Abstract
c-di-GMP is a cellular second messenger that regulates diverse bacterial processes, including swimming, biofilm formation and virulence. However, in Stenotrophomonas maltophilia, a nosocomial pathogen that frequently infects immunodeficient or immunoincompetent patients, the regulatory function of c-di-GMP remains unclear. Here we show that BsmR is a negative regulator of biofilm development that degrades c-di-GMP through its EAL domain. Increasing BsmR expression resulted in significant increase in bacterial swimming and decrease in cell aggregation. BsmR regulates the expression of at least 349 genes. Among them, 34 involved in flagellar assembly and a flagellar-assembly-related transcription factor (fsnR) are positively regulated. Although BsmR is a response regulator of the two-component signaling system, its role in biofilm formation depends on the expression level of its respective gene (bsmR), not on the protein’s phosphorylation level. A transcription factor, BsmT, whose coding gene is located in the same tetra-cistronic operon as bsmR, was shown to directly bind to the promoter region of the operon and, through a positive regulatory loop, modulate bsmR transcription. Thus, our results revealed that the c-di-GMP signaling pathway controls biofilm formation and swimming in S. maltophilia, suggesting c-di-GMP signaling as a target in the development of novel antibacterial agents to resist this pathogen.
Subject terms: Gene regulation, Bacterial genetics
Introduction
Stenotrophomonas maltophilia is a gram-negative bacterium belonging to the family Xanthomonadaceae. As an obligate aerobe it thrives in diverse ecological niches, including foods, soil, water systems, and plant roots1, 2. Moreover, it can persist for prolonged periods even in nutrient-poor aqueous environments3–6. In recent years, S. maltophilia has been identified not only as a globally emerging pathogen but also as an important opportunistic pathogen in hospital-acquired infections, accounting for ~65% of nosocomial infections7. A key element of the resilience and difficult eradication of S. maltophilia is the formidable capability of this bacterium to form biofilm, defined as a bacterial aggregate colonizing solid–liquid interfaces8, 9. Compared with planktonic cells, the bacteria embedded in a biofilm are 10–1,000 times more resistant to antimicrobial agents10–12. Biofilms of S. maltophilia have been found in various clinical settings and medical implants, mostly in immunocompromised or immunosuppressed hospitalized patients, and especially those in the intensive care unit (ICU). Mortality rates from S. maltophilia infections range from 23% to 77%9, 13–20. Although the World Health Organization recommends the treatment of these infections with trimethoprim-sulfamethoxazole, the susceptibility of S. maltophilia has rapidly decreased over the space of a decade, from >98% to 30–40%8, 21, 22. An effective antimicrobial strategy to combat S. maltophilia infections would be to interfere with its ability to form biofilms. However, the success of this approach depends on an understanding of the regulatory mechanisms in S. maltophilia biofilm development.
The environmental factors affecting S. maltophilia biofilm formation have been investigated, but the molecular basis of their regulatory mechanisms remains incompletely understood23–26 and thus far only a few related S. maltophilia genes have been experimentally studied. For example, several structural genes associated with the cell envelope, including those encoding the proteins involved in lipopolysaccharide/exopolysaccharide-coupled biosynthesis (rmlA, rmlC, and xanB) and the pump-encoding genes macABCsm and smeYZ have been identified as necessary for biofilm formation27–29. In addition, the genes encoding three transcription regulators (fleQ, fsnR, and bfmA) also control biofilm development. FleQ binds to the putative ATPase FleN to form a complex that directs flagellar gene expression30. FsnR, designated as a response regulator with transcription-regulating activity, binds directly to the promoter regions of gene clusters involved in flagellar assembly to activate their transcriptional initiation31, 32.
Besides the aforementioned regulatory factors, recent studies have identified bis-3′, 5′-cyclic diguanosine monophosphate (c-di-GMP) as an important cellular second messenger broadly distributed among bacteria and critical to the control of bacterial physiology, especially biofilm development and motility33. c-di-GMP activates downstream cascades by binding to specific protein effectors or riboswitches embedded in the leader regions of mRNAs. The turnover of intracellular c-di-GMP concentrations is modulated by two classes of enzymes, diguanylate cyclases (DGCs) and phosphodiesterases (PDEs). DGCs are GGDEF-domain-containing proteins that catalyze c-di-GMP biosynthesis from two molecules of GTP. PDEs are EAL- or HD-GYP-domain-containing proteins catalyzing the hydrolysis of c-di-GMP into pGpG or GMP. The processes associated with c-di-GMP turnover have been extensively documented in various animal pathogens, but none of the 27 GGDEF, EAL, or HD-GYP proteins (Supplementary Fig. S1) of S. maltophilia have been experimentally investigated. Their regulatory functions during biofilm development are therefore completely unknown.
In this study, we demonstrated the role of BsmR, an EAL-domain-containing response regulator (RR) in S. maltophilia CGMCC 1.1788, in controlling biofilm formation and swimming motility. Gradient increase in the expression of bsmR resulted in gradient increase in swimming motility and decrease in biofilm formation. BsmR degrades c-di-GMP to activate the expression of the transcriptional regulator of FsnR, which positively controls the transcription of two flagellar synthesis gene clusters to promote bacterial swimming motility. Unlike other RRs, the PDE activity of BsmR is independent of the protein’s phosphorylation level but does depend on the transcriptional level of bsmR. The latter is controlled by BsmT, encoded by a gene located in the same operon as bsmR. By autoregulation, BsmT directly binds to the promoter region of its own operon and activates bsmR expression (Fig. 1). In the present study, we dissected a c-di-GMP signaling cascade to show that this second messenger also regulates S. maltophilia biofilm formation. Our results will facilitate the development of new approaches to interfere with S. maltophilia biofilm formation, by targeting the c-di-GMP pathway.
Figure 1.
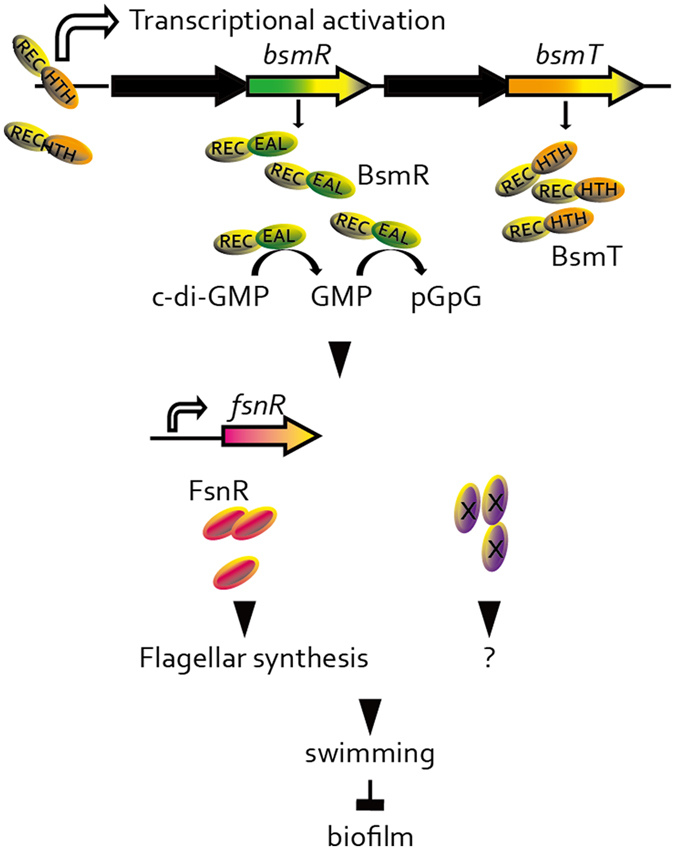
Working model. BsmR degrades c-di-GMP to decrease its cellular level, which activates the expression of FsnR, a transcriptional regulator controlling flagellar synthesis, to promote bacterial swimming motility and diminish the biofilm formation. Although as a response regulator, the PDE activity and the regulatory role of BsmR on bacterial swimming motility and biofilm formation do not depend on the phosphorylation level of BsmR but on its expression level, which is controlled by BsmT, another response regulator encoded by the bsmR operon. BsmT, working as a transcriptional regulator, directly binds to the promoter region of the bsmR operon and positively regulates the expression of all four genes in the bsmR operon, including bsmR. REC: conserved receiver domain; HTH: helix-turn-helix DNA binding domain; EAL: EAL domain; hallow arrows: transcription activation; ×: unknown regulatory elements;?: unknown regulatory role of ×.
Results
BsmR controls bacterial swimming motility and biofilm formation independent of its phosphorylation level
Bioinformatics analysis revealed that the S. maltophilia ATCC 13637 genome encodes 27 proteins involved in c-di-GMP metabolism, including 18 GGDEF-domain-containing proteins, 3 EAL domain-containing proteins, and 6 proteins containing both the GGDEF and EAL domains (Supplementary Fig. S1). Among them, DP16_RS18245 contains an N-terminal REC domain and a C-terminal EAL domain, marking it as a putative RR with diguanylate PDE activity. A mutational analysis demonstrated that DP16_RS18245 is involved in bacterial swimming motility and the regulation of biofilm formation. As shown in Fig. 2a–d, an in-frame deletion of this gene (strain ΔbsmR) did not cause recognizable phenotypic changes in biofilm formation or swimming motility. However, the overexpression of this mutant gene in S. maltophilia, achieved by transforming strain ΔbsmR with a recombinant broad-host-range plasmid (pBBR1MCS2::DP16_RS18245), yielding strain ΔbsmR-OXbsmR whose expression level of bsmR was 4255 fold of that of the WT strain (Fig. S3), resulted in a significant increase in the bacterial swimming zone, to 152.2% of the level of the WT strain. Overexpression also led to a decrease in biofilm development, to 4.0% of the WT strain level. In addition, the regulatory role of bsmR on biofilm formation and swimming motility was specific since overexpression of any other EAL-domain containing proteins encoded by S. maltophillia ATCC 13637 did not remarkably decreased the biofilm formation or increased the swimming motility, although some slight but significant decrease in the biofilm formation and increase in the swimming motility were identified (Fig. S2). The growth of both the mutant (ΔbsmR) and the overexpressing (ΔbsmR-OXbsmR) strains was similar to that of the WT strain, indicating that the phenotypic alterations were not caused by differences in growth rates (Fig. 2e). Together, these results suggested that DP16_RS18245 is a negative regulator of biofilm formation and a positive regulator of bacterial swimming motility. Therefore, DP16_RS18245 was named bsmR (biofilm and swimming motility regulator). Considering the possibility that overexpression systems might cause artificial phenotypes, a dose-dependent assay was used to verify the role of BsmR. Strains used in this assay were constructed by transforming the recombinant plasmid MpBBR1MCS2::bsmR which was achieved by replacing the lacZ promoter of pBBR1MCS2::bsmR with araC gene and the arabinose P BAD promoter, into ΔbsmR strain. As shown in Fig. 2f–h, the gradient increase in biofilm quantity and the gradient decrease in swimming zones were identified when the gradient concentration of IPTG was used to suppress the activity of the arabinose P BAD promoter. Parallelly, the expression level of bsmR was shown to be a gradient decrease accompanying the increase in IPTG concentration. The results of the dose-dependent assay verified the roles for bsmR as a negative regulator of biofilm formation and a positive regulator of swimming motility.
Figure 2.
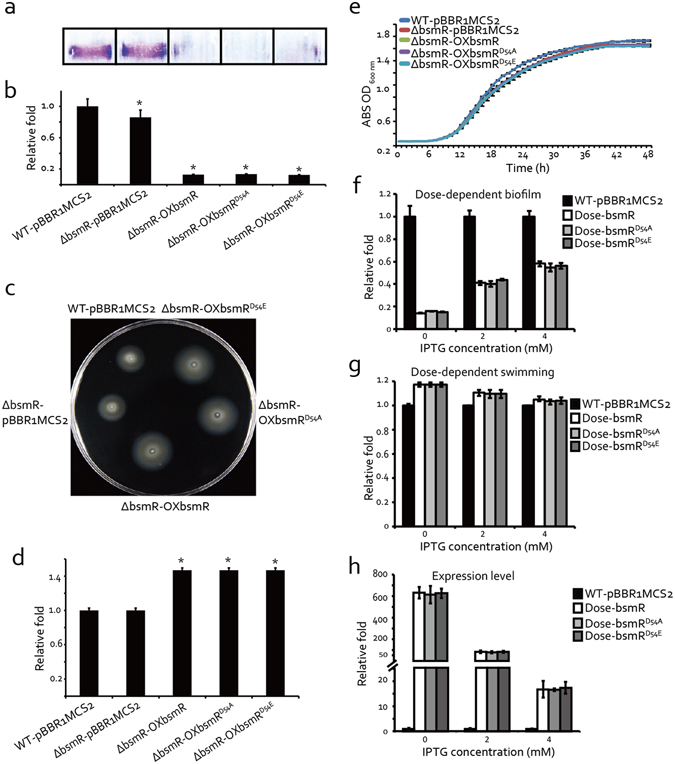
bsmR negatively regulates bacterial swimming motility and diminishes biofilm formation. (a) Biofilms formed by different bacterial strains and evaluated by crystal violet staining. (b) Relative quantification of the biofilm in (a) at 590 nm using a Tecan Infinite 200 Pro scanner. The experiment was repeated 4 times. (c) Swimming motility of different bacterial strains in rich NYG medium containing 0.15% agar. (d) Relative quantification of the diameters of the bacterial swimming zones. The data are the average of three separate measurements. (e) Growth curves of bacterial strains grown in rich NYG medium at 28 °C. ABS: absorbance. (f) Dose-dependent effect on biofilm formation. Relative quantification of the biofilm was measured when different concentrations of IPTG was used to suppress the expression of bsmR/bsmR D54A/bsmR D54E. (g) Dose-dependent effect on the swimming motility. Relative quantification of the diameters of the swimming zones was measured when different concentrations of IPTG was used to suppress the expression of bsmR/bsmR D54A /bsmR D54E. (h) The expression level of bsmR/bsmR D54A /bsmR D54E along with the addition of IPTG. Relative quantification of bsmR/bsmR D54A /bsmR D54E expression level using qRT-PCR assays. WT-pBBR1MCS2: wild-type strain containing the blank pBBR1MCS2 vector; ΔbsmR-pBBR1MCS2: bsmR in-frame deletion mutant containing the blank pBBR1MCS2 vector; ΔbsmR-OXbsmR: bsmR in-frame deletion mutant containing the recombinant pBBR1MCS2-bsmR vector; ΔbsmR-OXbsmRD54A: bsmR in-frame deletion mutant containing the recombinant pBBR1MCS2-bsmRD54A vector; ΔbsmR-OXbsmRD54E: bsmR in-frame deletion mutant containing the recombinant pBBR1MCS2-bsmRD54E vector; Dose-bsmR: bsmR in-frame deletion mutant containing the recombinant MpBBR1MCS2-bsmR; Dose-bsmRD54E: bsmR in-frame deletion mutant containing the recombinant MpBBR1MCS2-bsmRD54E; Dose-bsmRD54A: bsmR in-frame deletion mutant containing the recombinant MpBBR1MCS2-bsmRD54A. All data are representatives of at least triplicate repeatable experiments. All values are the means ± standard deviations. *p < 0.05, as determined by ANOVA.
In proteins of the two-component signal transduction system (TCS), the REC domain of a RR contains an invariant Asp residue as a phosphorylation site, and the level of RR phosphorylation is usually essential for TCS regulation34. To genetically determine the role of phosphorylation in BsmR regulation, two S. maltophilia recombinant strains were constructed using the ΔbsmR background: Strain ΔbsmR-OXbsmRD54A was constructed by transforming ΔbsmR with the pBBR1MCS2::bsmRD54A plasmid, in which nucleotides encoding the phosphorylation site Asp54 were substituted by those encoding an Ala residue. In this strain, the dephosphorylation of BsmR was constitutive. In strain ΔbsmR-OXbsmRD54E, pBBR1MCS2::bsmRD54E, in which the phosphorylation site was altered to encode a Glu, resulted in the constitutive phosphorylation of BsmR. Genetic analyses showed that the properties of the two recombinant strains with respect to biofilm development and bacterial swimming were similar to those of the bsmR-overexpressing strain (ΔbsmR-OXbsmR) (Fig. 2a–d). In addition, no significant difference was identified in the dose-dependent assays between the two recombinant strains and the bsmR-overexpressing strain (Fig. 2f–h). Collectively, these results indicated that BsmR controls both bacterial swimming motility and biofilm formation in a phosphorylation-independent manner.
bsmR is located in a tetra-cistronic operon
In bacteria, genes located in an operon are usually functionally associated35–37. According to the operon structure algorithms DOOR38 and MicrobesOnline Operon Predictions39, 40, bsmR was predicted to be in a genomic neighborhood that included a putative operon containing three other genes: DP16_RS18255, DP16_RS18250, and DP16_RS18240 (Fig. 3a). To verify the prediction, RT-PCR analyses were employed to detect transcripts generated from the intergenic regions between these genes. In repeated experiments, stable RT-PCR products representing positive amplification of the intergenic transcripts between DP16_RS18255-DP16_RS18250, DP16_RS18250-bsmR, and bsmR-DP16_RS18240 were identified (Fig. 3c), supporting the inclusion of these four genes within an operon (DP16_RS18255-DP16_RS18250-bsmR-DP16_RS18240).
Figure 3.
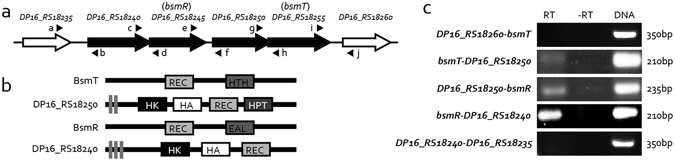
RT-PCR analyses demonstrate DP16_RS18240-bsmR-DP16_RS18250-bsmT as a tetra-cistronic operon. (a) Genomic localization of the bsmR gene. Arrows indicate the genes and their transcriptional directions. The black arrows indicate genes predicted to be in an operon. Black triangles around the arrows indicate the positions of the primers used in the RT-PCR assay. Gene names are listed above. (b) Putative secondary protein structures of BsmT, DP16_RS18250, DP16_RS18240, and BsmR. Protein structures were predicted by searching the Pfam database. (c) bsmR operon structure analysis by RT-PCR. RT: Amplification was carried out using cDNA transcribed from total RNAs as the template. −RT: cDNA synthesis in the absence of reverse transcriptase (negative control); DNA: PCR amplification with total DNA of S. maltophilia as the template (positive control). The data are representatives of triplicate repeatable experiments.
Among the four proteins encoded by this operon, DP16_RS18255 (named BsmT, biofilm and swimming motility related transcriptional regulator) is a RR with a typical REC domain and a helix-turn-helix domain. It is a putative transcription factor. Both DP16_RS18250 and DP16_RS18240 are histidine kinases (HKs) with conserved transmitter domains (Fig. 3b). In bacteria, a HK and its cognate RR constitute a typical TCS, the dominant bacterial signaling system. Since paired HK-RRs are usually encoded by neighboring genes in the genome34, 41, we proposed that the four operon-encoded proteins constitute two TCSs. To verify this hypothesis, four C-terminal His6-tagged recombinant proteins, BsmT-His6, DP16_RS18250-His6, BsmR-His6, and DP16_RS18240-His6, were expressed and then purified by Ni-nitrilotriacetic acid (Ni-NTA) affinity chromatography for use in an in vitro phosphorylation assay. However, under the assay conditions, two HKs (DS16_RS18250 and DP16_RS18240) failed to yield a phosphorylation band or phosphorylate BsmR or BsmT (Supplementary Fig. S5). These results suggested that the half-life of their phosphorylated state was too short to be detected or that unknown factors are necessary to activate their phosphorylation or stabilize their phosphorylation states, which should be investigated in the future study.
bsmR modulates cellular c-di-GMP levels to control bacterial swimming motility and biofilm formation
The N-terminal EAL domain of BsmR has putative PDE activity and was therefore expected to degrade the bacterial second messenger c-di-GMP. This finding was confirmed by expressing and then purifying recombinant BsmR-His6 for use in an in vitro c-di-GMP degradation assay. BsmR-His6 proteins were incubated with synthesized 32P-labeled c-di-GMP and the reactions were stopped at specific time points by the addition of an equal volume of 0.5 mM EDTA. The c-di-GMP levels in the reactions were then analyzed by thin layer chromatography. As shown in Fig. 4a, BsmR-His6 gradually degraded c-di-GMP such that after 30 min the c-di-GMP in the reaction was completely hydrolyzed to GMP. However, substitution of the active residue (Glu117) in the EAL domain by an Ala (recombinant protein BsmRE117A-His6) completely eliminated the PDE activity of BsmR. By adopting a FRET-based biosensor approach we were able to determine the concentration of c-di-GMP in bacterial cells. The FRET-based biosensor YFP-YcgR-CFP, in which the c-di-GMP receptor YcgR was inserted between CFP and YFP (cyan and yellow fluorescent proteins)42, 43, was used to transform Sma strains via the broad-host-range vector pBBR1MCS1 (recombinant plasmid pBBR1MCS1::yfp-ycgR-cfp). Binding of the biosensor to cellular c-di-GMP induced a conformational change that decreased FRET efficiency. The cellular c-di-GMP concentration was then determined by comparing the FRET efficiency of the cellular level with that of the in vitro YFP-YcgR-CFP protein in reactions with various concentrations of c-di-GMP. As shown in Fig. 4b, the overexpression of BsmR significantly decreased the cellular c-di-GMP level, to 50% of that of the WT strain, whereas there was no recognizable impact by overexpressing the inactive form of BsmR (BsmRE117A), which was consistent with the results of the cellular c-di-GMP level quantification by LC-MS/MS (Fig. 4c). Additionally, the gradual increase in cellular c-di-GMP level was identified when series concentrations of IPTG ranging from 0 to 4 mM were used to suppress the expression of bsmR (Fig. 4h). These results provide in vitro and in vivo evidence that BsmR is a PDE able to degrade c-di-GMP via its EAL domain.
Figure 4.
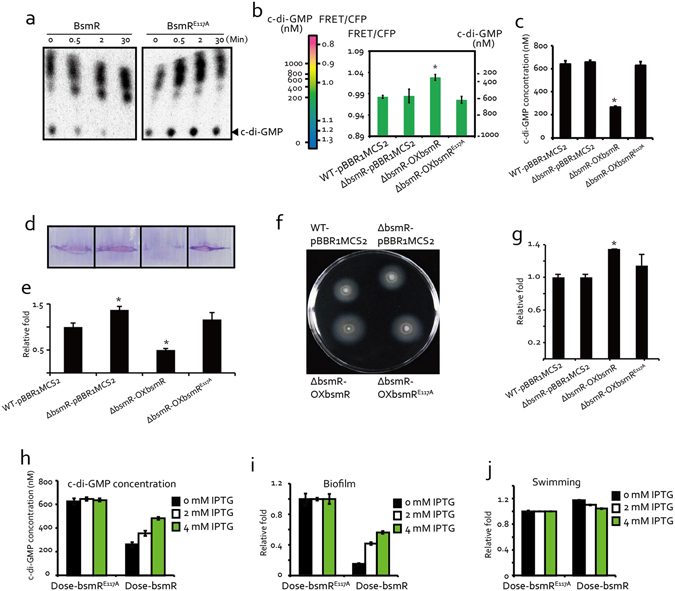
BsmR degrades c-di-GMP to regulate bacterial swimming motility and biofilm formation. (a) BsmR protein degrades c-di-GMP in vitro. Purified BsmR protein was incubated at 28 °C in reaction buffer together with the synthesized 32P-labeled c-di-GMP. At the indicated time points, aliquots of equal volume were retrieved and separated by thin layer chromatography. (b) Overexpressing bsmR significantly decreased the cellular c-di-GMP level. The FRET-based c-di-GMP biosensor was used to measure the amounts of intracellular c-di-GMP. The table of FRET values and c-di-GMP concentrations was generated by incubating the purified c-di-GMP biosensor with increasing concentrations of c-di-GMP. (c) Quantification of cellular c-di-GMP level by LC-MS/MS. (d) Biofilm of the bacterial strains stained with crystal violet. (e) Quantification of the relative bacterial levels in the biofilm shown in (d). (f) Swimming motility of the indicated strains grown on rich NYG medium plates containing 0.15% agar. (g) Relative quantification of bacterial swimming zone diameters. (h) Quantification of the cellular c-di-GMP level of strains induced by different concentrations of IPTG. (i) Relative quantification of the biofilm formed by strains induced by different concentrations of IPTG. (j) Relative quantification of the diameters of the swimming zones formed by strains induced by different concentrations of IPTG. The data are representatives of three independent experiments. Each value is the average of three replicates. Error bars represent the standard deviation (n ≥ 3). *p < 0.05, as determined by AVONA. The data are representative of at least triplicate repeatable experiments.
To determine whether the PDE of BsmR is an essential regulator of bacterial swimming motility and biofilm formation, a strain overexpressing the inactive form of BsmR (ΔbsmR-OXbsmRE117A) was constructed using the genetic background of the bsmR mutant. As shown in Fig. 4d–g, compared with the in-frame deletion mutant strain of bsmR, biofilm formation and bacterial swimming of ΔbsmR-OXbsmRE117A strain cells were unaffected; in fact, the levels of both were similar to those of the WT strain. By contrast, the overexpression of bsmR resulted in significant alterations in both phenotypes. Additionally, the dose-dependent effects of cellular c-di-GMP level controlled by BsmR were also detected. As shown in Fig. 4h–j, the cellular c-di-GMP level was gradually increased along with the addition of increasing concentrations of IPTG to suppress the BsmR expression level. Parallelly, the gradient increase in biofilm quantities and decrease in biofilm swimming zones were identified. On the contrary, no dose-dependent effects of BsmRE117A were identified. These results suggested that the degradation of cellular c-di-GMP by BsmR is critical to the regulation of swimming and cell aggregation of S. maltophilia.
Dissection of the bsmR regulon by high-throughput RNA sequencing (RNA-seq)
To understand how BsmR regulates bacterial swimming and biofilm formation, RNA-seq analysis was used to dissect the bsmR regulon. Genome-wide transcription levels in WT-pBBR1MCS2 and ΔbsmR-OXbsmR strains were compared as follows. Total RNAs were extracted from bacterial cells grown in rich NYG medium to an optical density at 600 nm (OD600) of 0.4 and used to generate cDNA by reverse transcription with random hexamer primers. The newly generated cDNAs were used for adaptor ligation and subsequent PCR amplification, followed by high-throughput Illumina sequencing. A comparison of the gene expression levels of the WT-pBBR1MCS2 and ΔbsmR-OXbsmR strains revealed that the overexpression of bsmR caused the up-regulation of 282 genes and the down-regulation of 67 genes (threshold > 2 fold, Fig. 5a and Supplementary Table S3). To verify the results, qRT-PCR assays were used to determine the expression level of bsmR and 9 other randomly selected genes among the putative target genes of bsmR. As shown in Fig. S3, in the overexpression strain (ΔbsmR-OXbsmR), the expression level of bsmR was 4255 fold of that in the WT-pBBR1MCS2 strain. Besides, 8 genes, including DP16_RS11225, DP16_RS11175, DP16_RS11180, DP16_RS11185, DP16_RS11190, DP16_RS11195, DP16_RS11260 and DP16_RS11360 were significantly up-regulated and 1 gene, DP16_RS09460, was significantly down-regulated in the overexpression strain (ΔbsmR-OXbsmR), which was consistent with the results of RNA-seq.
Figure 5.
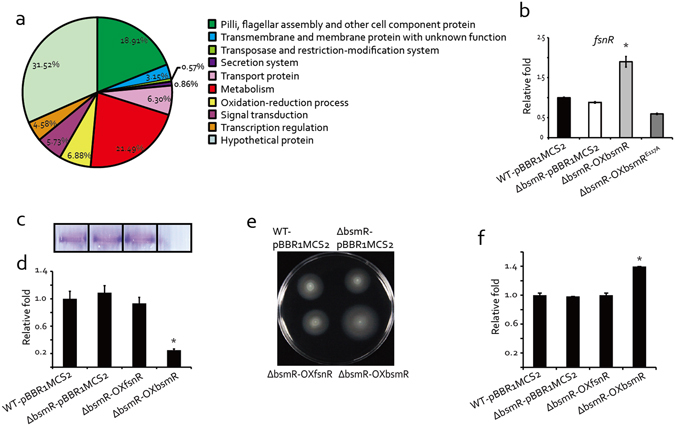
Dissection of the BsmR regulon by RNA-seq. (a) bsmR regulon analysis by high-throughput RNA sequencing. The putative target genes were functionally categorized into 10 groups (Supplementary Table S3). (b) qRT-PCR assay shows the positive regulation of fsnR by bsmR. cDNA was reverse-transcribed from the total RNAs of the indicated bacterial strains. The tmRNA level served as the internal control. (c) A biofilm formed by the bacterial strains and stained with crystal violet. (d) Relative quantification of the biofilm levels of the bacterial strains. (e) Swimming motility of the indicated bacterial strains on rich NYG medium plates containing 0.15% agar. (f) Relative quantification of bacterial swimming zone diameters. Each value is the average of three replicates. Error bars represent standard deviation (n ≥ 3). *p < 0.05, as determined by ANOVA. The data are representatives of at least triplicate repeatable experiments.
The 349 genes identified by RNA-seq analysis were functionally classified into 10 groups: cell component proteins, transmembrane and membrane proteins, transposase and restriction-modification, secretion, transport proteins, metabolism, oxidation-reduction, signal transduction, transcriptional regulation, and hypothetical proteins (Fig. 5a and Supplementary Table S3). Except the largest group, hypothetical proteins, containing 110 genes with a proportion of 31.52% of the genes regulated by BsmR under the assay conditions, there are two larger groups, metabolism proteins and cell component proteins, suggesting that BsmR plays an important role in modulating the cellular biophysical and biochemical reactions as well as cell component synthesis and assembly, which is the precondition to alter bacterial behavior. It was noteworthy that 34 genes associated with flagellar assembly (ratio 34/39, namely 87.2% of the genes involved in flagellar assembly in S. maltophilia ATCC 13637) were up-regulated in the bsmR-overexpressing strain, which suggested that bsmR positively controls bacterial flagellar assembly to modulate swimming motility. Additionally, in the signal transduction group, 12 chemotaxis-related genes, including the up-regulated chemotaxis gene cluster cheA-cheW-cheB-cheY-cheZ, were identified. Previous studies demonstrated that the chemotaxis signaling pathway controls the flagellar motor as well as bacterial motility44, 45. For example, in Escherichia coli, phosphorylated CheY binds to FliM, a switch protein located at the base of the flagellar body, to shift the direction of flagellar rotation from counterclockwise to clockwise46, 47. Taken together, these findings are consistent with the role for BsmR as a regulator of bacterial motility, by controlling both flagellar assembly and flagellar motor movements. Furthermore, a group of 24 genes involved in oxidation-reduction process were shown to be regulated by BsmR (Fig. 5a and Supplementary Table S3), which suggested another role for BsmR as a modulator of the cellular redox state. Besides the functional genes, some regulatory genes were also identified, including 4 sigma factors and 11 transcriptional regulators (Supplementary Table S3). Among them, there is a response regulator coding gene, DP16_RS11195, previously reported to encode the transcriptional regulator FsnR32. This positive regulator of bacterial swimming mobility directly binds to the promoter sequences of 10 flagella-associated genes to activate their transcription: DP16_RS19075, DP16_RS11295 (flhB), DP16_RS11250, DP16_RS11215 (fliE), DP16_RS11200, DP16_RS11175 (flgD), DP16_RS11160 (flgC), DP16_RS11100, DP16_RS11095 (flgA), and DP16_RS11070. We therefore hypothesized that BsmR activates fsnR transcription to modulate the activity of these genes. As shown in Fig. 5b, overexpressing bsmR significantly increased the fsnR mRNA level to 189.6% of that of the WT strain (Fig. 5b), indicating that fsnR is positively regulated by BsmR. However, epistasis analyses showed that the overexpression of fsnR against the bsmR in-frame deletion background had no influence on bacterial swimming motility or biofilm formation (Fig. 5c–f). This was probably because, besides fsnR, which modulates only a small portion of bsmR-targeted flagella-related genes (ratio 10/34), other transcriptional regulators or regulatory events might be activated simultaneously to cooperatively modulate bacterial motility and biofilm formation. For example, FliA, one of the sigma factors positively regulated by BsmR, was reported to modulate the swimming and biofilm formation by controlling the flagellum number in Rhodobacter sphaeroides 48. And DP16_RS09435, a transcriptional regulator negatively regulated by BsmR, is homologous to the CRP/FNR family protein which is a c-di-GMP effector reported to regulate biofilm formation in Burkholderia cenocepacia 49.
bsmT is involved in the regulation of bacterial swimming motility and biofilm formation
Since the bsmR operon contains four regulatory genes, we asked whether the other three genes are also involved in regulating bacterial swimming motility and biofilm formation, and whether there is a regulatory relationship between the four genes. In-frame deletion mutants and overexpressing strains of the other three genes were constructed and used in genetic analyses. As shown in Fig. 6a–d, the in-frame deletion of bsmT did not affect either bacterial swimming or biofilm production, but overexpressing bsmT significantly enhanced bacterial swimming motility, evidenced by the increase in the diameter of the swimming zone of strain ΔbsmT-OXbsmT to 115.6% of that of the WT strain. Moreover, in this strain bacterial biofilm formation was negligible, as it was only 4.6% of that measured in the WT strain. This pattern of phenotypic change resembled that of the bsmR-overexpressing strain. However, neither the deletion nor the overexpression of the other two HK genes, DP16_RS18250 and DP16_RS18240, resulted in remarkable differences with the WT strain with respect to swimming motility and biofilm formation (Fig. S4). Again, the similar growth curves of all aforementioned recombinant strains ruled out the influence of bacterial growth on either of these activities (Fig. 6e and Fig. S4e,j). In addition, a dose-dependent assay was also carried out to exclude the potential artificial phenotypes caused by the overexpression of bsmT. As shown in Fig. 6f–h, the dose-dependent effects of biofilm quantities and swimming zones were also identified along with the decrease of the expression level of bsmT, which supported the view that bsmT, similar to bsmR, modulates biofilm formation and swimming motility in the bacterial cells. Thus, within the bsmR operon, only bsmR and bsmT are involved in the regulation of bacterial swimming motility and biofilm formation.
Figure 6.
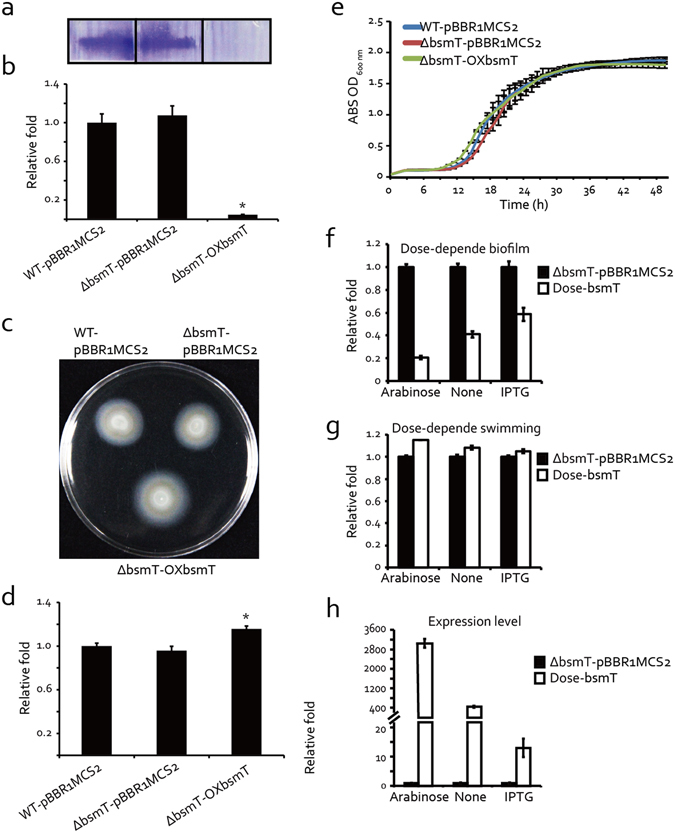
Phenotypic characterization shows that BsmT is a positive regulator of bacterial swimming motility and a negative regulator of biofilm formation. (a) Biofilm formation quantified by crystal violet staining. (b) Relative quantification of biofilm formation measured at 590 nm using a Tecan Infinite 200 Pro scanner. (c) Swimming motility analyses of bacterial strains on rich NYG medium plates containing 0.15% agar. (d) Relative quantification of bacterial swimming zone diameters. (e) Growth curves of bacterial strains grown on rich NYG medium at 28 °C. ABS: absorbance. (f) Dose-dependent effect on biofilm formation. Relative quantification of the biofilm was measured when 0.1% arabinose and 4 mM IPTG were used to suppress the expression of bsmT. (g) Dose-dependent effect on the swimming motility. Relative quantification of the diameters of the swimming zones was measured when 0.1% arabinose and 4 mM IPTG were used to suppress the expression of bsmT. (h) The expression level of bsmT along with the addition of 0.1% arabinose and 4 mM IPTG. Relative quantification of bsmT expression level using qRT-PCR assays. WT-pBBR1MCS2: wild-type strain containing a blank pBBR1MCS2 vector; ΔbsmT-pBBR1MCS2: blank pBBR1MCS2 vector-containing strain carrying an in-frame deletion of the gene bsmT; ΔbsmT-OXbsmT: the strain overexpressing bsmT in its in-frame deletion mutant; Dose-bsmT: bsmT in-frame deletion mutant containing the recombinant MpBBR1MCS2-bsmT. All data are representatives of at least triplicate repeatable experiments. The values are the means ± standard deviation (n = 3). *p < 0.05, as determined by ANOVA.
bsmT activates the expression of bsmR in a positive feedback manner
Since both bsmR and bsmT control bacterial swimming and biofilm formation, epistatic analysis was carried out to determine their regulatory relationship by constructing a double mutant of the two genes [strain Δ(bsmT-bsmR)-pBBR1MCS2] and then overexpressing bsmR and bsmT, respectively, in the resulting double mutant [strains Δ(bsmT-bsmR)-OXbsmR and Δ(bsmT-bsmR)-OXbsmT, respectively]. As shown in Fig. 7a–d, biofilm formation and swimming motility were similar in the double-mutant and WT strains. However, overexpressing bsmR against the double-mutant background caused a significant increase in the bacterial swimming zone, to 147.5% of that of the WT. It also sharply decreased biofilm formation, to 23.4% of that by the WT strain. The overexpression of bsmT, by contrast, did not cause a recognizable phenotypic difference with the double mutant and WT strains. There were no discrepancies in the growth of the tested strains (Fig. 7e). Accordingly, this experiment demonstrated that bsmR and bsmT function in the same signaling pathway to modulate bacterial swimming and biofilm formation, and that bsmR is in a regulatory position located downstream of bsmT.
Figure 7.
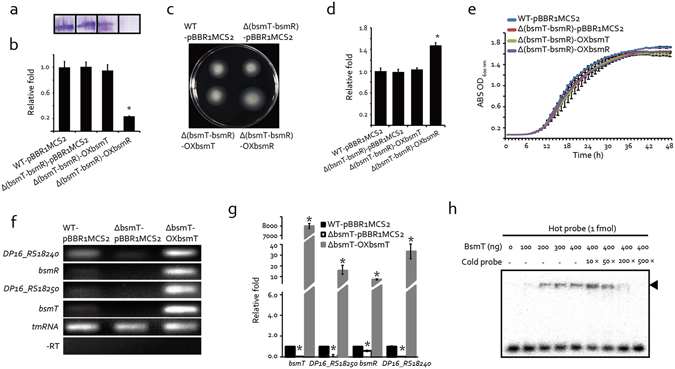
bsmT is epistatic to bsmR and regulates bsmR transcription through a positive feedback loop. (a) Biofilm levels of the bacterial strains quantified by crystal violet staining. (b) Relative quantification of biofilm formation. (c) Swimming motility analyses of bacterial strains grown on rich NYG plates containing 0.15% agar. (d) Relative quantification of the bacterial swimming zone diameters of the strains in (c). (e) Growth curves of bacterial strains grown in rich NYG medium at 28 °C. (f,g) bsmT positively regulates transcription of the bsmR operon. mRNA amounts were determined by semiquantitative RT-PCR (f) and quantitative RT-PCR (g), respectively. In (f), the bands represent PCR amplification products obtained using the cDNA generated from the total RNAs of the indicated strains. The tmRNA amplification product served as the loading control. −RT represents the negative control to detect the presence of contaminating DNA. (h) Electrophoretic mobility shift assay shows the direct binding of BsmT to the promoter region of the bsmR operon. 1 fmol of 32P-labeled double-stranded DNA corresponding to the promoter region of the bsmR operon was incubated at 28 °C in reaction buffer together with BsmT protein. Increasing amounts of unlabeled DNA served as the competitor. The black arrow indicates the position of the protein-DNA complex. (b,d,e and g) Each value is the average of three replicates. Error bars represent the standard deviations (n = 3). *p < 0.05, as determined by ANOVA. The qRT-PCR results (g) are representatives of triplicate repeatable experiments.
Since the phosphorylation state of BsmR does not seem to regulate swimming or biofilm formation, and bsmT encodes a transcriptional regulator located in an operon together with bsmR, we proposed that the expression level of bsmR is critical in the regulation of swimming motility and biofilm formation and that bsmT positively regulates bsmR expression via a feedback loop. This hypothesis was verified by using semi-qRT-PCR and qRT-PCR to estimate the bsmR transcription level. As shown in Fig. 7f,g, in the WT and ΔbsmT mutant strains, all four genes of the bfmR operon were transcribed at extremely low levels. However, overexpressing bsmT significantly increased the mRNA levels of the four genes, by 6.3- to 198.6-fold compared to the WT levels. This result suggested that BsmT autoregulates the transcription of its own operon. Interestingly, the mRNA level of DP16_RS18240 was higher than those of bsmR, bsmT and DP16_RS18250 in the WT-pBBR1MCS2 strain, indicating that additional cis-regulatory elements drive DP16_RS18240 expression. It was noteworthy that transcript level of bsmR, bsmT and DP16_RS18250 was almost undetectable in the WT-pBBR1MCS2 strain (Fig. 7f), suggesting their expression remains a low level under the assay conditions. And to confirm the regulatory relationship between BsmT and the promoter region of the bsmR operon, possible protein-DNA interactions were examined in an electrophoretic mobility shift assay (EMSA). The results showed that recombinant BsmT-His6 formed stable protein-DNA complexes with the 32P-labeled DNA probe corresponding to the 5′ promoter sequence of the bsmT-DP16_RS18250-bsmR-DP16_RS18240 operon. The addition of unlabeled probe resulted in effective competition with the labeled probe in binding to BsmT-His6 (Fig. 7h). Taken together, these results demonstrate that BsmT directly binds to the promoter region of its own operon and thereby regulates the transcription of all four genes (including bsmR) by a positive feedback loop.
Discussion
The spatial and temporal control of c-di-GMP in bacterial cells is important to its modulation of diverse physiological processes and behaviors50, 51. However, the role of c-di-GMP in the nosocomial pathogen S. maltophilia is unclear. Our study provides genetic and biochemical evidence of c-di-GMP degradation by the BsmR protein, both in vitro and in vivo, by its EAL domain (Fig. 4a–c,h). As a regulatory protein of S. maltophilia, BsmR was shown to modulate the expression of 349 genes under the tested condition. These genes include the previously identified transcription factor FsnR, which is critical to the regulation of flagellar assembly (Fig. 5a,b, Supplementary Table S3). Although bsmR inactivation did not remarkably impact bacterial swimming or biofilm formation, the gradient increase in expression of the respective protein resulted in gradient increase in cellular swimming capability and decrease in biofilm development (Fig. 2f–h). We also determined that the transcription factor BsmT whose coding gene is located in the same tetra-cistronic operon as bsmR, binds directly to the promoter region of the operon and positively regulates bsmR transcription. Epistatic analysis confirmed that BsmR is located downstream of BsmT, where it regulates biofilm formation and bacterial swimming (Fig. 7). To our knowledge, BsmR is the first experimentally investigated protein involved in c-di-GMP turnover and the control of biofilm formation in S. maltophilia.
BsmR is a RR with an EAL domain as the output region. In general, RR phosphorylation is critical in regulating the biochemical activity of the corresponding output region, via a mechanism involving a conformational change34. For example, PleD, a RR with a GGDEF domain, controls flagellar motor activity in the gram-negative bacterium Caulobacter crescentus. The DGC activity of PleD is activated by phosphorylation-triggered dimerization52, 53. In E. coli, the RR CheB contains a methylesterase region responsible for the regulation of chemotaxis by the bacterium. The phosphorylation of CheB in response to a diverse range of chemoeffector concentrations activates its methylesterase region, which in turn modulates bacterial motility54, 55. However, RR regulation independent of phosphorylation has also been reported. For example, the RR VieA of Vibrio cholerae contains an EAL domain. VieA negatively regulates biofilm formation and virulence by decreasing the cellular c-di-GMP level. Its PDE activity is independent of the phosphorylation level because in vitro phosphorylation by acetyl phosphate did not affect the ability of the enzyme to degrade c-di-GMP, and neither a mutation of the conserved phosphorylation site nor the deletion of the REC-domain-coding sequence altered the control of downstream vps genes56–58. Subsequent analysis revealed that the PDE activity of VieA is activated by intracellular Mg2+ and Mn2+ but inhibited by Ca2+ and Zn2+ 59, 60. The results of our analysis suggest that, similar to VieA, the PDE activity of BsmR is independent of its phosphorylation state since no significant difference of the dose-dependent effects between BsmR and its phosphorylation site mutated proteins, was identified with respect to bacterial swimming motility and biofilm formation (Fig. 2f–h). The positive correlation between the bsmR transcription level and cellular c-di-GMP level revealed by the qRT-PCR assay and LC-MS/MS assay (Figs 2h and 4d) suggests that bsmR expression level is an important regulatory determinant of BsmR PDE activity.
The role of bacterial swimming in biofilm formation is complex and depends on the different stages of biofilm development. 1) In the early stage of cell aggregation, bacteria must move towards the liquid-solid inter-face, a process driven by flagellar activity (swimming). 2) However, after becoming fixed to the solid surface, where they continue to grow, bacteria no longer require motility, which instead must be inhibited to avoid dispersal of the biofilm. 3) When the biofilm reaches the mature stage, swimming motility is re-activated to release the bacterial cells so that they are able to colonize new habitats8. Our results showed that the overexpression of bsmR substantially increased swimming of S. maltophilia, while strongly reducing biofilm formation (Fig. 2). Thus, the abnormally increased swimming motility caused by the up-regulation of bsmR acted as a negative force hindering bacterial aggregation on a solid surface. This implies the need for the tight regulation of BsmR activity during biofilm formation. Besides the transcription factor BsmT that plays a positive role in activating bsmR transcription, inhibitory factors of BsmR are needed during the cellular aggregation stage of biofilm development. The identification of these inhibitors and of the mechanism underlying the integration of the positive and negative control of BsmR activity will require further studies.
As revealed by RNA-seq analysis, the overexpression of BsmR altered the expression of 349 genes, which suggests that BsmR is a global regulator of the physiological processes of S. maltophilia (Fig. 5a, Supplementary Table S3). Among these genes, BsmR modulates the transcriptions of 45 that are associated with signal transduction and cellular regulation, including 3 HKs, 2 RRs, 9 transcription factors, and 3 alternative sigma factors. This result places BsmR-triggered c-di-GMP degradation in the center of a sophisticated network that also serves to regulate biofilm development by S. maltophilia.
In the development of novel antibacterial agents, the targeting of biofilm formation is likely to be an effective therapeutic strategy, since disruption of this critical process, which involves cell-cell communication, adhesion to a solid surface, the assembly of an extracellular polysaccharide matrix, and, later, biofilm dispersal, will increase the susceptibility of bacteria to established antibiotics61, 62. The decreased cell aggregation achieved by activating BsmR PDE activity demonstrates the potential of chemical agonists of the BsmR PDE to block S. maltophilia biofilm development and thus nosocomial infections, given that these agonists are unlikely to harm human health and they do not activate the expression of bacterial virulence factors. An in-depth analysis of the BsmR regulatory cascade will allow the screening and identification of agonists able to control S. maltophilia biofilm formation and therefore infections by this bacterium.
Methods
Bacterial strains, plasmids, and culture conditions
The bacterial strains and plasmids used in this study are listed in Supplementary Table S1. S. maltophilia strains were cultured at 28 °C in rich NYG medium (5 g tryptone/l, 3 g yeast extract/l, 20 g glycerol/l, pH 7.0) or 210 medium (5 g sucrose/l, 8 g casein enzymatic hydrolysate/l, 4 g yeast extract/l, 3 g K2HPO4/l, 0.3 g MgSO4·7H2O/l, pH 7.0). If necessary, strains were grown to an OD600 nm = 0.4 before the cells were harvested. Escherichia coli strain DH5α was used as the host to prepare all recombinant plasmids. E. coli strain BL21 (DE3) and pET30a (Novagen) vectors were used to express the His6-tagged recombinant proteins. In-frame deletion mutants were constructed by homologous, double-crossover recombination using the suicide vector pK18mobsacB. To construct genetically complementary strains, the recombinant broad-host-range vector pBBR1MCS2, carrying full-length sequences of the genes of interest (under the control of the Plac promoter), was electroporated into competent cells of S. maltophilia wild-type or mutant strains. Electroporation was performed in a Bio-Rad Pulser XCellTM (Bio-Rad, Hercules, CA, USA) at 18 kV/cm, 25 μF, and 200 Ω. Strains carrying point mutations were constructed using the Fast Mutagenesis System (Transgene Biotech, Beijing, China). All other general molecular biology operations were carried out according to standard molecular cloning protocols63. The antibiotics ampicillin (100 μg/ml), kanamycin (50 μg/ml), chloramphenicol (34 μg/ml) were added as needed. The primers used in the constructions are listed in Supplementary Table S2.
Biofilm assays
S. maltophilia biofilm mass was quantified by crystal violet staining as previously reported64, 65. Briefly, S. maltophilia strains were cultured overnight at 28 °C in rich NYG medium with the addition of different concentrations of IPTG or arabinose, diluted to an OD600 nm = 0.4, inoculated into 96-well polystyrene plates, and incubated at 28 °C for 6 h without shaking. The wells were washed rigorously with water, stained with 0.1% crystal violet for 20 min without shaking, washed rigorously with water, and dried. The crystal violet stain was solubilized in 95% ethanol and the released amount quantified using Tecan Infinite 200 Pro at 590 nm.
Bacterial swimming motility assay
The assayed strains were cultured overnight at 28 °C in rich NYG medium with the addition of different concentrations of IPTG or arabinose, washed twice with 10 mM MgCl2, and diluted to an OD600 nm = 0.4. Three microliters of the diluted cultures were then used to inoculate rich NYG medium plates containing 0.15% agar. The diameters of the swimming zones were measured after incubation of the plates at 28 °C for 24 h.
RNA extraction and semi-quantitative and quantitative RT-PCR assays
Total bacterial RNA was extracted using TRIzol (Invitrogen) following the manufacturer’s instructions and then quantified using a NanoDrop spectrophotometer (Thermo Fisher). Contaminating DNAs in the total RNAs were removed using DNA-free DNase (Life Technology). The first strand of the cDNA was generated using random primers (Promega) and Superscript III reverse transcriptase (Invitrogen). cDNA of the transfer mRNA served as the loading control for semi-quantitative and quantitative RT-PCR. In the PCRs, samples lacking reverse transcriptase during first strand cDNA synthesis served as the negative control to evaluate potential DNA contamination, and the DNA template of the WT strain as the positive control. The primers used in the PCR are listed in Supplementary Table S2.
High-throughput RNA sequencing assay
S. maltophilia strains were grown in rich NYG medium and incubated at 28 °C to OD600 nm = 0.4. The cells were harvested by centrifugation and total RNA was then extracted. Three biological repeats of each sample were prepared. Contaminating DNA was removed using DNA-free DNase (Life Technology). mRNAs were enriched by removing ribosomal RNAs with the MICROBEnrich kit (Ambion, Austen, TX, USA) following the manufacturer’s instructions. High-throughput RNA sequencing was carried out on an Illumina HiSeqTM 2000. Raw data were evaluated by the quality checks reported previously before further analysis66. (i) The distribution of nucleotide-level quality scores of more than 85% reads of both samples were more than 20, which means a high quantity of the sequencing run. (ii) The average and distribution of GC content of both samples were less than 60%, which would not induce biases in the quantification. (iii) 85.84% and 78.28% reads of ΔbsmR-OXbsmR and WT-pBBR1MCS2, respectively, were mapped to the gene sequences of S. maltophilia ATCC 13637, suggesting good quanlities of the samples. (iv) No significant decrease of the coverage from the 3′ to the 5′ ends of the transcripts was identified, suggesting no significant RNA degradation during sequencing. (v) rRNA Reads of ΔbsmR-OXbsmR and WT-pBBR1MCS2 were 1.78% and 5.25% of total reads, respectively, which would not affect the quantification. After the evaluation, all collected clean reads were aligned with the complete genomic sequence of S. maltophilia ATCC 13637 using SOAPaligner/SOAP2 using the standard of no more than 5 mismatches. Differentially expressed genes were screened by SOAPaligner/SOAP2 and the results were verified by Bowtle.
Protein expression and purification
All recombinant proteins were expressed with a C-terminal His6-tag. Expression was achieved using pET30a (Novagen) vectors and E. coli BL21 (DE3) cells. The primers used in the expression vector constructions are listed in Supplementary Table S2. The proteins were expressed and purified according to the manufacturer’s instructions. In brief, the expression strains were inoculated in rich LB medium (10 g tryptone/L, 5 g yeast extract/L, 10 g NaCl/L) at 37 °C, grown to an OD600 nm = 0.4–0.8, and induced at 16 °C for 16 h with 5 mM IPTG. The bacterial cultures were collected, sonicated, centrifuged, and used in Ni-nitrilotriacetic acid (Ni-NTA) affinity chromatography to collect the His6-tagged recombinant proteins according to the mannual. Then using Centricon YM-10 columns the proteins were stored in storage buffer (Tris-HCl 50 mM, EDTA 0.5 mM, NaCl 50 mM, glycerol 5%, pH 8.0) until further use.
In vitro phosphroylation assay
In vitro phosphorylation was assayed as described previously67. In brief, the purified C-terminal-His6-tagged membrane proteins DP16_RS18240 and DP16_RS18250 were incubated at 28 °C with 100 μM ATP containing 10 μCi [γ-32P]-ATP (PerkinElmer, USA) in 20 μl of reaction buffer (50 mM Tris-HCl, pH 7.8; 2 mM DTT; 25 mM NaCl; 25 mM KCl; 5 mM MgCl2) using SreS as the control to prove the assay was working. To examine whether there was phosphotransfer from the two kinases to BsmR and BsmT, purified BsmR and BsmT proteins were added into the reaction system, respectively. Then aliquots of the samples for electrophoresis were retrieved at the indicated time points. The reactions in those samples were immediately stopped by the addition of 6 × SDS loading buffer [250 mM Tris-HCl pH 6.8, 10% (w/v) SDS, 0.5% (w/v) bromophenol blue, 50% (v/v) glycerol, 25 mM PMSF]. SDS-PAGE was carried out on 12% acrylamide gels, after which the gels were placed into Ziploc bags, exposed to phosphor screens for 1 h at room temperature, and scanned at 25-μM resolution using the PhosphorImage system Typhoon FLA7000 (Amersham Biosciences, Bath, UK). Protein locations and amounts were determined by staining the gels with Coomassie bright blue after autoradiography.
In vivo phosphorylation assay
The in vivo phosphorylation assay was carried out following the Phos-tag Acrylamide Kit (Nard, USA). Briefly, S. maltophilia strains were grown in rich NYG medium and incubated at 28 °C to OD600 nm = 0.4. The cells were harvested by centrifugation and lysed with 1 M formic acid. 30 s of vortexing were required for the complete lysis of the cells. Each lysate was solubilized by the addition of 6 × SDS loading butter and neutralized to pH 6.0 by the addition of 5 N NaOH. Samples, together with the purified unphosphorylated BsmR and BsmT proteins, were loaded on Phos-tag acrylmnide gels and run at 4 °C. After the separation samples were transferred to nitrocellulose membranes by a Bio-Rad semidry transfer. And the monoclonal antibody of His6 tag conjugated with HRP and ClarityTM western ECL substrates (Bio-Rad) were used to image BsmR and BsmT proteins.
In vitro phosphodiesterase assays
The method was described in previous studies68–70. The 32P-labeled c-di-GMP used in this assay was generated by incubating diguanylate cyclase tDGCR158A with [α-32P]-GTP (PerkinElmer, USA) at 45 °C in 20 μl of c-di-GMP synthesis buffer (50 mM Tris-Cl, pH 8.0; 20 mM MgCl2; 250 mM NaCl; 1 mM DTT). The reaction was stopped by heating at 95 °C for 10 min. PDE assays were carried out by incubating the purified proteins with the synthesized c-di-GMP at 28 °C in reaction buffer (250 mM NaCl; 25 mM Tris, pH 8.0; 10 mM MgCl2; 5 mM β-mercaptoethanol). Aliquots of the samples were chronologically retrieved at the indicated time points and mixed with an equal volume of 0.5 M EDTA, pH 8.0, to stop the reactions. The c-di-GMP in the samples was separated by thin-layer chromatography on a polygram CEL 300 PEI cellulose thin layer chromatography plate. The plates were then placed into a Ziploc bag and exposed to a phosphor screen for 1 h before they were scanned at 25-μM resolution using the PhosphorImage system Typhoon FLA7000 (Amersham Biosciences, Bath, UK).
Cellular c-di-GMP quantification by LC-MS/MS
S. maltophilia strains were grown in rich NYG medium and incubated at 28 °C to OD600 nm = 0.4. The cells were harvested by centrifugation and resuspended in lysis buffer (40% acetonitrile, 40% methyl alcohol, 20% H2O). 30 s vortex and 15 min incubation on ice were needed to ensure the complete lysis. After that a 10-minute heating at 95 °C was carried out to inactivate all possible enzymatic activities of the lysate. Then the suspension were collected by centrifugation and evaporated to dryness by a vacuum freeze drier ZLS-1 (HeXi, Hunan, China). The pellet was solubilized using water and the c-di-GMP level was quantified by reverse phase-coupled HPLC-MS/MS. To calculate the c-di-GMP concentration in bacterial cells, bacterial cells used to extract c-di-GMP were numbered by diluting the bacterial culture and plated them on NYG plates for counting.
In vivo FRET-based c-di-GMP biosensor assay
This method was previously described43. The FRET-based c-di-GMP biosensor reporting system was constructed by inserting the complete coding sequence of the fused YPet-YcgR-CyPet protein into the broad-host vector pBBR1MCS1, as previously reported. The recombinant vector was used to transform S. maltophilia strains by electroporation. The primers used in the constructions are listed in Supplementary Table S2. The recombinant protein Ypet-YcgR-CyPet was expressed, purified, and incubated with increasing concentrations of c-di-GMP (0–1,000 nM) in PBS buffer (pH 7.4). FRET values (528 nm/496 nm) were measured at 28 °C using a Synergy H4 (BioTech, USA) system with excitation at 425 nm and emission scanning from 460 to 560 nm in 2-nm intervals. S. maltophilia strains containing the FRET-based c-di-GMP biosensor system were grown in rich NYG medium to an OD600 nm = 0.4 and used in FRET value measurements. The constructed table of FRET values and c-di-GMP concentrations was used to roughly determine the c-di-GMP concentrations of the S. maltophilia strains.
Electronic supplementary material
Acknowledgements
The work was financially supported by National Natural Science Foundation of China (grant 3140071), and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant XDB11040700).
Author Contributions
W.L., X.Q.T., J.W.W., L.L.D., and F.F.W. conducted the experiments. W.L., W.Q., Z.L., and F.F.W. designed the experiments and analyzed the data. W.Q., Z.L. and F.F.W. wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/9/2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
Contributor Information
Zhong Liu, Email: liuzhong@sjtu.edu.cn.
Fang-Fang Wang, Email: wangff@im.ac.cn.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04763-w
References
- 1.Palleroni NJ, Bradbury JF. Stenotrophomonas, a New Bacterial Genus for Xanthomonas-Maltophilia (Hugh 1980) Swings Et-Al 1983. Int. J. Syst. Bacteriol. 1993;43:606–609. doi: 10.1099/00207713-43-3-606. [DOI] [PubMed] [Google Scholar]
- 2.Swings J, Devos P, Vandenmooter M, Deley J. Transfer of Pseudomonas-Maltophilia Hugh 1981 to the Genus Xanthomonas as Xanthomonas-Maltophilia (Hugh 1981) Comb-Nov. Int. J. Syst. Bacteriol. 1983;33:409–413. doi: 10.1099/00207713-33-2-409. [DOI] [Google Scholar]
- 3.Berg G. Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biot. 2009;84:11–18. doi: 10.1007/s00253-009-2092-7. [DOI] [PubMed] [Google Scholar]
- 4.Berg G, Eberl L, Hartmann A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 2005;7:1673–1685. doi: 10.1111/j.1462-2920.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 5.Furushita M, Okamoto A, Maeda T, Ohta M, Shiba T. Isolation of multidrug-resistant Stenotrophomonas maltophilia from cultured yellowtail (Seriola quinqueradiata) from a marine fish farm. Appl. Environ. Microb. 2005;71:5598–5600. doi: 10.1128/Aem.71.9.5598-5600.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hejnar P, Bardon J, Sauer P, Kolar M. Stenotrophomonas maltophilia as a part of normal oral bacterial flora in captive snakes and its susceptibility to antibiotics. Vet. Microbiol. 2007;121:357–362. doi: 10.1016/j.vetmic.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Mah TFC, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/S0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 8.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Fujita J, et al. Clinical features of Stenotrophomonas maltophilia pneumonia in immunocompromised patients. Resp. Med. 1996;90:35–38. doi: 10.1016/S0954-6111(96)90242-5. [DOI] [PubMed] [Google Scholar]
- 10.Prosser BL, Taylor D, Dix BA, Cleeland R. Method of Evaluating Effects of Antibiotics on Bacterial Biofilm. Antimicrob. Agents. Ch. 1987;31:1502–1506. doi: 10.1128/AAC.31.10.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans RC, Holmes CJ. Effect of Vancomycin Hydrochloride on Staphylococcus-Epidermidis Biofilm Associated with Silicone Elastomer. Antimicrob. Agents. Ch. 1987;31:889–894. doi: 10.1128/AAC.31.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gristina AG, Hobgood CD, Webb LX, Myrvik QN. Adhesive Colonization of Biomaterials and Antibiotic-Resistance. Biomaterials. 1987;8:423–426. doi: 10.1016/0142-9612(87)90077-9. [DOI] [PubMed] [Google Scholar]
- 13.Sefcick A, Tait RC, Wood B. Stenotrophomonas maltophilia: an increasing problem in patients with acute leukaemia. Leukemia Lymphoma. 1999;35:207. doi: 10.3109/10428199909145721. [DOI] [PubMed] [Google Scholar]
- 14.Ewig S, et al. Evaluation of antimicrobial treatment in mechanically ventilated patients with severe chronic obstructive pulmonary disease exacerbations. Crit. Care Med. 2000;28:692–697. doi: 10.1097/00003246-200003000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Krcmery V, Koprnova J, Harniciarova A. Stenotrophomonas maltophilia bacteremia. Scand. J. Infect. Dis. 2004;36:400–400. doi: 10.1080/00365540410020154. [DOI] [PubMed] [Google Scholar]
- 16.Sakhnini E, Weissmann A, Oren I. Fulminant Stenotrophomonas maltophilia soft tissue infection in immunocompromised patients: An outbreak transmitted via tap water. Am. J. Med. Sci. 2002;323:269–272. doi: 10.1097/00000441-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura A, et al. Diagnostic Value of PCR Analysis of Bacteria and Fungi from Blood in Empiric-Therapy-Resistant Febrile Neutropenia. J. Clin. Microbiol. 2010;48:2030–2036. doi: 10.1128/Jcm.01700-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen MH, Muder RR. Meningitis Due to Xanthomonas-Maltophilia - Case-Report and Review. Clin. Infect. Dis. 1994;19:325–326. doi: 10.1093/clinids/19.2.325. [DOI] [PubMed] [Google Scholar]
- 19.Looney WJ, Narita M, Muhlemann K. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect. Dis. 2009;9:312–323. doi: 10.1016/S1473-3099(09)70083-0. [DOI] [PubMed] [Google Scholar]
- 20.Safdar A, Rolston KV. Stenotrophomonas maltophilia: Changing spectrum of a serious bacterial pathogen in patients with cancer. Clin. Infect. Dis. 2007;45:1602–1609. doi: 10.1086/522998. [DOI] [PubMed] [Google Scholar]
- 21.Pompilio A, et al. Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: the role of cell surface hydrophobicity and motility. Fems. Microbiol. Lett. 2008;287:41–47. doi: 10.1111/j.1574-6968.2008.01292.x. [DOI] [PubMed] [Google Scholar]
- 22.Toleman MA, Bennett PM, Bennett DMC, Jones RN, Walsh TR. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg. Infect. Dis. 2007;13:559–565. doi: 10.3201/eid1304.061378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Bonaventura, G., Spedicato, A., D’Antonio, D., Robuffo, I. & Piccolomini, R. Biofilm formation by Stenotrophomonas maltophilia: Modulation by quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrob. Agents. Ch. 48, 151–160, doi:10.1128/Aac.48.1.151-160.2004 (2004). [DOI] [PMC free article] [PubMed]
- 24.Di Bonaventura G, Stepanovic S, Picciani C, Pompilio A, Piccolomini R. Effect of environmental factors on biofilm formation by clinical Stenotrophomonas maltophilia isolates. Folia. Microbiol. 2007;52:86–90. doi: 10.1007/BF02932144. [DOI] [PubMed] [Google Scholar]
- 25.Stoodley P, Dodds I, Boyle JD, Lappin-Scott HM. Influence of hydrodynamics and nutrients on biofilm structure. J. Appl. Microbiol. 1999;85:19s–28s. doi: 10.1111/j.1365-2672.1998.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 26.Garcia, C. A., Alcaraz, E. S., Franco, M. A. & de Rossi, B. N. P. Iron is a signal for Stenotrophomonas maltophilia biofilm formation, oxidative stress response, OMPs expression, and virulence. Front. Microbiol. 6, doi:Artn 92610.3389/Fmicb.2015.00926 (2015). [DOI] [PMC free article] [PubMed]
- 27.Huang TP, Somers EB, Wong ACL. Differential biofilm formation and motility associated with lipopolysaccharide/exopolysaccharide-coupled biosynthetic genes in Stenotrophomonas maltophilia. J. Bacteriol. 2006;188:3116–3120. doi: 10.1128/Jb.188.8.3116-3120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YT, Huang YW, Liou RS, Chang YC, Yang TC. MacABCsm, an ABC-type tripartite efflux pump of Stenotrophomonas maltophilia involved in drug resistance, oxidative and envelope stress tolerances and biofilm formation. J. Antimicrob. Chemoth. 2014;69:3221–3226. doi: 10.1093/jac/dku317. [DOI] [PubMed] [Google Scholar]
- 29.Lin YT, Huang YW, Chen SJ, Chang CW, Yang TC. The SmeYZ Efflux Pump of Stenotrophomonas maltophilia Contributes to Drug Resistance, Virulence-Related Characteristics, and Virulence in Mice. Antimicrob. Agents. Ch. 2015;59:4067–4073. doi: 10.1128/Aac.00372-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JG, et al. Crystallization of the N-terminal regulatory domain of the enhancer-binding protein FleQ from Stenotrophomonas maltophilia. Acta. Crystallogr. F. 2014;70:326–330. doi: 10.1107/S2053230x14001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang XM, Wang FF, Zhang H, Zhang Q, Qian W. Genome-Wide Identification of Genes Necessary for Biofilm Formation by Nosocomial Pathogen Stenotrophomonas maltophilia Reveals that Orphan Response Regulator FsnR Is a Critical Modulator. Appl. Environ. Microb. 2015;81:1200–1209. doi: 10.1128/Aem.03408-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng L, et al. Systematic Mutational Analysis of Histidine Kinase Genes in the Nosocomial Pathogen Stenotrophomonas maltophilia Identifies BfmAK System Control of Biofilm Development. Appl. Environ. Microb. 2016;82:2444–2456. doi: 10.1128/Aem.03951-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross P, et al. Regulation of Cellulose Synthesis in Acetobacter-Xylinum by Cyclic Diguanylic Acid. Nature. 1987;325:279–281. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- 34.West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 2001;26:369–376. doi: 10.1016/S0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- 35.Telenti A, et al. The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat. Med. 1997;3:567–570. doi: 10.1038/Nm0597-567. [DOI] [PubMed] [Google Scholar]
- 36.Baumler AJ, Tsolis RM, Heffron F. The lpf fimbrial operon mediates adhesion of Salmonella typhimurium to murine Peyer’s patches. P. Natl. Acad. Sci. USA. 1996;93:279–283. doi: 10.1073/pnas.93.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osbourn AE, Field B. Operons. Cell. Mol. Life Sci. 2009;66:3755–3775. doi: 10.1007/s00018-009-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao XZ, et al. DOOR 2.0: presenting operons and their functions through dynamic and integrated views. Nucleic. Acids Res. 2014;42:D654–D659. doi: 10.1093/nar/gkt1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price MN, Huang KH, Alm EJ, Arkin AP. A novel method for accurate operon predictions in all sequenced prokaryotes. Nucleic. Acids Res. 2005;33:880–892. doi: 10.1093/nar/gki232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price MN, Alm EJ, Arkin AP. Interruptions in gene expression drive highly expressed operons to the leading strand of DNA replication. Nucleic. Acids Res. 2005;33:3224–3234. doi: 10.1093/nar/gki638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourguignon T, et al. Molecular Mechanism of the Two-Component Suicidal Weapon of Neocapritermes taracua Old Workers. Mol. Biol. Evol. 2016;33:809–819. doi: 10.1093/molbev/msv273. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi T, Galiacy SD, Briscoe G, Erickson HP. An experimental study of GFP-based FRET, with application to intrinsically unstructured proteins. Protein Sci. 2007;16:1429–1438. doi: 10.1110/ps.072845607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christen M, et al. Asymmetrical Distribution of the Second Messenger c-di-GMP upon Bacterial Cell Division. Science. 2010;328:1295–1297. doi: 10.1126/science.1188658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bren A, Eisenbach M. How signals are heard during bacterial chemotaxis: Protein-protein interactions in sensory signal propagation. J. Bacteriol. 2000;182:6865–6873. doi: 10.1128/Jb.182.24.6865-6873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourret RB, Stock AM. Molecular information processing: Lessons from bacterial chemotaxis. J. Biol. Chem. 2002;277:9625–9628. doi: 10.1074/jbc.R100066200. [DOI] [PubMed] [Google Scholar]
- 46.Kuo SC, Koshland DE. Roles of Chey and Chez Gene-Products in Controlling Flagellar Rotation in Bacterial Chemotaxis of Escherichia-Coli. J. Bacteriol. 1987;169:1307–1314. doi: 10.1128/jb.169.3.1307-1314.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bren A, Eisenbach M. The N terminus of the flagellar switch protein, FliM, is the binding domain for the chemotactic response regulator, CheY. J. Mol. Biol. 1998;278:507–514. doi: 10.1006/jmbi.1998.1730. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson DA, Chacko SJ, Venien-Bryan C, Wadhams GH, Armitage JP. Regulation of Flagellum Number by FliA and FlgM and Role in Biofilm Formation by Rhodobacter sphaeroides. J. Bacteriol. 2011;193:4010–4014. doi: 10.1128/Jb.00349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fazli M, et al. The CRP/FNR family protein Bcam1349 is a c-di-GMP effector that regulates biofilm formation in the respiratory pathogen Burkholderia cenocepacia. Mol. Microbiol. 2011;82:327–341. doi: 10.1111/j.1365-2958.2011.07814.x. [DOI] [PubMed] [Google Scholar]
- 50.Sondermann H, Shikuma NJ, Yildiz FH. You’ve come a long way: c-di-GMP signaling. Curr. Opin. Microbiol. 2012;15:140–146. doi: 10.1016/j.mib.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the First 25 Years of a Universal Bacterial Second Messenger. Microbiol. Mol. Biol. R. 2013;77:1–52. doi: 10.1128/Mmbr.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul R, et al. Activation of the diguanylate cyclase PleD by phosphorylation-mediated dimerization. J. Biol. Chem. 2007;282:29170–29177. doi: 10.1074/jbc.M704702200. [DOI] [PubMed] [Google Scholar]
- 53.Aldridge P, Paul R, Goymer P, Rainey P, Jenal U. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus. Mol. Microbiol. 2003;47:1695–1708. doi: 10.1046/j.1365-2958.2003.03401.x. [DOI] [PubMed] [Google Scholar]
- 54.Lupas A, Stock J. Phosphorylation of an N-Terminal Regulatory Domain Activates the Cheb Methylesterase in Bacterial Chemotaxis. J. Biol. Chem. 1989;264:17337–17342. [PubMed] [Google Scholar]
- 55.Hughes CA, Mandell JG, Anand GS, Stock AM, Komives EA. Phosphorylation causes subtle changes in solvent accessibility at the interdomain interface of methylesterase CheB. J. Mol. Biol. 2001;307:967–976. doi: 10.1006/jmbi.2001.4523. [DOI] [PubMed] [Google Scholar]
- 56.Tamayo R, Tischler AD, Camilli A. The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J. Biol. Chem. 2005;280:33324–33330. doi: 10.1074/jbc.M506500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 2004;53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tischler AD, Camilli A. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 2005;73:5873–5882. doi: 10.1128/Iai.73.9.5873-5882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell, S. L., Ismail, A. M., Kenrick, S. A. & Camilli, A. The VieB auxiliary protein negatively regulates the VieSA signal transduction system in Vibrio cholerae. Bmc. Microbiol. 15, doi:ARTN 5910.1186/s12866-015-0387-7 (2015). [DOI] [PMC free article] [PubMed]
- 60.Dey AK, Bhagat A, Chowdhury R. Host Cell Contact Induces Expression of Virulence Factors and VieA, a Cyclic di-GMP Phosphodiesterase, in Vibrio cholerae. J. Bacteriol. 2013;195:2004–2010. doi: 10.1128/Jb.02127-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 62.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Ag. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Sambrook, J., Fritsch, E. F., Maniatis, T. Molecular Cloning. (Cold Spring Harbor Laboratory Press, 1989).
- 64.Musken M, Di Fiore S, Romling U, Haussler S. A 96-well-plate-based optical method for the quantitative and qualitative evaluation of Pseudomonas aeruginosa biofilm formation and its application to susceptibility testing. Nat. Protoc. 2010;5:1460–1469. doi: 10.1038/nprot.2010.110. [DOI] [PubMed] [Google Scholar]
- 65.O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 66.‘t Hoen PAC, et al. Reproducibility of high-throughput mRNA and small RNA sequencing across laboratories. Nat. Biotechnol. 2014;32:291–291. doi: 10.1038/nbt.2702. [DOI] [PubMed] [Google Scholar]
- 67.Wang FF, et al. A three-component signalling system fine-tunes expression kinetics of HPPK responsible for folate synthesis by positive feedback loop during stress response of Xanthomonas campestris. Environ. Microbiol. 2014;16:2126–2144. doi: 10.1111/1462-2920.12293. [DOI] [PubMed] [Google Scholar]
- 68.Lindenberg S, Klauck G, Pesavento C, Klauck E, Hengge R. The EAL domain protein YciR acts as a trigger enzyme in a c-di-GMP signalling cascade in E. coli biofilm control. Embo J. 2013;32:2001–2014. doi: 10.1038/emboj.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christen M, Christen B, Folcher M, Schauerte A, Jenal U. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 2005;280:30829–30837. doi: 10.1074/jbc.M504429200. [DOI] [PubMed] [Google Scholar]
- 70.Rao F, et al. Enzymatic synthesis of c-di-GMP using a thermophilic diguanylate cyclase. Anal. Biochem. 2009;389:138–142. doi: 10.1016/j.ab.2009.03.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


