ABSTRACT
Johne's disease, a chronic gastrointestinal inflammatory disease caused by Mycobacterium avium subspecies paratuberculosis, is endemic in dairy cattle and other ruminants worldwide and remains a challenge to diagnose using traditional serological methods. Given the close phylogenetic relationship between M. avium subsp. paratuberculosis and the human pathogen Mycobacterium tuberculosis, here, we applied a whole-proteome M. tuberculosis protein array to identify seroreactive and diagnostic M. avium subsp. paratuberculosis antigens. A genome-scale pairwise analysis of amino acid identity levels between orthologous proteins in M. avium subsp. paratuberculosis and M. tuberculosis showed an average of 62% identity, with more than half the orthologous proteins sharing >75% identity. Analysis of the M. tuberculosis protein array probed with sera from M. avium subsp. paratuberculosis-infected cattle showed antibody binding to 729 M. tuberculosis proteins, with 58% of them having ≥70% identity to M. avium subsp. paratuberculosis orthologs. The results showed that only 4 of the top 40 seroreactive M. tuberculosis antigens were orthologs of previously reported M. avium subsp. paratuberculosis antigens, revealing the existence of a large number of previously unrecognized candidate diagnostic antigens. Enzyme-linked immunosorbent assay (ELISA) testing of 20 M. avium subsp. paratuberculosis recombinant proteins, representing reactive and nonreactive M. tuberculosis orthologs, further confirmed that the M. tuberculosis array has utility as a screening tool for identifying candidate antigens for Johne's disease diagnostics. Additional ELISA testing of field serum samples collected from dairy herds around the United States revealed that MAP2942c had the strongest seroreactivity with Johne's disease-positive samples. Collectively, our studies have considerably expanded the number of candidate M. avium subsp. paratuberculosis proteins with potential utility in the next generation of rationally designed Johne's disease diagnostic assays.
KEYWORDS: antigens, Mycobacterium, protein array
INTRODUCTION
Johne's disease is a chronic intestinal disease caused by infections in animals that are exposed to Mycobacterium avium subspecies paratuberculosis early in life (1). Despite the significant economic losses associated with Johne's disease in dairy cattle and sheep, progress in controlling infection has been significantly impeded by the lack of reliable and easy to use tests for detecting early infection. Over time, this results in infected animals shedding M. avium subsp. paratuberculosis into the environment and transmitting disease while appearing healthy. Extant enzyme immunoassays apply cumbersome and antiquated approaches to preparing immunodiagnostic antigens that comprise whole-cell M. avium subsp. paratuberculosis extracts. Thus, by diluting sensitive and specific antigens buried within these complex extracts, the resulting assays have low levels of sensitivity for the detection of animals at early stages of infection. To address this shortcoming, we have initiated a program of protein antigen discovery based on the complete genome sequence of M. avium subsp. paratuberculosis.
The search continues for ideal antigens that can be used in antibody-based serological tests to control many infectious diseases. Animal producers want a test that predicts infection accurately and early, while private industry will only develop cheap, marketable tests. The latter eliminates DNA-based tests, and thus, researchers have focused on antibody-based tests because of lower cost. The use of purified recombinant proteins as antigens that specifically detect M. avium subsp. paratuberculosis will be among the critical diagnostic tools in Johne's disease detection, especially in the early, subclinical stages of disease. Our group has previously used protein arrays to screen for seroreactive antigens during early (subclinical) and late (clinical) stages of Johne's disease (2). Animals appear healthy in the subclinical stage, but they shed small numbers of bacteria in their feces intermittently, thus serving as a transmission source for herd mates. Animals in the clinical stage show disease signs, including weight loss, diarrhea, and consistent fecal shedding of bacteria. However, it can take several years for clinical signs to appear, making transmission difficult to stop in herds.
The M. avium subsp. paratuberculosis protein microarray is a tool that allows simultaneous determination of antibody responses to each spotted protein using only a small amount of serum and provides a fast, efficient approach to identify the most immunodominant proteins for low-cost diagnosis of Johne's disease. Furthermore, the immunodominant proteins identified by this approach may then be used to develop M. avium subsp. paratuberculosis peptide-based enzyme-linked immunosorbent assays (ELISAs) that identify infected animals in both clinical and subclinical stages of disease with high sensitivity and specificity. M. avium subsp. paratuberculosis protein arrays were previously constructed from a collection of greater than 600 expressed and purified M. avium subsp. paratuberculosis recombinant proteins (3). Early antigens were identified using an experimental infection model to track the developing humoral immune response in calves. Three antigens were identified for which antibodies were detected in calves by 70 days postinfection (4). Antigens during the later stages of Johne's disease were also identified in naturally infected cattle (2). However, these antigens are only the best of the subproteome represented on the protein array. The question that remains is, are they the best in the entire proteome?
Even if all of the recombinant M. avium subsp. paratuberculosis proteins that are currently available were spotted and analyzed on protein arrays, they would still comprise less than 20% of the predicted M. avium subsp. paratuberculosis proteome (n = 4,350), demonstrating that a large fraction of potential antigen candidates have yet to be screened. Given the time and costs associated with cloning, expressing, and purifying additional proteins from M. avium subsp. paratuberculosis, we explored the possibility of leveraging the availability of the whole-proteome microarray from Mycobacterium tuberculosis to screen for antigens useful in Johne's disease diagnostics. M. tuberculosis is the causative agent of human tuberculosis (TB) and is a related pathogen belonging to the same genus as M. avium subsp. paratuberculosis. The M. tuberculosis protein array has over 4,000 proteins spotted, which covers 99% of the M. tuberculosis proteome (5). This novel approach is the most ambitious unbiased screen of antigens ever undertaken for Johne's disease.
An M. tuberculosis proteome array was constructed and used previously to determine changes in the humoral immune response in patients with TB (5). Moreover, the array has been successfully applied to biomarker identification of active M. tuberculosis infection in a global collection of human serum and plasma samples (5). The principal finding was the identification of the immunoproteome, a set of antigens comprising 10% of the M. tuberculosis proteome that was highly reactive in M. tuberculosis-exposed individuals. Systems immunology analysis yielded a smaller subset of antigens associated with active M. tuberculosis infections with primarily extracellular secretory functions. Diagnostic targets were further downselected after integrated analysis of humans and experimentally infected cynomolgus macaques (5). Collectively, these data led us to examine the utility of the M. tuberculosis protein array for Johne's disease antibody screening.
RESULTS
Genome comparison of M. avium complex and M. tuberculosis complex strains.
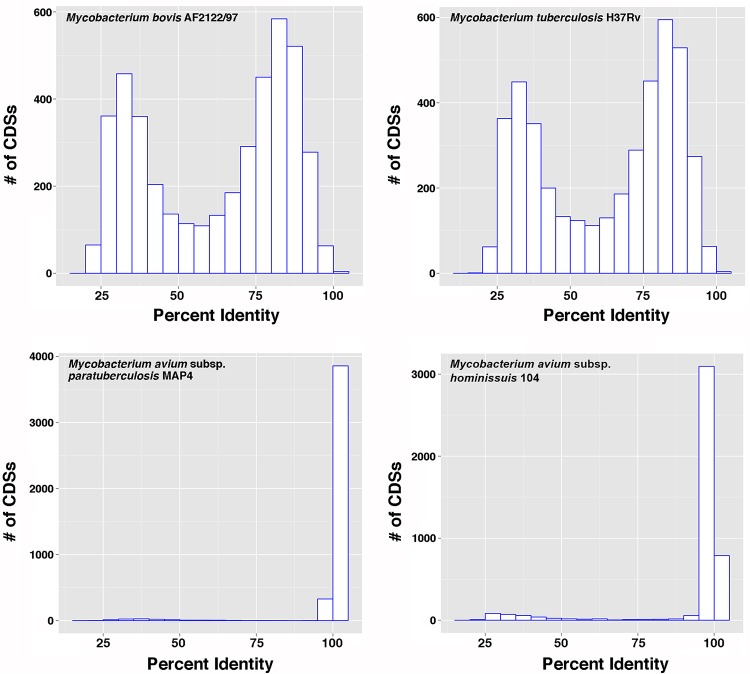
The M. avium complex (MAC) consists of eight species, including M. avium (6). Within the M. avium species are four subspecies, M. avium subsp. hominissuis, M. avium subsp. avium, M. avium subsp. silvaticum, and M. avium subsp. paratuberculosis. All four subspecies are virtually identical at the genomic level (7, 8). Likewise, species within the M. tuberculosis complex are all highly similar based on genomic and proteomic comparisons (9). However, these two mycobacterial complexes are more distantly related to each other and are easily distinguishable. Thus, before investing in a strategy to leverage the M. tuberculosis protein array for Johne's disease studies, a genome scale comparative pairwise analysis of amino acid identities between orthologous MAC and M. tuberculosis complex proteins was performed. Analysis of Mycobacterium bovis and M. tuberculosis species from the M. tuberculosis complex showed a biphasic histogram with an average of 62% identity (range, 19% to 100%) among the protein-encoding genes with M. avium subsp. paratuberculosis strain K-10, and more than half of the orthologous proteins shared greater than 75% identity (Fig. 1), whereas the majority of coding DNA sequences (CDSs) from the MAP4 strain were at 100% identity and M. avium subsp. hominissuis strain 104 was at 95% percent identity or greater (Fig. 1). A complete list of percentages of identity of CDSs for sequenced mycobacterial genomes, using the MAP4 genome (10) as a reference, is presented in Table S1 in the supplemental material. Further bioinformatics analyses confirmed that the M. tuberculosis protein array contained more than 800 orthologs of M. avium subsp. paratuberculosis proteins that have been previously expressed (3). An additional 1,898 M. tuberculosis proteins with >60% identity to M. avium subsp. paratuberculosis orthologs are present on the M. tuberculosis array but are not among the M. avium subsp. paratuberculosis proteins that have been previously analyzed on a protein array (2, 4). Collectively, these data strengthened the hypothesis that the M. tuberculosis protein array might serve as a useful tool for screening over 1,800 additional candidate antigens for Johne's disease detection.
FIG 1.
Distribution of pairwise comparisons of M. avium subsp. paratuberculosis strain K-10 with the mycobacterial species indicated on each graph. The histogram bars represent the frequency of CDS orthologs at a given percent identity.
Protein array analysis of Johne's disease serum samples.
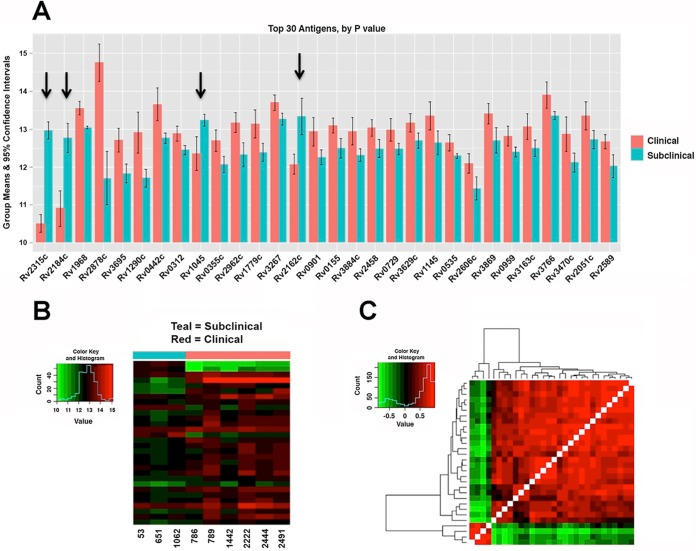
To directly test the applicability of the M. tuberculosis array, 9 serum samples from cows with Johne's disease (6 clinical and 3 subclinical) and 3 serum samples from healthy control cows were probed using the M. tuberculosis whole-proteome array. For comparison, an M. avium subsp. paratuberculosis subproteomic array was also exposed to a subset of these serum samples. The log2-transformed signal intensities of both the M. tuberculosis and M. avium subsp. paratuberculosis arrays are shown as box and whisker plots in Fig. 2. The raw and normalized signal intensities of the M. tuberculosis protein array are shown in Fig. S1 in the supplemental material. The results revealed a total of 729 M. tuberculosis antigens that were reactive to at least one of the serum samples from cows with Johne's disease and had a mean intensity greater than 10 (see Table S2 in the supplemental material), 552 of which had an M. avium subsp. paratuberculosis ortholog with identity above 70%, suggesting that this approach has identified hundreds of new seroreactive M. avium subsp. paratuberculosis orthologs as potential antigen candidates for future analysis. The top 30 M. tuberculosis proteins showing differential antibody responses between cows in clinical and subclinical stages of disease are shown in Fig. 3A with additional information about each protein listed in Table S3 in the supplemental material. While most antigens had stronger antibody responses in cows in the clinical stage of disease, four of the proteins showed significantly greater reactivity with antibodies from animals in the subclinical stage of disease, suggesting potential early detection antigens (Fig. 3A). Three of these proteins are annotated as hypothetical proteins (Rv1045, Rv2184c, and Rv2315c), while Rv2162c is a PE-PGRS family protein (11). Collectively, antibody reactivity is stronger for animals in the clinical stage of disease than for animals in the subclinical stage of disease (Fig. 3B), and correlation of antibody responses is strong overall, but the four proteins mentioned above (Rv1045, Rv2315c, Rv2184c, and Rv2162c) lacked correlated responses, which may be indicative of unique diagnostic markers (Fig. 3C).
FIG 2.
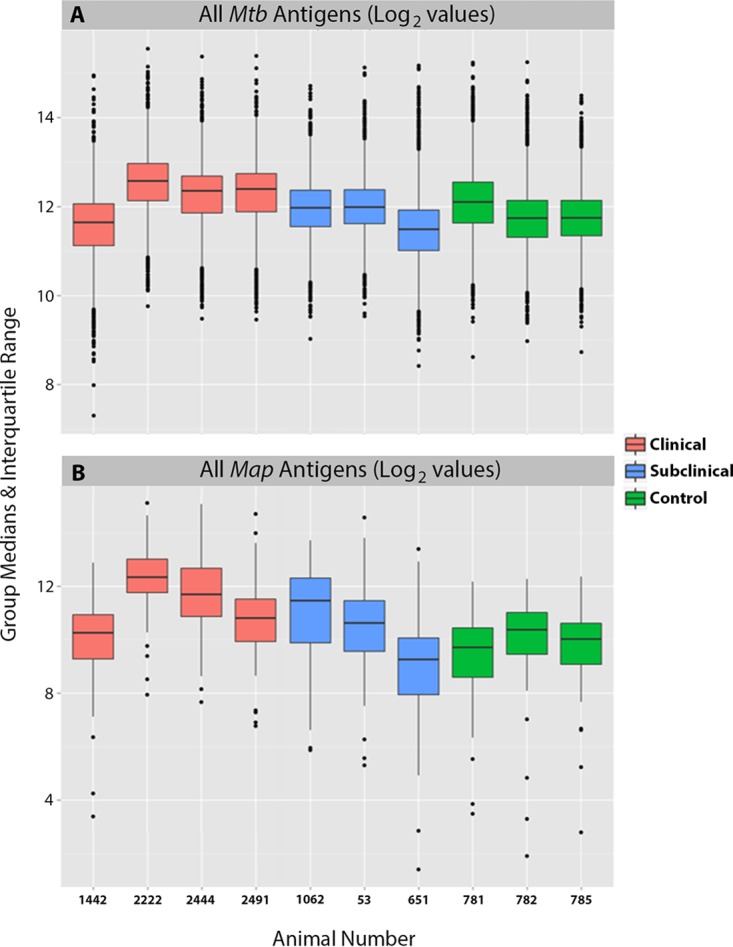
Box plots showing the overall distribution of antibody responses to M. tuberculosis and M. avium subsp. paratuberculosis proteins. Log2-transformed signal intensities for each animal are shown in the box plots for M. tuberculosis proteins (A) and M. avium subsp. paratuberculosis proteins (B). The black horizontal bars, boxes, whiskers, and dots indicate the medians, ranges, 1.5 times the interquartile ranges, and outliers, respectively. The boxes are colored based on the health status of the animals.
FIG 3.
M. tuberculosis proteins showing differential antibody reactivity by disease state. (A) Differences among the top 30 antigens between cows in the clinical and subclinical stages of disease ordered from left (lowest P value) to right (highest P value, based on Student's t test). See Table S3 in the supplemental material for confidence intervals, P values, and adjusted P values. The bars represent the means of log2-transformed data, and the error bars represent 95% confidence intervals. The arrows mark candidate antigens that are higher in animals in the subclinical stage of disease. (B) Heat map of the log2-transformed signals for the top 30 antigens ordered by subclinical and clinical groups. Serum sample numbers are along the bottom, and genes are stacked in the same order as in panel A. (C) Hierarchical clustering of the correlations between antigen reactivities. Red shows highly correlated responses, and green is uncorrelated responses, which may highlight diagnostic markers.
Several M. tuberculosis proteins that showed the strongest antibody reactivity with Johne's disease serum were also orthologous to known M. avium subsp. paratuberculosis antigens. For example, the strongest antibody response among cows with clinical Johne's disease was that of Rv1860, a proline-rich secreted protein showing a mean signal intensity of 15.3 (see Table S2 in the supplemental material). The M. avium subsp. paratuberculosis ortholog of this protein is MAP1569, which has been observed as a strong antigen in multiple serological screening approaches (12–15). The second strongest antigen among the cows in the clinical stage of disease was Rv2878c, which is homologous to MAP2942c, another known antigen in M. avium subsp. paratuberculosis (16). Other published M. avium subsp. paratuberculosis antigens identified as orthologs in Table S2 are MAP0210c (16), MAP0900 (2), and MAP2121c (17). However, M. avium subsp. paratuberculosis orthologs for most of the M. tuberculosis antigens identified in this study have not been described in the literature, suggesting they are new candidate diagnostic antigens for Johne's disease. Finally, 6 of the top 10 proteins identified from the clinical disease stage were also ranked among the top 10 in the subclinical disease stage (see Table S2), suggesting that these antigens may be detected throughout the progression of this chronic disease.
The top 20 proteins with the highest infected-to-negative ratios on the M. avium subsp. paratuberculosis partial protein array are shown in Table 1. A 6.40 clinical-to-negative ratio was observed for MAP0856c, the gene for which is present on a genomic island unique to M. avium subsp. paratuberculosis (18, 19), making it a particularly interesting diagnostic candidate. The strongest ratio overall is 14.39 from MAP2121c, a membrane protein that has been suggested as an antigen in the past (17, 20, 21) and whose ortholog (Rv3347c) was ranked 33rd on the M. tuberculosis array (see Table S2 in the supplemental material). Other known M. avium subsp. paratuberculosis antigens that appear among the top 10 clinical-to-negative ratios in Table 1 are MAP2942c (16) and MAP1272c (22). The top 20 M. tuberculosis proteins with the highest ratios are listed in Table S4 in the supplemental material.
TABLE 1.
M. avium subsp. paratuberculosis proteins with the highest infection-to-control ratios
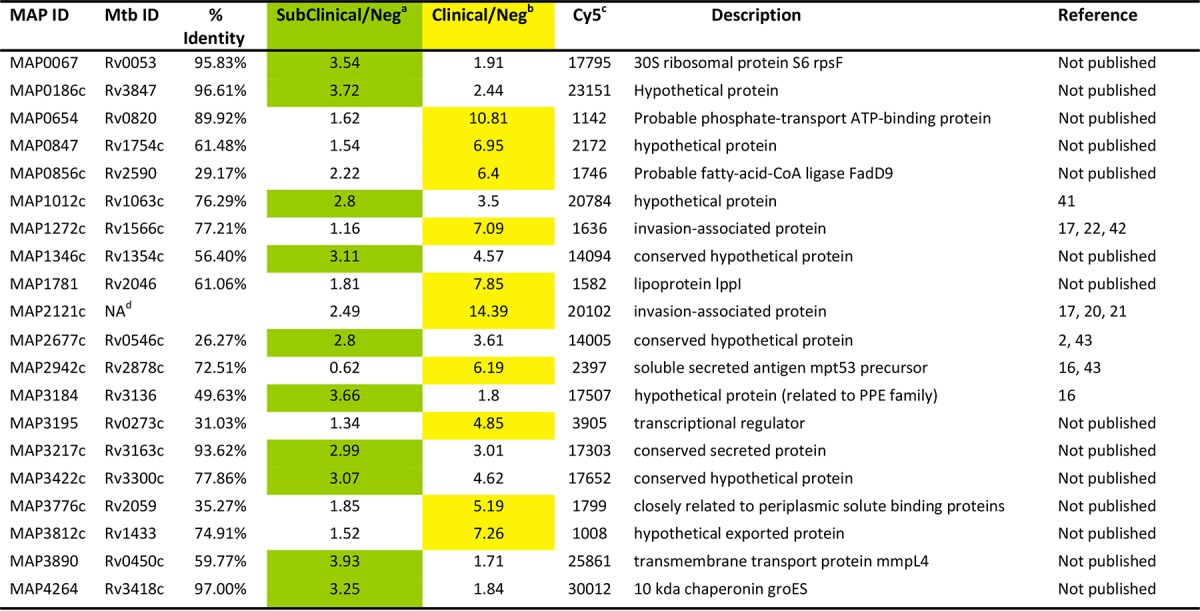
Ratio of average intensities of sera from cows in the subclinical stage of disease to the average intensity of sera from negative cows. Green highlighting shows antigens with stronger subclinical antibody responses than clinical antibody responses.
Ratio of average intensities of sera from cows in the clinical stage of disease to the average intensity of sera from negative cows. Yellow highlighting shows antigens with stronger clinical antibody responses than subclinical antibody responses.
Cy5–anti-MBP used to determine spot intensities/local correction of signal.
NA, no ortholog in M. tuberculosis (Mtb).
Correlation of M. avium subsp. paratuberculosis and M. tuberculosis proteins.
Correlation between the seroreactivity of antigens on the M. avium subsp. paratuberculosis protein array and orthologs on the M. tuberculosis array was next examined. By taking the log2-transformed data, Pearson's rho was 0.25 among all unique matches, suggesting poor correlation (Fig. 4A). However, correlation among the top antigens identified on the M. avium subsp. paratuberculosis and M. tuberculosis arrays was stronger, with a value of 0.41 and good clustering even among proteins that had relatively low levels of identity (Fig. 4B). Although preliminary, these data suggest that M. tuberculosis orthologs on the M. tuberculosis arrays react to sera from M. avium subsp. paratuberculosis-infected cows in a manner similar to what is observed with the purified proteins spotted on the M. avium subsp. paratuberculosis array.
FIG 4.
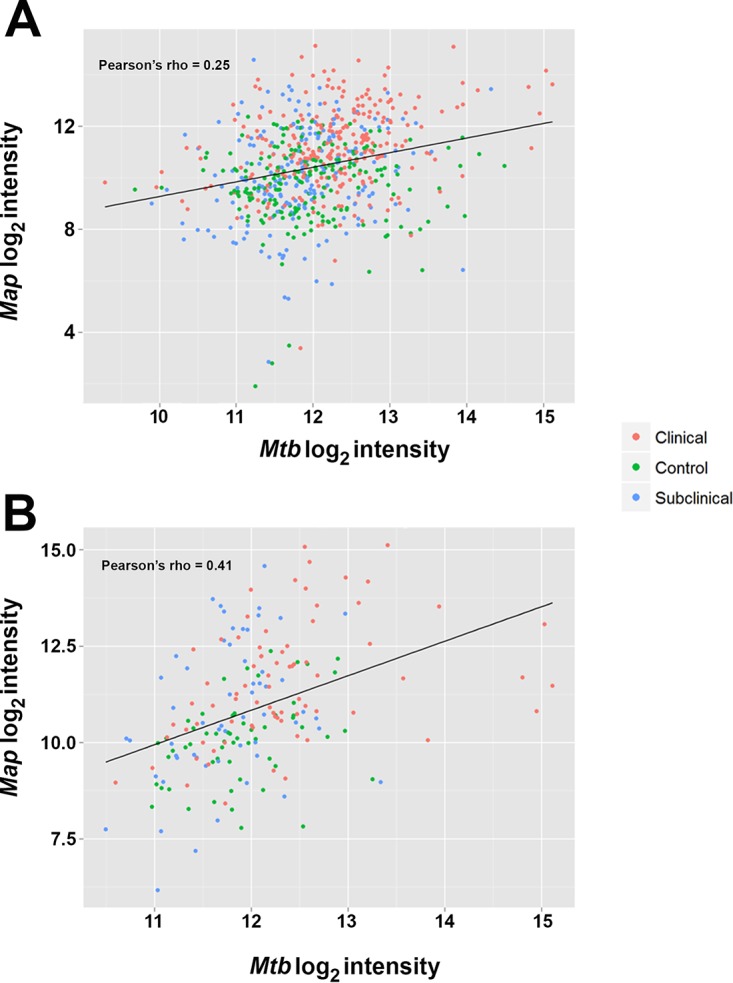
Correlation between M. avium subsp. paratuberculosis (Map) and M. tuberculosis (Mtb) proteins. (A) All orthologous genes. (B) The top antigens. Pearson's correlation is indicated in each plot.
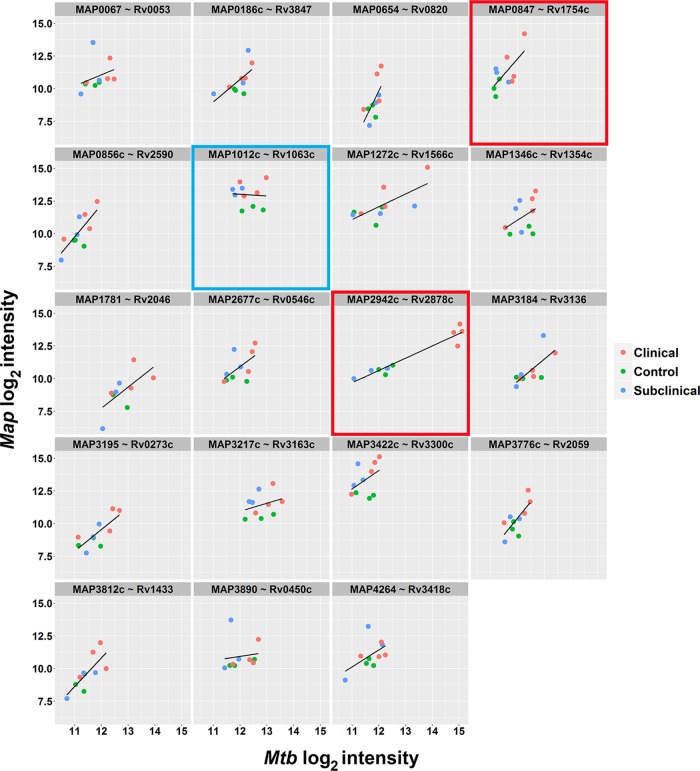
The distribution of reactivity for the top M. avium subsp. paratuberculosis antigens listed in Table 1, along with the corresponding M. tuberculosis proteins, was examined at the antigen level, along with how they correlated among disease statuses (Fig. 5). Representative subplots are highlighted and show antigens that display good seroreactive correlation with both M. avium subsp. paratuberculosis and M. tuberculosis proteins (Fig. 5, red boxes), as well as examples where the M. avium subsp. paratuberculosis antigen was a better predictor for clinical disease (Fig. 5; blue box). Those that displayed good correlation also had high amino acid identity. For example, the M. avium subsp. paratuberculosis protein MAP4264 was 97% identical to Rv3418c in M. tuberculosis (Fig. 5).
FIG 5.
Correlation of seroreactive M. avium subsp. paratuberculosis antigens with M. tuberculosis proteins. The lattice plots show log2-transformed data for M. avium subsp. paratuberculosis antibody responses (y axis) versus M. tuberculosis antibody responses (x axis). Each subplot represents one of the top reactive M. avium subsp. paratuberculosis antigens (from Table 1) with the corresponding orthologous M. tuberculosis antigen. Individual subjects are plotted for each antigen combination and color coded by clinical group. A line of best fit is drawn for each subplot. The plots boxed in red indicate good seroreactive correlation with both M. avium subsp. paratuberculosis and M. tuberculosis proteins. The plot boxed in blue indicates where M. avium subsp. paratuberculosis antigen was the best predictor of Johne's disease. MAP2121c is missing because there is no M. tuberculosis ortholog for the gene.
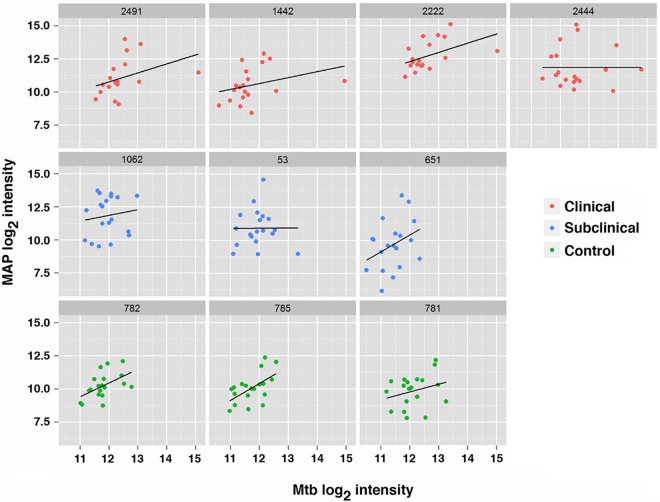
Next, the distribution of reactivity for the antigens listed in Table 1 at the individual animal level from the M. avium subsp. paratuberculosis array and their correlation with the M. tuberculosis array were examined (Fig. 6). The results show, on average, good correlation between the two arrays for each individual animal, as indicated by positive slopes. However, two animals (53 and 2444) showed poor correlation, with slopes near zero (Fig. 6). Collectively, these results suggest that the M. tuberculosis arrays will provide a mechanism for the triage of M. avium subsp. paratuberculosis orthologs, especially those with high identity but that are not seroreactive with sera from infected animals or those that appear to be equally reactive with negative sera and infected cows, representing targets that can be excluded from the list that may be predictive from an M. avium subsp. paratuberculosis diagnostic assay development standpoint.
FIG 6.
Correlation of the top seroreactive M. avium subsp. paratuberculosis antigens with M. tuberculosis proteins analyzed by individual cow. The lattice plots show log2-transformed data for M. avium subsp. paratuberculosis antibody responses (y axis) against antibody responses to M. tuberculosis proteins (x axis). Each subplot represents the responses from one study cow, and the dots represent the top reactive M. avium subsp. paratuberculosis antigens with the corresponding orthologous M. tuberculosis antigens. Individual subjects are plotted for each antigen combination and color coded by clinical group. A line of best fit is drawn for each subplot. MAP2121c was not included because there is no M. tuberculosis ortholog for the gene.
Reactivity of M. avium subsp. paratuberculosis orthologs by ELISA.
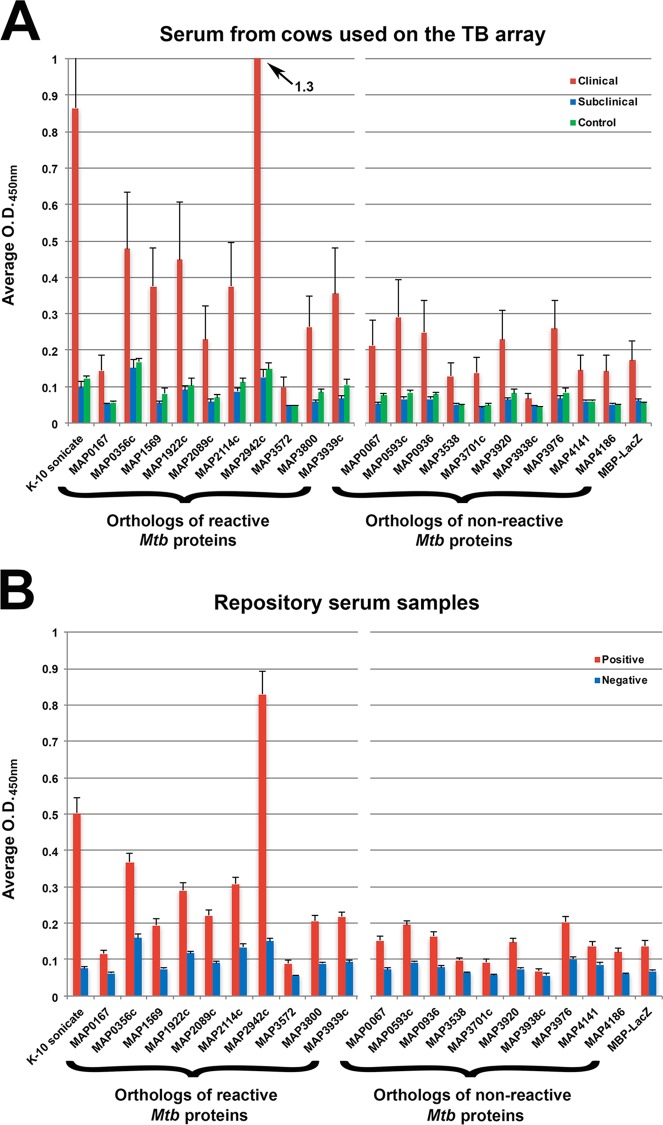
To test the ability of the M. tuberculosis protein array to discriminate and rank strong antigens for Johne's disease antibody tests, 10 M. avium subsp. paratuberculosis recombinant protein orthologs corresponding to M. tuberculosis reactive proteins (see Table S2 in the supplemental material) were selected, along with 10 recombinant protein orthologs corresponding to low-reactive or nonreactive M. tuberculosis proteins (not listed in Table S2). ELISAs with the same serum samples from the protein array study were used to evaluate these 20 M. avium subsp. paratuberculosis proteins. MAP2942c was clearly the strongest antigen among the proteins, with an average optical density (OD) reading of 1.3 (Fig. 7A). This was also the only recombinant protein that demonstrated higher reactivity than the whole-cell extract (Fig. 7A, K-10 sonicate). The M. tuberculosis ortholog for the protein is Rv2878c, which ranked second among all the antigens tested with sera from cows with clinical disease (Table S2).
FIG 7.
The humoral immune response against M. avium subsp. paratuberculosis recombinant proteins confirms strong antigens identified from the M. tuberculosis array. Serum immunoglobin levels from healthy and Johne's disease cattle were analyzed by ELISA. Shown are the mean optical density values for each protein against labeled serum samples. The M. avium subsp. paratuberculosis orthologs to reactive M. tuberculosis proteins (from Table S2 in the supplemental material) are grouped on the left, and orthologs to nonreactive M. tuberculosis proteins (not listed in Table S2) are grouped on the right. The error bars represent the standard errors of the mean (SEM). (A) ELISA analysis was performed using the same serum set as in the M. tuberculosis array study (Table 2). (B) An expanded serum set from well-characterized dairy herds around the United States was used to further test the same recombinant protein set (see Table S5 in the supplemental material). A total of 30 negative and 42 positive serum samples were analyzed. The results are shown as means and SEM and were compared using analysis of variance (ANOVA) for the 10 reactive versus 10 nonreactive M. avium subsp. paratuberculosis orthologs for the positive and negative repository serum samples.
The same 20 recombinant proteins were further tested using a set of well-characterized serum samples from dairy cows around the United States. The MAP2942c protein was once again the strongest antigen in this serum set. Comparing M. avium subsp. paratuberculosis orthologs of the M. tuberculosis reactive and nonreactive proteins showed significance (analysis of variance [ANOVA]; P < 0.05) for both the positive and negative serum repository samples (Fig. 7B). The mean sample-to-positive (S/P) ratios were significantly higher (P < 0.05) among the reactive M. avium subsp. paratuberculosis orthologs in the clinical and positive serum groups as measured by ELISA (Fig. 8). Collectively, these data demonstrate that M. avium subsp. paratuberculosis orthologs of the antigenic M. tuberculosis proteins are able to distinguish M. avium subsp. paratuberculosis-infected cows from healthy cows.
FIG 8.
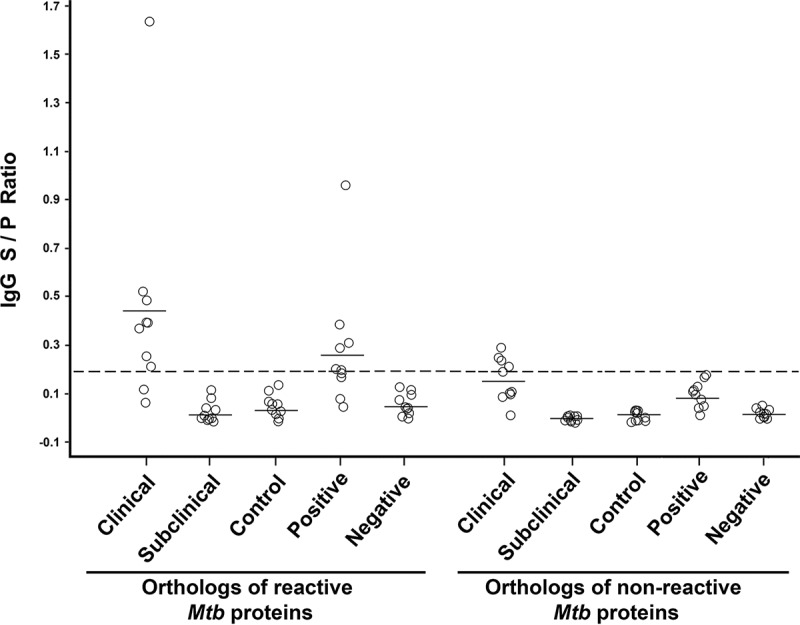
Distribution of reactive and nonreactive M. avium subsp. paratuberculosis othologous proteins with Johne's disease-positive and -negative serum samples. The results are expressed as the sample-to-positive ratio, where ≥0.2 was considered positive. The circles represent M. avium subsp. paratuberculosis recombinant proteins. The horizontal bars represent the means. The cutoff value of 0.2 is indicated by the dashed horizontal line.
DISCUSSION
Although M. avium subsp. paratuberculosis and M. tuberculosis are related mycobacterial species, belonging to their own complexes within the genus, the results of our investigations suggest there is sufficient antigenic cross-reactivity to leverage the M. tuberculosis array for Johne's disease studies. By using the M. tuberculosis protein array to test sera from cows with Johne's disease, known M. avium subsp. paratuberculosis antigens were confirmed and hundreds of new potential antigens were identified. This study may represent the largest, unbiased screen of antigens ever undertaken for Johne's disease. Furthermore, the screen, combined with a large collection of recombinant proteins, makes it possible to construct a diagnostic platform that could range from ELISA to miniature lateral-flow-based tests.
The results of this study have the side benefit of documenting a majority of the M. bovis proteins that cross-react with M. avium subsp. paratuberculosis, since M. bovis is so closely related to M. tuberculosis. This is important, because M. bovis is also a pathogen of cattle that has important regulatory consequences (23–25), and vaccination efforts against either pathogen ideally should not interfere with diagnostic tests for the other (26, 27). The data obtained in this study make it possible to systematically determine cross-reactive antigens, as well as antigens that may distinguish each pathogen. Armed with this new information, vaccine and diagnostic strategies for either disease are better informed.
An important component of this study is the assembly of well-characterized serum samples to enable identification of potent antigens for early and late diagnosis of Johne's disease. Sera were obtained from cows, along with longitudinal clinical information so that they could be stratified by the various stages of Johne's disease. Although the repository serum samples could not be included in the M. tuberculosis array study due to costs associated with printing and processing, these additional, well-characterized sera enabled further refinement of the best antigens in the ELISA study.
Through this approach, antigens were identified in both clinical and subclinical groups in comparison with the ratio of intensities to those of the negative-control group. As expected, different patterns of serum antibody responses were observed for cattle in clinical and subclinical stages of disease, with overall stronger responses noted for cows in the clinical stage of disease. These results corroborate the paradigm of a progressive increase in antibody response to M. avium subsp. paratuberculosis infection as animals transition to more advanced stages of disease (28). Among the top 30 M. tuberculosis antigens, ranked by P value, only 4 showed stronger antibody reactivity in cows in the subclinical stage of disease. Notably, three of these antigens are annotated as hypothetical proteins, suggesting that proteins that are antigenic in the subclinical stage not only are rare but also not well characterized.
From observed differences between the two disease stages, a dynamic antibody response to some proteins detected during subclinical Johne's disease disappears during the clinical stage, and the immunodominant antigens recognized by clinical sera may not be recognized by subclinical-stage antibody responses. The hypothesis still needs to be tested on an expanded set of samples from large-scale serum collections that have recently been established for Johne's disease studies. Data obtained from the current study indicated these expanded studies are feasible and hence will be undertaken.
According to one study describing antibody responses to the M. tuberculosis proteome, 484 out of 4,099 M. tuberculosis proteins were recognized as antigenic by sera from 240 TB patients (5), and we estimate that the M. avium subsp. paratuberculosis proteome will have a similar number when tested with sera from Johne's disease cattle. Using these 484 M. tuberculosis proteins, we searched the M. avium subsp. paratuberculosis genome and found 361 orthologs, accounting for 74.6% of the potential M. avium subsp. paratuberculosis antigens identified in the M. tuberculosis study. About 70% of these orthologs are not present on the M. avium subsp. paratuberculosis array but will be included in a second-generation M. avium subsp. paratuberculosis protein array. Bioinformatics analysis could then be used to compare epitopes and further narrow the number of M. avium subsp. paratuberculosis proteins missing from a whole-immunoproteome analysis.
We hypothesized that the most immunodominant proteins would be identified by comparison between infected cattle and negative controls, as was done previously using the M. tuberculosis microarray, where 13 proteins were identified as associated with active TB (5). A major challenge with the current technology is to accurately classify M. avium subsp. paratuberculosis-infected animals in different disease statuses, particularly true-negative animals. However, another group has developed a Bayesian methodology (29, 30) that allows us to estimate the sensitivity and specificity of a new test even if we do not know the gold standard. This technique has been applied to the development of a multiplex assay for the detection of antibodies to Borrelia burgdorferi in horses (31), and it will be employed in future studies with a larger set of Johne's disease cows.
Antibody screening with M. tuberculosis microarrays has identified new antigenic proteins that are not in our collection of M. avium subsp. paratuberculosis recombinant proteins. However, we realize that the proteins identified with an M. tuberculosis microarray may not all be good candidates because the high identity of amino acid sequences may contribute to cross-reactivity. Likewise, nonreactive proteins on the M. tuberculosis microarray may not be indicative of nonreactivity in M. avium subsp. paratuberculosis orthologs. Whereas negative responses may represent true negatives in corresponding M. avium subsp. paratuberculosis orthologs (which will be a majority of cases), sera may not recognize M. tuberculosis proteins, not because the corresponding orthologs in M. avium subsp. paratuberculosis are not immunodominant, but because the two orthologs are too different at the amino acid level.
Despite this, proteins showing the highest intensity were readily identified. The most prominent protein from the ELISA study was MAP2942c, termed a Mpt53 secreted antigen, which has been annotated as a disulfide oxidoreductase and was previously identified by screening an expression library with sera from a cow in the clinical stage of disease (16). In addition, the gene encoding this protein showed increased transcription in the presence of iron (32) and the rhodamine agent D157070 (33). MAP2942c had the second highest intensity in clinical disease but was among the weakest reactors in animals in the subclinical stage of disease, based on the M. tuberculosis array. Likewise, MAP1569, another secreted antigen, was strongest during clinical disease but 145th during subclinical disease (see Table S2 in the supplemental material). The protein has been shown to activate dendritic cells and stimulate gamma interferon (IFN-γ) production (34). Membrane proteins, fatty acid-coenzyme A (CoA) synthase, and cytochrome c oxidase are among the strongest reactors during subclinical disease.
The analysis showed that 1,916 M. tuberculosis proteins had at least 75% amino acid identity to M. avium subsp. paratuberculosis; however, there are 1,805 M. avium subsp. paratuberculosis proteins with identities to M. tuberculosis proteins below 60%. Even though 327 of these low-identity proteins are already represented on an existing M. avium subsp. paratuberculosis array (unpublished data), the remainder represent proteins that are not likely amenable to analysis by using only the M. tuberculosis array. Nonetheless, the results suggest that the M. tuberculosis protein microarray can be successfully applied to M. avium subsp. paratuberculosis antibody screening, since it will add greatly to the number of testable candidate antigens. To overcome these limitations and until whole-proteome M. avium subsp. paratuberculosis arrays become available, future studies will need to consider applying sophisticated bioinformatics and statistical analysis tools to analyze the M. avium subsp. paratuberculosis orthologs of all positive and negative spots to make a meaningful determination on selection of M. avium subsp. paratuberculosis protein candidates to pursue in antigen-based diagnostic assays.
MATERIALS AND METHODS
Comparative genome analysis.
The genome sequence of M. avium subsp. paratuberculosis strain K-10, a bovine isolate from a Wisconsin dairy herd, was compared to those of M. avium subspecies hominissuis strain 104, M. bovis AF2122/97, and M. tuberculosis H37Rv. M. avium subsp. paratuberculosis strain MAP4, a human isolate (10), was also used in these analyses. The number of amino acid matches was determined by pairwise analysis for all CDSs in each genome and plotted as percent identity on a histogram.
M. tuberculosis and M. avium subsp. paratuberculosis protein array content and spotting.
Antigen Discovery, Inc. (ADI), Irvine, CA, has developed protein microarrays for antibody screening of M. tuberculosis, Brucella, and malaria infections (5, 35, 36), as well as other prominent diseases. Therefore, the M. tuberculosis array was fabricated by ADI as previously described (5, 37). Briefly, using M. tuberculosis H37Rv genomic DNA as a template, all the open reading frames were amplified using custom PCR primers. Genes greater than 3 kb in length were amplified as multiple overlapping fragments. PCR products were cloned into a linearized T7 vector, pXI, using in vivo recombination cloning. Using individually purified plasmids, M. tuberculosis proteins were expressed in an Escherichia coli-based in vitro transcription and translation (IVTT) system (5 Prime, Gaithersburg, MD). The resulting IVTT reactions were printed as single spots without further purification on custom 3-pad nitrocellulose-coated Avid slides (Grace Bio-Labs, Bend, OR) using an OmniGrid Accent microarray printer (Digilabs, Inc., Marlborough, MA) in 4-by-4 subarray format, with each subarray comprising 18 by 18 spots. The total number of M. tuberculosis proteins spotted was 3,963, representing 3,864 genes (3,722 genes in the current NCBI gene set and 142 pseudogenes from prior gene sets). Among them, 48 genes of >3 kb were fragmented into a total of 133 segments. Each subarray included negative-control spots carrying IVTT reaction mixtures without DNA templates, purified protein spots of previously identified M. tuberculosis biomarkers, and positive-control spots for hybridization.
For the M. avium subsp. paratuberculosis array, 108 purified M. avium subsp. paratuberculosis K-10 recombinant proteins were selected (shown in red in Table S1 in the supplemental material) from a recombinant protein collection (3) based on the available quantity and identity to M. tuberculosis orthologs. In addition, 33 control spots were included for duplicate printing onto SuperEpoxy 2 glass slides (ArrayIt, Sunnyvale, CA) using an OmniGrid 100 microarray printer. The control spots included unconjugated bovine IgG (serially diluted) and mouse IgG for secondary-antibody binding. Additional controls included defined peptides, such as maltose binding protein (MBP) and cell extracts from M. avium subsp. avium, M. avium subsp. hominissuis, Mycobacterium intracellulare, Mycobacterium smegmatis, and Mycobacterium scrofulaceum. The negative controls included rabbit IgG, phosphate-buffered saline (PBS), and array-printing buffer. All M. avium subsp. paratuberculosis recombinant proteins were expressed as MBP fusions and affinity purified as described previously (38). Epoxy-activated slides were used, since they enable high-density printing and strong protein attachment via covalent cross-linking and high-resolution detection (39). Proteins were spotted at concentrations of 0.10 to 0.20 μg/μl. To determine maximum spot intensities and local background correction, M. avium subsp. paratuberculosis arrays were probed with monoclonal antibodies to the maltose binding protein tag. The amounts of protein spotted varied slightly, and this was taken into account in our analysis. All protein array slides were stored for no more than 3 days in a desiccator cabinet at 4°C until they were ready for probing with bovine serum samples.
Bovine serum samples.
Serum samples used on the M. tuberculosis protein arrays were from Holstein cows, including six in the clinical stage of disease and three in the subclinical stage, along with three healthy control cows (Table 2). The same set of serum samples, with the exception of 786 and 789, was also used on the M. avium subsp. paratuberculosis protein arrays. All of these cows were housed at the USDA National Animal Disease Center in Ames, IA, and have an approved IACUC protocol. Furthermore, all the cows with Johne's disease acquired the infection naturally. All the cows have documented health histories and are tested quarterly by ELISA, IFN-γ response, and fecal culture. Table 2 shows the results of these tests at the time the bleed was taken for this study. Control cows were defined as Johne's disease free by negative serum ELISA (IDEXX, Westbrook, ME), IFN-γ (Bovigam; ThermoFisher Scientific, Waltham, MA), and fecal PCR tests. Animals in the clinical stage of disease showed Johne's disease symptoms, which included shedding bacteria in the feces, and a positive serum ELISA result using an M. avium subsp. paratuberculosis antibody test kit (IDEXX). The subclinical stage of disease is not as clearly defined, but animals in this stage are typically IFN-γ positive and may be intermittently shedding low numbers of bacteria in their feces. All animals were skin test negative for bovine TB.
TABLE 2.
Health status of cattle used in this study
| Cow identifier | Disease status | Test result |
|||
|---|---|---|---|---|---|
| IDEXX ELISAa | IFN-γb | Fecal PCRc |
|||
| IS900 | ISMap02 | ||||
| 781 | Control | 0.073 | 0.063/0.077 | Neg | Neg |
| 782 | Control | 0.048 | 0.059/0.145 | Neg | Neg |
| 785 | Control | 0.058 | 0.113/0.148 | Neg | Neg |
| 53 | Subclinical | 0.046 | 0.120/0.474 | Neg | 27.95 |
| 651 | Subclinical | 0.023 | 0.124/0.279 | Neg | Neg |
| 1062 | Subclinical | 0.053 | 0.141/1.468 | Neg | Neg |
| 786 | Clinical | 2.94 | 0.044/0.084 | 26.28 | 16.62 |
| 789 | Clinical | 2.26 | 0.196/0.598 | 22.43 | Neg |
| 1442 | Clinical | 2.62 | 0.043/0.083 | 26.13 | 18.1 |
| 2222 | Clinical | 2.96 | 0.178/1.479 | 25.83 | 20.66 |
| 2444 | Clinical | 3.07 | 0.089/0.124 | 30.05 | 20.64 |
| 2491 | Clinical | 3.26 | 0.088/0.103 | 33.74 | 19.4 |
A negative ELISA result is defined as less than 0.1.
Values are reported as no stimulation/antigen stimulation. A negative IFN-γ test result is anything less than 0.11.
A negative (Neg) fecal PCR result is a threshold cycle (CT) value higher than 35. IS900 has 17 copies per genome, while ISMap02 has 6 copies per genome.
While the same serum samples were also used in the ELISA validation experiment, an additional 73 well-characterized bovine serum field samples from the Johne's Disease Integrated Program Repository were also included in this experiment (see Table S5 in the supplemental material). The repository samples were classified into two groups based on three commercial ELISAs, as well as fecal culture and fecal PCR (Table S5). The positive group consisted of 42 samples that were fecal culture positive and ELISA positive and the negative group consisted of 30 samples from negative low-exposure cows.
Probing protein microarrays.
Prior to incubation with serum, the M. tuberculosis and M. avium subsp. paratuberculosis arrays were rehydrated and blocked for 30 min using protein array blocking buffer (10485356; Maine Manufacturing, Sanford, ME) at room temperature. Serum/plasma samples were diluted 1:200 in blocking buffer and incubated on arrays at 4°C overnight with gentle agitation. The arrays were washed 3 times in wash buffer (Tris-buffered saline [TBS] with 0.05% Tween 20). Bound IgG antibodies were detected with either a biotinylated anti-bovine secondary antibody (Jackson ImmunoResearch, West Grove, PA), followed by incubation with SureLight P-3 fluorochrome conjugated to streptavidin (Columbia Biosciences, Columbia, NY) for M. tuberculosis arrays or Cy3-conjugated anti-goat IgG (Jackson ImmunoResearch, West Grove, PA) for M. avium subsp. paratuberculosis arrays. The M. avium subsp. paratuberculosis array consisted of MBP-tagged recombinant proteins; thus, Cy5–anti-MBP was used to determine spotting efficiencies. After three washes to remove unbound detection antibodies, the slides were dried and scanned in a GenePix 4300A microarray scanner (Molecular Devices, San Diego, CA) for M. tuberculosis arrays or a GenePix 4000B for M. avium subsp. paratuberculosis arrays. Fluorescence intensity values for each spot were quantified using a GenePix 6.0 (Molecular Devices, Sunnyvale, CA) utilizing local background subtraction for each spot, and the data were exported in comma-separated-value (CSV) format. Each spot on the array is comprised of several hundred pixels. The raw values from array scans are median intensities of all the pixels in the printed spots after background subtraction for each protein or negative control.
Recombinant M. avium subsp. paratuberculosis antigen ELISA.
Polysorb plates (Nunc; 96 well) were coated with 50 μl/well of 1 μg/ml recombinant M. avium subsp. paratuberculosis protein or MBP/LacZ or 5 μg/ml M. avium subsp. paratuberculosis total antigen sonicate extract in carbonate-bicarbonate buffer (0.06 M, pH 9.4). The plates were sealed and incubated overnight at 4°C and then washed three times with 0.01 M PBS, pH 7.2, containing 0.05% Tween 20 (PBS-T) (Sigma-Aldrich, St. Louis, MO, USA). The wells were blocked by adding 200 μl/well of PBS-T containing 1% bovine serum albumin (PBS-T–BSA) and incubating at room temperature for 1 h before washing the plate three times with PBS-T. Serum samples, diluted 1:250 in PBS-T–BSA, were added to duplicate wells (100 μl/well) and incubated at room temperature for 1 h before washing three times with PBS-T. Then, 100 μl/well of anti-goat IgG peroxidase conjugate (Vector Laboratories, Burlingame, CA, USA) diluted 1:20,000 in PBS-T–BSA was added to all the wells and incubated at room temperature for 1 h before the plates were again washed three times with PBS-T. Finally, 100 μl/well of tetramethylbenzidine (TMB) SureBlue solution (KPL, Gaithersburg, MD, USA) was added, and the reaction was allowed to progress for 10 to 15 min at room temperature with no light before the reaction was stopped with 100 μl/well of TMB stop solution (KPL). Spectrophotometric reading of all the wells was performed at 450 nm using a SpectraMax 340PC384 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Readings were analyzed by the S/P ratio, which was calculated as follows: S/P ratio = (sample OD − negative-control OD)/(positive-control OD − negative-control OD). Samples were considered positive if the S/P ratio was greater than 0.20.
Data analysis.
The intensity data files in CSV format were read in, processed, and analyzed using an automated data analysis pipeline developed at ADI (Irvine, CA) that is implemented in R (http://www.r-project.org). Spot intensity measurements were converted into a single data matrix of intensities with the local background subtracted. For each sample, quality checks were performed for possible missing spots, contamination, and unusual background variation. The data were also inspected for the presence of subtle systematic effects and biases (probing day, slide, pad, print order, etc.). Once the data passed quality assurance, the final data set utilized for analysis was obtained by log2 transformation of raw intensities for variance stabilization without further adjustment due to low, homogeneous background levels. An antigen was classified as reactive to a given sample if antibody target signal intensity values were at least twice the sample's median IVTT negative control. Data were modeled using parametric (Student's t test) and nonparametric (Wilcoxon's rank sum test and area under the concentration-time curve [AUC] estimation) tests for between-group comparisons. The data were visualized using bar graphs, box plots, and heat maps for antibody levels. Hierarchical clustering of between-group antibody correlations was used to identify sets of antibody targets with diagnostic potential. Correlation of antibody responses between M. avium subsp. paratuberculosis and M. tuberculosis orthologs was assessed using Pearson's correlation coefficient (rho). Qualification of means was done, with 95% confidence intervals, and interquartile ranges were used for medians. P values were adjusted for the false-discovery rate (FDR) using the Benjamini-Hochberg method, and P values of <0.05 were considered significant (40).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Janis K. Hansen at the National Animal Disease Center.
This work was supported by the USDA Agricultural Research Service and USDA-NIFA award 2014-06417 to V.K., J.J.C., and J.P.B. The development of the M. tuberculosis proteome microarray was supported in part by the Foundation for Innovative New Diagnostics.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00081-17.
REFERENCES
- 1.Biet F, Boschiroli ML. 2014. Non-tuberculous mycobacterial infections of veterinary relevance. Res Vet Sci 97(Suppl):S69–S77. doi: 10.1016/j.rvsc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Bannantine JP, Paustian ML, Waters WR, Stabel JR, Palmer MV, Li L, Kapur V. 2008. Profiling bovine antibody responses to Mycobacterium avium subsp. paratuberculosis infection by using protein arrays. Infect Immun 76:739–749. doi: 10.1128/IAI.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannantine JP, Stabel JR, Bayles DO, Geisbrecht BV. 2010. Characteristics of an extensive Mycobacterium avium subspecies paratuberculosis recombinant protein set. Protein Expr Purif 72:223–233. doi: 10.1016/j.pep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Bannantine JP, Bayles DO, Waters WR, Palmer MV, Stabel JR, Paustian ML. 2008. Early antibody response against Mycobacterium avium subspecies paratuberculosis antigens in subclinical cattle. Proteome Sci 6:5. doi: 10.1186/1477-5956-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, Cirillo DM, Michel G, Talbot EA, Perkins MD, Felgner PL, Liang X, Gennaro ML. 2010. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A 107:14703–14708. doi: 10.1073/pnas.1009080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cayrou C, Turenne C, Behr MA, Drancourt M. 2010. Genotyping of Mycobacterium avium complex organisms using multispacer sequence typing. Microbiology 156:687–694. doi: 10.1099/mic.0.033522-0. [DOI] [PubMed] [Google Scholar]
- 7.Bannantine JP, Baechler E, Zhang Q, Li L, Kapur V. 2002. Genome scale comparison of Mycobacterium avium subsp. paratuberculosis with Mycobacterium avium subsp. avium reveals potential diagnostic sequences. J Clin Microbiol 40:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannantine JP, Zhang Q, Li LL, Kapur V. 2003. Genomic homogeneity between Mycobacterium avium subsp. avium and Mycobacterium avium subsp. paratuberculosis belies their divergent growth rates. BMC Microbiol 3:10. doi: 10.1186/1471-2180-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakham F, Aouane O, Ussery D, Benjouad A, Ennaji MM. 2012. Computational genomics-proteomics and phylogeny analysis of twenty one mycobacterial genomes (tuberculosis and nontuberculosis strains). Microb Inform Exp 2:7. doi: 10.1186/2042-5783-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannantine JP, Li L, Mwangi M, Cote R, Raygoza Garay JA, Kapur V. 2014. Complete genome sequence of Mycobacterium avium subsp. paratuberculosis, isolated from human breast milk. Genome Announc 2:e01252-13. doi: 10.1128/genomeA.01252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meena LS. 2015. An overview to understand the role of PE_PGRS family proteins in Mycobacterium tuberculosis H37 Rv and their potential as new drug targets. Biotechnol Appl Biochem 62:145–153. doi: 10.1002/bab.1266. [DOI] [PubMed] [Google Scholar]
- 12.Facciuolo A, Kelton DF, Mutharia LM. 2013. Novel secreted antigens of Mycobacterium paratuberculosis as serodiagnostic biomarkers for Johne's disease in cattle. Clin Vaccine Immunol 20:1783–1791. doi: 10.1128/CVI.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souza GS, Rodrigues AB, Gioffre A, Romano MI, Carvalho EC, Ventura TL, Lasunskaia EB. 2011. Apa antigen of Mycobacterium avium subsp. paratuberculosis as a target for species-specific immunodetection of the bacteria in infected tissues of cattle with paratuberculosis. Vet Immunol Immunopathol 143:75–82. doi: 10.1016/j.vetimm.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Cho D, Sung N, Collins MT. 2006. Identification of proteins of potential diagnostic value for bovine paratuberculosis. Proteomics 6:5785–5794. doi: 10.1002/pmic.200600207. [DOI] [PubMed] [Google Scholar]
- 15.Cho D, Shin SJ, Talaat AM, Collins MT. 2007. Cloning, expression, purification and serodiagnostic evaluation of fourteen Mycobacterium paratuberculosis proteins. Protein Expr Purif 53:411–420. doi: 10.1016/j.pep.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Willemsen PT, Westerveen J, Dinkla A, Bakker D, van Zijderveld FG, Thole JE. 2006. Secreted antigens of Mycobacterium avium subspecies paratuberculosis as prominent immune targets. Vet Microbiol 114:337–344. doi: 10.1016/j.vetmic.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Munir S, Bannantine JP, Sreevatsan S, Kanjilal S, Kapur V. 2007. Rapid expression of Mycobacterium avium subsp. paratuberculosis recombinant proteins for antigen discovery. Clin Vaccine Immunol 14:102–105. doi: 10.1128/CVI.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paustian ML, Kapur V, Bannantine JP. 2005. Comparative genomic hybridizations reveal genetic regions within the Mycobacterium avium complex that are divergent from Mycobacterium avium subsp. paratuberculosis isolates. J Bacteriol 187:2406–2415. doi: 10.1128/JB.187.7.2406-2415.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semret M, Alexander DC, Turenne CY, de Haas P, Overduin P, van Soolingen D, Cousins D, Behr MA. 2005. Genomic polymorphisms for Mycobacterium avium subsp. paratuberculosis diagnostics. J Clin Microbiol 43:3704–3712. doi: 10.1128/JCM.43.8.3704-3712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leite FL, Reinhardt TA, Bannantine JP, Stabel JR. 2015. Envelope protein complexes of Mycobacterium avium subsp. paratuberculosis and their antigenicity. Vet Microbiol 175:275–285. doi: 10.1016/j.vetmic.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Bannantine JP, Huntley JF, Miltner E, Stabel JR, Bermudez LE. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 149:2061–2069. doi: 10.1099/mic.0.26323-0. [DOI] [PubMed] [Google Scholar]
- 22.Bannantine JP, Lingle CK, Stabel JR, Ramyar KX, Garcia BL, Raeber AJ, Schacher P, Kapur V, Geisbrecht BV. 2012. MAP1272c encodes an NlpC/P60 protein, an antigen detected in cattle with Johne's disease. Clin Vaccine Immunol 19:1083–1092. doi: 10.1128/CVI.00195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP, Waters WR. 2010. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg Dis 57:205–220. doi: 10.1111/j.1865-1682.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 24.More SJ, Radunz B, Glanville RJ. 2015. Lessons learned during the successful eradication of bovine tuberculosis from Australia. Vet Rec 177:224–232. doi: 10.1136/vr.103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien DJ, Schmitt SM, Fitzgerald SD, Berry DE. 2011. Management of bovine tuberculosis in Michigan wildlife: current status and near term prospects. Vet Microbiol 151:179–187. doi: 10.1016/j.vetmic.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 26.Coad M, Clifford DJ, Vordermeier HM, Whelan AO. 2013. The consequences of vaccination with the Johne's disease vaccine, Gudair, on diagnosis of bovine tuberculosis. Vet Rec 172:266. doi: 10.1136/vr.101201. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald SD, Bolin SR, Boland KG, Lim A, Kaneene JB. 2011. Overt Mycobacterium avium subsp. paratuberculosis infection: an infrequent occurrence in archived tissue from false TB reactor cattle in Michigan, USA. Vet Med Int 2011:910738. doi: 10.4061/2011/910738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stabel JR. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet Microbiol 77:465–473. doi: 10.1016/S0378-1135(00)00331-X. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Turnbull BW, Grohn YT, Nielsen SS. 2006. Estimating receiver operating characteristic curves with covariates when there is no perfect reference test for diagnosis of Johne's disease. J Dairy Sci 89:3038–3046. doi: 10.3168/jds.S0022-0302(06)72577-2. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Turnbull BW, Nielsen SS, Grohn YT. 2011. Bayesian analysis of longitudinal Johne's disease diagnostic data without a gold standard test. J Dairy Sci 94:2320–2328. doi: 10.3168/jds.2010-3675. [DOI] [PubMed] [Google Scholar]
- 31.Wagner B, Freer H, Rollins A, Erb HN, Lu Z, Grohn Y. 2011. Development of a multiplex assay for the detection of antibodies to Borrelia burgdorferi in horses and its validation using Bayesian and conventional statistical methods. Vet Immunol Immunopathol 144:374–381. doi: 10.1016/j.vetimm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Janagama HK, Kumar S, Bannantine JP, Kugadas A, Jagtap P, Higgins L, Witthuhn B, Sreevatsan S. 2010. Iron-sparing response of Mycobacterium avium subsp. paratuberculosis is strain dependent. BMC Microbiol 10:268. doi: 10.1186/1471-2180-10-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bull TJ, Linedale R, Hinds J, Hermon-Taylor J. 2009. A rhodanine agent active against non-replicating intracellular Mycobacterium avium subspecies paratuberculosis. Gut Pathog 1:25. doi: 10.1186/1757-4749-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JS, Shin SJ, Collins MT, Jung ID, Jeong YI, Lee CM, Shin YK, Kim D, Park YM. 2009. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein activates dendritic cells and induces a Th1 polarization. Infect Immun 77:2979–2988. doi: 10.1128/IAI.01411-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang L, Leng D, Burk C, Nakajima-Sasaki R, Kayala MA, Atluri VL, Pablo J, Unal B, Ficht TA, Gotuzzo E, Saito M, Morrow WJ, Liang X, Baldi P, Gilman RH, Vinetz JM, Tsolis RM, Felgner PL. 2010. Large scale immune profiling of infected humans and goats reveals differential recognition of Brucella melitensis antigens. PLoS Negl Trop Dis 4:e673. doi: 10.1371/journal.pntd.0000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felgner PL, Roestenberg M, Liang L, Hung C, Jain A, Pablo J, Nakajima-Sasaki R, Molina D, Teelen K, Hermsen CC, Sauerwein R. 2013. Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci Rep 3:3549. doi: 10.1038/srep03549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunnath-Velayudhan S, Davidow AL, Wang HY, Molina DM, Huynh VT, Salamon H, Pine R, Michel G, Perkins MD, Xiaowu L, Felgner PL, Flynn JL, Catanzaro A, Gennaro ML. 2012. Proteome-scale antibody responses and outcome of Mycobacterium tuberculosis infection in nonhuman primates and in tuberculosis patients. J Infect Dis 206:697–705. doi: 10.1093/infdis/jis421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bannantine JP, Paustian ML. 2006. Identification of diagnostic proteins in Mycobacterium avium subspecies paratuberculosis by a whole genome analysis approach. Methods Mol Biol 345:185–196. [DOI] [PubMed] [Google Scholar]
- 39.Zhu H, Snyder M. 2003. Protein chip technology. Curr Opin Chem Biol 7:55–63. doi: 10.1016/S1367-5931(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hockberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300. [Google Scholar]
- 41.Hughes V, Bannantine JP, Denham S, Smith S, Garcia-Sanchez A, Sales J, Paustian ML, McLean K, Stevenson K. 2008. Immunogenicity of proteome-determined Mycobacterium avium subsp. paratuberculosis-specific proteins in sheep with paratuberculosis. Clin Vaccine Immunol 15:1824–1833. doi: 10.1128/CVI.00099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurung RB, Begg DJ, Purdie AC, Bannantine JP, Whittington RJ. 2013. Antigenicity of recombinant maltose binding protein-Mycobacterium avium subsp. paratuberculosis fusion proteins with and without factor Xa cleaving. Clin Vaccine Immunol 20:1817–1826. doi: 10.1128/CVI.00596-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leroy B, Roupie V, Noel-Georis I, Rosseels V, Walravens K, Govaerts M, Huygen K, Wattiez R. 2007. Antigen discovery: a postgenomic approach to paratuberculosis diagnosis. Proteomics 7:1164–1176. doi: 10.1002/pmic.200600988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.