Abstract
Concordance between mutations in the primary papillary thyroid carcinoma (PTC) and the paired x lymph node metastasis may elucidate the potential role of molecular targeted therapy in advanced stages. BRAF and NRAS mutations in primary PTC (n = 253) with corresponding metastatic lymph node (n = 46) were analyzed utilizing StripAssays (ViennaLab Diagnostics). Statistical analysis was performed using (SPSS, Inc.), version 24.0 with a p-value of <0.05, and concordance via kappa agreement. BRAF mutation frequency in conventional PTC (cPTC): 56.8%, papillary thyroid microcarcinoma (PTMC): 36.5%, PTMC-FV: 2.7% and PTC-FV: 4.1%. NRAS mutation frequency in PTC-FV: 28.6%, PTMC: 28.6%, PTMC-FV: 23.8%, and cPTC: 19.0%. BRAF mutation correlation with older age in cPTC (42.6 versus 33.6) years (p < 0.001) was the only significant clinicopathologic parameter. BRAF mutations were concordant in the primary and its corresponding lymph node deposits in PTC with a kappa of 0.77 (p-value < 0.0001). BRAF mutations are predominant in cPTC and PTMC while NRAS mutations in PTC-FV. BRAF mutation is conserved in metastatic lymph node deposits, thus BRAF is an early mutational pathogenetic driver. Therefore, targeted therapy is potential in recurrent and advanced stage disease.
Introduction
Papillary thyroid carcinoma (PTC) is the most common malignant thyroid cancer, accounting for 1.5% of all cancers in the United States1 and up to 6% in the Arab countries2. Around 50% of PTCs present with lymph node metastasis and approximately 5–7% show distant metastasis usually involving the lungs and bones3. Prognostic clinicopathologic factors in PTC, regarding recurrence and metastasis, include age, gender, tumor size, infiltrative growth pattern, multifocality, and extrathyroidal extension4. Recently, genetic aberrations have been postulated to be contributing factors to the clinical and behavioral metastatic risks of PTCs5–7. These include enzymes of the mitogen-activated protein kinase (MAPK) signaling pathway, specifically BRAF and RAS genes8. PTCs harboring the BRAF mutation are usually characterized by a T1799A point mutation in the v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) resulting in a valine-to-glutamic acid switch at codon 600 (V600E)9.
Several studies reported an association of the BRAF mutational status with a number of PTC clinicopathologic parameters inclusive of recurrence and worse prognosis10, 11. For instance, Nikiforova et al. correlated between BRAF status on one hand and both advanced age (5th decade) and extrathyroidal extension on the other hand12, while others reported no significant correlation13, 14. Furthermore, there is conflicting evidence with respect to BRAF mutational analysis on cytology smears as a guide for further surgical management. Alternative studies reported a prediction of lymph node status based on BRAF cytology15, 16, while Barabaro et al. identified no significant association17. Yet, there is increasing evidence that coexistence of this BRAF mutation with other promoter mutations, specifically TERT promoter mutations, might form a genetic background defining PTC with the worst clinicopathologic parameters and outcomes18. Specifically, the C228T TERT promoter mutation has been shown to be associated with the BRAF V600E mutation, which was prevalent in the aggressive types of thyroid cancer19.
In the era of targeted therapy, which is based on the understanding of tumor molecular biology, it is critical to determine the molecular profiles in both the primary and metastatic sites as well. The use of anti-BRAF therapy is currently under investigation in clinical trials for cases of advanced surgically unresectable and/or radioresistant thyroid cancer cases20. Therefore, we aimed in this study to determine the BRAF and NRAS molecular signature concordance rates between the four main different primary PTC subtypes and the corresponding paired metastatic lymph node deposits in order to elucidate the potential clinical implication of selective molecular targeted therapy in advanced stage PTC. Additionally, we sought to determine the frequencies and types of BRAF and NRAS mutations in a cohort of Lebanese patients and correlate between the findings and the various clinicopathologic features of individual PTC subtypes: conventional PTC (cPTC), papillary thyroid microcarcinoma (PTMC) defined as tumors measuring ≤1 cm in maximum diameter, follicular variant of PTMC (PTMC-FV) and the follicular variant of PTC (PTC-FV).
Materials and Methods
Patient Selection
All patients enrolled in this retrospective clinical study gave informed consents for both participation and publication of identifying information/images (when applicable). The study with all its experimental protocols was conducted under the Institutional Review Board (IRB) approvals of the American University of Beirut Medical Center (AUBMC) and Hammoud Hospital University Medical Center (HHUMC). All experiments were performed in accordance with relevant guidelines and regulations. Archived formalin fixed paraffin embedded (FFPE) tissues of 312 PTC patients were collected from the Departments of Pathology and Laboratory Medicine, AUBMC and HHUMC, Beirut, Lebanon, between the period of January 2001 and December 2011.
Patients Tissue Sampling
Out of the 312 PTC cases, 253 PTC cases with available paraffin blocks and a minimal tumor size of 1 mm underwent mutational analysis. All the 253 PTC cases were analyzed for BRAF and KRAS, and only 202 with available extracted DNA underwent analysis for NRAS mutations (Fig. 1). As a negative control, 15 cases of multinodular goiter were used. Demographic (age and gender) and prognostic histopathologic features (tumor size, lymphovascular invasion, extrathyroidal invasion, focality, and lymph node metastasis) were evaluated and correlated with the molecular aberrations. Lymph node dissection was performed on128 cases of the 253, out of which 62 had metastatic lymph node deposits. Yet, only 46 cases with available paraffin blocks and a minimal tumor size of 1 mm underwent mutational analysis. Patients’ consents were waivered by the IRB because this is a retrospective study.
Figure 1.
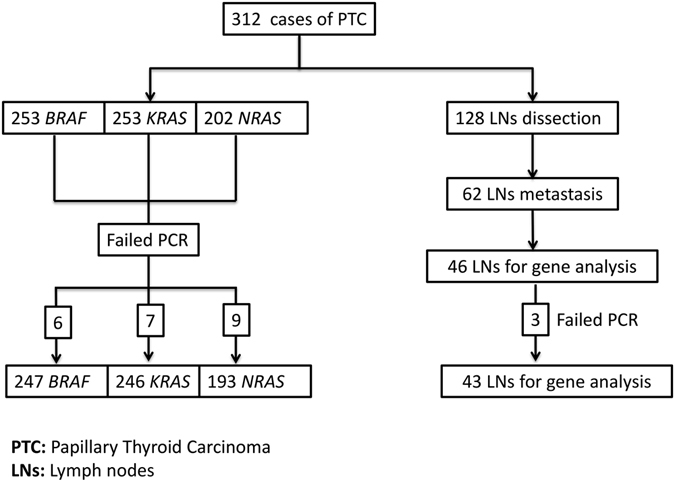
Stratification of the 312 cases of PTC included in our study.
DNA Extraction and Quantification
DNA was extracted from 253PTC cases utilizing the QIAamp FFPE DNA extraction kit (Qiagen, California, USA), and quantified via the Qubit fluorometer (Thermofisher Scientific, USA). The extracted DNA was stored at −20 °C until further use.
BRAF, KRAS, and NRAS Analysis Using Reverse Hybridization
The BRAF, KRAS, and NRAS StripAssays (ViennaLab Diagnostic GmbH, Vienna, Austria) were utilized to detect different point mutations and deletions in the genes coding for BRAF and NRAS. The detection sensitivity for mutant alleles is 1%, performed according to the manufacturer’s instructions. Mutational analysis was performed by polymerase chain reaction (PCR) and reverse hybridization as follows: first, a multiplex PCR amplification using biotinylated oligonucleotide primers was performed for BRAF, KRAS, and NRAS gene sequence amplification; second, reverse hybridization of the amplification products was ensued via a test strip, which contains allele-specific oligonucleotide probes for mutations and controls immobilized on a parallel array; and finally, bound biotinylated sequences were visualized using streptavidin-alkaline phosphatase conjugate and enzymatic color development. Positive control samples included defined mutated cell line DNA or clones.
Statistical Analysis
Data were entered into a Microsoft Excel datasheet, and then transferred to the Statistical Package for Social Science software (SPSS, Inc.), version 24.0, which was used for data management, cleaning, and analyses. Descriptive statistics was carried out and reported as number and percent for categorical variables, whereas the mean and standard deviation (±) for continuous ones. Association between mutation and demographic, clinical and pathological data was assessed using the Chi square test or Fisher’s exact test for categorical variables, and student’s independent t-test or Mann Whitney test for continuous ones. Moreover, to assess for the agreement between the primary tumor and its corresponding lymph node metastasis, kappa agreement was calculated and reported along with the p-value. Statistical significance was specified at 0.05 levels.
Results
Thyroid Cancer Patients’ Demographics
AUBMC thyroid carcinoma database from 2001 to 2011 revealed 385 thyroid cancers with PTC as the predominant type constituting 91.7% (321/385) of the cases. The overall female-to-male ratio of the PTC cases was 2.5:1. Approximately 26% of the patients were <30 years old, 56% were between 31–49 years old, and 18% were >50 years old. The frequency of each PTC histopathologic subtype was as follows: 123 cases of cPTC (49%), 76 cases of PTMC (30.0%), 15 cases of PTMC-FV (5.9%), and 39 cases of PTC-FV (15.5%) (Table 1, Fig. 2).
Table 1.
Frequency of Primary BRAF and NRAS mutations in cPTC, PTMC, PTMC-FV and PTC-FV.
| Variables | BRAF mutation n (%) n = 148 | No BRAF mutation n (%) n = 99 | P-value | NRAS mutation n (%) n = 21 | No NRAS mutation n (%) n = 172 | P-value |
|---|---|---|---|---|---|---|
| PTC Subtype | <0.0001* | <0.0001* | ||||
| cPTC | 84 (56.8) | 36 (36.4) | 4 (19.0) | 97 (56.4) | ||
| PTMC | 54 (36.5) | 21 (21.2) | 6 (28.6) | 49 (28.5) | ||
| PTMC-FV | 4 (2.7) | 11 (11.1) | 5 (23.8) | 8 (4.7) | ||
| PTC-FV | 6 (4.1) | 31 (31.3) | 6 (28.6) | 18 (10.5) |
*Significant difference.
Figure 2.
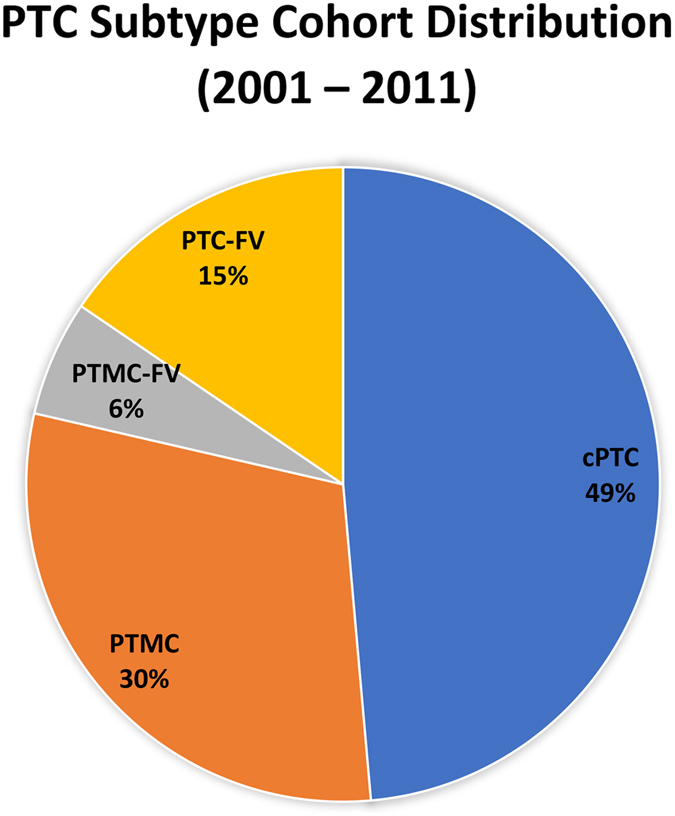
Pie graph showing the frequency of each PTC histopathologic subtype: 123 cases of cPTC (49%), 76 cases of PTMC (30%), 15 cases of PTMC-FV (6%), and 39 cases of PTC-FV (15%).
BRAF and NRAS Mutational Frequency
The frequency of BRAF and NRAS mutations varied among the different histopathologic subtypes of PTC. In cPTC and PTMC subtypes, BRAF mutations were predominant, while NRAS were less common. Conversely, in PTMC-FV and PTC-FV, NRAS mutations were more common than BRAF mutations with statistical significance of p < 0.0001. The BRAF V600E mutation subtype comprised 98.0% of the total BRAF mutated PTC cases followed by BRAF V600M identified in two cases: one cPTC case and a second cPTC case with a concomitant BRAF V600E and V600M. The NRAS mutational subtypes included c.182 A > G (p.Q61R) c.181 C > A (p.Q61K), c.34 G > A (p.G12S) and c.38 G > A (p.G13D). Concomitant BRAF and NRAS mutations were detected in five PTC cases inclusive of one cPTC, two PTMC and two PTMC-FV cases. KRAS mutations were detected in only 4 out of 246 cases tested; therefore, no further statistical analysis was performed. No mutations were detected in all adenomatous goiter cases (n = 15).
Clinicopathologic Correlation of Mutations with PTC Histopathologic Subtypes
The mutational status of BRAF and NRAS in the four PTC variants (cPTC, PTMC, PTMC-FV and PTC/FV) was compared to the clinicopathological parameters, including age, gender, tumor size, extracapsular extension, lymphovascular involvement, lymph node metastasis, and multifocality.
BRAF mutation in cPTC was significantly correlated to older age (BRAF mutated cPTC, mean age = 42.6 ± 14.5 years vs. wild-type cPTC, mean age = 33.6 ± 15.1 years, p = 0.005). A trend towards higher incidence of BRAF mutation was found in patients with higher tumor stage (p = 0.054). There was no significance association with respect to BRAF and NRAS mutations in the remaining cPTC clinicopathologic features (Table 2).
Table 2.
Clinicopathological features of c-PTC cases with respect to BRAF and NRAS mutations.
| Variables | BRAF mutation n (%) n=84 | No BRAF mutation n (%) n=36 | P-value | NRAS mutation n (%) n=4 | No NRAS mutation n (%) n=97 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.005* | 0.520 | ||||||||
| Mean ± SD | 42.6±14.5 | 33.6±15.1 | 36.2±16.9 | 39.2±15.1 | ||||||
| Gender | 0.120 | 0.306 | ||||||||
| Female | 58 | (69.0) | 30 | (83.3) | 2 | (50.0) | 71 | (73.2) | ||
| Male | 26 | (31.0) | 6 | (16.7) | 2 | (50.0) | 26 | (26.8) | ||
| Stage | 0.054 | 0.612 | ||||||||
| I | 62 | (73.8) | 31 | (86.1) | 3 | (75.0) | 77 | (79.4) | ||
| II | 2 | (2.4) | 3 | (8.3) | 0 | (0.0) | 5 | (5.2) | ||
| III | 14 | (17.6) | 1 | (2.8) | 1 | (25.0) | 10 | (10.3) | ||
| IV | 4 | (4.8) | 0 | (0.0) | 0 | (0.0) | 4 | (4.1) | ||
| Not available | 2 | (2.4) | 1 | (2.8) | 0 | (0.0) | 1 | (1.0) | ||
| Focality | 0.338 | 0.659 | ||||||||
| Unifocal | 54 | (64.3) | 24 | (66.7) | 2 | (50.0) | 61 | (62.9) | ||
| Multifocal | 29 | (34.5) | 10 | (27.8) | 2 | (50.0) | 33 | (34.0) | ||
| Not available | 1 | (1.2) | 2 | (5.6) | 0 | (0.0) | 3 | (3.1) | 0.592 | |
| Size | 0.071 | |||||||||
| ≤3 | 72 | (85.7) | 26 | (72.2) | 3 | (75.0) | 78 | (80.4) | ||
| >3 | 11 | (13.1) | 7 | (19.4) | 1 | (25.0) | 15 | (25.0) | ||
| Not available | 1 | (1.2) | 3 | (8.3) | 0 | (0.0) | 4 | (4.1) | ||
| Extrathyroidal extension | 0.324 | 1.000 | ||||||||
| Present | 34 | (40.5) | 13 | (36.1) | 2 | (50.0) | 40 | (41.2) | ||
| Absent | 49 | (58.3) | 21 | (58.3) | 2 | (50.0) | 54 | (55.7) | ||
| Not available | 1 | (1.2) | 2 | (5.6) | 0 | (0.0) | 3 | (3.1) | ||
| Lymphovascular invasion | 0.196 | 0.252 | ||||||||
| Present | 26 | (31.0) | 15 | (44.4) | 0 | (0.0) | 35 | (36.1) | ||
| Absent | 35 | (41.7) | 16 | (41.7) | 3 | (75.0) | 35 | (36.1) | ||
| Not available | 23 | (27.4) | 5 | (13.9) | 1 | (25.0) | 27 | (27.8) | ||
| Lymphnodes status | 1.000 | 0.026 | ||||||||
| Positive | 35 | (41.7) | 15 | (41.7) | 0 | (0.0) | 45 | (34.0) | ||
| Negative | 24 | (28.6) | 11 | (30.6) | 1 | (25.0) | 33 | (46.4) | ||
| Not available | 25 | (29.8) | 10 | (27.8) | 3 | (75.0) | 19 | (19.6) | ||
*Significant difference.
In PTMC and PTMC-FV, clinicopathologic parameters were not significantly correlated with neither BRAF nor NRAS mutations. However, there was a higher trend for BRAF mutation with multifocality in PTMC (Tables 3 and 4). Similarly, PTC-FV did not correlate with any clinicopathologic feature; however, we noticed that BRAF mutations were exclusive to tumors sizes smaller than or equal to 3 cm, absence of extrathyroidal extension, and absence of lymphovascular invasion, while NRAS mutations were exclusive to females and absence of extrathyroidal extension. (Fig. 3, Table 5).
Table 3.
Clinicopathological features of PTMC cases with respect to BRAF and NRAS mutations.
| Variables | BRAF mutation n (%) n = 54 | No BRAF mutation n (%) n = 21 | P-value | NRAS mutation n (%) n = 6 | No NRAS mutation n (%) n = 49 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.42 | 0.88 | ||||||||
| Mean ± SD | 46.6 ± 11.8 | 47.3 ± 14.9 | 45.0 ± 17.3 | 47.1 ± 11.6 | ||||||
| Gender | 0.330 | 0.298 | ||||||||
| Female | 43 | (79.6) | 19 | (90.5) | 4 | (66.7) | 41 | (83.7) | ||
| Male | 11 | (20.4) | 2 | (9.5) | 2 | (33.3) | 8 | (16.3) | ||
| Stage | 1.000 | 0.378 | ||||||||
| I | 50 | (92.6) | 20 | (95.2) | 5 | (83.3) | 46 | (93.9) | ||
| II | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| III | 3 | (5.6) | 1 | (4.8) | 1 | (16.7) | 2 | (4.1) | ||
| IV | 1 | (1.9) | 0 | (0.0) | 0 | (0.0) | 1 | (2.0) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Focality | 0.428 | 0.204 | ||||||||
| Unifocal | 32 | (59.3) | 15 | (71.4) | 5 | (83.3) | 25 | (51.0) | ||
| Multifocal | 22 | (40.7) | 6 | (28.6) | 1 | (16.7) | 24 | (49.0) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Extrathyroidal extension | 1.000 | 0.619 | ||||||||
| Present | 11 | (20.4) | 4 | (19.0) | 2 | (33.3) | 11 | (22.4) | ||
| Absent | 43 | (79.6) | 17 | (81.0) | 4 | (66.7) | 38 | (77.6) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Lymphovascular invasion | 0.850 | 0.339 | ||||||||
| Present | 2 | (3.7) | 0 | (0.0) | 0 | (0.0) | 1 | (2.0) | ||
| Absent | 46 | (85.2) | 18 | (85.7) | 4 | (66.7) | 41 | (83.7) | ||
| Not available | 6 | (11.1) | 3 | (14.3) | 2 | (33.3) | 7 | (14.3) | ||
| Lymphnodes status | 0.196 | 1.000 | ||||||||
| Positive | 9 | (16.7) | 2 | (9.5) | 1 | (16.7) | 7 | (14.3) | ||
| Negative | 14 | (25.9) | 2 | (9.5) | 1 | (16.7) | 13 | (26.5) | ||
| Not available | 31 | (57.4) | 17 | (81.0) | 4 | (66.7) | 29 | (59.2) | ||
*Significant difference.
Table 4.
Clinicopathological features of PTMC-FV cases with respect to BRAF and NRAS mutations.
| Variables | BRAF mutation n (%) n = 4 | No BRAF mutation n (%) n = 11 | P-value | NRAS mutation n (%) n = 5 | No NRAS mutation n (%) n = 8 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 1.000 | 0.724 | ||||||||
| Mean ± SD | 46.7 ± 14.7 | 47.3 ± 13.2 | 46.0 ± 14.2 | 44.4 ± 12.4 | ||||||
| Gender | 1.000 | 0.385 | ||||||||
| Female | 4 | (100.0) | 9 | (81.8) | 4 | (80.0) | 8 | (100.0) | ||
| Male | 0 | (0.0) | 2 | (18.2) | 1 | (20.0) | 0 | (0.0) | ||
| Stage | 0.476 | 1.000 | ||||||||
| I | 3 | (75.0) | 10 | (90.9) | 4 | (80.0) | 7 | (87.5) | ||
| II | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| III | 1 | (25.0) | 1 | (9.1) | 1 | (20.0) | 1 | (12.5) | ||
| IV | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Focality | 0.604 | 0.565 | ||||||||
| Unifocal | 3 | (75.0) | 6 | (54.5) | 4 | (80.0) | 4 | (50.0) | ||
| Multifocal | 1 | (25.0) | 5 | (45.5) | 1 | (20.0) | 4 | (50.0) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | ||||||
| Extrathyroidal extension | 0.476 | 1.000 | ||||||||
| Present | 1 | (25.0) | 1 | (9.1) | 1 | (20.0) | 1 | (12.5) | ||
| Absent | 3 | (75.0) | 10 | (90.9) | 4 | (80.0) | 7 | (87.5) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Lymphovascular invasion | 0.267 | NA | ||||||||
| Present | 1 | (25.0) | 0 | (0.0) | (0.0) | (0.0) | ||||
| Absent | 3 | (75.0) | 11 | (100.0) | 5 | (100.0) | 8 | (100.0) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Lymphnodes status | 1.000 | 1.000 | ||||||||
| Positive | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Negative | 1 | (25.0) | 2 | (18.2) | 1 | (20.0) | 2 | (25.0) | ||
| Not available | 3 | (75.0) | 9 | (81.8) | 4 | (80.0) | 6 | (75.0) | ||
*Significant difference.
Figure 3.
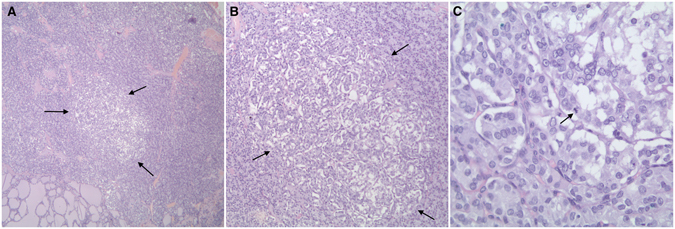
Histopathological examination of two PTC tissues. H&E staining (x100) shows A case of multifocal PTC-FV with a PTMC-FV focus that was positive for the NRAS mutation. (A) and (B) Note that the microscopic focus was unencapsulated (arrows, 40x and 100x). (C) Note the follicular architecture, irregular nuclei with clearing and grooves with a mitotic figure (arrows, 400x).
Table 5.
Clinicopathological features of PTC-FV cases with respect to BRAF and NRAS mutations.
| Variables | BRAF mutation n (%) n = 6 | No BRAF mutation n (%) n = 31 | P-value | NRAS mutation n (%) n = 6 | No NRAS mutation n (%) n = 18 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.952 | 0.974 | ||||||||
| Mean ± SD | 40.8 ± 17.4 | 45.5 ± 12.9 | 41.5 ± 11.7 | 41.4 ± 12.9 | ||||||
| Gender | 0.653 | 0.280 | ||||||||
| Female | 4 | (66.7) | 23 | (74.2) | 6 | (100.0) | 13 | (72.2) | ||
| Male | 2 | (33.3) | 8 | (25.8) | 0 | (0.0) | 5 | (27.8) | ||
| Stage | 1.000 | 1.000 | ||||||||
| I | 4 | (66.7) | 17 | (54.8) | 4 | (66.7) | 12 | (66.7) | ||
| II | 1 | (16.7) | 8 | (25.8) | 1 | (16.7) | 3 | (16.7) | ||
| III | 1 | (16.7) | 5 | (16.1) | 1 | (16.7) | 2 | (11.1) | ||
| IV | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Not available | 0 | (0.0) | 1 | (3.2) | 0 | (0.0) | 1 | (5.6) | ||
| Focality | 0.383 | 1.000 | ||||||||
| Unifocal | 4 | (66.7) | 13 | (41.9) | 2 | (33.3) | 8 | (44.4) | ||
| Multifocal | 2 | (33.3) | 18 | (58.1) | 4 | (66.7) | 10 | (55.6) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Size | 0.293 | 0.724 | ||||||||
| ≤3 | 6 | (100.0) | 20 | (64.5) | 4 | (66.7) | 13 | (72.2) | ||
| >3 | 0 | (0.0) | 10 | (32.3) | 2 | (33.3) | 4 | (22.2) | ||
| Not available | 0 | (0.0) | 1 | (3.2) | 0 | (0.0) | 1 | (5.6) | ||
| Extrathyroidal extension | 0.571 | 0.546 | ||||||||
| Present | 0 | (0.0) | 7 | (22.6) | 0 | (0.0) | 3 | (16.7) | ||
| Absent | 6 | (100.0) | 24 | (77.4) | 6 | (100.0) | 15 | (83.3) | ||
| Not available | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Lymphovascular invasion | 0.097 | 0.251 | ||||||||
| Present | 0 | (0.0) | 7 | (22.6) | 2 | (33.3) | 1 | (5.6) | ||
| Absent | 5 | (83.3) | 24 | (77.4) | 4 | (66.7) | 16 | (88.9) | ||
| Not available | 1 | (16.7) | 0 | (0.0) | 0 | (0.0) | 1 | (5.6) | ||
| Lymphnodes status | 0.307 | 1.000 | ||||||||
| Positive | 1 | (16.7) | 1 | (3.2) | 0 | (0.0) | 2 | (11.1) | ||
| Negative | 1 | (16.7) | 11 | (35.5) | 2 | (33.3) | 7 | (38.9) | ||
| Not available | 4 | (66.7) | 19 | (61.3) | 4 | (66.7) | 9 | (50.0) | ||
*Significant difference.
BRAF Mutational concordance between primary PTC and paired lymph nodes metastasis
BRAF mutations were concordant in the primary and its corresponding lymph node deposits in PTC with a kappa of 0.77 (p-value < 0.0001) (Fig. 4, Table 6). Agreement coefficients for mutational concordance between primary and paired lymph node deposits were not calculated for NRAS mutations due to the small number of NRAS mutated cases and their corresponding lymph node metastasis.
Figure 4.
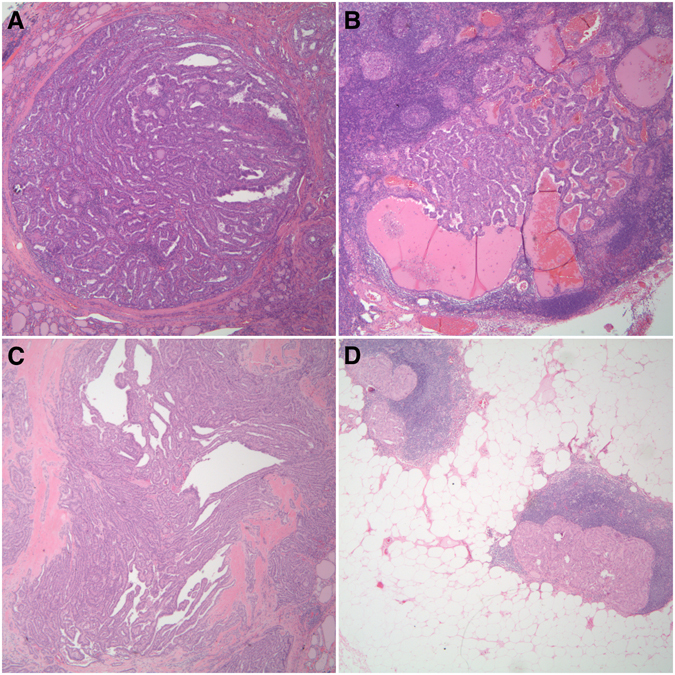
Histopathological examination of two PTC tissues. H&E staining (x100) shows (A) and (B) Representative case of mutant BRAF in primary cPTC (size = 3 cm and age = 28 years-old) and the corresponding paired lymph node metastasis (40x). (C) Primary PTMC (size = 0.7 cm and age = 33 years) with a mutant BRAF and (D) paired lymph node metastasis (40x).
Table 6.
Agreement in BRAF mutation in between primary PTC tumor and the corresponding metastatic lymph nodes.
| Primary PTC | Kappa (P-value) | |||
|---|---|---|---|---|
| No BRAFmutation | BRAFMutation | |||
| LN Metastasis | No BRAF mutation | 17 (94.4%) | 4 (16.0%) | 0.77 (<0.0001) |
| BRAF Mutation | 1 (5.6%) | 21 (84.0%) | ||
| Total | 18 | 25 | ||
Concordance in BRAF mutation between primary PTC and the corresponding metastatic lymph nodes.
Discussion
The current study evaluated the concordance rates of BRAF and NRAS mutations between primary PTC tumors and paired metastatic lymph node deposits of the four most common subtypes of PTC: cPTC, PTMC, PTMC-FV and PTC-FV. In addition, the mutational BRAF and NRAS statuses were correlated with the different clinicopathologic parameters.
BRAF and RAS mutations are the most common in PTC21, 22. In this series, we found that BRAF mutation incidence, approximated to be 60%, was closer to the higher edge of the worldwide reported range (36–69%), while NRAS was lower with approximately 11% vs. 30% reported in literature23–26. Comparably, we found that BRAF mutations were more prevalent than NRAS mutations in cPTC (56.8% vs. 50%) and PTMC (36.5% vs. 40%), whereas NRAS mutations showed a higher incidence than BRAF mutations in PTMC-FV (23.8%) and PTC-FV (28.6%). Interestingly, we identified a significantly elevated NRAS mutational frequency within PTMC (28.6%) similar to PTC-FV (28.6%); a finding higher than that reported by Schulten et al. (5.4%)27. Besides, among the 75 patients with PTMC evaluated in our cohort, 54 had BRAF mutation-positive (72% of PTMCs) while 21 had negative BRAF mutation (28% of PTMCs). Our results are in accordance with what has been reported in worldwide literature in this regards, where a study by Sun et al. showed that out of 86 PTMC cases, around 65% were positive for BRAF mutation28.
Clinicopathologic parameters’ correlation with BRAF and NRAS mutations is controversial among different studies12–14. In a cohort of 129 PTMCs tested for BRAF V600E mutation and their correlation with the clinicopathologic features of patients, results showed no significant differences in age, sex, tumor size, location, and multifocality between the BRAF V600E mutated and non-mutated microcarcinomas9. However, there was significantly higher prevalence of infiltrative tumor borders, tumor-associated stromal desmoplasia/fibrosis and/or sclerosis, classic nuclear features of PTC, and cystic change in mutated microcarcinomas9. Similarly, results from another study demonstrated significant association between BRAF mutation-positive tumors and the following features: infiltrative growth, stromal fibrosis, psammoma bodies, plump eosinophilic tumor cells, and classic fully developed nuclear features of PTC, but not other clinicopathological parameters24. In addition, BRAF mutational status has been correlated with recurrence of PTMCs, suggesting its importance in stratifying patients for surgical management28, 29. On the other hand, several papers concluded that BRAF positivity is not significantly associated with most clinicopathologic features redolent of aggressiveness, including tumor multicentricity, lymphovascular invasion, extranodal extension, central neck involvement, advanced stage (stage III or IV), and distant metastasis30, 31.
In our cases, the only significant clinicopathologic correlation found was between advanced age and BRAF mutation in cPTC (p < 0.005), a potential causal link between older age and an advanced stage disease presentation. While Rodolico et al. identified BRAF mutations in 41% of PTMCs and an association with a higher age (mean = 53 years) and lymph node metastasis32, we reported a frequency of 36.5% BRAF mutated cases in PTMCs but with no statistically significant correlation with the various clinicopathologic parameters. Yet, a trend towards higher incidence of BRAF mutation was found in patients with higher tumor stage (p = 0.054). That being said, the clinical benefit of selective molecular targeted therapy in aggressive and advanced stage PTMC is still questionable33.
PTC-FV, which was initially described by Lindsay et al.34 and categorized by Chem and Rosai due to the morphologic and biological overlap with PTC35, represents a unique molecular subgroup of PTC cases. At the molecular level, and in contrast to cPTC and PTMC, PTC-FV exhibits a RAS family mutation. The Cancer Genome Atlas clustered PTC into two main morphologically and molecularly distinct groups, namely BRAF driven and RAS mutated tumors36. Nikiforov et al. recommends that the encapsulated variant of PTC-FV is best classified as “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP) due to the low risk malignant behavior. Only cases with the infiltrative pattern retain the PTC-FV term37. One case of PTC-FV harbored lymph node metastasis and was negative for the BRAF or NRAS mutation, while none of the PTMC-FV cases exhibited lymph node metastasis. The literature on lymph node metastasis in PTC-FV varies greatly among different studies and ranges between14% and 94%38.
Locoregional lymph node metastasis in PTC may be found in up to 46.8%39. In high-risk patients, characterized by older age, tumor size >3 cm, and extracapsular extension, the number and size of lymph node metastasis affects prognosis and survival. Locoregional recurrence, with a follow-up of three decades, can reach up to 30%40–42. The current study showed a highly significant concordance rate of 84% for BRAF mutation in primary PTC and corresponding paired lymph node metastasis. Similarly, Walts et al. and Vasco et al. reported concordance rates of 95.2% and 81% respectively for primary PTCs and the corresponding paired metastatic lymph node deposits43, 44. This implies that BRAF mutation is conserved in both the primary and paired metastatic lymph nodes, thus supporting the hypothesis of a driver mutational role in the pathogenesis of PTC, particularly cPTC and PTMC, a finding reinforced by the genomic analysis of PTC via the Cancer Genome Atlas Research Network 36. Therefore, does BRAF testing predict central lymph node metastasis and an aggressive PTC phenotype? Actually, the positive predictive value and negative predictive values of BRAF mutational testing in PTC as a marker of central lymph node metastasis were estimated to be 47% and 91%, respectively45. Hence, the utility of BRAF as a prognostic marker may be confined to the cPTC subtype46.
Argumentatively, there is a potential role of selective molecular targeted therapy in recurrent and advanced metastatic PTC cases that are surgically unresectable and radioresistant. Phase II clinical trials utilizing Selumetinib, a tyrosine kinase inhibitor targeting BRAF mutations in PTC, were conducted without any significant survival benefit47. Currently, a study by Dadu et al. involving treatment of advanced cPTC stage disease exhibited a 47% partial response and a 53% stable disease over a minimal 6-month period48. The BRAF status of the paired lymph node deposits was not determined in the study by Dadu et al. An interesting prospective study may identify responders versus non-responders with respect to metastatic lymph node BRAF status. In our study, NRAS mutations within metastatic lymph nodes were detected only in cPTC and PTMC, but the numbers are too low to conclude a significant concordance rate in either.
This study carries a number of limitations that relate to the relatively small number of cases evaluated, especially PTMC-FV and PTC-FV cases, and accordingly data may not apply to the different subtypes of PTC. Besides, the study is also limited by being retrospective in nature.
Conclusion
In conclusion, BRAF mutation is conserved in the primary and paired metastatic lymph node deposits in cPTC and PTMC. Testing for the BRAF mutation within lymph nodes is recommended in order to identify responders to the selective tyrosine kinase inhibitors in advanced stage cPTC. The high prevalence of BRAF and NRAS in PTMC and PTMC-FV with the absent significant clinicopathologic correlation undermines the role of BRAF testing in such a predominantly curable malignant thyroid disease. Finally, NRAS and BRAF testing in PTC-FV comprise a potentially diagnostically reassuring result. Further prospective studies are required to assess BRAF status within primary and paired lymph nodes for patients treated with selective targeted therapy in advanced stage cPTC.
Acknowledgements
We would like to express our gratitude thanks to the Department of Pathology and Laboratory Medicine at AUBMC and HHUMC for their support in the conduction of this study. This research was supported by the Lebanese Council for Scientific Research (CNRS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
N.F. worked on study conception and design, and contributed to the writing of the hypothesis, data collection, pathological slides review, selection of tissue for the molecular analysis, and data analysis. M.J. worked also on the pathological slides review, data analysis, histology figures, and statistics. M.N., G.K., and C.O. performed the molecular analysis experiments and molecular data analyses. H.T. performed the statistical analyses. H.B. performed data analyses and worked on the figures illustrations. F.F. and T.A. collected part of the clinical data of patients as well. G.Z. worked on study concept and design. R.M. was responsible for the molecular data analysis, study supervision and conduction of the whole project. All authors contributed to the drafting of the manuscript, and critically revised and edited the manuscript prior to approving the final draft. All authors approved the final draft of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Najla Fakhruddin and Mark Jabbour contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Salim EI, et al. Cancer epidemiology and control in the arab world-past, present and future. Asian Pac J Cancer Prev. 2009;10:3–16. [PubMed] [Google Scholar]
- 3.LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24:S1–S9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 4.Carcangiu ML, et al. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer. 1985;55:805–828. doi: 10.1002/1097-0142(19850215)55:4<805::AID-CNCR2820550419>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab. 2012;97:4559–4570. doi: 10.1210/jc.2012-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing M, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. Jama. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers MB, McKim KL, Parsons BL. A subset of papillary thyroid carcinomas contain KRAS mutant subpopulations at levels above normal thyroid. Mol Carcinog. 2014;53:159–167. doi: 10.1002/mc.21953. [DOI] [PubMed] [Google Scholar]
- 8.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 9.Virk RK, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype-phenotype correlation. Mod Pathol. 2013;26:62–70. doi: 10.1038/modpathol.2012.152. [DOI] [PubMed] [Google Scholar]
- 10.Prescott JD, et al. BRAF V600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery. 2012;152:984–990. doi: 10.1016/j.surg.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elisei R, et al. The BRAF V600E mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012;97:4390–4398. doi: 10.1210/jc.2012-1775. [DOI] [PubMed] [Google Scholar]
- 12.Nikiforova MN, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 13.Trovisco V, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch. 2005;446:589–595. doi: 10.1007/s00428-005-1236-0. [DOI] [PubMed] [Google Scholar]
- 14.Eloy C, Santos J, Soares P, Sobrinho-Simões M. The preeminence of growth pattern and invasiveness and the limited influence of BRAF and RAS mutations in the occurrence of papillary thyroid carcinoma lymph node metastases. Virchows Arch. 2011;459:265–276. doi: 10.1007/s00428-011-1133-7. [DOI] [PubMed] [Google Scholar]
- 15.Chung SY, et al. Cytomorphological factors and BRAF mutation predicting risk of lymph node metastasis in preoperative liquid-based fine needle aspirations of papillary thyroid carcinoma. Acta Cytol. 2013;57:252–258. doi: 10.1159/000343617. [DOI] [PubMed] [Google Scholar]
- 16.Rossi ED, et al. BRAF (V600E) mutation analysis on liquid‐based cytology‐processed aspiration biopsies predicts bilaterality and lymph node involvement in papillary thyroid microcarcinoma. Cancer Cytopathol. 2013;121:291–297. doi: 10.1002/cncy.21258. [DOI] [PubMed] [Google Scholar]
- 17.Barbaro D, et al. The BRAF V600E mutation in papillary thyroid cancer with positive or suspected pre-surgical cytological finding is not associated with advanced stages or worse prognosis. Endocrine. 2014;45:462–468. doi: 10.1007/s12020-013-0029-5. [DOI] [PubMed] [Google Scholar]
- 18.Xing M, et al. BRAF V600E and TERT Promoter Mutations Cooperatively Identify the Most Aggressive Papillary Thyroid Cancer With Highest Recurrence. Journal of Clinical Oncology. 2014;32:2718–2726. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, et al. TERT Promoter Mutations and Their Association with BRAF V600E Mutation and Aggressive Clinicopathological Characteristics of Thyroid Cancer. The Journal of Clinical Endocrinology and Metabolism. 2014;99:E1130–E1136. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roch, H.-L. An Open-Label, Multi-Center Phase II Study of the BRAF Inhibitor Vemurafenib in Patients With Metastatic or Unresectable Papillary Thyroid Cancer (PTC) Positive for the BRAF V600 Mutation and Resistant to Radioactive Iodine http://clinicaltrials.gov (2011).
- 21.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nature reviews. Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ming J, et al. Association between BRAF and RAS mutations, and RET rearrangements and the clinical features of papillary thyroid cancer. International Journal of Clinical and Experimental Pathology. 2015;8:15155–15162. [PMC free article] [PubMed] [Google Scholar]
- 23.Xing M. BRAF mutation in thyroid cancer. Endocrine-related cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein A, et al. Papillary thyroid carcinomas with and without BRAF V600E mutations are morphologically distinct. Histopathology. 2012;60:1052–1059. doi: 10.1111/j.1365-2559.2011.04149.x. [DOI] [PubMed] [Google Scholar]
- 25.Yip, L. et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: a study of 1510 patients. Annals of surgery262, 519-525 discussion 524-515 doi:10.1097/sla.0000000000001420 (2015). [DOI] [PMC free article] [PubMed]
- 26.Penna, G. C. et al. Molecular Markers Involved in Tumorigenesis of Thyroid Carcinoma: Focus on Aggressive Histotypes. Cytogenetic and genome research doi:10.1159/000456576 (2017). [DOI] [PubMed]
- 27.Schulten H-J, et al. Comprehensive survey of HRAS, KRAS, and NRAS mutations in proliferative thyroid lesions from an ethnically diverse population. Anticancer Res. 2013;33:4779–4784. [PubMed] [Google Scholar]
- 28.Sun Y, et al. Correlation between the BRAF(v600E) gene mutation and factors influencing the prognosis of papillary thyroid microcarcinoma. International Journal of Clinical and Experimental Medicine. 2015;8:22525–22528. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, et al. BRAF(V600E) Is Correlated with Recurrence of Papillary Thyroid Microcarcinoma: A Systematic Review, Multi-Institutional Primary Data Analysis, and Meta-Analysis. Thyroid. 2016;26:248–255. doi: 10.1089/thy.2015.0391. [DOI] [PubMed] [Google Scholar]
- 30.Gouveia C, et al. Lack of association of braf mutation with negative prognostic indicators in papillary thyroid carcinoma: The university of california, san francisco, experience. JAMA Otolaryngology–Head & Neck Surgery. 2013;139:1164–1170. doi: 10.1001/jamaoto.2013.4501. [DOI] [PubMed] [Google Scholar]
- 31.Lee DY, et al. Predicting Extrathyroidal Extension in Patients With Papillary Thyroid Microcarcinoma According to a BRAF Mutation. Clin Exp Otorhinolaryngol. 2017;10:174–181. doi: 10.21053/ceo.2015.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodolico V, et al. BRAFV600E mutation and p27kip1 expression in papillary carcinomas of the thyroid ≤1 cm and their paired lymph node metastases. Cancer. 2007;110:1218–1226. doi: 10.1002/cncr.22912. [DOI] [PubMed] [Google Scholar]
- 33.Kuo EJ, Goffredo P, Sosa JA, Roman SA. Aggressive variants of papillary thyroid microcarcinoma are associated with extrathyroidal spread and lymph-node metastases: a population-level analysis. Thyroid. 2013;23:1305–1311. doi: 10.1089/thy.2012.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsay S. Carcinoma of the Thyroid Gland: A Clinical and Pathologic Study of 293 Patients at the University of California Hospital. Calif Med. 1960;93:261–261. [Google Scholar]
- 35.Chem KT, Rosai J. Follicular variant of thyroid papillary carcinoma: a clinicopathologic study of six cases. Am J Surg Pathol. 1977;1:123–130. doi: 10.1097/00000478-197706000-00003. [DOI] [PubMed] [Google Scholar]
- 36.The Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikiforov YE, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA oncology. 2016;2:1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanchard C, et al. Factors predictive of lymph node metastasis in the follicular variant of papillary thyroid carcinoma. Br J Surg. 2013;100:1312–1317. doi: 10.1002/bjs.9210. [DOI] [PubMed] [Google Scholar]
- 39.Scheumann GF, et al. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994;18:559–567. doi: 10.1007/BF00353765. [DOI] [PubMed] [Google Scholar]
- 40.Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139–148. doi: 10.1016/S0039-6060(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 41.Shah PK, et al. Regional recurrence after lymphadenectomy for clinically evident lymph node metastases from papillary thyroid cancer: a cohort study. Ann Surg Oncol. 2012;19:1453–1459. doi: 10.1245/s10434-011-1890-1. [DOI] [PubMed] [Google Scholar]
- 42.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 43.Walts AE, Pao A, Sacks W, Bose S. BRAF genetic heterogeneity in papillary thyroid carcinoma and its metastasis. Hum Pathol. 2014;45:935–941. doi: 10.1016/j.humpath.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Vasko V, et al. High prevalence and possible de novo formation of BRAF mutation in metastasized papillary thyroid cancer in lymph nodes. J Clin Endocrinol Metab. 2005;90:5265–5269. doi: 10.1210/jc.2004-2353. [DOI] [PubMed] [Google Scholar]
- 45.Howell GM, et al. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20:47–52. doi: 10.1245/s10434-012-2611-0. [DOI] [PubMed] [Google Scholar]
- 46.Henke LE, et al. BRAF mutation is not predictive of long‐term outcome in papillary thyroid carcinoma. Cancer Med. 2015;4:791–799. doi: 10.1002/cam4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayes DN, et al. Phase II efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18:2056–2065. doi: 10.1158/1078-0432.CCR-11-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dadu R, et al. Efficacy and tolerability of vemurafenib in patients with BRAFV600E-positive papillary thyroid cancer: MD Anderson Cancer Center off label experience. J Clin Endocrinol Metab. 2014;100:E77–E81. doi: 10.1210/jc.2014-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]


