Abstract
N-glycosylations can regulate the adhesive function of integrins. Great variations in both the number and distribution of N-glycosylation sites are found in the 18 α and 8 β integrin subunits. Crystal structures of αIIbβ3 and αVβ3 have resolved the precise structural location of each N-glycan site, but the structural consequences of individual N-glycan site on integrin activation remain unclear. By site-directed mutagenesis and structure-guided analyses, we dissected the function of individual N-glycan sites in β3 integrin activation. We found that the N-glycan site, β3-N320 at the headpiece and leg domain interface positively regulates αIIbβ3 but not αVβ3 activation. The β3-N559 N-glycan at the β3-I-EGF3 and αIIb-calf-1 domain interface, and the β3-N654 N-glycan at the β3-β-tail and αIIb-calf-2 domain interface positively regulate the activation of both αIIbβ3 and αVβ3 integrins. In contrast, removal of the β3-N371 N-glycan near the β3 hybrid and I-EGF3 interface, or the β3-N452 N-glycan at the I-EGF1 domain rendered β3 integrin more active than the wild type. We identified one unique N-glycan at the βI domain of β1 subunit that negatively regulates α5β1 activation. Our study suggests that the bulky N-glycans influence the large-scale conformational rearrangement by potentially stabilizing or destabilizing the domain interfaces of integrin.
Introduction
Glycosylation not only adds extra molecular mass to a protein and helps maintain protein stability, folding, and solubility, but also contributes to another level of structural and functional diversity1, 2. The attachment of carbohydrate moieties to the amide nitrogen of asparagine (Asn, N) residue, a process named N-linked glycosylation, is one of the most abundant post-translational modifications of protein2, 3. It has been widely appreciated that protein N-glycans play important roles in many cellular processes such as cell adhesion and migration by modulating the function of cell adhesion molecules including integrins4–10. Aberrant N-glycosylations have been observed under pathological conditions such as inflammation and cancer progression and metastasis4, 11–17, underscoring the importance of understanding the molecular function of N-glycans.
Integrins are α/β heterodimeric cell surface glycoproteins that mediate a wide range of biological functions such as development, immune response, and blood clotting18. The combination of 18 α and 8 β subunits results in 24 integrin members in human18 (Fig. 1). Each subunit of integrin contains a large extracellular domain with multiple subdomains, a single transmembrane domain and generally a short cytoplasmic domain. The integrin extracellular domain can be divided into the headpiece and the leg domains (Fig. 1A). Recent structural and functional studies have revealed that integrins can undergo a transition from a bent conformation in the resting state to an extended conformation in the active state as a result of the headpiece extension, headpiece opening and leg domain separation19. Such long-range conformational rearrangements are critical for the upregulation of integrin affinity to bind the extracellular ligands19. Both α and β integrin subunits are the major carriers of N-glycans (Fig. 1). The importance of integrin N-glycans has been evidenced by the functional effects on integrin expression, cell adhesion, spreading and migration upon the loss or gain of N-glycan sites or the changes in N-glycan contents8, 14, 20–23. Given the large-scale conformational changes of integrin and the bulky N-glycans attached to the moving domains of integrin, it is tempting to speculate that the N-glycans might influence the structural changes and thus the activation of integrins. In line with this possibility, a recent study on EGF receptor (EGFR) demonstrated that the N-glycosylation is critical for the ectodomain conformational rearrangement and its orientation relative to the cell membrane24. However, how the individual N-glycan regulates integrin conformation and ligand binding has not been well studied.
Figure 1.
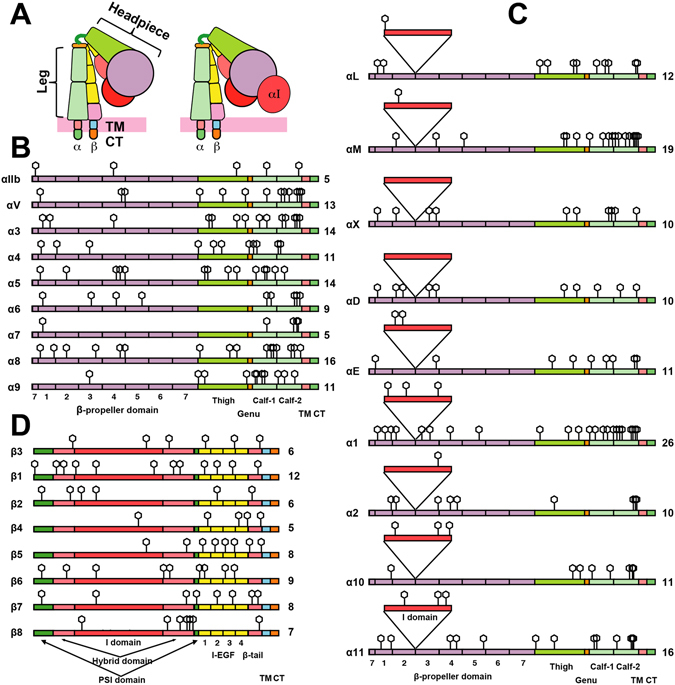
Integrin structure and N-linked glycosylation. (A) Cartoon models of integrin in the bent conformation. The domains are color-coded as same as panels B–D. (B,C) The distribution of potential N-glycan sites in the integrin α subunits without (B) or with (C) the αI-domain. The 7 blades of β-propeller domain are labeled. (D) The distribution of potential N-glycan sites in the integrin β subunits. The numbers of predicted N-linked glycosylation sites are shown on the right for each integrin subunit.
Among the integrin family, the αIIbβ3 and αVβ3 integrins have been very well characterized both structurally and functionally25–30. αIIbβ3 is essential for platelet-mediated hemostasis and thrombosis31, 32, while αVβ3 is important in tumor angiogenesis, metastasis, and inflammation33, 34. It has been reported that the αVβ3 glycosylation differs significantly between primary and metastatic melanoma cells14. Although the glycan structures are shaved and only partially resolved in the crystal structures of αIIbβ3 and αVβ3, the precise location of each individual N-glycan site can be readily defined. Many of these N-glycan sites lie in the domain interfaces that will be rearranged or disrupted from the bent to the extended conformational transition during integrin activation. The goal of this study is to investigate the effect of loss of individual N-glycan sites on β3 integrin activation and relate the function of the N-glycan sites to their structure location. We also extended our study to α5β1 integrin and identified one N-glycan site of β1 subunit that negatively regulates α5β1 ligand binding.
Results
Distribution of the N-linked glycans on integrins
The potential N-linked glycosylation sites of integrins were predicted based on the presence of the consensus NXT/S sequons (X is any amino acids except proline). As shown in Fig. 1, the putative N-linked glycosylation sites are distributed among almost all the extracellular subdomains of both α and β subunits within the headpiece and leg domains (Fig. 1). Of the 18 human α integrins, half of them contain an extra ligand-binding αI (inserted) domain (Fig. 1A–C). The numbers of N-linked glycosylation sites range from 5 (αIIb and α7) to 16 (α8) among the αI-less α-subunits (Fig. 1B), and from 10 (α2, αD and αX) to 26 (α1) among the αI-containing α-subunits (Fig. 1C). The β-subunits have relatively less N-glycan sites compared with the α-subunits, ranging from 5 (β4) to 12 (β1) sites (Fig. 1D). The locations, as well as the numbers of N-glycan sites, vary substantially even within the same subdomains among both α and β subunits. Interestingly, the N-glycan sites are mostly abundant in the leg domains of both α and β subunits, especially in the calf-1 and calf-2 domains of α-subunits (Fig. 1B,C). The αM and α1 subunits have the most abundant N-glycans (12 sites) in their calf-1 and -2 domains (Fig.1C). Some of the N-glycan sites, such as the ones adjacent to the transmembrane domains of both α and β subunits are relatively conserved (Fig. 1B–D).
Effect of the loss of individual N-glycan sites of αIIb subunit on αIIbβ3 integrin expression and ligand binding
αIIbβ3 integrin has been used as a prototype in understanding integrin structure and function32. The αIIb subunit has 5 predicted N-glycan sites: two in the β-propeller domain, one in the thigh domain, one in the calf-1 domain and one in the calf-2 domain (Fig 2A). Three of them have been visualized in the crystal structure (Fig. 2A). We removed the individual N-glycan site of αIIb subunit one by one by the glutamine substitution. Each Asn to Gln mutant of αIIb was co-expressed with wild-type β3 subunit in HEK293FT cells. The αIIbβ3 integrin activation was measured by the binding of ligand-mimetic mAb PAC-1 in the physiological Ca2+/Mg2+ condition or in the external integrin activator, Mn2+. Overall, the individual Asn to Gln substitution of αIIb subunit had no significant effect on PAC-1 binding to αIIbβ3 when activated by Mn2+ (Fig. 2B). The αIIb-N931Q mutation slightly reduced PAC-1 binding (Fig. 2B). Compared with the wild-type αIIb subunit, the cell surface expression of αIIbβ3 was decreased up to 20% among the αIIb N15Q, N249Q, N570Q and N680Q mutations. However, the αIIb-N931Q mutation dramatically decreased the cell surface expression to more than 50% (Fig. 2B). We also performed the Asn to Ser mutation, given most of the αIIb N-glycan sites locate at a loop region and the serine residue is potentially modified by O-linked glycans. Interestingly, when co-expressed with wild-type β3 in HEK293FT cells, the αIIb-N15S and the αIIb-N931S mutations significantly reduced Mn2+-induced PAC-1 binding (Fig. 2C). There is an increase of PAC-1 binding with the αIIb-N680S mutation, although it is not statistically significant (Fig. 2C). The αIIb-N249S and αIIb-N570S mutations had no effect on PAC-1 binding (Fig. 2C). The Ser substitutions, especially the αIIb-N931S mutation, had less effect on the αIIbβ3 cell surface expression than the Gln substitutions (Fig. 2B,C). Overall, individual deletion of the αIIb N-glycans had little or no effect on the Mn2+-induced ligand binding of αIIbβ3 integrin. Of note, the decreased ligand binding by the αIIb-N15S mutation is due to the Ser substitution not the loss of N-glycan since the αIIb-N15Q and αIIb-N15R mutation had no such obvious effect (Fig. 2B).
Figure 2.
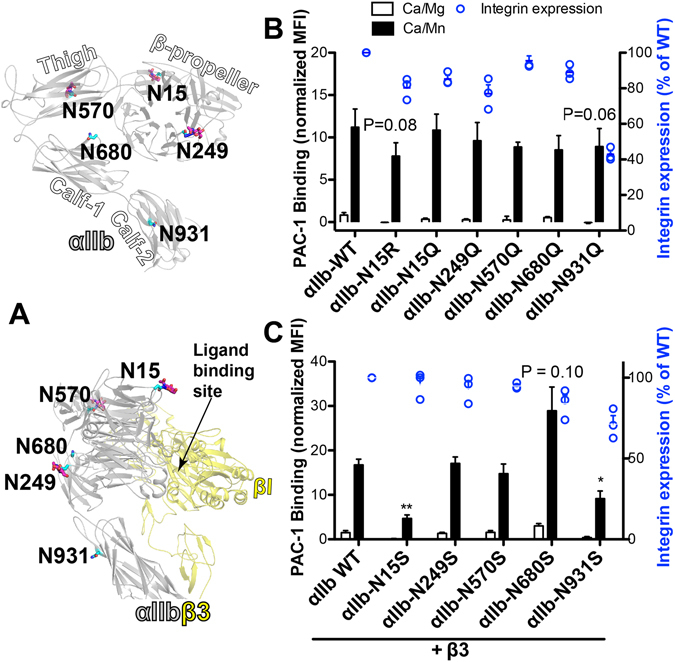
Effect of the individual N-glycan deletion of αIIb subunit on αIIbβ3 ligand binding. (A) Locations of αIIb N-glycan sites in the crystal structure of αIIbβ3 (PDB code 3FCS) at the bent conformation. Asn residues are shown as sticks with carbons in cyan. N-glycan residues resolved in the crystal structure are shown as sticks with carbons in magenta. Oxygens and nitrogens are red and blue, respectively. It should be noted that the N-glycan residues visualized in the crystal structure are trimmed ones. The native N-glycan chains could be much longer and more complex. (B,C) Ligand-mimetic mAb PAC-1 binding of the indicated single mutations of αIIb co-expressed with β3 in HEK293FT cells in the presence of 1 mM Ca2+/ Mg2+ (Ca/Mg) or 0.2 mM Ca2+ plus 2 mM Mn2+ (Ca/Mn). PAC-1 binding was measured by flow cytometry and presented as mean fluorescence intensity (MFI) normalized to integrin expression (AP3 binding). Data are means ± s.e.m. (n ≥ 3). Two-tailed t-tests were used to compare the wild type (WT) and the mutants in the Ca/Mn condition. *P < 0.05; **P < 0.01.
Effect of the loss of each individual N-glycan site of β3 subunit on αIIbβ3 integrin expression and ligand binding
The β3 subunit has 6 N-linked glycan sites: one in the βI domain, two in the hybrid domain, one in the I-EGF1 domain, one in the I-EGF3 domain and one in the β-tail domain (Fig. 3A), all of which have been resolved in the crystal structure (Fig. 3A). All these N-glycans can move with their attached subdomains during the extension of β3 integrin (Fig. 3B). Similar to the αIIb Asn to Gln mutations, most of the β3 Asn to Gln single mutations had little effect on the cell surface expression of αIIbβ3 in HEK293FT cells (Fig. 3C). Remarkably, the β3 N320Q, N559Q and N654Q mutations, located at the βI, I-EGF3, and β-tail domains, respectively, all significantly reduced the Mn2+-induced PAC-1 binding (Fig. 3A–C). In contrast, both the N371Q and N452Q mutations, located at the hybrid and I-EGF1 domains, respectively, significantly enhanced the Mn2+-induced PAC-1 binding (Fig. 3A–C). The β3-N320Q mutation in the βI domain had the most dramatic negative effect on PAC-1 binding among all the mutations. However, the combined mutation β3-N320Q-N559Q did not further decrease PAC-1 binding, but only further reduced the cell surface expression of αIIbβ3 (Fig. 3C).
Figure 3.
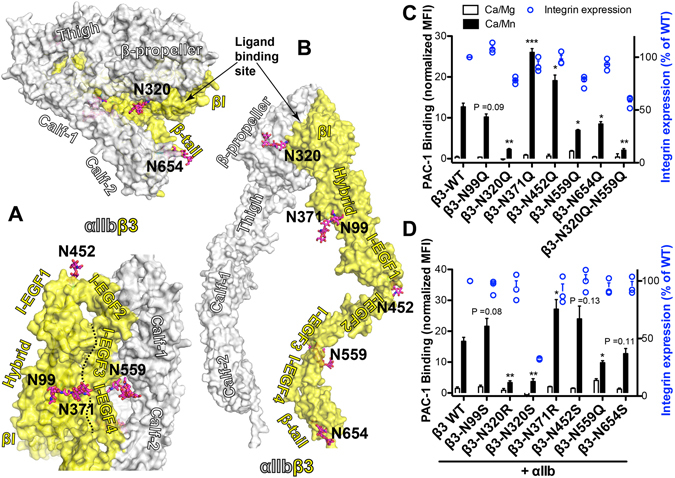
Effect of the individual N-glycan deletion of β3 subunit on αIIbβ3 ligand binding. (A) Locations of the β3 N-glycan sites in the crystal structure of αIIbβ3 (PDB code 3FCS) at the bent conformation shown as the solvent accessible surface in two views. The boundary of the hybrid/I-EGF3-4 domain interface is indicated as a black dotted line. (B) Model of the high affinity extended conformation of αIIbβ3. Asn residues are shown as sticks with carbons in cyan. N-glycan groups resolved in the crystal structure are shown as sticks with carbons, oxygens, and nitrogens in magenta, red, and blue, respectively. (C,D) Ligand-mimetic mAb PAC-1 binding of the indicated single mutations of β3 co-expressed with αIIb in HEK293FT cells in the presence of 1 mM Ca2+/Mg2+ (Ca/Mg) or 0.2 mM Ca2+ plus 2 mM Mn2+ (Ca/Mn). PAC-1 binding was measured by flow cytometry and presented as mean fluorescence intensity (MFI) normalized to integrin expression (AP3 binding). Data are means ± s.e.m. (n ≥ 3). Two-tailed t-tests were used to compare the wild type (WT) and the mutants in the Ca/Mn condition. *P < 0.05; **P < 0.01; ***P < 0.001.
To test whether the mutational effect on the αIIbβ3 PAC-1 binding is specific to the glutamine substitution, we also mutated the Asn to either Ser or Arg. As seen with the β3-N320Q mutation, both the β3 N320R and N320S mutations remarkably reduced the Mn2+-induced PAC-1 binding, although the β3-N320S mutation also dramatically reduced the cell surface expression of αIIbβ3 (Fig. 3D). In addition, the β3 N371R and N452S mutations also increased PAC-1 binding, while the β3-N654S mutation decreased PAC-1 binding (Fig. 3D). The β3 N99Q and N99S mutations had no significant effect on Mn2+-induced αIIbβ3 PAC-1 binding (Fig. 3C,D).
We next tested the effect of selected N-glycan mutations of αIIb and β3 subunits on the binding of the physiological ligand human fibrinogen (Fg). Consistent with the PAC-1 binding assay, the β3-N320R, β3-N559Q and αIIb-N15S all decreased the Mn2+-induced Fg binding to αIIbβ3 expressed in HEK293FT cells (Fig. 4A). However, the combined mutations, αIIb-N15S/β3-N320R and αIIb-N15S/β3-N559Q did not further reduce Fg binding (Fig. 4A). When measured by the anti-αIIb mAb 10E5, the anti-αIIbβ3 complex-specific mAb AP2, and the anti-β3 mAb AP3, these N-glycan mutations showed little effect on the cell surface expression of αIIbβ3 integrin (Fig. 4B), suggesting that these N-glycans may not affect the αIIb and β3 heterodimerization. Taken together, these data demonstrate the importance of individual N-glycan sites in regulating αIIbβ3 ligand binding.
Figure 4.
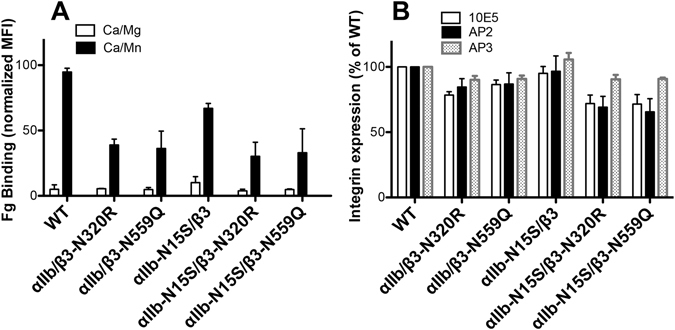
N-glycan deletions decreased αIIbβ3 integrin activation from outside the cell. (A) Fibrinogen (Fg) binding to HEK293FT cells transfected with the indicated αIIbβ3 constructs. Ligand binding was done in the presence of 1 mM Ca2+/Mg2+ (Ca/Mg) or 0.2 mM Ca2+ plus 2 mM Mn2+ (Ca/Mn). (B) Cell surface expression of αIIbβ3 integrin constructs reported by anti-αIIb mAb 10E5, anti-αIIbβ3 complex-specific mAb AP2, and anti-β3 mAb AP3. The ligand or mAb binding was measured by flow cytometry. Data are means ± s.e.m. (n ≥ 3).
Effect of the loss of individual N-glycan sites on αIIbβ3 activation from inside the cell
Integrin activation can be triggered from both outside and inside the cell, namely outside-in and inside-out signaling35. Having found that the loss of individual N-glycan sites can exert either negative or positive effect on αIIbβ3 activation induced by Mn2+ from outside the cell, we asked whether it has the similar effect on integrin inside-out activation, in which the signals are initiated from the cytoplasmic domain and transmitted to the ligand-binding site through large-scale conformational changes. The active mutations in the cytoplasmic domains such as the αIIb-R995A and αIIb-F993A or the overexpression of talin-1 head (TH) domain have been used to mimic integrin inside-out activation36–38. When co-expressed with the active αIIb-R995A mutation, the β3 N99Q, N320Q, N559Q and N654Q mutations all significantly reduced the constitutive PAC-1 binding to αIIbβ3 (Fig. 5A), while the β3 N371Q and N452Q mutations significantly enhanced PAC-1 binding (Fig. 5A). The αIIb-F993A mutation rendered αIIbβ3 more active than the αIIb-R995A mutation did (Fig. 5A,B). Likewise, the β3 N320R and N559Q mutations significantly reduced αIIb-F993A-mediated αIIbβ3 activation (Fig. 5B). The β3-N371R mutation did not further enhance the αIIb-F993A-mediated PAC-1 binding probably because the PAC-1 binding already reached the maximal level (Fig. 5B). Interestingly, the β3-N559Q mutation in the I-EGF3 domain exerted the most profound defect on αIIbβ3 inside-out activation (Fig. 5A,B).
Figure 5.
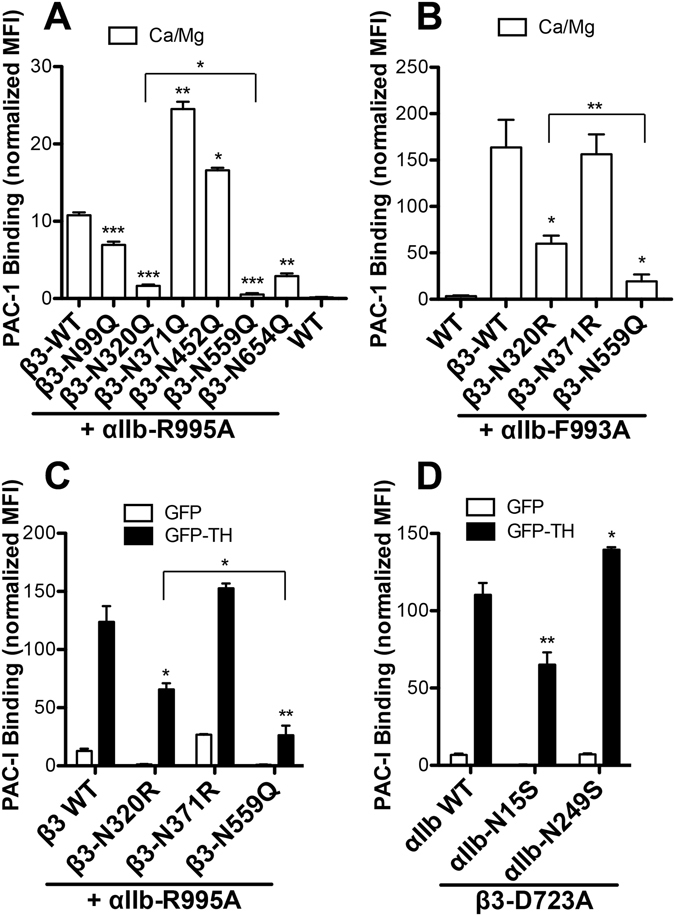
N-glycan deletions decreased αIIbβ3 integrin activation from inside the cell. (A,B) PAC-1 binding of HEK293FT cells transfected with the indicated β3 constructs and the αIIb-R995A or αIIb-F993A mutant. (C,D) PAC-1 binding of the αIIbβ3 constructs co-expressed with EGFP or EGFP-Talin1-head (TH) in the HEK293FT cells. The binding was done in the presence of 1 mM Ca2+/Mg2+ (Ca/Mg) and measured by flow cytometry. Data are means ± s.e.m. (n ≥ 3). Two-tailed t-tests were used to compare the wild type (WT) and the mutants in the same condition. *P < 0.05; **P < 0.01; ***P < 0.001.
We next tested the effect of the loss of single N-glycan sites on TH-induced αIIbβ3 activation. We performed this assay in the presence of αIIb-R995A or β3-D723A mutation, which has been shown to greatly enhance the TH-induced αIIbβ3 activation39. Consistent with the data above, the β3 N320R and N559Q mutations significantly reduced, while the β3 N371R mutation slightly increased TH-induced PAC-1 binding (Fig. 5C). As seen above, the β3-N559Q had a more remarkable effect than the β3-N320R mutation on TH-mediated αIIbβ3 activation (Fig. 5C). These data demonstrate that the N-glycans can exert both negative and positive effect on αIIbβ3 inside-out activation. Consistent with the Mn2+-induced PAC-1 binding, the αIIb-N15S mutation reduced TH-mediated αIIbβ3 activation (Fig. 5D). In contrast, the αIIb-N249S mutation increased TH-mediated αIIbβ3 activation (Fig. 5D). However, it should be noted again that the negative effect of αIIb-N15S mutation might not be directly due to the loss of N-glycan.
Effect of N-glycan deletions on αIIbβ3 integrin conformational change
The integrin affinity for ligand binding is tuned by the long-range conformational changes during integrin activation19. The ligand binding to the headpiece induces integrin ectodomain extension from the outside-in direction. On the other hand, activators from inside the cell also induce integrin conformational rearrangement, resulting in the affinity increase for the extracellular ligands. Since our data show that some of the N-glycans affect ligand binding of αIIbβ3 integrin, which requires integrin conformational changes, it is tempting to speculate that the N-linked glycans might exert their effect through regulating the conformations of integrin. To test this possibility, we used two conformation-specific mAbs, 319.4 and 370.3, to report the active conformations of β3 and αIIb, respectively. Eptifibatide (Ept), a high-affinity ligand-mimetic inhibitor that binds both the resting and active αIIbβ3, was used as a ligand to induce integrin conformational change from outside. Ept induced the binding of both 319.4 and 370.3 mAbs to αIIbβ3 (Fig. 6). The β3 N320R and N559Q mutations significantly reduced the Ept-induced binding of both mAbs to αIIbβ3 (Fig. 6A,B), while the β3-N371R mutation significantly enhanced the Ept-induced mAb 319.4 but not 370.3 binding (Fig. 6A,B). However, there was no obvious effect of the αIIb N15S, N249S and N931S mutations on the Ept-induced mAb binding (Fig. 6C,D), although the N15S and N931S mutations reduced the Mn2+-mediated PAC-1 binding (Fig. 2C). These data indicate that individual N-glycans can affect the ligand-induced conformational rearrangement of αIIbβ3 integrin.
Figure 6.
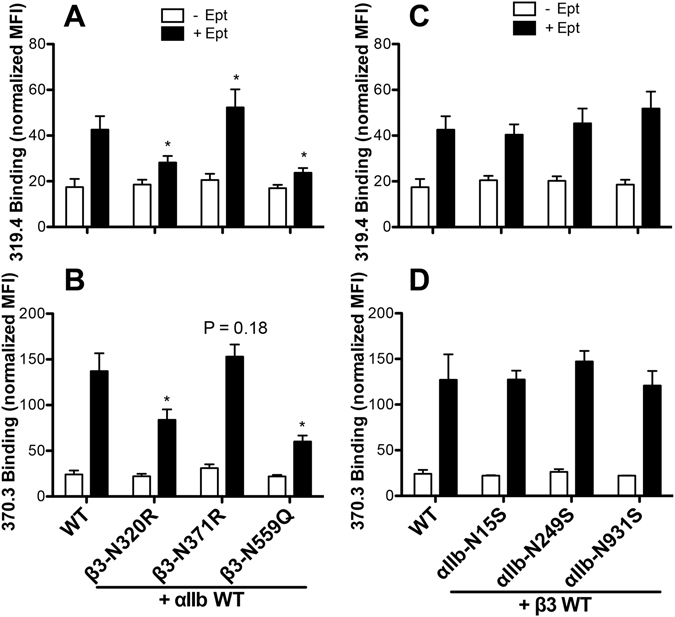
Effect of N-glycan deletions on ligand-induced conformational change of αIIbβ3 integrin. (A–D) The binding of the active conformation-specific anti-β3 mAb 319.4 (A,C) and the anti-αIIb mAb 370.3 (B,D) to the indicated αIIbβ3 constructs expressed in HEK293FT cells in the absence or presence of ligand-mimetic drug eptifibatide (Ept) plus 1 mM Ca2+/Mg2+. The mAb binding was measured by flow cytometry and presented as a normalized MFI to integrin expression (AP3 binding). Data are means ± s.e.m. (n = 3). Two-tailed t-tests were used to compare the wild type (WT) and the mutants in the same condition. *P < 0.05.
To test the effect of N-glycans on the conformational change of αIIbβ3 integrin upon inside-out activation, we used the active cytoplasmic mutations β3-K716A40, 41 and αIIb-R993A. These mutations constitutively induced the binding of mAbs 319.4 and 370.3 to αIIbβ3 (Fig. 7), indicating the conformational changes of integrin from the inside-out direction. The presence of αIIb N15S, N249S and N931S mutations did not affect the β3-K716A-mediated binding of anti-β3 mAb 319.4 (Fig. 7A). However, the αIIb N15S and N931S but not N249S mutations reduced the β3-K716A-mediated binding of anti-αIIb mAb 370.3 (Fig. 7B). In contrast, the β3 N320R and N559Q mutations reduced the αIIb-R993A-mediated binding of both 319.4 (Fig. 7C) and 370.3 (Fig. 7D) mAbs. The β3-N371R mutation had no obvious effect on the αIIb-R993A-mediated binding of both mAbs (Fig. 7C,D) probably because the αIIb-R993A mutation already induced the maximum level of αIIbβ3 activation as shown in the PAC-1 binding assay (Fig. 5B). These data demonstrate the importance of N-glycans in integrin conformational rearrangement during inside-out activation.
Figure 7.
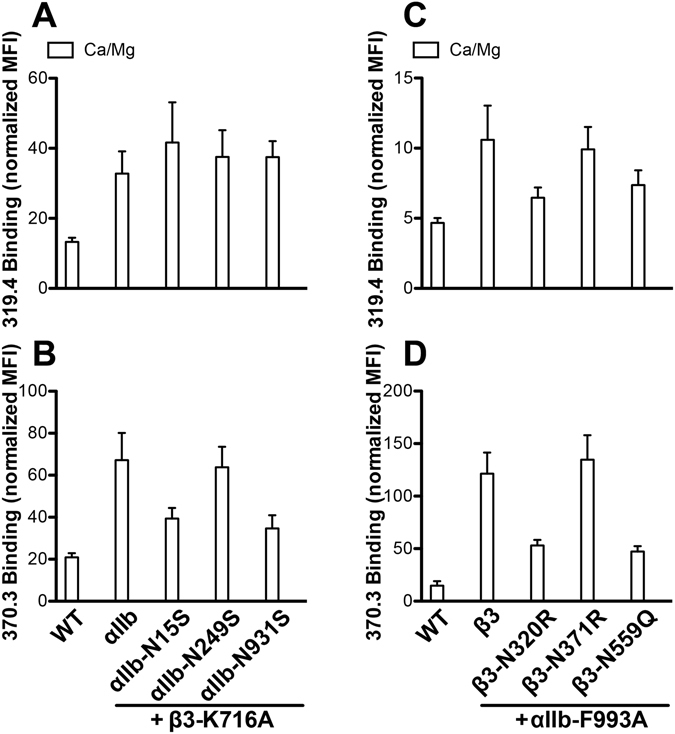
Effect of N-glycan deletions on the αIIbβ3 conformational change induced by inside-out activation. (A,B) Binding of the anti-β3 mAb 319.4 or anti-αIIb mAb 370.3 to the HEK293FT cells transfected with the αIIb WT or the glycan mutants and the active β3-K716A mutant, which mimics integrin inside-out activation. (C,D) Binding of the anti-β3 mAb 319.4 or anti-αIIb mAb 370.3 to the HEK293FT cells transfected with the β3 WT or the glycan mutants and the active αIIb-F993A mutant, which mimics integrin inside-out activation. Data are means ± s.e.m. (n = 3).
Effect of N-glycan deletions on the activation of αVβ3 integrin
The β3 subunit also forms a heterodimer with αV subunit. Changes in the complexity of N-linked glycosylation have been observed in αVβ3 integrins during the metastatic progression of tumor cells14. Having established the functional role of the individual N-glycan sites in αIIbβ3 integrin activation, we further studied the effect of N-glycan deletions on the function of αVβ3 integrin. The attachments of N-glycans have been confirmed in the αVβ3 crystal structure for most of the N-glycan sites (Fig. 8A,B). Human fibronectin (Fn) was used as a physiological ligand for αVβ3. To avoid the effect from the α5β1 integrin, which is the major Fn receptor, we used the HEK293FT cells with both endogenous α5 and β1 subunits being knocked out by the CRISPR/Cas9 technology. Consistent with the αIIbβ3 integrin, the β3-N559Q and the β3-N654Q/S mutation reduced, while the β3-N371Q/R mutation increased Mn2+-mediated Fn binding to αVβ3 integrin (Fig. 8C). However, the β3-N99Q/S, β3-N452Q/S, and even the β3-N320Q/R mutation had no obvious effect on αVβ3 Fn binding (Fig. 8C). When co-expressed with the activating αV-GAAKR mutation, which mimics αVβ3 inside-out activation42, the β3-N559Q but not β3-N320R mutation remarkably dampened Fn binding (Fig. 8D). This is also in contrast with the αIIbβ3 integrin, in which both β3-N559Q and β3-N320R mutations greatly reduced the inside-out activation of αIIbβ3 (Fig. 5B). These data indicate that certain individual N-glycans of β3 subunit may exert different effects on the function of αIIbβ3 and αVβ3 integrins.
Figure 8.
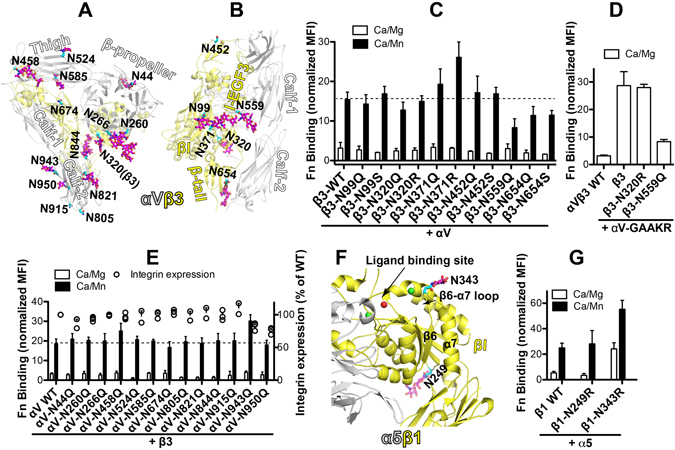
Effect of N-glycan deletions on αVβ3 and α5β1 ligand binding. (A) Locations of αV N-glycan sites in the crystal structure of αVβ3 (PDB code 4G1E). (B) Locations of β3 N-glycan sites in the crystal structure of αVβ3 (PDB code 4G1E). Asn residues are shown as sticks with carbons in cyan. N-glycan residues resolved in the crystal structure are shown as sticks with carbons in magenta. Oxygens and nitrogens are red and blue, respectively. (C) Fibronectin (Fn) binding of HEK293FT-α5β1-KO cells transfected with the β3 WT or the glycan mutants and αV WT. (D) Fn binding of HEK293FT-α5β1-KO cells transfected with the indicated β3 constructs and the αV-GAAKR mutant that mimics integrin inside-out activation. (E) Fn binding of HEK293FT-α5β1-KO cells transfected with the αV WT or the glycan mutants and β3 WT. (F) Locations of selected N-glycans at the βI domain of β1 integrin in the crystal structure of α5β1 headpiece (PDB code 4WJK). Asn and glycans are shown as sticks. Color codes are the same as panels A and B. Metal ions at the ligand-binding site are shown as spheres. (G) Fn binding of HEK293FT-α5β1-KO cells transfected with the β1 WT or the selected glycan mutants and α5 WT. Fn binding was done in the presence of 1 mM Ca2+/Mg2+ (Ca/Mg) or 0.2 mM Ca2+ plus 2 mM Mn2+ (Ca/Mn). Data are means ± s.e.m. (n = 3).
Next, we studied the effect of removal of each individual N-glycan site in αV subunit on the activation of αVβ3 integrin. αV subunit has 13 potential N-glycan sites, 10 of which have been confirmed in the crystal structure (Fig. 8A). As shown in Fig. 8E, compared with the wild-type αVβ3, most of the individual N-glycan deletions by the N to Q substitutions had no obvious effect either on the cell surface expression or the Fn binding of αVβ3 (Fig. 8E). Among all the mutations, only the αV-N458Q and the αV-N943Q moderately increased Mn2+-induced Fn binding to αVβ3 (Fig. 8E), and only the αV-N943Q and the αV-N950Q mutations mildly reduced the cell surface expression of αVβ3 (Fig. 8E). We also did the Ser substitutions for each N-glycan site of αV subunit, but no obvious effect was observed on Mn2+-induced αVβ3 Fn binding (Data not shown).
One N-glycan site in the βI domain of β1 subunit negatively regulates α5β1 integrin activation
Among the integrin β subunits, β1 subunit has the most abundant putative N-glycan sites (Fig. 1D). When paired with α5 subunit, the α5β1 heterodimer has 26 potential N-glycan sites. The function of N-glycans in the α5β1 complex formation and cell surface expression or in α5β1-mediated cell adhesion, migration, and interaction with other cell surface receptors has been studied using mutagenesis approach8. By comparing the locations of the putative N-glycan sites among the integrin β subunit (Fig. 1D), we found that the β1 subunit has an N-glycan site of β1-N343 uniquely residing at the β6-α7 loop of βI domain, which has been determined in the α5β1 headpiece crystal structure (Fig. 8F). A hallmark structural change of the βI domain during integrin activation is the downward movement of the β6-α7 loop and the α7-helix27, 30. Therefore, we hypothesized that the unique N-glycan of β1-N343 at the β6-α7 loop might play a role in regulating α5β1 activation. When co-expressed with the wild-type α5 subunit in the HEK293FT-α5β1-KO cells, the β1-N343R mutation greatly enhanced the Fn binding to α5β1 both in Ca/Mg and Ca/Mn conditions (Fig. 8G). As a control, the β1-N249R mutation distal to the ligand-binding site (Fig. 8F) had no effect on α5β1 Fn binding (Fig. 8G). Thus, the loss of the N-glycan at the β1 β6-α7 loop facilitates α5β1 integrin activation, indicating a negative regulation by this unique N-glycan of β1 subunit.
Discussion
The great variation in the number and distribution of N-linked glycosylation sites among integrin subunits add another level of heterogeneity to the very complicated integrin family (Fig. 1). An increasing body of evidence indicates that integrin N-glycans contribute to cell adhesion and migration probably by affecting integrin expression, internalization, and association with other cell surface molecules5, 15, 16. However, a direct connection between integrin N-glycans and activation-dependent ligand binding has been missing. In addition, previous work studied the functional effect of either the overall changes in integrin glycosylation or the combined N-glycan sites such as within the same integrin subdomains20, 43, 44, but the function of each individual N-glycan site has not been well documented. In this study, using the structurally well-characterized αIIbβ3, αVβ3, and α5β1 as model integrins, we found that the loss of certain individual N-glycan site either reduced or enhanced integrin activation reported by the changes in the binding of ligands or active conformation-specific mAbs, indicating that the N-linked glycosylation can exert both positive and negative effects on integrin function.
Among the N-glycan mutations of the αIIb subunit, only the αIIb-N15S mutation largely reduced the Mn2+- and TH-induced αIIbβ3 activation. It seems that the negative effect on integrin activation is not due to the loss of N-glycan of αIIb-N15 since the αIIb-N15R and αIIb-N15Q mutations didn’t have much effect on αIIbβ3 ligand binding. A previous study showed that the αIIb-N15Q mutation inhibited pro-αIIb maturation, complex formation, and degradation45, but we didn’t see an obvious effect on the cell surface expression of αIIbβ3 with this mutation. The αIIb-N15 resides at the blade 7 of β-propeller domain close to the interface formed by the αIIb β-propeller and the β3 βI domain. It is not readily known why the αIIb-N15S mutation affects αIIbβ3 ligand binding. A similar N-glycan site of αV integrin, αV-N44, locates at the blade 1 of αV β-propeller domain, but its mutation to Gln or Ser had no effect on Mn2+-induced αVβ3 Fn binding. Thus, the negative effect of αIIb-N15S on αIIbβ3 ligand binding should be specific to the Ser residue, probably due to the gain of O-linked glycosylation as indicated by the change of molecular weight of αIIb-subunit (data not shown), which may affect ligand binding directly or indirectly through affecting αIIbβ3 conformational change. The N-glycans and O-glycans differ in the composition and the formation of the branches within the glycan structures, which determine the interaction with other molecules12. Moreover, the N-glycans also vary significantly in length and complexity12. Although the complete deletion of αIIb-N15 N-glycan had no effect on αIIbβ3 ligand binding, the negative effect of αIIb-N15S mutation by the potential gain-of-O-glycan suggests that the structure variations of αIIb-N15 N-glycan may regulate the αIIbβ3 function, which is clearly worth further investigation.
Remarkably, almost all the β3 single N-glycan deletion mutations affected the αIIbβ3 ligand binding induced either by Mn2+ or by the activating mutations that mimic integrin inside-out activation. Our structural analysis revealed an interesting pattern of the N-glycan location and its impact on integrin ligand binding. All the three β3 N-glycans, including N320, N559, and N654, which positively regulate αIIbβ3 activation, locate at the αIIb-β3 inter-chain interfaces. The β3-N320 glycan locates near the interface of αIIb β-propeller and β3 βI domain and inserts into the interface between the headpiece and the leg domains in the bent conformation of αIIbβ3 (Fig. 3A). Similarly, the β3-N559 glycan lodges in the interface of αIIb calf-1/2 and β3 I-EGF3 domains. The β3-N654 glycan resides at the interface of αIIb calf-2 and β3 β-tail domains (Fig. 3A). All of these interfaces are disrupted during integrin activation due to the headpiece extension and leg domain separation (Fig. 3B)19. The hydrophilic and bulky glycan groups may destabilize these interfaces at the bent conformation by repulsive interactions and therefore facilitate integrin conformational change. Consequently, deletion of these wedge-like glycans dampens αIIbβ3 ligand binding and conformational change potentially due to the stabilization of the bent inactive conformation of integrin. This is consistent with the previous studies showing that introducing an artificial N-glycan site into the βI and hybrid domain interface of β3 subunit46 or into the thigh and calf-1 domain interface of αIIb subunit47 renders αIIbβ3 constitutively active by enforcing integrin extension. In contrast, the β3 N-glycans, attaching to N371 and N452, which negatively regulate αIIbβ3 activation, are located close to the intra-chain interfaces of β3 subunit. Unlike the β3-N559 glycan that inserts into the domain interface, the β3-N371 glycan lies on the interface formed by the β3 hybrid and I-EGF3/4 domains (Fig. 3A), which is disrupted upon the β3 extension (Fig. 3B). The loss of β3-N371 glycan increased αIIbβ3 activation, indicating that this glycan contributes to stabilizing the interface highly possibly by acting like a door bolt. In addition, the loss of the β3-N99 N-glycan, which is adjacent to the N-glycan of β3-N371 but farther away from the hybrid/I-EGF interface, slightly decreased αIIbβ3 activation. The close contacts between β3-N371 and β3-N99 N-glycans may influence the conformation of the β3-N371 N-glycan, which in turn affects the hybrid/I-EGF interface. Upon deletion of the β3-N99 N-glycan, the conformation of the β3-N371 N-glycan may further stabilize the hybrid/I-EGF interface at the bent conformation. The β3-N452 glycan resides at the opposite side of the acute angle formed by the I-EGF1 and I-EGF2 domains (Fig. 3A). This angle opens to almost 180° after integrin extension (Fig. 3B), which places the β3-N452 glycan at the newly formed I-EGF1/2 domain interface (Fig.3B). As a result, the bulky N-glycan of β3-N452 may exert repulsive tension to the interface and thus destabilize the extended conformation. Indeed, the removal of β3-N452 N-glycan facilitated αIIbβ3 activation. Thus, our data demonstrate a location-specific contribution of individual N-glycan sites in regulating integrin activation largely through affecting integrin conformational changes.
A direct link between integrin N-glycans and the conformational regulation has been missing until a recent study, in which the effect of N-glycans on the conformational equilibria of α5β1 integrin was elegantly investigated48. Our data are consistent with their results showing that the complex N-glycans stabilize the extended active conformation relative to the bent resting conformation. It was also proposed that the bulky N-glycans might exert their regulatory roles by crowding or repulsive interactions within the domain interfaces48, which is in agreement with the structure interpretations of our data. However, only the combined effects of the overall changes of N-glycans on integrin affinity were examined in the α5β1 study and the results might be a mixture of both negative and positive effects, although the positive effect was obviously dominated48. Our data show that individual N-glycan sites exert different effects on the β3 integrin activation, arguing the importance of investigating the functional role of individual N-glycans in integrin activation.
Although αVβ3 integrin shares the same β3 subunit with αIIbβ3, it is influenced differently by the loss of certain β3 N-glycan sites. Particularly, the β3-N320 mutation greatly reduced αIIbβ3 activation but had no effect on αVβ3 activation. Moreover, the αV-N844 glycan, although buried at the interface between the β3 βI and β-tail domain in the bent conformation of αVβ3 (Fig. 8A), also had no effect on Mn2+-mediated αVβ3 Fn binding, consistent with a recent study29. The requirement of large-scale conformational changes for αVβ3 ligand binding remains controversial29, 49, but an increasing body of data supports the importance of integrin extension and headpiece opening in αVβ3 activation29. However, since the function of αIIbβ3 is to mediate platelet aggregation for hemostasis, it requires the activity of αIIbβ3 to be strictly regulated31, while the fundamental function of αVβ3 is to mediate cell adhesion and migration that are essential for cell survival and proliferation34, and thus the activation of αVβ3 might not be as strictly regulated as αIIbβ3 integrin. The different effect of β3 N-glycans on αVβ3 and αIIbβ3 activation might be attributed to the differences in their biological function. Indeed, the differences in the affinity regulation between αVβ3 and αIIbβ3 had been reported previously50, 51.
The natural variants that cause the loss- or gain-of-function due to individual glycosylation mutations have not been reported for β3 integrins. Nevertheless, a recent study showed that aberrant glycosylation of both αV and β3 subunits were observed between primary and metastatic melanoma cells, which modify the integrin-mediated tumor cell migration14. However, it is not known which N-glycan site is changed during the tumor cell progression. Moreover, N-glycans can have different types depending on their contents, including the high-mannose, hybrid, and complex N-glycans. It has been shown that the composition of N-glycans such as branching and sialylation regulates the function of N-glycans2. Considering the importance of individual N-glycan sites in integrin activation as determined in the present study, it will be of great interest to determine if the heterogeneity of certain individual N-glycans affects their regulatory roles in integrin function. Of the 13 N-glycan sites of αV subunit, deletion of each individual site had little effect on αVβ3 expression and activation. It will be interesting to know if the simultaneous removal of multiple N-glycan sites will affect integrin function as shown in the study of α5β1 integrin20, 44.
Among the 24 human integrins, the function of N-glycans of α5β1 has been relatively well characterized. The N-glycan sites that are important for α5β1 heterodimer formation and biological function have been determined in both α5 and β1 subunits20, 44. Recent studies have mapped the important biological function to several individual N-glycan sites. For example, the N-glycan sites at the α5 β-propeller domain are important for α5β1-mediated cell adhesion and migration21, 52; one of the N-glycans at the α5 calf-1 domain is important for the complex formation with EGFR and the inhibition of EGFR signaling53; the N-glycan at the β1 β-tail domain is important for β1 activation and interaction with other cell membrane proteins including syndecan-4 and EGFR54; the 3 N-glycan sites at the β1 βI domain are important for α5β1 complex formation and cell spreading44. Furthermore, recent kinetic studies of the correlation between α5β1 affinity and conformation suggest that the complex-type N-glycans of α5β1 help stabilize the high-affinity conformation48. In the current study, we identified one N-glycan site at β1-N343 in the βI domain, which negatively regulates β1 activation since the removal of this glycan site rendered α5β1 constitutively active. The β1-N343 glycan may directly regulate the conformational change of βI domain by restraining the movement of the β6-α7 loop, or directly regulate ligand binding due to its proximity to the ligand binding site as proposed based on the structure modeling studies55, 56. We expect to identify more location-specific functions of individual N-glycan sites in α5β1 and other integrins. These N-glycan sites may work in concert to balance the biological activity of integrin.
Understanding of integrin conformation and affinity regulation has been greatly assisted by the mutagenesis studies. Critical residues and interactions that are important to integrin activation have been identified based on the loss- or gain-of-function mutations57. By site-directed mutagenesis, our study provides evidence that individual N-linked carbohydrate residues, depending on their structural locations, regulate integrin activation at least in part through influencing the conformational rearrangement. Exactly how these N-glycan “hotspots” are modified and regulated and how they contribute to integrin affinity and biological function need to be studied in more detail using both structural and cell biology approaches.
Materials and Methods
DNA constructs
DNA constructs of human αIIbβ3, αVβ3, α5β1, and EGFP-tagged mouse talin-1-head (EGFP-TH) were as described42, 57, 58. All the mutations were introduced by PCR using PfuTurbo DNA polymerase following the protocol of the QuikChange XL site-directed mutagenesis kit (Agilent Technologies). The introduced mutations were confirmed by DNA sequencing (Retrogen).
Antibodies and protein ligands
PAC-1 (BD Bioscience) is a ligand-mimetic mAb (IgM) specific for activated αIIbβ3 integrin59. AP3 is a conformation-independent anti-β3 mAb60 and was conjugated with Alexa Fluor 488 (ThermoFisher Scientific) or Sulfo-NHS-Biotin (ThermoFisher Scientific). 10E5 is an anti-αIIb mAb27, 61. 319.4 and 370.3 are mAbs that recognize the active conformations of β3 and αIIb, respectively57. Human fibrinogen (Fg) (Enzyme Research Laboratories) and human fibronectin (Fn) (Sigma-Aldrich) were conjugated with Alexa Fluor 647 (ThermoFisher Scientific). PE-labeled MAR4 (BD Bioscience) is a non-functional anti-β1 integrin mAb. VC5 is anti-α5 integrin mAb (BD Bioscience). Alexa Fluor 647 conjugated goat anti-mouse IgM and PE-conjugated streptavidin were from ThermoFisher Scientific.
Cell lines
HEK293FT cells (ThermoFisher Scientific) were cultured in DMEM plus 10% FBS at 37 °C with 5% CO2. The α5 and β1 subunits double-knockout HEK293FT (HEK293FT-α5β1-KO) cells were generated by the CRISPR/Cas9 gene editing technology using the α5 and β1 CRISPR/Cas9 KO plasmids from Santa Cruz Biotechnology. In brief, cells were transfected with the α5 KO plasmids for 5–7 days. The α5-negative cells were selected by single cell sorting after staining with the anti-α5 mAb VC5. The established α5-KO cells were transfected with the β1 KO plasmids and the β1-negative cells were selected by single cell sorting after staining with the anti-β1 mAb MAR4. Single cell clones with the lowest expression of both α5 and β1 subunits were selected for the following experiments.
Soluble ligand binding assay
PAC-1 and Fg binding of HEK293FT cells transfected with αIIbβ3 were as described39, 57. For EGFP-TH-induced ligand binding, HEK293FT cells were co-transfected with integrin constructs and EGFP or EGFP-TH for at least 24 hours. Ligand binding was performed in HBSGB buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 5.5 mM glucose, and 1% BSA) with 5 μg/ml PAC-1 or 50 μg/ml Alexa Fluor 647-labeled Fg in the presence of 10 μM eptifibatide (αIIbβ3-specific inhibitor) or 1 mM Ca2+/Mg2+ (Ca/Mg) or 0.2 mM Ca2+ plus 2 mM Mn2+ (Ca/Mn) at 25 °C for 30 min. Cells were then washed and incubated on ice for 30 min with the detecting reagents: 10 μg/ml Alexa Fluor 488-labeled AP3 (for Fg binding) or Alexa Fluor 488-labeled AP3 plus Alexa Fluor 647-labeled goat anti-mouse IgM (for PAC-1 binding). For EGFP-TH-induced PAC-1 binding, cells were washed and incubated on ice with biotin-labeled AP3 plus PE-labeled streptavidin and Alexa Fluor 647-labeled goat anti-mouse IgM. Human Fn binding to αVβ3 or α5β1 integrins were performed with HEK293FT-α5β1-KO transfectants. Cells were first incubated with 50 μg/ml Alexa Fluor 647-labeled Fn in the presence of 10 μM cilengitide (αVβ3-specific inhibitor), 5 mM EDTA (for α5β1), Ca/Mg or Ca/Mn, and then washed and incubated with 10 μg/ml Alexa Fluor 488-labeled AP3 (for αVβ3) or PE-labeled MAR4 (for α5β1). Integrin positive or integrin and EGFP double-positive cells were acquired for calculating the mean fluorescence intensity (MFI) by flow cytometry using Accuri C6 cytometer (BD Biosciences). Ligand binding was presented as normalized MFI, i.e. ligand MFI (after subtracting the ligand MFI in the inhibitor or EDTA condition) as a percentage of integrin MFI. By this calculation, the binding of ligands was normalized to total integrin expression.
Conformation-specific antibody binding
Binding of the active conformation-specific anti-β3 mAb 319.4 and anti-αIIb mAb 370.3 to the HEK293FT transfectants was performed as described39, 57. In brief, cells were first incubated with 10 μg/ml biotin-labeled 319.4 or 370.3 in the HBSGB buffer containing 1 mM Ca2+/Mg2+ in the absence or presence of 10 μM αIIbβ3-specific ligand-mimetic inhibitor eptifibatide at 25 °C for 30 mins, and then washed and incubated with 10 μg/ml Alexa fluor 488-labeled AP3 and Alexa fluor 647-labeled streptavidin on ice for 30 min. AP3 positive cells (expressing αIIbβ3 integrin) were acquired for calculating the MFI by flow cytometry. The 319.4 or 370.3 mAb binding was presented as normalized MFI, i.e. streptavidin MFI as a percentage of AP3 MFI. By this calculation, the binding of conformation-specific mAbs was normalized to total integrin expression.
Statistical analysis
The statistical analysis was performed using the GraphPad Prism software. Two-tailed Student’s t-test was used to calculate the p values for comparing the two experimental groups, for example, the wild type and the mutant data under the same condition. The assays were repeated independently at least three times for statistical analysis.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
We thank Drs. Daniel Bougie, Richard Aster, Peter Newman, and Barry Coller for providing antibodies; David Calderwood for providing the DNA construct of EGFP-tagged mouse talin-1-head domain. This work was supported by grants HL122985 and HL131836 (to J. Zhu) from the Heart, Lung, and Blood Institute of the National Institute of Health. X. Cai was a visiting scholar supported by the fund from the College of Animal Science and Veterinary Medicine, Qingdao Agricultural University.
Author Contributions
X.Cai, A.M.M.Thinn, Z.Wang, H.Shan, and J.Zhu performed the experiments and analyzed the data. J.Zhu designed the study and wrote the manuscripts. All the authors contributed to the manuscript preparation.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hu Shan, Email: shanhu67@163.com.
Jieqing Zhu, Email: Jieqing.Zhu@bcw.edu.
References
- 1.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nature reviews. Molecular cell biology. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi M. N-linked protein glycosylation in the ER. Biochimica et biophysica acta. 2013;1833:2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Scott DW, Patel RP. Endothelial heterogeneity and adhesion molecules N-glycosylation: implications in leukocyte trafficking in inflammation. Glycobiology. 2013;23:622–633. doi: 10.1093/glycob/cwt014. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi N, Korekane H. Branched N-glycans and their implications for cell adhesion, signaling and clinical applications for cancer biomarkers and in therapeutics. BMB Rep. 2011;44:772–781. doi: 10.5483/BMBRep.2011.44.12.772. [DOI] [PubMed] [Google Scholar]
- 6.Janik ME, Litynska A, Vereecken P. Cell migration-the role of integrin glycosylation. Biochimica et biophysica acta. 2010;1800:545–555. doi: 10.1016/j.bbagen.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu J, Isaji T, Sato Y, Kariya Y, Fukuda T. Importance of N-glycosylation on α5β1 integrin for its biological functions. Biol Pharm Bull. 2009;32:780–785. doi: 10.1248/bpb.32.780. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, et al. Branched N-glycans regulate the biological functions of integrins and cadherins. FEBS J. 2008;275:1939–1948. doi: 10.1111/j.1742-4658.2008.06346.x. [DOI] [PubMed] [Google Scholar]
- 10.Lertkiatmongkol P, et al. The Role of Sialylated Glycans in Human Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1)-mediated Trans Homophilic Interactions and Endothelial Cell Barrier Function. J Biol Chem. 2016;291:26216–26225. doi: 10.1074/jbc.M116.756502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao YY, et al. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008;99:1304–1310. doi: 10.1111/j.1349-7006.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi N, Kizuka Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv Cancer Res. 2015;126:11–51. doi: 10.1016/bs.acr.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Pochec E, et al. Aberrant Glycosylation of αVβ3 Integrin is Associated with Melanoma Progression. Anticancer Res. 2015;35:2093–2103. [PubMed] [Google Scholar]
- 15.Takahashi M, Kizuka Y, Ohtsubo K, Gu J, Taniguchi N. Disease-associated glycans on cell surface proteins. Mol Aspects Med. 2016;51:56–70. doi: 10.1016/j.mam.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Hoja-Lukowicz D, Przybylo M, Duda M, Pochec E, Bubka M. On the trail of the glycan codes stored in cancer-related cell adhesion proteins. Biochimica et biophysica acta. 2017;1861:3237–3257. doi: 10.1016/j.bbagen.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Kremser ME, et al. Characterisation of α3β1 and αVβ3 integrin N-oligosaccharides in metastatic melanoma WM9 and WM239 cell lines. Biochimica et biophysica acta. 2008;1780:1421–1431. doi: 10.1016/j.bbagen.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Hynes RO. Integrins: bi-directional, allosteric, signalling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 19.Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Current opinion in cell biology. 2012;24:107–115. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaji T, et al. N-glycosylation of the β-propeller domain of the integrin α5 subunit is essential for α5β1 heterodimerization, expression on the cell surface, and its biological function. J. Biol. Chem. 2006;281:33258–33267. doi: 10.1074/jbc.M607771200. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, et al. An N-glycosylation site on the β-propeller domain of the integrin α5 subunit plays key roles in both its function and site-specific modification by beta1,4-N-acetylglucosaminyltransferase III. J Biol Chem. 2009;284:11873–11881. doi: 10.1074/jbc.M807660200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjan A, Bane SM, Kalraiya RD. Glycosylation of the laminin receptor (α3β1) regulates its association with tetraspanin CD151: Impact on cell spreading, motility, degradation and invasion of basement membrane by tumor cells. Exp Cell Res. 2014;322:249–264. doi: 10.1016/j.yexcr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Nicolaou N, et al. Gain of glycosylation in integrin α3 causes lung disease and nephrotic syndrome. J Clin Invest. 2012;122:4375–4387. doi: 10.1172/JCI64100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaszuba K, et al. N-Glycosylation as determinant of epidermal growth factor receptor conformation in membranes. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:4334–4339. doi: 10.1073/pnas.1503262112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong J-P, et al. Crystal structure of the extracellular segment of integrin αVβ3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong JP, et al. Crystal structure of the complete integrin αVβ3 ectodomain plus an α/β transmembrane fragment. J Cell Biol. 2009;186:589–600. doi: 10.1083/jcb.200905085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao T, Takagi J, Wang J-h, Coller BS, Springer TA. Nature. 2004. Structural basis for allostery in integrins and binding of fibrinogen-mimetic therapeutics; pp. 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, et al. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong X, et al. αVβ3 Integrin Crystal Structures and their Functional Implications. Biochemistry. 2012;51:8814–8828. doi: 10.1021/bi300734n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Zhu J, Springer TA. Complete integrin headpiece opening in eight steps. J Cell Biol. 2013;201:1053–1068. doi: 10.1083/jcb.201212037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin αIIbβ3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coller BS. αIIbβ3: structure and function. Journal of thrombosis and haemostasis: JTH. 2015;13(Suppl 1):S17–25. doi: 10.1111/jth.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkinson SJ, Ellison TS, Steri V, Gould E, Robinson SD. Redefining the role(s) of endothelial αVβ3-integrin in angiogenesis. Biochemical Society transactions. 2014;42:1590–1595. doi: 10.1042/BST20140206. [DOI] [PubMed] [Google Scholar]
- 34.Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25:234–240. doi: 10.1016/j.tcb.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nature reviews. Molecular cell biology. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadokoro S, et al. Talin binding to integrin β tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 37.Calderwood DA, et al. The talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 38.Hughes PE, O’Toole TE, Ylanne J, Shattil SJ, Ginsberg MH. The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J. Biol. Chem. 1995;270:12411–12417. doi: 10.1074/jbc.270.21.12411. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Wang Z, Thinn AM, Ma YQ, Zhu J. The dual structural roles of the membrane distal region of the α-integrin cytoplasmic tail during integrin inside-out activation. J Cell Sci. 2015;128:1718–1731. doi: 10.1242/jcs.160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J, et al. The structure of a receptor with two associating transmembrane domains on the cell surface: integrin αIIbβ3. Mol. Cell. 2009;34:234–249. doi: 10.1016/j.molcel.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim C, et al. Basic amino-acid side chains regulate transmembrane integrin signalling. Nature. 2012;481:209–213. doi: 10.1038/nature10697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J, Boylan B, Luo B-H, Newman PJ, Springer TA. Tests of the extension and deadbolt models of integrin activation. J. Biol. Chem. 2007;282:11914–11920. doi: 10.1074/jbc.M700249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. Aberrant N-glycosylation of β1 integrin causes reduced α5β1 integrin clustering and stimulates cell migration. Cancer Res. 2002;62:6837–6845. [PubMed] [Google Scholar]
- 44.Isaji T, Sato Y, Fukuda T, Gu J. N-glycosylation of the I-like domain of β1 integrin is essential for β1 integrin expression and biological function: identification of the minimal N-glycosylation requirement for α5β1. J Biol Chem. 2009;284:12207–12216. doi: 10.1074/jbc.M807920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell WB, Li J, French DL, Coller BS. αIIbβ3 biogenesis is controlled by engagement of αIIb in the calnexin cycle via the N15-linked glycan. Blood. 2006;107:2713–2719. doi: 10.1182/blood-2005-07-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo B-H, Springer TA, Takagi J. Stabilizing the open conformation of the integrin headpiece with a glycan wedge increases affinity for ligand. Proc. Natl. Acad. Sci. USA. 2003;100:2403–2408. doi: 10.1073/pnas.0438060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamata T, et al. Structural requirements for activation in αIIbβ3 integrin. J Biol Chem. 2010;285:38428–38437. doi: 10.1074/jbc.M110.139667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, et al. Conformational equilibria and intrinsic affinities define integrin activation. EMBO J. 2017;36:629–645. doi: 10.15252/embj.201695803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 50.Mor-Cohen R, et al. Unique disulfide bonds in epidermal growth factor (EGF) domains of β3 affect structure and function of αIIbβ3 and αVβ3 integrins in different manner. J Biol Chem. 2012;287:8879–8891. doi: 10.1074/jbc.M111.311043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamata T, Handa M, Sato Y, Ikeda Y, Aiso S. Membrane-proximal α/β stalk interactions differentially regulate integrin activation. J. Biol. Chem. 2005;280:24775–24783. doi: 10.1074/jbc.M409548200. [DOI] [PubMed] [Google Scholar]
- 52.Hang Q, et al. A key regulator of cell adhesion: Identification and characterization of important N-glycosylation sites on integrin α5 for cell migration. Mol Cell Biol. 2017;37:e00558–16. doi: 10.1128/MCB.00558-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hang Q, et al. N-Glycosylation of integrin α5 acts as a switch for EGFR-mediated complex formation of integrin α5β1 to α6β4. Sci Rep. 2016;6:33507. doi: 10.1038/srep33507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou S, et al. Importance of membrane-proximal N-glycosylation on integrin β1 in its activation and complex formation. FASEB J. 2016;30:4120–4131. doi: 10.1096/fj.201600665R. [DOI] [PubMed] [Google Scholar]
- 55.Nagae M, et al. Crystal structure of α5β1 integrin ectodomain: Atomic details of the fibronectin receptor. J Cell Biol. 2012;197:131–140. doi: 10.1083/jcb.201111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Pan D, Bellis SL, Song Y. Effect of altered glycosylation on the structure of the I-like domain of β1 integrin: a molecular dynamics study. Proteins. 2008;73:989–1000. doi: 10.1002/prot.22126. [DOI] [PubMed] [Google Scholar]
- 57.Zhang C, et al. Modulation of integrin activation and signaling by α1/α1’-helix unbending at the junction. J Cell Sci. 2013;126:5735–5747. doi: 10.1242/jcs.137828. [DOI] [PubMed] [Google Scholar]
- 58.Bouaouina M, Lad Y, Calderwood DA. The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate β1 and β3 integrins. J Biol Chem. 2008;283:6118–6125. doi: 10.1074/jbc.M709527200. [DOI] [PubMed] [Google Scholar]
- 59.Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- 60.Kouns WC, et al. Further characterization of the loop structure of platelet glycoprotein IIIa: partial mapping of functionally significant glycoprotein IIIa epitopes. Blood. 1991;78:3215–3223. [PubMed] [Google Scholar]
- 61.Coller BS, Peerschke EI, Scudder LE, Sullivan CA. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J. Clin. Invest. 1983;72:325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


