appl Microbiol. 2001;91:725–734. doi: 10.1046/j.1365-2672.2001.01419.x. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez A., Autio W.R., McLandsborough L.A. Effect of surface roughness and stainless steel finish on Listeria monocytogenes attachment and biofilm formation. J Food Prot. 2008;71:170–175. doi: 10.4315/0362-028x-71.1.170. [DOI] [PubMed] [Google Scholar]
- 20.Qin Z., Ou Y., Yang L. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007;153:2083–2092. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]