Abstract
Electronegative low-density lipoprotein (LDL) has been shown to increase coronary artery disease risk in hemodialysis patients, but its effect on the risk of peripheral artery disease (PAD) remains unclear. We separated plasma LDL from 90 uremia patients undergoing hemodialysis into 5 subfractions (L1–L5) according to charge by using fast-protein liquid chromatography with an anion-exchange column and examined the distribution of L5—the most electronegative LDL subfraction—in total LDL (i.e. L5%). During a 5-year period, we followed up with these patients until the occurrence of ischemic lower-extremity PAD. During the follow-up period, ischemic lower-extremity PAD developed in 24.4% of hemodialysis patients. L5% was higher in hemodialysis patients in whom ischemic lower-extremity PAD occurred (3.03% [IQR, 2.36–4.54], n = 22) than in hemodialysis patients in whom PAD did not occur (1.13% [IQR, 0.90–1.83], n = 68) (p < 0.001). Furthermore, L5% significantly increased the adjusted hazard ratio of ischemic lower-extremity PAD (1.54 [95% CI, 1.14–2.10]) (p = 0.005). Flow-mediated dilation was negatively associated with L5% (p < 0.001). Additionally, in vivo experiments from mice showed that L5 compromised endothelium-dependent vascular relaxation through a nitric oxide–related mechanism. Our findings indicate that increased L5% may be associated with the occurrence of ischemic lower-extremity PAD in hemodialysis patients.
Introduction
Non-traumatic peripheral artery disease (PAD) is common among uremia patients, with an incidence up to 10-fold higher than that in non-uremia patients1. Lower-extremity PAD is a primary cause of mortality in uremia patients, as well the leading cause of amputation2. Results from the Dialysis Outcomes and Practice Pattern Study showed that the prevalence of PAD was 12% to 38% among different countries3, and the world-wide prevalence of PAD continues to increase4. The traditional risk factors associated with the pathogenesis of PAD are similar to those associated with systemic atherosclerosis, including age, smoking, diabetes, hypertension, and high body mass index (BMI)5–8. Serum hypercalcemia, hyperphosphatemia, and hyperparathyroidism are also important risk factors of PAD, especially in patients with chronic renal failure or uremia9, 10. Recently, genetic factors have also been found to be associated with the occurrence of PAD11.
Dyslipidemia, especially high levels of plasma cholesterol or low-density lipoprotein-cholesterol (LDL-C), is another traditional factor of atherosclerosis, but its role in the development of coronary artery disease in uremia patients remains controversial12–14. Similarly, the role of dyslipidemia in the development of PAD in uremia patients also remains unclear15, 16.
LDL is a heterogeneous substance composed of molecules differing in size, density, chemical composition, and electric charge. Importantly, LDL with increased electronegativity has been shown to be more atherogenic than its electropositive LDL counterpart17. We have previously shown that LDL can be separated into 5 subfractions (designated L1-L5) with increasing electronegativity by using fast-protein liquid chromatography with an anion-exchange column. L5, the most electronegative LDL subfraction18, has been studied extensively for its role in the pathogenesis of atherosclerosis and has been shown to be toxic to both cardiomyocytes and endothelial cells19. Furthermore, a high percentage of L5 in total LDL (ie, L5%) has been associated with acute myocardial infarction and coronary artery disease20. L5 from patients with increased cardiovascular risks such as diabetes and hypercholesterolemia decreases nitric oxide formation in endothelial cells, which impairs vascular relaxation and leads to endothelial dysfunction21, 22.
Recently, we showed that L5% is higher in patients with early chronic kidney disease or end-stage renal disease than in individuals with normal kidney function23, 24. Moreover, L5 stimulates platelet activation and enhances vascular thrombosis25, which promote ischemic atherosclerotic vascular disease formation. However, the association between L5 and PAD in hemodialysis patients has not been explored. We conducted a cohort study to examine the occurrence of ischemic lower-extremity PAD among hemodialysis patients and estimated the hazard ratio of L5% on PAD.
Results
Increased L5% in hemodialysis patients with ischemic lower-extremity PAD
L5 distribution data were collected for 91 patients, but one patient was lost to follow-up 3 months after blood collection because of transferring to another dialysis center for maintenance hemodialysis therapy. Of the remaining 90 hemodialysis patients included in the study, ischemic lower-extremity PAD developed in 22 (24.4%) patients [PAD (+)] during the 5-year follow-up period but not in the other 68 patients [PAD (−)]. As shown in Table 1, the average follow-up time was shorter in the PAD (+) group than in the PAD (−) group. Patient age was higher in the PAD (+) group than in the PAD (−) group. Furthermore, PAD (+) patients had a higher percentage of diabetes mellitus (DM) and ischemic heart disease (IHD). Blood plasma levels of triglyceride, cholesterol, and LDL-C were also significantly higher in PAD (+) patients. Importantly, L5% and Hs-CRP levels were higher in the PAD (+) group than in the PAD (−) group. Other variables analyzed were not statistically different between groups (Table 1).
Table 1.
Characteristics of patients with or without new onset ischemic lower-extremity peripheral artery disease during follow-up.
| Variable | PAD (−) (n = 68) | PAD (+) (n = 22) | P value |
|---|---|---|---|
| Age (years) | 52.5 (44.2–61.0) | 59.5 (56.2–63.0) | 0.015 |
| Sex (male) (%) | 84.3% | 70.7% | 0.15 |
| HD Vintage (months) | 51.0 (37.3–67.8) | 42.0 (31.3–67.3) | 0.17 |
| BMI (kg/m2) | 22.2 (21.2–24.0) | 22.7 (21.3–26.0) | 0.29 |
| IHD (%) | 29.4% | 68.1% | <0.001 |
| Hypertension (%) | 76.4% | 95.4% | 0.06 |
| DM (%) | 10.3% | 50.0% | 0.001 |
| Smoking (%) | 13.2% | 4.5% | 0.44 |
| Triglyceride (mg/dl) | 134 (98–199) | 256 (199–345) | <0.001 |
| Cholesterol (mg/dl) | 176 (150–192) | 212 (176–224) | <0.001 |
| HDL-C (mg/dl) | 39 (34–44) | 39 (31–48) | 0.88 |
| LDL-C (mg/dl) | 98 (78–115) | 111 (90–149) | 0.021 |
| L5% | 1.13 (0.90–1.83) | 3.03 (2.36–4.54) | <0.001 |
| Hs-CRP (mg/dl) | 0.34 (0.16–0.75) | 1.18 (0.79–2.07) | <0.001 |
| Calcium (mg/dl) | 9.5 (9.1–10.1) | 9.6 (9.2–10.2) | 0.58 |
| Phosphate (mg/dl) | 5.2 (4.4–5.9) | 5.6 (4.6–6.1) | 0.22 |
| Ca × P | 49.2 (43.2–56.7) | 52.5 (43.1–60.7) | 0.33 |
| iPTH (pg/mL) | 198 (153–300) | 247 (177–297) | 0.35 |
| BUN (mg/dl) | 64.5 (58.2–72.5) | 67.0 (56.2–75.2) | 0.84 |
| Creatinine (mg/dl) | 10.2 (9.7–11.4) | 10.8 (10.1–11.4) | 0.46 |
Data are presented as the median value (interquartile range) or as the percentage. BMI = body mass index; BUN = blood urea nitrogen; Ca × P, calcium-phosphorous product; DM = diabetes mellitus; HD = hemodialysis; HDL-C = high-density lipoprotein-cholesterol; Hs-CRP = high sensitivity-C reactive protein; IHD = ischemic heart disease; iPTH = intact parathyroid hormone; LDL-C = low-density lipoprotein-cholesterol.
The effect of L5% on the hazard ratio for the occurrence of ischemic lower-extremity PAD
To identify variables that enhance the risk of ischemic lower-extremity PAD, we used the Cox-proportional hazard method. Similar to other studies4, 5, 7, 26, we found that history of IHD, male sex, older age, DM, LDL-C levels, and BMI significantly increased the crude hazard ratio for the occurrence of ischemic lower-extremity PAD. However, when we adjusted for all of these factors, we found that only IHD and L5% significantly increased the hazard ratio for the occurrence of ischemic lower-extremity PAD (Table 2).
Table 2.
Hazard ratios of different variables on ischemic lower-extremity PAD in hemodialysis patients.
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| L5% | 1.75 (1.43–2.13) | <0.001 | 1.54 (1.14–2.10) | 0.005 |
| IHD | 0.18 (0.07–0.48) | 0.001 | 4.85 (1.59–14.76) | 0.005 |
| Cholesterol | 1.02 (1.01–1.03) | <0.001 | 1.01 (0.99–1.03) | 0.28 |
| LDL-C | 1.02 (1.01–1.04) | 0.002 | 1.01 (0.99–1.04) | 0.19 |
| Hs-CRP | 2.16 (1.34–3.47) | <0.001 | 0.90 (0.46–1.78) | 0.51 |
| DM | 0.17 (0.07–0.44) | <0.001 | 1.36 (0.38–4.85) | 0.63 |
| Age | 1.06 (1.01–1.11) | 0.008 | 1.03 (0.98–1.10) | 0.19 |
| HD Vintage | 0.98 (0.96–1.01) | 0.079 | — | — |
| Sex | 0.40 (0.14–1.10) | 0.076 | — | — |
| Hypertension | 0.19 (0.02–1.47) | 0.11 | — | — |
| Smoking | 2.93 (0.39–21.85) | 0.29 | — | — |
| Calcium | 1.11 (0.57–2.13) | 0.76 | — | — |
| Phosphate | 1.20 (0.73–1.96) | 0.46 | — | — |
| BMI | 1.15 (0.98–1.34) | 0.075 | — | — |
Model 1 = crude hazard ratio; Model 2 = adjusted for L5%, IHD, LDL-C, Hs-CRP, DM, and age. BMI = body mass index; DM = diabetes mellitus; HD = hemodialysis; HR = hazard ratio; Hs-CRP = high-sensitivity C-reactive protein; IHD = ischemic heart disease; LDL-C = low-density lipoprotein-cholesterol; PAD = peripheral artery disease.
Plasma L5% in patients with IHD history
We grouped patients according to IHD history or the presence of ischemic lower-extremity PAD. The median L5% (interquartile range) values of these subgroups are shown in Table 3. Patients with both IHD history and lower-extremity PAD had the highest median value of L5%, whereas patients without either IHD history or lower-extremity PAD had the lowest median value of L5%. The L5% of all patients with IHD history was 2.13% (1.21–2.80) (n = 35), whereas the L5% of all patients without IHD history was 1.18% (0.88–2.30) (n = 55). Thus, patients with IHD history had a significantly higher L5% than did patients without IHD history (p = 0.007).
Table 3.
Plasma L5% of hemodialysis patients with or without IHD history and ischemic lower-extremity PAD.
| IHD history(−) | IHD history(+) | |
|---|---|---|
| Ischemic lowerextremity PAD(−) | 1.12% (0.86–1.63)(n = 48) | 2.10% (1.12–2.61)(n = 20) |
| Ischemic lowerextremity PAD(+) | 2.28% (2.16–3.78)(n = 7) | 3.49% (2.43–5.14)(n = 15) |
Data are shown as the median (interquartile range) L5%. IHD, ischemic heart disease; PAD, peripheral artery disease. (+) and (−) indicate the presence or absence, respectively.
The effect of L5% on the hazard ratio for the occurrence of ischemic lower-extremity PAD in patients with IHD
We further used the Cox-proportional hazard method to identify variables that increased the risk of ischemic lower-extremity PAD in patients with IHD history. In patients with IHD history, the L5% was higher in patients who developed ischemic lower-extremity PAD during the follow-up period [2.49% (95% CI, 2.28–4.63) (n = 15)] than in patients who did not [1.25% (95% CI, 1.08–2.06) (n = 20)] (p < 0.001). Furthermore, L5%, cholesterol, and LDL-C levels significantly increased the crude hazard ratio for the occurrence of ischemic lower-extremity PAD in patients with IHD. When we adjusted for all of these factors, L5% was the only variable that significantly increased the hazard ratio for the occurrence of ischemic lower-extremity PAD in patients with IHD history (Table 4).
Table 4.
Hazard ratios of different variables on ischemic lower-extremity PAD in hemodialysis patients with a history of IHD.
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| L5% | 1.34 (1.03–1.75) | 0.028 | 1.55 (1.01–1.86) | 0.043 |
| Cholesterol | 1.02 (1.01–1.04) | 0.003 | 1.00 (0.99–1.04) | 0.09 |
| LDL-C | 1.03 (1.02–1.05) | 0.032 | 1.01 (0.98–1.04) | 0.45 |
| DM | 0.41 (0.13–1.30) | 0.13 | — | — |
| Hs-CRP | 1.48 (0.76–2.89) | 0.24 | — | — |
| Age | 0.98 (0.93–1.04) | 0.61 | — | — |
| HD vintage | 0.98 (0.96–1.01) | 0.13 | — | — |
| Sex | 0.52 (0.18–1.47) | 0.93 | — | — |
| Hypertension | 0.39 (0.04–3.21) | 0.09 | — | — |
| Smoking | 3.47 (0.46–25.98) | 0.34 | — | — |
| Calcium | 1.60 (0.74–2.57) | 0.58 | — | — |
| Phosphate | 1.20 (0.65–2.19) | 0.55 | — | — |
| BMI | 0.98 (0.83–1.17) | 0.89 | — | — |
Model 1 = crude hazard ratio; Model 2 = adjusted for L5%, cholesterol, and LDL-C. BMI = body mass index; DM = diabetes mellitus; HD = hemodialysis, HR = hazard ratio; Hs-CRP = high-sensitivity C-reactive protein; LDL-C = low-density lipoprotein-cholesterol; PAD = peripheral artery disease.
Kaplan-Meier analysis using an L5% cutoff value for ischemic lower-extremity PAD
To find the best cutoff value for ischemic lower-extremity PAD, we used receiver operating characteristic analysis. The area under the curve was 0.940 (95% CI, 0.895–0.985) (p < 0.001). When we set the L5% cutoff value at 2.239, we observed a sensitivity of 0.909 and a specificity of 0.853 for ischemic lower-extremity PAD (Fig. 1a).
Figure 1.
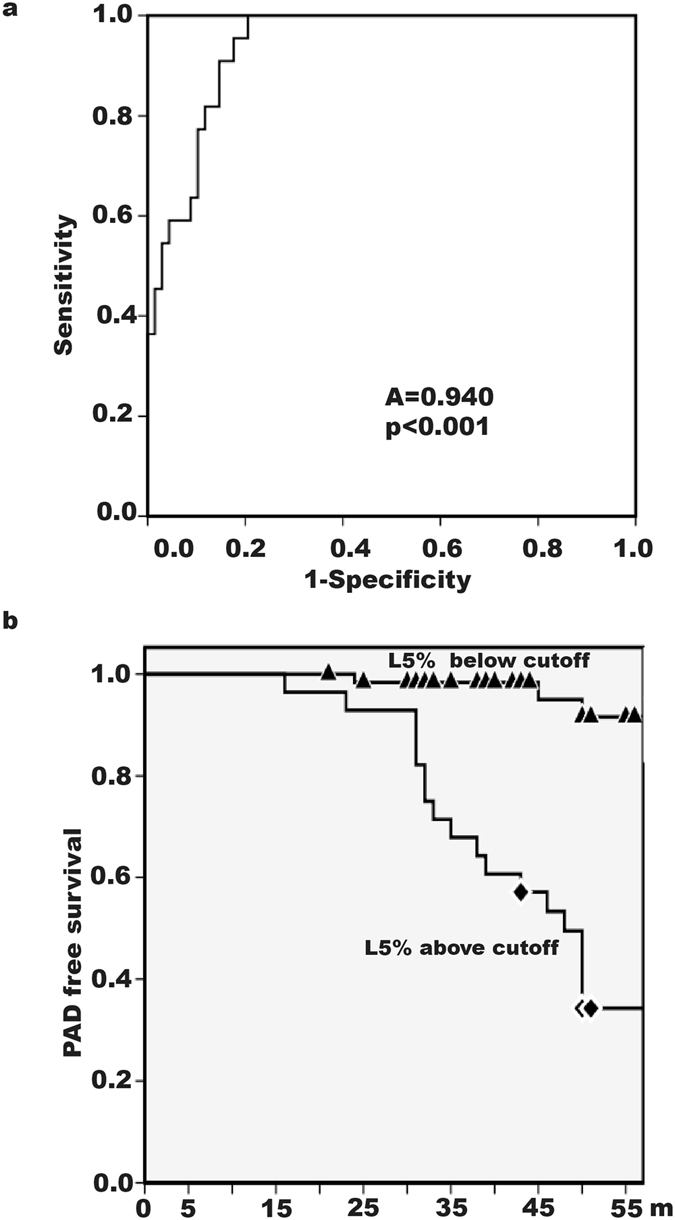
Receiver operating characteristic (ROC) analysis and ischemic lower-extremity peripheral arterial disease (PAD)-free survival in hemodialysis patients. (a) ROC analysis showed an area under the curve of 0.940 (p < 0.001). An L5% cutoff value of 2.239 was associated with ischemic lower-extremity PAD with a sensitivity of 0.909 and a specificity of 0.853. A = area under curve. (b) Patients with an L5% below 2.239 had a higher ischemic lower-extremity PAD-free survival rate than did patients with an L5% above 2.239 (log-rank test, p < 0.001).
When we grouped all 90 patients according to whether their L5% was above or below the cutoff value, Kaplan-Meier analysis showed that the ischemic lower-extremity PAD–free survival rate in patients with an L5% below 2.239 was significantly higher than that in patients with an L5% above 2.239 (p < 0.001, log-rank test) (Fig. 1b).
Positive association between L5% and Hs-CRP levels in hemodialysis patients
Because we found that age, IHD, DM, and levels of triglyceride, cholesterol, LDL-C, and Hs-CRP were significantly higher in the PAD (+) group than in the PAD (−) group, we performed linear regression analysis to characterize the association of L5% with these variables in hemodialysis patients. In univariate regression analysis, IHD, DM, and levels of triglyceride, cholesterol, LDL-C, and Hs-CRP were significantly associated with L5%. In multivariate regression analysis in which we adjusted for all of these factors, only Hs-CRP level was positively associated with L5% (p = 0.004) (Table 5).
Table 5.
Univariate and multivariate linear regression analysis for identifying independent factors associated with L5% in hemodialysis patients.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| B (SE) | P value | B (SE) | P value | |
| Age | 0.013 (0.013) | 0.33 | −0.002 (0.012) | 0.85 |
| IHD | 0.624 (0.298) | 0.039 | 0.166 (0.284) | 0.56 |
| DM | 1.257 (0.280) | <0.001 | 0.479 (0.304) | 0.12 |
| Triglyceride | 0.007 (0.001) | <0.001 | 0.002 (0.002) | 0.26 |
| Cholesterol | 0.015 (0.004) | <0.001 | 0.010 (0.006) | 0.10 |
| LDL-C | 0.013 (0.005) | 0.018 | −0.007 (0.008) | 0.37 |
| Hs-CRP | 0.989 (0.187) | 0.001 | 0.611 (0.204) | 0.004 |
| IHD | 0.624 (0.298) | 0.039 | 0.166 (0.284) | 0.56 |
DM = diabetes mellitus; Hs-CRP = high-sensitivity C-reactive protein; IHD = ischemic heart disease; LDL-C = low-density lipoprotein-cholesterol; B = regression coefficient; SE = standard error.
Negative correlation of flow-mediated dilation (FMD) with L5% and decreased FMD in patients with ischemic lower-extremity PAD
Age, IHD, DM, smoking, hypertension, dyslipidemia or hypercholesterolemia, hyperphosphotemia, and high Hs-CRP levels are factors associated with FMD27–33. We performed univariate regression analysis and found that L5%, IHD, DM, and cholesterol levels were significantly associated with FMD. However, in multivariate regression analysis, only L5% was significantly associated with FMD (p < 0.001) (Table 6). The median value of FMD in the PAD (+) group was 4.44% (IQR, 3.10–5.22), whereas the median FMD value in the PAD (−) group was 6.10% (IQR, 5.22–8.30). Thus, the median value of FMD was significantly lower in PAD (+) hemodialysis patients than in PAD (−) hemodialysis patients (p < 0.001).
Table 6.
Univariate and multivariate linear regression analysis for identifying independent factors associated with FMD in hemodialysis patients.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| B (SE) | P value | B (SE) | P value | |
| L5% | −1.016 (0.155) | <0.001 | −0.992 (0.191) | <0.001 |
| Age | −0.015 (0.024) | 0.52 | −0.004 (0.023) | 0.85 |
| IHD | −1.535 (0.512) | 0.004 | −0.918 (0.498) | 0.07 |
| DM | −1.321 (0.529) | 0.014 | −0.073 (0.533) | 0.90 |
| Smoking | −0.957 (0.827) | 0.25 | −0.874 (0.002) | 0.24 |
| Hypertension | −0.248 (0.669) | 0.71 | 0.289 (0.598) | 0.63 |
| Cholesterol | −0.021 (0.006) | 0.001 | −0.006 (0.006) | 0.37 |
| Phosphate | −0.541 (0.283) | 0.06 | −0.237 (0.255) | 0.36 |
| Hs-CRP | −0.658 (0.370) | 0.08 | 0.639 (0.379) | 0.56 |
DM = diabetes mellitus; FMD = flow-mediated dilation; Hs-CRP = high-sensitivity C-reactive protein; IHD = ischemic heart disease; B = regression coefficient; SE = standard error.
L5-induced decrease in vascular relaxation through a phospho-eNOS–related mechanism
L5% was higher in rats with chronic kidney disease (CKD) induced by 5/6 nephrectomy than in control rats (Fig. 2a). When aortas with intact endothelium from CKD rats and control rats were subjected to an ex vivo tension study, aortas from CKD rats showed an impaired acetylcholine-induced relaxation response (Fig. 2b). However, the acetylcholine-induced relaxation response was abolished in both control and CKD rats when the vascular endothelium was denuded (Fig. 2b). When we treated C57BL/6 mice with chronic venous injections of L5 or normal saline, the expression of both eNOS and phospho-eNOS protein was lower in the aortic endothelium of L5-treated mice than in that of control mice (Fig. 2c).
Figure 2.
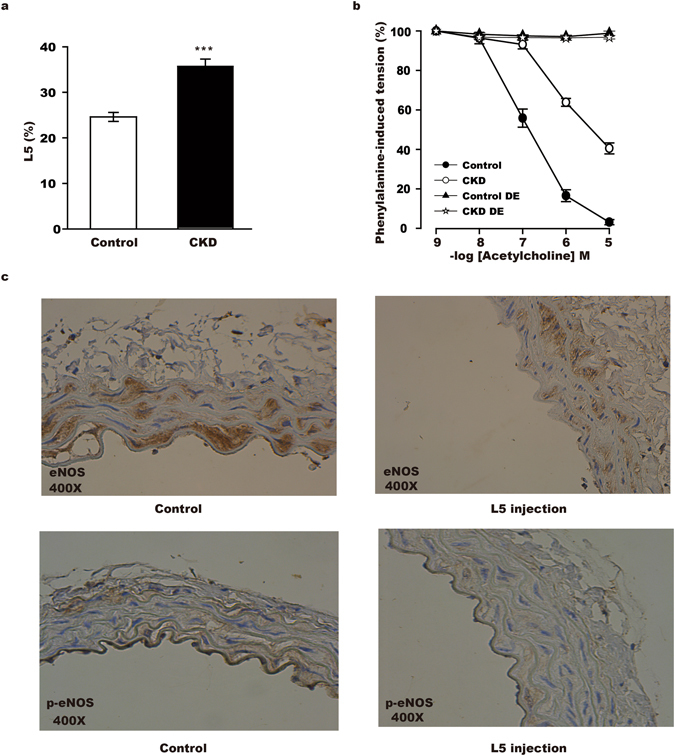
L5-induced decrease in vascular relaxation through a phospho-eNOS–related mechanism (a) The plasma L5 level (L5%) was higher in CKD rats than in control rats (35.7 ± 1.6% vs. 24.6. ± 1.0%, n = 6 per group) (***p < 0.001). (b) Phenylalanine-induced aortic tension, which is reversed by the addition of acetylcholine, was lower in control rats (open circle, n = 6) than in CKD rats (black circle, n = 6). Aortic tension is presented as the difference in area under the curve (AUC) (AUC open circle vs. AUC black circle, p < 0.001). The ability of acetylcholine to reverse phenylalanine-induced aortic tension was abolished in both control rats (black triangle, n = 6) and CKD rats (empty star, n = 6) when the aortic endothelium was denuded (DE) (AUC black triangle vs. AUC empty star, p = 0.10). The non-parametric method of integrated AUC measure was used to compare groups. (c) Representative immunohistochemistry results show that the expression of eNOS and phospho-eNOS (p-eNOS) in the aortic endothelium was lower in mice injected with L5 (n = 4, right panel) than in mice injected with normal saline (n = 4, left panel).
Discussion
In our study, we showed that L5% was significantly higher in hemodialysis patients in whom ischemic lower-extremity PAD developed than in hemodialysis patients in whom ischemic lower-extremity PAD did not develop and that L5% increased the hazard ratio for the occurrence of ischemic lower-extremity PAD in this patient population. The positive association of L5% with ischemic lower-extremity PAD was observed in hemodialysis patients, regardless of whether they had a history of IHD, although our findings indicated that hemodialysis patients with IHD history had a higher risk of ischemic lower-extremity PAD than did hemodialysis patients without IHD. Furthermore, we identified a negative correlation of L5% with FMD. Our findings suggest that L5% may be associated with the occurrence of PAD in uremia patients on hemodialysis.
We found that the percentage of ischemic lower-extremity PAD was higher in hemodialysis patients with older age, DM, IHD history, or hyperlipidemia—all traditional risk factors for PAD in non-uremia patients. However, no difference was seen between hemodialysis patients who were smokers or non-smokers or between patients with or without hypertension. Compared to the general adult population, the prevalence of smokers in our cohort of hemodialysis patients was low, whereas the prevalence of hypertension was high, which may explain these findings.
History of IHD is an important risk factor of PAD34. The results of our Cox-regression analysis showed that L5% and IHD history had a significantly higher hazard ratio for the occurrence of ischemic lower-extremity PAD than did all other traditional risk factors for PAD. Furthermore, L5% was higher in patients with a history of IHD than in patients without a history of IHD. In our subgroup analysis among patients with IHD history, L5% was higher in the PAD (+) group than in the PAD (−) group. These results suggest that L5% has a high hazard ratio for ischemic lower-extremity PAD, even in IHD patients.
There are several possible mechanisms that may underlie L5-mediated endothelial dysfunction and PAD occurrence in hemodialysis patients. L5 LDL contains apolipoprotein B and is rich in apolipoprotein CIII and triglycerides18, 24. Similar to other LDL that is rich in apolipoprotein CIII or triglycerides, L5 is highly atherogenic35, 36. Our previous studies have shown that plasma L5 levels are increased in patients with high cardiovascular disease risks, such as patients with familial hypercholesterolemia or diabetes and in smokers and hemodialysis patients18, 21, 24, 37. Furthermore, L5 from hypercholesterolemic patients was shown to enhance the production of CRP by endothelial cells via the activation of lectin-like oxidized LDL receptor-1 (LOX-1)38. CRP is a marker of systemic inflammation and also of PAD39. CRP has been shown to impair endothelial-dependent vasodilatation40 and to enhance LOX-1 expression through FcγRs (Fc-gamma receptors), which can further compromise endothelial function41. Consistent with the results of the study with L5 from hypercholesterolemic patients38, we found that L5% was positively associated with Hs-CRP levels in hemodialysis patients with PAD, indicating that increased Hs-CRP levels may be a possible mechanism underlying L5-mediated endothelial dysfunction and PAD occurrence in hemodialysis patients.
In addition, L5 can induce endothelial cell apoptosis through LOX-1 and reduce the formation of nitric oxide in endothelial cells, which leads to endothelial dysfunction21. Similarly, through LOX-1, L5 can activate platelet activation and promote thrombogenesis25. Both endothelial dysfunction and enhanced thrombogenesis predispose patients to atherosclerotic vascular disease including PAD42. Endothelial cell dysfunction can be manifested by poor FMD24. Poor FMD is a marker of atherosclerotic vascular disease43, 44 and can also predict an atherosclerotic vascular event28. In our multivariate regression analysis, L5% was significantly associated with poor FMD and a high cumulative rate of ischemic lower-extremity PAD, whereas traditional risk factors were not (Table 6). Previously, we showed that L5 can inhibit eNOS phosphorylation in human aortic endothelial cells and impair vascular relaxation via a nitric oxide–mediated mechanism. Therefore, through the suppression of phospho-eNOS in endothelial cells, L5 impaired vascular relaxation and resulted in a decrease in FMD24. In the present study, we showed that the expression of both eNOS and phospho-eNOS protein was suppressed in endothelial cells of the terminal aorta in mice that received chronic venous injections of L5 (Fig. 2c). We used the terminal portion of the aorta in place of arteries in the extremities because its location and size made it easier to retrieve than vessels in the limbs of mice.
Compatible with the negative correlation that we observed between L5 and FMD, L5 has been associated with oxidative stress45. Previous studies have shown that oxidative stress is associated with PAD in hemodialysis patients. Reducing oxidative stress in these patients with propionyl L-carnitine can significantly increase nitric oxide formation, decrease vasoconstrictive endothelin-1 production, and improve vascular hemodynamic flow46, 47. Together, these findings suggest that the association between L5% and lower-extremity PAD in uremia patients may in part be attributed to the promotion of oxidative stress by L5 LDL.
Lipid-lowering agents have been shown to have anti-inflammatory effects and protective effects on endothelial function48, 49. Previously, studies in which lipid-lowering agents were examined for their ability to lower cardiovascular mortality in hemodialysis patients showed no efficacy, even though serum cholesterol and LDL-C levels were successfully decreased by these agents12, 13. Therefore, lipid-lowering agents that specifically target the lowering of L5% may be more important than cholesterol-lowering in the prevention of atherosclerotic vascular disease.
A limitation of our study was the sample size. Because 20 ml of blood are required to measure L5%, in addition to another 20 ml of blood for other laboratory tests, recruiting patients to donate blood for the LDL distribution study can be challenging. However, because this was a cohort study with a 5-year duration of observation, the study design, in part, compensated for the small sample size. Furthermore, all but one of our patients for whom L5 distribution data were collected completed the study. We included only patients who had been receiving hemodialysis at our center for more than 6 months, and only the one patient who was lost to follow up transferred to another dialysis center. Notably, at our hospital, the rate of kidney transplantation is lower than 2% per year.
We found no difference in the severity of vascular calcification between PAD (+) and PAD (−) groups. In addition, serum phosphate, calcium, or intact parathyroid hormone levels, which are known to contribute to vascular calcification when elevated10, 50, did not increase the hazard ratios for ischemic lower-extremity PAD in our hemodialysis patients. However, our study did not contain sufficient data to rule out the possibility that lower extremity artery calcification resulted in PAD. We also did not score coronary artery or abdominal aorta calcifications with computed tomography.
In conclusion, ischemic lower-extremity PAD is common in hemodialysis patients, and our study findings indicate that increased levels of L5% may be associated with its occurrence in this patient population. Furthermore, our findings suggest that hemodialysis patients with or without IHD are both at risk of ischemic lower-extremity PAD in the presence of high L5 levels in the blood.
Methods
Patients
Our study was an observational cohort study, which included 90 adult patients with uremia who underwent maintenance hemodialysis therapy twice a week for at least 6 months at China Medical University Hospital (CMUH). The study was performed according to the STROBE guidelines for observational studies. Hemodialysis therapy and blood collection were performed after written, informed patient consent was obtained. This study protocol was approved by the CMUH institutional review board (reference number: CMUH104-REC2-160) and adhered to the Declaration of Helsinki.
Ischemic lower-extremity PAD was defined as intermittent claudication, rest pain of the feet or sole, lower-leg tissue loss from an arterial insufficiency ulcer, or gangrene of the feet or sole. The diagnosis criteria for PAD followed the Fontaine Classification, and we accepted patients only with stage II, III, and IV PAD51. The follow-up of recruited patients started from the day of blood collection and continued until ischemic lower-extremity PAD or mortality occurred. Our follow-up period was from January 1, 2010 to December 31, 2015. We excluded patients with pre-existing ischemic PAD and patients who were lost to follow-up because of transferring to another dialysis center for dialysis therapy within 6 months of blood sampling for the L5 distribution study.
For all patients included in the study, we obtained a complete medical history and routine laboratory test results on the day of blood collection. Medical history included smoking, hypertension, DM, and previous IHD. The diagnosis of IHD was made by a cardiologist on the basis of clinical symptoms and imaging studies such as myocardial scintigraphy or coronary angiography. Routine laboratory tests were performed with mid-week predialysis fasting venous blood and included measurements of serum calcium, phosphate, blood urea nitrogen, creatinine, cholesterol, triglyceride, LDL-C, high-density lipoprotein-cholesterol, intact parathyroid hormone (iPTH), and high-sensitivity C-reactive protein (Hs-CRP).
Isolation of L5
Blood was separated into red blood cells and plasma immediately after collection, and LDL was isolated from plasma with ultracentrifugation24. The isolated LDL was then loaded in a fast-protein liquid chromatography machine with an anion-exchange column. LDL subfractions L1 to L5 were eluted sequentially, as previously described18.
Diagnosis of ischemic lower-extremity PAD
Ischemic lower-extremity PAD was diagnosed by using the patient’s ischemic pain history and the ankle-brachial index (ABI) test. ABI measurements were performed by using an ABI-form device (Colin VP1000; Omron Healthcare, Inc., Kyoto, Japan), with the patient fully resting in a supine position. PAD was defined by having an ABI value less than 0.952. In all patients, the PAD diagnosis and the site of vascular occlusion were further confirmed by using duplex sonography.
Flow-mediated dilation measurement
FMD was measured on the day of blood collection by inflating a sphygmomanometer cuff around the mid-forearm to 250 mmHg. The cuff was then deflated 5 min later. The diameter of the brachial artery was measured after cuff inflation and deflation from a B-mode ultrasound image (Logiq e, GE Healthcare, Wauwatosa, WI, USA). FMD was defined as the percent increase of brachial artery diameter after cuff deflation53.
Aortic ring tension assay
All animal studies adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals Eighth Edition. All animal study protocols were approved by Institutional Animal Use and Care Committee (protocol number: 2016393). Twelve 2-month-old Sprague–Dawley rats were used in this study; 6 underwent 5/6 nephrectomy to induce CKD as previously described54, whereas 6 control rats did not. Three months later, rats were euthanized, blood was collected, and the thoracic aorta was excised. L5 was isolated, and aortas were dissected into four 3-mm-long rings. We recorded the vascular tension by using a data acquisition system (PowerLab, ADInstruments Ltd., Denver, CO, USA). Increasing concentrations of acetylcholine (10 nM–10 μM) were applied to the rings during the sustained phase (considered as 100%) of phenylephrine (0.3 μM)-induced aortic ring contraction. In some aortic ring preparations, endothelium was denuded by using a cotton swab as previously described24.
Immunohistochemistry
Two-month-old C57BL/6 male mice received daily venous injections of L5 (2 mg/kg)(n = 4) or normal saline (control)(n = 4) for one month. At the end of the experiment, mice were euthanized, and the abdominal aorta was excised and fixed in 10% formalin. The terminal part of the abdominal aorta was cut into pieces and embedded in paraffin. Immunohistochemistry was performed by incubating aortic sections with mouse anti-eNOS antibody (1:50) (Abcam, Cambridge, MA, USA) or mouse anti–phospho-eNOS antibody (1:50) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C overnight and then with horseradish peroxidase-conjugated secondary antibody (1:500) (Santa Cruz Biotechnology) at room temperature. Images were captured by using a Nikon E600 light microscope (Nikon, Kokyo, Japan; magnification, 400X).
Statistical analysis
Univariate analysis of quantitative variables was performed by using the Mann-Whitney U test, and data were expressed as the median value with the interquartile range (IQR, 25%–75%). For categorical variables, the chi-square test or Fisher’s exact test was used. The Cox-proportional hazard method was used to estimate the hazard ratio with a 95% CI for the development of ischemic low-extremity PAD after adjusting for covariate factors. Receiver operating characteristic analysis was performed to find the best cutoff value of L5% for ischemic lower-extremity PAD. Univariate and multivariate linear regression studies were performed to identify factors significantly associated with L5% and FMD. All calculations were performed by using IBM SPSS Statistics version 21.0 for Mac (SPSS Inc., Chicago, IL, USA), and all statistical analyses were reviewed by a biostatistician.
Acknowledgements
The authors thank MacArthur M. Elayda, MD, PhD, of the Texas Heart Institute for reviewing our biostatistics analyses. This study was supported in part by grants from China Medical University Hospital, Taiwan (DMR-104-014), Kaohsiung Medical University (KMU) (KMU-TP104D013), KMU Hospital (KMUH) (101-1M23), KMU Alumni Association of America (KMUH-10221), the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW 104-TDU-B-212-113002), the Stroke Biosignature Program Grant from Academia Sinica in Taiwan (BM10601010036), and the National Health Research Institutes (NHRI-EX104-10305S1). These funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Author Contributions
C.T.C. and C.H.C.: study design and manuscript writing; M.Y.S. and K.C.C.: data analysis; A.S.L., W.Y.C., C.M.C., and C.C.W.: performed experiments; N.S.: manuscript editing and writing. All authors approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56:1524–1533. doi: 10.1046/j.1523-1755.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- 2.O’Hare A, Johansen K. Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol. 2001;12:2838–2847. doi: 10.1681/ASN.V12122838. [DOI] [PubMed] [Google Scholar]
- 3.Rajagopalan S, et al. Peripheral arterial disease in patients with end-stage renal disease: observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Circulation. 2006;114:1914–1922. doi: 10.1161/CIRCULATIONAHA.105.607390. [DOI] [PubMed] [Google Scholar]
- 4.Fowkes FG, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 5.Tendera M, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 7.Ix JH, et al. Association of body mass index with peripheral arterial disease in older adults: the Cardiovascular Health Study. Am J Epidemiol. 2011;174:1036–1043. doi: 10.1093/aje/kwr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, et al. Risk factors for peripheral arterial disease among patients with chronic kidney disease. Am J Cardiol. 2012;110:136–141. doi: 10.1016/j.amjcard.2012.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campean V, et al. Atherosclerosis and vascular calcification in chronic renal failure. Kidney Blood Press Res. 2005;28:280–289. doi: 10.1159/000090182. [DOI] [PubMed] [Google Scholar]
- 10.Hagstrom E, et al. Parathyroid hormone and calcium are independently associated with subclinical vascular disease in a community-based cohort. Atherosclerosis. 2015;238:420–426. doi: 10.1016/j.atherosclerosis.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Matsukura M, et al. Genome-Wide Association Study of Peripheral Arterial Disease in a Japanese Population. PLoS One. 2015;10:e0139262. doi: 10.1371/journal.pone.0139262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanner C, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 13.Fellstrom BC, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 14.Baigent C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Hare AM, Hsu CY, Bacchetti P, Johansen KL. Peripheral vascular disease risk factors among patients undergoing hemodialysis. J Am Soc Nephrol. 2002;13:497–503. doi: 10.1681/ASN.V132497. [DOI] [PubMed] [Google Scholar]
- 16.Ogata H, et al. Detection of peripheral artery disease by duplex ultrasonography among hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2199–2206. doi: 10.2215/CJN.09451209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanova EA, Bobryshev YV, Orekhov AN. LDL electronegativity index: a potential novel index for predicting cardiovascular disease. Vasc Health Risk Manag. 2015;11:525–532. doi: 10.2147/VHRM.S74697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CY, et al. Isolation, characterization, and functional assessment of oxidatively modified subfractions of circulating low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2003;23:1083–1090. doi: 10.1161/01.ATV.0000071350.78872.C4. [DOI] [PubMed] [Google Scholar]
- 19.Lee AS, et al. Electronegative low-density lipoprotein induces cardiomyocyte apoptosis indirectly through endothelial cell-released chemokines. Apoptosis. 2012;17:1009–1018. doi: 10.1007/s10495-012-0726-1. [DOI] [PubMed] [Google Scholar]
- 20.Chang PY, et al. Aspirin protects human coronary artery endothelial cells against atherogenic electronegative LDL via an epigenetic mechanism: a novel cytoprotective role of aspirin in acute myocardial infarction. Cardiovasc Res. 2013;99:137–145. doi: 10.1093/cvr/cvt062. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, et al. Electronegative LDL impairs vascular endothelial cell integrity in diabetes by disrupting fibroblast growth factor 2 (FGF2) autoregulation. Diabetes. 2008;57:158–166. doi: 10.2337/db07-1287. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, et al. Mediation of electronegative low-density lipoprotein signaling by LOX-1: a possible mechanism of endothelial apoptosis. Circ Res. 2009;104:619–627. doi: 10.1161/CIRCRESAHA.108.190116. [DOI] [PubMed] [Google Scholar]
- 23.Chang KC, et al. Increased LDL electronegativity in chronic kidney disease disrupts calcium homeostasis resulting in cardiac dysfunction. J Mol Cell Cardiol. 2015;84:36–44. doi: 10.1016/j.yjmcc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Chang CT, et al. Electronegative Low-density Lipoprotein Increases Coronary Artery Disease Risk in Uremia Patients on Maintenance Hemodialysis. Medicine (Baltimore) 2016;95:e2265. doi: 10.1097/MD.0000000000002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan HC, et al. Highly electronegative LDL from patients with ST-elevation myocardial infarction triggers platelet activation and aggregation. Blood. 2013;122:3632–3641. doi: 10.1182/blood-2013-05-504639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch AT, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 27.Maruhashi T, et al. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart. 2013;99:1837–1842. doi: 10.1136/heartjnl-2013-304739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos-Garcia D, et al. Impaired brachial flow-mediated dilation is a predictor of a new-onset vascular event after stroke. Cerebrovasc Dis. 2011;32:155–162. doi: 10.1159/000328651. [DOI] [PubMed] [Google Scholar]
- 29.Tousoulis D, et al. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013;62:667–676. doi: 10.1016/j.jacc.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 30.Johnson HM, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010;55:1988–1995. doi: 10.1016/j.jacc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimbo D, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension. 2010;55:1210–1216. doi: 10.1161/HYPERTENSIONAHA.109.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens KK, et al. Phosphate as a cardiovascular risk factor: effects on vascular and endothelial function. Lancet. 2015;385(Suppl 1):S10. doi: 10.1016/S0140-6736(15)60325-7. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe S, et al. Elevated C-reactive protein levels and enhanced high frequency vasomotion in patients with ischemic heart disease during brachial flow-mediated dilation. PLoS One. 2014;9:e110013. doi: 10.1371/journal.pone.0110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CS, et al. Hemodialysis Is Associated With Increased Peripheral Artery Occlusive Disease Risk Among Patients With End-Stage Renal Disease: A Nationwide Population-Based Cohort Study. Medicine (Baltimore) 2015;94:e1164. doi: 10.1097/MD.0000000000001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenson RS, et al. Genetics and Causality of Triglyceride-Rich Lipoproteins in Atherosclerotic Cardiovascular Disease. J Am Coll Cardiol. 2014;64:2525–2540. doi: 10.1016/j.jacc.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 36.Hsu JF, et al. Low-density lipoprotein electronegativity is a novel cardiometabolic risk factor. PLoS One. 2014;9:e107340. doi: 10.1371/journal.pone.0107340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang D, et al. Electronegative LDL circulating in smokers impairs endothelial progenitor cell differentiation by inhibiting Akt phosphorylation via LOX-1. J Lipid Res. 2008;49:33–47. doi: 10.1194/jlr.M700305-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Chu CS, et al. Electronegative low-density lipoprotein increases C-reactive protein expression in vascular endothelial cells through the LOX-1 receptor. PLoS One. 2013;8:e70533. doi: 10.1371/journal.pone.0070533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rein P, et al. Systemic inflammation is higher in peripheral artery disease than in stable coronary artery disease. Atherosclerosis. 2015;239:299–303. doi: 10.1016/j.atherosclerosis.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Hein TW, et al. Selective activation of lectin-like oxidized low-density lipoprotein receptor-1 mediates C-reactive protein-evoked endothelial vasodilator dysfunction in coronary arterioles. Circ Res. 2014;114:92–100. doi: 10.1161/CIRCRESAHA.114.301763. [DOI] [PubMed] [Google Scholar]
- 41.Stancel N, et al. Interplay between CRP, Atherogenic LDL, and LOX-1 and Its Potential Role in the Pathogenesis of Atherosclerosis. Clin Chem. 2016;62:320–327. doi: 10.1373/clinchem.2015.243923. [DOI] [PubMed] [Google Scholar]
- 42.Makin AJ, Chung NA, Silverman SH, Lip GY. Thrombogenesis and endothelial damage/dysfunction in peripheral artery disease. Relationship to ethnicity and disease severity. Thromb Res. 2003;111:221–226. doi: 10.1016/j.thromres.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Matsuzawa Y, et al. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2015;4:e002270–e002270. doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allan RB, Delaney CL, Miller MD, Spark JI. A comparison of flow-mediated dilatation and peripheral artery tonometry for measurement of endothelial function in healthy individuals and patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2013;45:263–269. doi: 10.1016/j.ejvs.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Chen CY, et al. The most negatively charged low-density lipoprotein L5 induces stress pathways in vascular endothelial cells. J Vasc Res. 2012;49:329–341. doi: 10.1159/000337463. [DOI] [PubMed] [Google Scholar]
- 46.Signorelli SS, et al. A randomised, controlled clinical trial evaluating changes in therapeutic efficacy and oxidative parameters after treatment with propionyl L-carnitine in patients with peripheral arterial disease requiring haemodialysis. Drugs Aging. 2006;23:263–270. doi: 10.2165/00002512-200623030-00008. [DOI] [PubMed] [Google Scholar]
- 47.Signorelli SS, et al. Propionyl-L-carnitine therapy: effects on endothelin-1 and homocysteine levels in patients with peripheral arterial disease and end-stage renal disease. Kidney Blood Press Res. 2006;29:100–107. doi: 10.1159/000094363. [DOI] [PubMed] [Google Scholar]
- 48.Shahbazian H, et al. Anti-inflammatory effect of simvastatin in hemodialysis patients. Jundishapur J Nat Pharm Prod. 2015;10:e17962. doi: 10.17795/jjnpp-17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirmizis D, et al. Effects of simvastatin on markers of inflammation, oxidative stress and endothelial cell apoptosis in patients on chronic hemodialysis. J Atheroscler Thromb. 2010;17:1256–1265. doi: 10.5551/jat.5710. [DOI] [PubMed] [Google Scholar]
- 50.Signorelli SS, Fiore V, Malaponte G. Inflammation and peripheral arterial disease: the value of circulating biomarkers (Review) Int J Mol Med. 2014;33:777–783. doi: 10.3892/ijmm.2014.1657. [DOI] [PubMed] [Google Scholar]
- 51.Fontaine R, Kim M, Kieny R. [Surgical treatment of peripheral circulation disorders] Helv Chir Acta. 1954;21:499–533. [PubMed] [Google Scholar]
- 52.Liu JH, et al. Comparing Survival between peritoneal dialysis and hemodialysis patients with subclinical peripheral artery disease: a 6-year follow-up. Int J Med Sci. 2013;10:434–440. doi: 10.7150/ijms.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang CC, et al. Skin Autofluorescence Is Associated with Endothelial Dysfunction in Uremic Subjects on Hemodialysis. PLoS One. 2016;11:e0147771. doi: 10.1371/journal.pone.0147771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma LJ, et al. Regression of glomerulosclerosis with high-dose angiotensin inhibition is linked to decreased plasminogen activator inhibitor-1. J Am Soc Nephrol. 2005;16:966–976. doi: 10.1681/ASN.2004060492. [DOI] [PubMed] [Google Scholar]


