Abstract
Pathogenic BRCA1/2 germline mutations confer high risks of breast and ovarian cancer to women of European ancestry. Characterization of BRCA1/2 mutations in other ethnic groups is also medically important. We comprehensively screened 68 Colombian breast/ovarian cancer families for small-range mutations, 221 families for large-genomic rearrangements, and 1,022 unselected breast cancer cases for Colombian founder mutations in BRCA1/2. The risk of cancer among relatives of mutation carriers and the mutation penetrance were estimated by survival analysis. Identified BRCA2 mutations included 6310delGA and the recurrent 1991del4 mutations. A novel large BRCA2 deletion was found in 0.9% of the screened families. Among unselected breast cancer cases, 3.3% tested positive for BRCA1/3450del4, 2.2% for BRCA1/A1708E, 1.1% for BRCA2/3034del4, and 0.4% for BRCA2/1991del4. Female relatives of carriers of BRCA1/2 founder mutations showed a 5.90 times higher risk of breast cancer, when the woman herself carried a BRCA1 mutation compared to a non-carrier (95% CI 2.01–17.3). The estimated cumulative risk of breast cancer by age 70 years for BRCA1 mutations carriers was 14% (95% CI 5–38) compared to 3% for the general Colombian population (relative risk of breast cancer 4.05). Together with known founder mutations, reported novel variants may ease a cost-effective BRCA1/2 screening in women with Colombian ancestry.
Introduction
About 10% of breast cancers are hereditary and can be attributed to germline mutations in breast cancer susceptibility genes, in particular BRCA1 and BRCA2 (BRCA1/2). Accurate estimates of the risk of breast and ovarian cancers for BRCA1/2 mutation carriers are crucial for genetic counselling. Preventive measures can be offered to women at high risk, such as intensified surveillance, prophylactic mastectomy and oophorectomy, and in some cases chemoprevention1. Several studies have investigated the risk of breast cancer in BRCA1/2 mutation carriers. However, penetrances are usually estimated for mixtures of mutations found in women of European ancestry. Estimated cumulative risks for European women vary between 45% and 85% for breast cancer, and 10% and 65% for ovarian cancer by age 70 years, depending on the type of population and the type of case ascertainment2–8. Examination of founder mutations in non-European ethnic groups permits to assess mutation-specific penetrance, and possible differences between them, which may be of clinical relevance.
In Colombia, breast cancer is the main cause of cancer-related death among women, with incidence and mortality age-standardised (world) rates of 35.7 and 10.8 cases per 100,000 person-years, respectively9. The genome of Colombian women is the result of genetic admixture between Native Americans, Spaniards who reached South America in the sixteenth century, Native African slaves who arrived in seventeenth century, and subsequent immigration, mainly from Europe10. Little is known about the contribution of BRCA1/2 mutations to hereditary breast cancer in Colombia. In a study on 53 breast/ovarian cancer families, we previously identified three common deleterious founder mutations, 3450del4 and A1708E in BRCA1, and 3034del4 in BRCA2 (BIC nomenclature (https://research.nhgri.nih.gov/bic/)11. The two founder BRCA1 mutations accounted for 100% of all BRCA1 mutations, and the identified founder BRCA2 mutation represented 40% of all BRCA2 mutations in this initial set of families. The overall prevalence of BRCA1/2 mutations was 50% in multiple case breast cancer families and 33% in breast and ovarian cancer families. Two studies of unselected breast (and ovarian) cancer patients reported BRCA1/2 mutation frequencies of 1.2% and 15%, respectively12, 13. In these studies, the two Colombian BRCA1 founder mutations accounted for 100% (breast cancer patients) and 92% (ovarian cancer patients) of all BRCA1 mutations.
The lack of data on possible large-genomic rearrangements (LGRs) in BRCA1/2, and the scarcity of data on the prevalence of BRCA1/2 mutations in Colombian familial and unselected breast cancer patients motivated the present study. We comprehensively screened 68 breast/ovarian cancer families for small-range mutations, 221 families for LGRs, and 1,022 unselected breast cancer cases for Colombian founder mutations in BRCA1/2. We conducted survival analyses to estimate the risk of cancer among relatives of carriers of BRCA1/2 mutations, and the penetrance of specific Colombian BRCA1/2 founder mutations.
Results
Spectra and Frequencies of BRCA1/2 Mutations in Breast/Ovarian Cancer Families
In total, 290 index cases from 289 breast/ovarian cancer families were investigated. Table 1 shows their high-risk group distribution. Twelve patients were diagnosed before 35 years of age; 242 belonged to families with at least two breast cancer cases; 34 patients to families with both, breast and ovarian cancer; one to a family with male breast cancer; and one to a family with at least one ovarian cancer. The median age of disease onset was 46 years (range 25–83) for female breast cancer (n = 288). The male patient was diagnosed with breast cancer at 69 years of age and the ovarian cancer patient at 53 years of age.
Table 1.
Distribution of examined families in high-risk groups and corresponding BRCA1/2 mutation frequencies.
| Risk Group | Family Phenotype | Screened for Small-Range Mutations | Screened for Large-Genomic Rearrangements | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of families | Number (%) with mutations in | Number of Families | Number (%) with mutations in | ||||||
| BRCA1 | BRCA2 | BRCA1/2 | BRCA1a | BRCA2 | BRCA1/2 | ||||
| Female BC families | 57 | 0 (0.0) | 6 (10.5) | 6 (10.5) | 196 | 0 (0.0) | 2 (1.0) | 2 (1.0) | |
| A1 | 1 case ≤ 35 years | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| A2 | Multiple cases | 57 | 0 (0.0) | 6 (10.5) | 6 (10.5) | 184 | 0 (0.0) | 2 (1.0) | 2 (1.0) |
| A3 | Breast-ovarian cancer families | 10 | 1 (10.0) | 0 (0.0) | 1 (10.0) | 24 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥1 BC and ≥ 1 OC, at any age | |||||||||
| B | Male BC families | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥1 case of male BC | |||||||||
| C | OC families | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥1 OC at any age | |||||||||
| All families | 68 | 1 (1.5) | 6 (8.8) | 7 (10.3) | 221 | 0 | 2 (0.9) | 2 (0.9) | |
BC, breast cancer; OC, ovarian cancer.
a72/221 breast/ovarian cancer families were screened for BRCA1.
Screening of the complete BRCA1/2 coding regions in 68 families revealed seven (10%) deleterious mutations: one in BRCA1 and six in BRCA2. Risk-group specific BRCA1/2 mutation frequencies are shown in Table 1. The recurrent BRCA2/1991del4 mutation accounted for 33% of all BRCA2 mutations identified in this family set (Table 2). All mutations were frame shift mutations. The BRCA2 6310delGA mutation has been previously reported once in the NCBI mutation database. Similar ages at diagnosis were found in BRCA1/2 mutation carriers (n = 8, median 53 years, range 38–65 years) and non-BRCA carriers (n = 60, median 56 years, range 32–83 years).
Table 2.
Small-range mutations and large genomic rearrangements in the BRCA1/2 genes in Colombian breast/ovarian cancer families.
| Family | Gene | Mutation Nomenclature | Classificationc | No. of BIC Entriesb | |||
|---|---|---|---|---|---|---|---|
| BICa: genomic level | HGVSb: genomic level | HGVSb: protein level | Totald | with Hispanic ancestrye | |||
| Deleterious small-range mutations | |||||||
| 295 | BRCA1 | 1793delA | c.1674delA | p.Gly559fs | M | 6 | 5 |
| 291,409 | BRCA2 | 1991del4 | c.1763_1766delATAA | p.Asn588fs | M | 5 | 1 |
| 382 | BRCA2 | 2929delC | c.2701delC | p.Ala902fs | M | f | 1 |
| 55/648g | BRCA2 | 6252insG | c.6024dupG | p.Gln2009fs | M | 2 | 2 |
| 399 | BRCA2 | 6306delAA | c.6078_6079delAA | p.Glu2028fs | M | 3 | 1 |
| 282 | BRCA2 | 6310delGA | c.6082_6083delGA | p.Glu2028fs | M | 2 | 1j |
| Sequence variants of no or uncertain clinical significance | |||||||
| 644 | BRCA2 | 451G > C | c.223G > C | p.Ala75Pro | benign | 52 | 8 |
| 643 | BRCA2 | IVS4 − 37T > A | c.426 − 37T > A | — | VUS | 2 | 1 |
| 23,639 | BRCA2 | IVS13 − 62A > G | c.7008 − 62A > G | — | benign/VUS | 6 | 1 |
| 237 | BRCA2 | IVS14 + 53C > T | c.7435 + 53C > T | — | benign | 39 | 1 |
| 430 | BRCA2 | IVS19+15T> C | c.8487+15T> C | — | benignh | Novel | 1l |
| 558 | BRCA2 | IVS21 − 19A > G | c.8755 − 19A > G | — | VUS | i | 1 |
| 31,92,482,545 | BRCA2 | IVS24 − 83G > A | c.9257 − 83 G > A | — | benign | 3 | 1 |
| 485 | BRCA2 | IVS24 − 143T > A | c.9257 − 143T > A | — | benign | j | 1 |
| Large genomic rearrangements | |||||||
| 0055,1465 | BRCA2/ex1-14delk | M | Novel | 1l | |||
aBIC, Breast Cancer Information Core database as of October 2016 (https://research.nhgri.nih.gov/bic/).
bNomenclature follows Human Genome Variation Society (HGVS) (https://www.hgvs.org/). Numbering starts at the first A of the first coding ATG. (located in exon 2) of NCBI GenBank Accession NM_007294.3 (BRCA1) and NM_000059.3 (BRCA2).
cM, deleterious mutation; VUS, variant of uncertain clinical significance.
dIncluding those with ancestry data and those from the present study.
eThe term “Hispanic” was used for individuals of Spanish, Mexican, Central and South American, Cuban, or Puerto Rican descent.
fReported in one multiple case breast cancer family from Spain (in Miramar MD, et al. Genetic analysis of BRCA1 and BRCA2 in breast/ovarian cancer families. from Aragon (Spain). Breast Cancer Res Treat 2008;112(2):353-8, p353).
gTwo probands in family 55/648.
hClassification based on in silico analyses.
iThree times reported in NCBI.
jOnce reported in NCBI.
kThe deletion breakpoints were not determined.
lIdentified in the present study.
In addition to the deleterious mutations, eight distinct BRCA2 sequence variants were identified. Seven variants had already been detected in recent studies and classified as benign or as variants of uncertain clinical significance (VUS). The novel variant, IVS19+15T > C, was predicted to be benign by 5/5 in silico prediction tools.
Multiplex ligation dependent probe amplification (MLPA) screening for LGRs in the BRCA1/2 genes was performed in index cases of 221 breast/ovarian cancer families. Table 1 shows their high-risk group distribution. One novel large BRCA2 deletion, comprising exons 1 to 14 (ex1-14del), was identified in two (0.9%) unrelated patients (Supplementary Figure 1). No LGRs were identified in BRCA1. Phenotypes of all families harbouring deleterious BRCA1/2 germline mutations are shown in Table 3.
Table 3.
Characteristics of the Colombian breast/ovarian cancer families harboring BRCA1/2 mutations and variants.
| Family | No. of Cancers | Age at Onset (years) | Other Cancers: Age at Onset (years) | ||
|---|---|---|---|---|---|
| Female BC (bilateral) | OC | BC | OC | ||
| Families carrying deleterious BRCA1 mutations | |||||
| 295 | 5 | 1 | 38*, 61, ?, ?, ? | 39 | Colon:65, prostate:83 |
| Families carrying deleterious BRCA2 mutations | |||||
| Small-range mutations | |||||
| 291 | 3 | — | 30, 35, 65* | — | Sarcoma:47 |
| 399 | 3 | — | 44*, 60, 74 | — | — |
| 409 | 3 | — | 43, 45*, 45 | — | 3x Skin:48, 50, 89, colon:33, lung:69 |
| 382 | 3 (1) | — | 47, 55*, 55/65 | — | Leukemia:55 |
| 55a/648 | 4 | — | 46*, 63*, 82, ? | — | Colon:31, 2x cervix:40, ?, stomach:41, esophagus:83 |
| 282 | 4 (1) | — | 34/65*, 60, 60, 68 | — | Colon:30, bladder:65, lung:73 |
| Ex1-14del mutations | |||||
| 1465 | 3 | — | 47, 51*, 61 | — | Liver:60, retinoblastoma:? |
| 0055 | 3 (1) | — | 45/48*, 48, 64 | — | — |
| Families carrying BRCA2 sequence variants | |||||
| 482 | 2 | 1 | 55, ? | 59* | — |
| 31 | 3 | — | 36, 38, 47* | — | Larynx:40 |
| 92 | 3 | — | 45, 50*, 70 | — | Larynx:75 |
| 430 | 3 | 1 | 38, 40, 45* | 79 | — |
| 545 | 3 | — | 35*, 37, ? | — | Lung:? |
| 558 | 3 | — | 58, 62, 68* | — | — |
| 644 | 3 | — | 55, 60, 63* | — | Liver:63, 2x colon:66, 70, pancreas:70 |
| 643 | 3 | — | 54, 55*, 55 | — | Thyroid:54 |
| 237 | 4 | — | 48, 63*, 66, 68 | — | — |
| 23 | 5 | — | 56, 57*, 58, 63, 75 | — | Brain:63, bone:79, larynx:80, prostate:86 |
| 485 | 6 | — | 37, 40, 49*, ?, ?, ? | — | Lung:?, liver:? |
| 639 | 6 | — | 40, 40, 40, 49, 50*, 50 | — | — |
*Proband, BC: breast cancer, OC: ovarian cancer.
aCase 648 from the Col-BCCC study turned out to be a member of family 55.
Haplotype analysis for the two recurrent BRCA2 mutations, 1991del4 and ex1-14del was performed on all mutation carriers at four BRCA2 flanking loci. BRCA2/1991del4 was identified in four breast cancer patients and ex1-14del in two breast cancer patients. All four 1991del4 mutation carriers shared the same haplotype. The two ex1-14del mutation carriers displayed a similar allelic pattern at three loci and a distinct pattern at one locus (Fig. 1).
Figure 1.
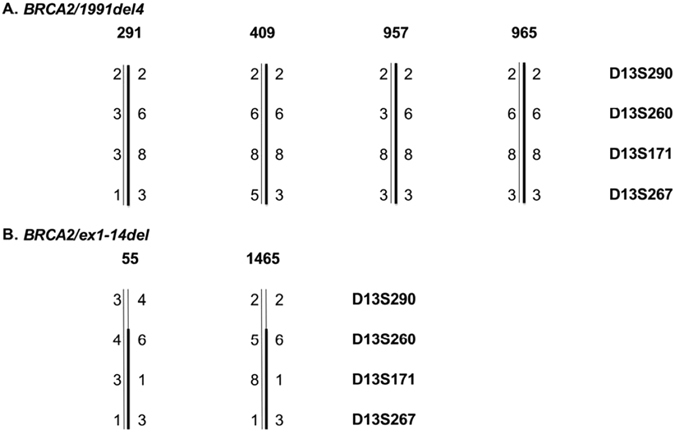
Haplotype analysis of BRCA2/1991del4 (A) and BRCA2/ex1-14del (B) on mutation carriers at four microsatellite BRCA2 flanking loci. Family numbers are given above the haplotype. Alleles are coded by numbers. D13S290: allele 2 (CA)12, allele 3 (CA)13, allele 4 (CA)14; D13S260: allele 3 (CA)20, allele 4 (CA)21, allele 5 (CA)22, allele 6: (CA)23; D13S171:allele: 1 (CA)13, allele 3 (CA)15, allele 8 (CA)20; D13S267: allele 1 (CA)32, allele 3 (CA)34, allele 5 (CA)36. Common haplotypes are indicated by a bold bar.
Spectra and Frequencies of BRCA1/2 Mutations in Unselected Breast Cancer Patients
The frequency of the four small-range BRCA1/2 Colombian founder mutations was assessed in 1,022 unselected breast cancer patients participating in the Colombian breast cancer case-control study (Col-BCCC) using PCR-based methods. In total, 71 (7%) BRCA1/2 mutations were identified: 56 (5.5%) in BRCA1 and 15 (1.5%) in BRCA2. Mutation frequencies are shown in Table 4. No mutations were detected in the 1,023 healthy Col-BCCC controls.
Table 4.
Frequencies of the four small-range BRCA1/2 founder mutations in unselected breast cancer patients and controls from Col-BCCC.
| Gene | Mutation Nomenclature | No. of Mutations (%) in Cases (n = 1,022) | No. of Mutations (%) in Controls (n = 1,023) | ||
|---|---|---|---|---|---|
| BICa: genomic level | HGVSb: genomic level | HGVSb: protein level | |||
| BRCA1 | 3450del4 | c.3331_3334delCAAG | p.Gln1111fs | 34 (3.3) | 0 (0) |
| BRCA1 | A1708E | c.5123C > A | p.Ala1708Glu | 22 (2.2) | 0 (0) |
| BRCA2 | 1991del4 | c.1763_1766delATAA | p.Asn588fs | 4 (0.4) | 0 (0) |
| BRCA2 | 3034del4 | c.2808_2811delACAA | p.Ala938fs | 11 (1.1) | 0 (0) |
| Total no. of mutations | 71 (7.0) | 0 (0) | |||
aBIC, Breast Cancer Information Core database as of October 2016 (https://research.nhgri.nih.gov/bic/).
bHGVS, Human Genome Variation Society (https://www.hgvs.org/).
Penetrance of Colombian BRCA1/2 Founder Mutations
The risk of breast cancer, ovarian cancer, and any type of cancer, including cervical cancer and melanoma, was first assessed in 251 female relatives of 73 carriers of founder mutations in BRCA1/2 (probands). Seventeen relatives of BRCA1/2 mutation carriers were diagnosed with primary breast cancer, four with primary ovarian cancer, two with melanoma and three with cervical neoplasms. Table 5 shows estimated hazard ratios (HR) of breast cancer from a multiple Cox proportional hazards model that in addition to the presence and type of BRCA1/2 mutations included the covariates birth year, type of recruitment (family or unselected case-control study), type of relationship with the proband as well as proband’s age at diagnosis. A minority of the relatives of BRCA1/2 mutation carriers (n = 38) was enrolled within the Col-BC/OC-Family study; the majority (n = 213) were recruited through unselected probands from the Col-BCCC study. The investigated cohort included eleven mothers, 86 sisters and 57 daughters (first-degree relatives) as well as 97 second- and third-degree relatives (aunts, nieces, grand-mothers, grand-daughters) of mutation carriers.
Table 5.
Estimated hazard ratios (HRs) of breast cancer in relatives of carriers of Colombian BRCA1/2 founder mutations stratified by birth year, proband’s age at diagnosis, mutated BRCA1/2 gene and BRCA1/2 mutation type.
| Variable | Level | Women | Events | HR | 95% CI | Pval | Cumulative Risk by Age 70 Years | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Birth year | Before 1960 | 65 | 6 | Ref. | 0.17 | 0.21 | 0.00–0.39 | |
| 1960–69 | 59 | 10 | 3.94 | 1.19–13.1 | 0.60 | 0.00–0.90 | ||
| 1970–79 | 53 | 1 | 2.67 | 0.25–28.3 | 0.47 | 0.00–0.89 | ||
| 1980+ | 74 | 0 | — | |||||
| Study type | Case-control | 213 | 13 | Ref. | 0.74 | 0.25 | 0.02–0.42 | |
| Family study | 38 | 4 | 1.22 | 0.39–3.79 | 0.29 | 0.00–0.52 | ||
| Relationship with proband | Other | 97 | 7 | 2.72 | 0.91–8.13 | 0.07 | 0.52 | 0.00–0.78 |
| Sister | 86 | 6 | Ref. | 0.23 | 0.00–0.44 | |||
| Daughter | 57 | 4 | 5.53 | 1.51–20.2 | 0.77 | 0.00–0.97 | ||
| Mother | 11 | 0 | — | |||||
| Proband’s age at diagnosis | Less than 40 | 59 | 3 | Ref. | 0.37 | 0.21 | 0.00–0.42 | |
| 40–44 | 57 | 4 | 1.01 | 0.23–4.52 | 0.21 | 0.00–0.41 | ||
| 45–49 | 70 | 3 | 1.09 | 0.22–5.44 | 0.23 | 0.00–0.46 | ||
| 50+ | 65 | 7 | 2.47 | 0.63–9.61 | 0.44 | 0.00–0.71 | ||
| BRCA1/2 mutation | None | 142 | 5 | Ref. | 0.01 | 0.13 | 0.00–0.25 | |
| BRCA1 | 80 | 10 | 5.90 | 2.01–17.3 | 0.55 | 0.04–0.79 | ||
| BRCA2 | 29 | 2 | 2.55 | 0.49–13.1 | 0.30 | 0.00–0.59 | ||
| Mutation type | None | 142 | 5 | Ref. | 0.02 | 0.13 | 0.00–0.26 | |
| 3450del4 | 54 | 7 | 5.63 | 1.78–17.8 | 0.55 | 0.00–0.80 | ||
| A1708E | 26 | 3 | 6.66 | 1.57–28.4 | 0.61 | 0.00–0.90 | ||
| 1991del4 | 7 | 1 | 8.45 | 0.97–73.2 | 0.70 | 0.00–0.98 | ||
| 3034del4 | 22 | 1 | 1.50 | 0.17–12.8 | 0.19 | 0.00–0.48 |
HR, hazard ratio; CI, confidence interval; Pval, P-value; Ref., reference.
In total, 109 relatives of mutation carriers were found to carry themselves BRCA1/2 mutations (80 in BRCA1 and 29 in BRCA2). Among first-degree relatives of mutation carriers, 44 carried BRCA1 and 18 BRCA2 mutations. Statistically significant risk differences were found between BRCA1/2 mutation carriers and non-carriers. The HR of breast cancer was 5.90 (95% CI 2.01 to 17.33) for BRCA1 and HR = 2.55 (95% CI 0.49 to 13.13) for BRCA2 mutation carriers compared to non-carriers. Risk differences were also noticed when relatives of probands were stratified by mutation type. The highest risk of breast cancer was found in carriers of the BRCA2/1991del4 mutation (HR = 8.45, 95% CI 0.97 to 73.24) followed by the two BRCA1 mutations A1708E (HR = 6.66, 95% CI 1.57 to 28.38) and 3450del4 (HR = 5.63, 95% CI 1.78 to 17.81), and the BRCA2/3034del4 mutation (HR = 1.50, 95% CI 0.17 to 12.84).
Separate analyses were performed to evaluate the cumulative risk of ovarian cancer by age 45 years in first-degree relatives of BRCA1/2 mutation carriers. It amounted to 5% for sisters (three ovarian cancer diagnoses) and 12% for daughters (one ovarian cancer diagnosis) of probands. Complete results for ovarian cancer and combined cancer types (breast, ovarian and cervical cancers, and melanoma) are provided in Supplementary Tables 1 and 2.
Penetrance analyses of the complete set of pedigrees using Mendel resulted in a HR of breast cancer by age 70 years equal to 2.81 (95% CI 1.47 to 5.35) for carriers of founder BRCA1 mutations compared to non-carriers. Together with a cumulative breast cancer risk of 3.3% in the general Colombian population, this translates into a 9% penetrance by age 70 years (95% CI 5 to 18). Visual inspection of pedigree deviances revealed four departing pedigrees, two of them in favour of the alternative (with two carriers each diagnosed at ages 32 and 41, and 29 and 38 years), and two of them in favour of the null hypotheses (with a non-carrier diagnosed at age 39 and one unaffected carrier at age 60 years) (Supplementary Figure 2). Exclusion of these four outlying families increased the HR of breast cancer by age 70 years to 4.05 (95% CI 1.43 to 11.4), and the corresponding cumulative risk of breast cancer by age 70 years to 14% (95% CI 5 to 38). The number of non-proband women diagnosed with breast cancer was too small to estimate the penetrance of BRCA2 mutations (two non-proband cases), and separate BRCA1 mutations (n = 7 for 3450del4 and n = 3 for A1708E).
Discussion
To our knowledge, this is the largest study on the prevalence of BRCA1/2 mutations in breast/ovarian cancer families and unselected breast cancer patients from Colombia. It is also the first report on the prevalence of LGRs in BRCA1/2, and on the cancer risk conferred by specific Colombian BRCA1/2 founder mutations. In the present study, seven deleterious small-range BRCA1/2 mutations were identified in 10% of Colombian breast/ovarian cancer families, including the recurrent BRCA2/1991del4 mutation, which showed a founder origin. This mutation has been identified previously in a single African American multiple case breast cancer family and more recently in a single breast and ovarian cancer family from Serbia14, 15. A novel LGR BRCA2/ex1-14del was found in 0.9% of Colombian breast/ovarian cancer families. A higher LGR frequency of 6.7% was recently reported in a large study on 1,560 Latin American/Caribbean breast/ovarian cancer families, which may be due to different selection criteria, since only families with at least three breast/ovarian cancer cases were screened in the present study, and to ethnic differences16.
Identified BRCA2/ex1-14del mutation carriers shared a conserved haplotype over an approximately 4 cM region spanning the BRCA2 locus implying that the mutation may have arisen from a common founder. BRCA2/ex1-14del, identified in Colombian breast/ovarian cancer families, represents the second founder LGR identified in a Hispanic population after BRCA1/ex9-12del, which is a common Mexican founder mutation17, 18.
Interestingly, none of the LGRs previously identified in Hispanic breast/ovarian cancer families and unselected breast cancer patients have been found in Colombians19–21. Previously identified LGRs include BRCA1/ex10dup and amplification of BRCA1 exons 3, 5 and 6 in Chileans22, BRCA1/ex9-12del in Mexican Americans18, BRCA1/ex8-9dup17, BRCA1/ex18-19del, and BRCA1/ex8-10del in Mexicans17, BRCA2/ex1-2del in Costa Ricans23, BRCA1/ex8-9del found in Bahamians24 and BRCA2/ex14del detected in male breast cancer patients from Brazil25. In short, it seems that the spectrum of LGRs varies among Latin Americans and among Latino Americans.
Combination of previously reported and present prevalences of small-range BRCA1/2 founder mutations result in 89% of all BRCA1 mutations attributed to A1708E and 3450del4, and 44% of all BRCA2 mutations due to the novel 1991del4 and 3034del4 mutations11. Inclusion of the two novel founder mutations BRCA2/1991del4 and BRCA2/ex1-14del in routine BRCA1/2 mutation testing would improve risk assessment and carrier detection in Colombian women.
The four most common BRCA1 mutations in Latin American breast cancer patients are: the ex9-12del mutation (1.45%) found in Mexican Americans and Mexicans, but not in Spaniards and South Americans19, 21, 26; 185delAG (0.9%) is found in many different regions of Latin America including Argentina, Brazil, Chile, Mexico and Peru; A1708E (0.58%) is found in Mexico, Spain and Colombia (one of the Colombian founder mutations)11, 18, 27; 3450del4 (0.15%), finally, is identified in patients from Brazil, Chile and Colombia (one of the Colombian founder mutations). For BRCA2, the most common mutations are H372N (0.88%), E49X (0.38%), 3492instT (0.37%), and 6174delT (0.32%). The H372N and 6174delT mutations have been found in Argentina, Brazil, Chile, Costa Rica, but not in Mexico, while 3492insT was found in Mexico and not in any other Latin American country23, 28. The 3034del4 (0.07%) was identified in Argentina, Colombia (one of the Colombian founder mutations) and Peru. These results demonstrate that certain mutations are specific of certain regions, whereas others are found throughout the whole Latin America. However, in consideration that organized genetic BRCA1/2 testing is not performed in most Latin American countries, further studies are required to investigate regional differences.
In the present study, the frequencies of the Colombian founder mutations (BRCA1/A1708E, BRCA1/3450del4, BRCA2/1991del4, BRCA2/3034del4) in 1,022 unselected breast cancer cases were 5.5% for BRCA1 and 1.5% for BRCA2. No mutations were identified in 1,023 controls. The cumulative frequency of the two BRCA1 founder mutations is likely slightly smaller than the frequency that would have been obtained in a complete gene screen, given that the two BRCA1 mutations account for approximately 90% of all BRCA1 mutations. In contrast, the 1.5% BRCA2 mutation frequency is likely underestimated, as the two screened BRCA2 mutations only account for about half of all BRCA2 mutations. Other studies on the prevalence of BRCA1/2 mutations in unselected breast cancer patients from Brazil (n = 402), Mexico (n = 810; n = 96), Colombia (Medellin) (n = 244), Cuba (n = 307) and Peru (n = 266) have reported frequencies in the range 0.3–11.4% for BRCA1 and 0.4–3.1% for BRCA2 mutations12, 13, 17, 29–32. Probably also these frequencies have been underestimated, given that BRCA1/2 genes were only partially screened, few mutations or panels of known mutations were tested, and screening was restricted to few LGRs or was not performed. A remarkably higher frequency of BRCA1 mutations equal to 24% has been reported in the Bahamas24, 33.
We found that the highest risk of breast cancer was associated with the frame shift mutation BRCA2/1991del4, but this result needs validation due to the small number of women investigated in the analysis. Both the estimated relative risk of breast cancer by age 70 years (4.05) and the corresponding cumulative risk for BRCA1 mutation carriers (14%) were lower than expected. Previously reported cumulative risks of breast cancer for BRCA1 mutation carriers vary largely between studies, with an average cumulative risk of 65%2. Possible reasons for the risk differences with earlier reports include the study design, applied methodology and the investigated Colombian population, which carries specific types of BRCA1 mutations and possibly particular genetic modifiers2–8. Risk differences could be also related to specific environmental modifiers, including the larger number of children, younger age at first birth, shorter height, less hormone use, and less alcohol consumption of Hispanic women compared to non-Hispanic white women34. External validation on an independent set of mutation-positive families is needed before interpreting screening implications.
Breast cancer is the most common cancer worldwide, and Latin America is not an exception, with rising incidence and mortality rates. Today, a commercial screening test for three previously identified founder small-range mutations is used. We propose to extend this panel by incorporating the novel founder mutations identified in this study (1991del4 and ex1-14del in BRCA2). These novel mutations should be also included in the panel of 114 recurrent Hispanic BRCA1/2 mutations (HISPANEL). A less-expensive BRCA1/2 testing tool would constitute a cost-effective strategy to further enhance control of breast and ovarian cancer in women with Colombian ancestry.
Methods
Ethical Approval
Informed consent was signed by all study participants. The research protocol was approved by the Ethics Committee of the Pontificia Universidad Javeriana in Bogota, Colombia. The methods were carried out in accordance with relevant guidelines and regulations.
Patients and Methods
Study Populations
Breast/ovarian cancer families were ascertained within a study (Col-BC/OC-Family) at the Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia from June 2004 to January 2008. Eighty-four index patients from 83 breast and/or ovarian cancer families, diagnosed with invasive breast or epithelial ovarian cancer, were selected for genetic testing after genetic counselling. The patient collective included 41 newly recruited families, and 42 index cases from 42 families, who previously tested negative for small-range BRCA1/2 mutations11.
Unselected patients were recruited within the Col-BCCC, which included 1,022 breast cancer cases and 1,023 controls. Cases were mainly recruited from hospitals in Bogota, Neiva and Villavicencio, which are located in the geographic centre of Colombia (the so called Andean region), during the period 03/2007 to 02/2011. Cases were women with a diagnosis of breast cancer after January 1st, 2004. Controls were recruited in the period of 06/2007 to 06/2011. Controls were healthy and unrelated women, who reported no family history of breast or any other type of cancer in two generations and who participated in the Colombian National Pap Smear Program35. Cases and controls were eligible if they were of Hispanic origin and resided in the study region. Controls were matched to cases by 2-year age classes.
DNA Isolation and BRCA1/2 Mutation Analyses
Genomic DNA was extracted from nine millilitre of peripheral blood collected into an EDTA tube using the salting out extraction method36. The entire coding regions and adjacent intronic splice junctions of BRCA1 (Genbank accession number U14680) and BRCA2 (Genbank accession number U43746) were screened in 69 index patients from 68 breast/ovarian cancer families (41 newly recruited within the Col-BC/OC-Family study, and 27 from the Col-BCCC study in A2, A3, C high-risk families). We used denaturating high performance liquid chromatography (DHPLC) analysis with the WAVE DNA fragment analysis system (Transgenomics, Omaha, NE, USA). PCR-primer pairs, PCR reactions set-up, cycling conditions, and DHPLC running conditions were as previously described37, 38. Each sample revealing variants was sequenced using an automated DNA CEQ 8000 sequencer (Beckman, Hilden, Germany) as previously described11.
Two hundred and twenty-one index patients from breast/ovarian cancer families (42 from the Col-BC/OC-Family study, who tested negative for small-range BRCA1/2 mutations, and 179 from the Col-BCCC study, who belonged to A1-A3, B high-risk families) were further screened: 72 for LGRs in BRCA1 and 221 for LGRs in BRCA2. MLPA analysis was performed using probe mix P002 and P087 for BRCA1 and P045 for BRCA2 according to the manufacturer’s instructions (MRC Holland, Amsterdam, the Netherlands). PCR was carried out using D4-labeled primers. Separation and relative quantification of the amplified product was obtained using the Beckman CEQ 8000XL DNA Analysis System (Beckman Coulter, Fullerton, USA). Variation in peak height was evaluated by comparing each sample with normal controls using SeqPilot software (JSI medical systems, Kippenheim, Germany). The presence of a deletion was confirmed by a second MLPA.
The prevalence of the four Colombian founder mutations was assessed in 1,022 unselected breast cancer cases and 1,023 healthy controls of the Col-BCCC study. Screening for BRCA1/3450del4, BRCA2/1991del4 (identified in present study), and BRCA2/3034del4 was performed by mismatch PCR. Screening for BRCA1/A1708E was performed by PCR-based restriction fragment length polymorphism (RFLP) analysis.
For BRCA1/3450del4, the PCR reaction was set up in 10 µl containing 25 ng genomic DNA, 1x PCR buffer (Green GoTaq Flexi Buffer, Promega, Madison, WI, USA), 1.5 mM MgCl2, 250 µM of dNTPs, 0.1 µM of each allele specific primer, 0.3 µM of the mismatch primer and 1 U DNA Polymerase (GoTaq Hot Start Polymerase, Promega). After an initial GoTaq activation step for 10 minutes at 95 °C, 30 cycles of PCR reactions consisting of 1 minute at 94 °C, 1 minute at 55 °C and 1 minute at 72 °C were carried out. This was followed by a final extension step of 7 minutes at 72 °C. Amplified PCR products were separated on a 3% agarose gel containing ethidium bromide and scored by UV visualisation. The fragment sizes of the wild type and mutant alleles were 287 bp and 162 bp, respectively
PCR reactions and conditions for BRCA2/1991del4 and BRCA2/3034del4 were as for BRCA1/3450del4 with the exception of using 2 mM and 3 mM MgCl2, respectively. The fragment sizes of the wild type and mutant alleles were 233 bp and 144 bp for BRCA2/1991del4 and 342 bp and 153 bp for BRCA2/3034del4.
PCR reactions and conditions for BRCA1/A1708E (C > A) were as for BRCA1/3450del4 with the exception of using two allele specific PCR primers, 3 mM MgCl2, and an annealing temperature of 60 °C. Amplified PCR products were digested with 15 U AciI in a total volume of 22 µl (New England BioLabs, Ipswich, MA, USA) for 12 hours at 37 °C, followed by 20 minutes at 60 °C to inactivate AciI and separated on a 3% agarose gel containing ethidium bromide. The fragment sizes of the wild type C allele were 226 bp and 126 bp and of the mutant A allele 352 bp.
Primer sequences are available upon request. All primers were synthesized by Integrated DNA Technologies, Coralville, IA, USA.
Haplotype Analysis
Individuals with identical BRCA2 germline mutations from apparently unrelated families were scored for allele sharing indicative of a common ancestor. Haplotype analysis was performed at four extragenic microsatellite loci D13S290, D13S260, D13S171, and D13S267) flanking the BRCA2 gene. Microsatellite alleles were identified by automated fluorescent-bases fragment detection from amplified PCR products using a CEQ 8000 XL DNA Analysis System (Beckman, Hilden, Germany).
In silico Analyses
The novel BRCA2 variant in intron 19 was evaluated for its potential effect on splicing using the splice prediction algorithms SpliceSiteFinder-like (http://www.umd.be/-searchSplice-Site.html), MaxEntScan (http://genes.mit.edu/burgelab/maxent/), NNSPLICE (http://www.fruitfly.org/seq_tools/splice.html), GeneSplicer (http://ccb.jhu.-edu/software/genesplicer/), and HumanSplice Finder (http://www.umd.be/HSF/). We used the Alamut software interface (Interactive Biosoftware) with default settings.
Statistical Analyses
Two different approaches were used to assess the risk of cancer conferred by Colombian BRCA1/2 founder mutations. First, a standard multiple Cox proportional hazards model was fitted to censored information on age at diagnosis from relatives of carriers of BRCA1/2 founder mutations (probands), and cumulative risks were estimated using Breslow method. We also estimated the penetrance of BRCA1 mutations with the Mendel package for statistical analyses, taking into account ascertainment by conditioning on proband diagnoses39, 40. In short, a Cox proportional hazards model was fitted to censored data on age at diagnosis from probands and their relatives, incorporating the background incidence of breast cancer in Colombia reported by GLOBOCAN as the baseline hazard function9. Mendel allows multiple probands per pedigree and conditions on specially appended pedigrees during parameter estimation in a modified segregation analyses. We visually inspected the deviance (twice the log-likelihood difference under the alternative and null hypotheses) of each pedigree in order to identify influential families in favour or against the null hypothesis of no risk increase.
Electronic supplementary material
Acknowledgements
We thank J. Leary, M. Thomassen, B. Fiebig, T. Caldes, J.N. Weitzel, I. Kostantopoulou, and D. Steinmann for providing BRCA1/2 mutation controls and M. Gilbert for expert technical assistance. We thank the Colombian Breast Cancer Study Group (COLBCS) for its contribution. COLBCS: researchers: D. Torres, I. Briceño (Pontificia Universidad Javeriana, Bogota); F. Gil (Unit of Clinical Epidemiology and Biostatistics, Pontificia Universidad Javeriana, Bogota); A. Beltran, V. Ariza, (Universidad Nacional, Bogota); clinicians: J. Caicedo, C. Ramirez, E. Quintero, S. Quintero, J. Robledo (Country Clinic, Bogota); M. Tawil, L. Torregrosa (Pontificia Universidad Javeriana, Bogota); J. German Olaya (Hospital Universitario Hernando Moncaleano Perdomo, Neiva); study coordinator: U. Hamann, DKFZ, Heidelberg. We thank all women who participated in this study. This work was supported by the Deutsches Krebsforschungszentrum, the Alexander von Humboldt Foundation (postdoctoral fellowship to D. Torres), Bonn, and the Pontificia Universidad Javeriana, Bogota.
Author Contributions
D.T. designed the study, participated in the recruitment of study participants, blood sample and data collection, performed the experiments, analysed and interpreted the data, and wrote the paper. J.L.B. and M.U.R. performed the experiments, analysed and interpreted data, and wrote the paper. I.B., A.B., V.Z., and COLBCS participated in the recruitment of study participants, blood sample and data collection. F.G. provided database management. U.H. designed and coordinated the study, analysed and interpreted the data, and wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
D. Torres and J. Lorenzo Bermejo contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05056-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Metcalfe KA, et al. An evaluation of needs of female BRCA1 and BRCA2 carriers undergoing genetic counselling. J Med Genet. 2000;37:866–874. doi: 10.1136/jmg.37.11.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniou A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. American journal of human genetics. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniou AC, et al. BRCA1 and BRCA2 mutation predictions using the BOADICEA and BRCAPRO models and penetrance estimation in high-risk French-Canadian families. Breast Cancer Res. 2006;8:R3. doi: 10.1186/bcr1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. American journal of human genetics. 1995;56:265–271. [PMC free article] [PubMed] [Google Scholar]
- 6.Evans DG, et al. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008;8:155. doi: 10.1186/1471-2407-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford D, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. American journal of human genetics. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King MC, Marks JH, Mandell JB, New York Breast Cancer Study, G Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, et al. Geographic patterns of genome admixture in Latin American Mestizos. PLoS genetics. 2008;4:e1000037. doi: 10.1371/journal.pgen.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres D, et al. High proportion of BRCA1/2 founder mutations in Hispanic breast/ovarian cancer families from Colombia. Breast Cancer Res Treat. 2007;103:225–232. doi: 10.1007/s10549-006-9370-1. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez JE, et al. Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from medellin, Colombia. Hered Cancer Clin Pract. 2014;12:11. doi: 10.1186/1897-4287-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez AO, et al. BRCA1 and BRCA2 mutations among ovarian cancer patients from Colombia. Gynecol Oncol. 2012;124:236–243. doi: 10.1016/j.ygyno.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Dobricic J, et al. Serbian high-risk families: extensive results on BRCA mutation spectra and frequency. J Hum Genet. 2013;58:501–507. doi: 10.1038/jhg.2013.30. [DOI] [PubMed] [Google Scholar]
- 15.Kanaan Y, et al. Inherited BRCA2 mutations in African Americans with breast and/or ovarian cancer: a study of familial and early onset cases. Hum Genet. 2003;113:452–460. doi: 10.1007/s00439-003-0999-0. [DOI] [PubMed] [Google Scholar]
- 16.Judkins T, et al. Clinical significance of large rearrangements in BRCA1 and BRCA2. Cancer. 2012;118:5210–5216. doi: 10.1002/cncr.27556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villarreal-Garza C, et al. Significant clinical impact of recurrent BRCA1 and BRCA2 mutations in Mexico. Cancer. 2015;121:372–378. doi: 10.1002/cncr.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weitzel JN, et al. Prevalence and type of BRCA mutations in Hispanics undergoing genetic cancer risk assessment in the southwestern United States: a report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol. 2013;31:210–216. doi: 10.1200/JCO.2011.41.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Hoya M, et al. Genomic rearrangements at the BRCA1 locus in Spanish families with breast/ovarian cancer. Clin Chem. 2006;52:1480–1485. doi: 10.1373/clinchem.2006.070110. [DOI] [PubMed] [Google Scholar]
- 20.Fachal L, Blanco A, Santamarina M, Carracedo A, Vega A. Large genomic rearrangements of BRCA1 and BRCA2 among patients referred for genetic analysis in Galicia (NW Spain): delimitation and mechanism of three novel BRCA1 rearrangements. PloS one. 2014;9:e93306. doi: 10.1371/journal.pone.0093306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres D, et al. Absence of the BRCA1 del (exons 9-12) mutation in breast/ovarian cancer families outside of Mexican Hispanics. Breast Cancer Res Treat. 2009;117:679–681. doi: 10.1007/s10549-009-0383-4. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez A, Faundez P, Carvallo P. Genomic rearrangements of the BRCA1 gene in Chilean breast cancer families: an MLPA analysis. Breast Cancer Res Treat. 2011;128:845–853. doi: 10.1007/s10549-011-1382-9. [DOI] [PubMed] [Google Scholar]
- 23.Dutil J, Colon-Colon JL, Matta JL, Sutphen R, Echenique M. Identification of the prevalent BRCA1 and BRCA2 mutations in the female population of Puerto Rico. Cancer Genet. 2012;205:242–248. doi: 10.1016/j.cancergen.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbari MR, et al. The spectrum of BRCA1 and BRCA2 mutations in breast cancer patients in the Bahamas. Clin Genet. 2014;85:64–67. doi: 10.1111/cge.12132. [DOI] [PubMed] [Google Scholar]
- 25.Timoteo AR, et al. Identification of a new BRCA2 large genomic deletion associated with high risk male breast cancer. Hered Cancer Clin Pract. 2015;13:2. doi: 10.1186/s13053-014-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porchia, L. M. et al. Common BRCA1 and BRCA2 Mutations among Latin American Breast Cancer Subjects: A Meta-Analysis. J Carcinog. Mutagen6.3 (2015).
- 27.Diez O, et al. Analysis of BRCA1 and BRCA2 genes in Spanish breast/ovarian cancer patients: a high proportion of mutations unique to Spain and evidence of founder effects. Hum Mutat. 2003;22:301–312. doi: 10.1002/humu.10260. [DOI] [PubMed] [Google Scholar]
- 28.Dutil J, et al. The spectrum of BRCA1 and BRCA2 alleles in Latin America and the Caribbean: a clinical perspective. Breast Cancer Res Treat. 2015;154:441–453. doi: 10.1007/s10549-015-3629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abugattas J, et al. Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Peru. Clin Genet. 2015;88:371–375. doi: 10.1111/cge.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes MC, et al. Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Brazil. Breast Cancer Res Treat. 2007;103:349–353. doi: 10.1007/s10549-006-9378-6. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez RC, et al. Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Cuba. Fam Cancer. 2008;7:275–279. doi: 10.1007/s10689-008-9187-7. [DOI] [PubMed] [Google Scholar]
- 32.Torres-Mejia G, et al. Recurrent BRCA1 and BRCA2 mutations in Mexican women with breast cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:498–505. doi: 10.1158/1055-9965.EPI-13-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donenberg T, et al. A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat. 2011;125:591–596. doi: 10.1007/s10549-010-1156-9. [DOI] [PubMed] [Google Scholar]
- 34.Hines LM, et al. Comparative analysis sof breast cancer risk factors among Hispanic and non-Hispanic white women. Cancer. 2010;116:3215–23. doi: 10.1002/cncr.25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pineros M, Cendales R, Murillo R, Wiesner C, Tovar S. [Pap test coverage and related factors in Colombia, 2005] Rev Salud Publica (Bogota) 2007;9:327–341. doi: 10.1590/S0124-00642007000300002. [DOI] [PubMed] [Google Scholar]
- 36.Laitinen, J., Samarut, J. & Holtta, E. A nontoxic and versatile protein salting-out method for isolation of DNA. Biotechniques17, 316, 318, 320–312 (1994). [PubMed]
- 37.Arnold, N. et al. A highly sensitive, fast, and economical technique for mutation analysis in hereditary breast and ovarian cancers. Hum Mutat14, 333–339 (1999). [DOI] [PubMed]
- 38.Gross E, Arnold N, Goette J, Schwarz-Boeger U, Kiechle M. A comparison of BRCA1 mutation analysis by direct sequencing, SSCP and DHPLC. Hum Genet. 1999;105:72–78. doi: 10.1007/s004399900092. [DOI] [PubMed] [Google Scholar]
- 39.Lange K, et al. Mendel: the Swiss army knife of genetic analysis programs. Bioinformatics. 2013;29:1568–1570. doi: 10.1093/bioinformatics/btt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lange K, Sinsheimer JS, Sobel E. Association testing with Mendel. Genetic epidemiology. 2005;29:36–50. doi: 10.1002/gepi.20073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


