ABSTRACT
Naturally acquired antibodies to Plasmodium falciparum schizont egress antigen 1 (PfSEA-1A) are associated with protection against severe malaria in children. Vaccination of mice with SEA-1A from Plasmodium berghei (PbSEA-1A) decreases parasitemia and prolongs survival following P. berghei ANKA challenge. To enhance the immunogenicity of PfSEA-1A, we identified five linear B-cell epitopes using peptide microarrays probed with antisera from nonhuman primates vaccinated with recombinant PfSEA-1A (rPfSEA-1A). We evaluated the relationship between epitope-specific antibody levels and protection from parasitemia in a longitudinal treatment-reinfection cohort in western Kenya. Antibodies to three epitopes were associated with 16 to 17% decreased parasitemia over an 18-week high transmission season. We are currently designing immunogens to enhance antibody responses to these three epitopes.
KEYWORDS: B-cell epitopes, malaria, PfSEA-1
INTRODUCTION
We previously demonstrated that naturally acquired antibodies to a highly invariant region of Plasmodium falciparum schizont egress antigen 1 (PfSEA-1A) (amino acids [aa] 810 to 1,023) are associated with protection from severe malaria in young children. Vaccination of mice with SEA-1A from Plasmodium berghei (PbSEA-1A) protects mice against P. berghei ANKA challenge. Under conditions where transmission is holoendemic, only 6% of 2-year-olds have detectable anti-PfSEA-1A IgG responses. This prevalence increases to 56% in 12- to 35-year-olds (1).
Poor immunogenicity has hampered the development of other malaria vaccine candidates (2, 3). To maximize the immunogenicity of PfSEA-1A-based immunogens at the preclinical stage, we are designing epitope-enhanced immunogens expressing multiple copies of selected linear B-cell epitopes that are the targets of protective antibody responses. To identify these protective B-cell epitopes, we generated polyclonal PfSEA-1A antibodies in nonhuman primates, used these antibodies to map linear B-cell epitopes within PfSEA-1A by peptide microarray, and then related the recognition of these epitopes to resistance to reinfection in a cohort of 12- to 35-year-olds living in a region of western Kenya where infection is holoendemic.
RESULTS
Currently, humans have not been vaccinated with PfSEA-1-containing constructs. To identify B-cell epitopes potentially recognized by PfSEA-1A-vaccinated humans, we vaccinated nonhuman primates with recombinant PfSEA-1A (rPfSEA-1A) and used sera from six PfSEA-1A-vaccinated and one adjuvant-only-vaccinated nonhuman primate to identify immunoreactive peptides on a high-density array of 15-mers spanning the PfSEA-1A region (aa 810 to 1,023). Of 273 total overlapping peptides, five distinct oligomers reacted strongly with the vaccinated serum (Fig. 1A; see also Table S1 in the supplemental material). The consensus sequence for these oligomers was determined by the reactivity of each nonhuman primate serum sample (see Table S2). We synthesized the five peptides (Thermo Fisher) represented in the reactive regions on the array, conjugated them to microspheres, and probed them with sera from rPfSEA-1-vaccinated (n = 7) and adjuvant-only-vaccinated (n = 7) nonhuman primates in our bead-based assay. As expected, adjuvant-only-vaccinated animals did not respond to any of the 5 PfSEA-1A epitopes. All seven rPfSEA-1A-vaccinated animals responded to one or more of the five unique epitopes, and the results from our bead-based assay were broadly concordant with the initial microarray results (Fig. 1B).
FIG 1.
Aotus monkeys vaccinated with rPFSEA-1A predominantly recognize 5 epitopes within PfSEA-1A. (A) Sera from six of the rPfSEA-1A-vaccinated primates (M13F, M7F, M3F, M8F, M4, and M1F) were tested and bound discrete peptide regions on an overlapping PfSEA-1A peptide (n = 273) microarray. Serum from primate M9F, which was vaccinated with adjuvant only, did not bind any of the peptides on the microarray (data not shown). (B) Epitope-conjugated beads were challenged with sera from rPfSEA-1A-vaccinated (n = 7) and adjuvant-only-vaccinated monkeys (n = 7). Fluorescence levels for each epitope were normalized by subtracting responses to BSA-conjugated beads.
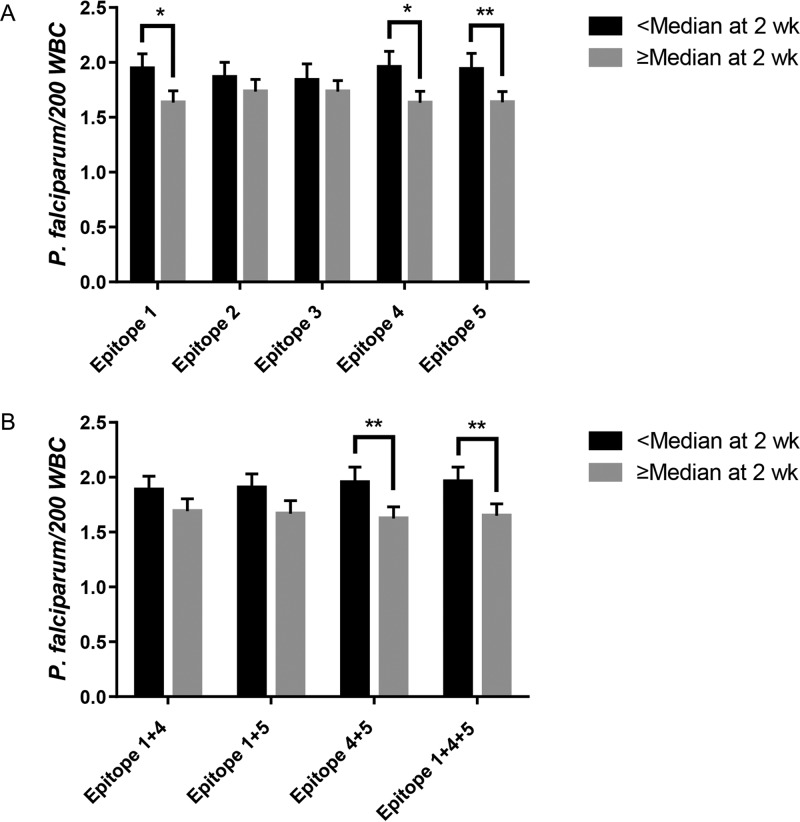
To determine if antibody responses to these immunodominant PfSEA-1A epitopes recognized by vaccinated nonhuman primates predicted protection in humans, we performed a secondary data analysis using our bead-based assay with data and serum samples previously collected from a cohort of Kenyan males as part of a treatment-reinfection study (n = 138). IgG responses to individual epitopes 1, 4, and 5 were each associated with significantly decreased parasitemia (16 to 17%) over an 18-week follow-up period (P < 0.05) (Fig. 2A) when analyzed as dichotomous antibody levels (high versus low) in generalized estimating equation (GEE) models.
FIG 2.
Antibody levels to PfSEA-1A epitopes 1, 4, and 5 predict resistance to parasitemia. Least-squares mean parasitemia for individuals with antibody levels greater than the median (n = 134) were compared to those with antibody levels less than or equal to the median (n = 134) throughout the entire follow-up period by a generalized estimating equation. *, P <0.05; **, P, <0.01. (A) Epitopes 1 to 5 considered individually. (B) Epitopes 1, 4, and 5 considered in combinations.
We next evaluated combinations of antibody reactivity to epitopes 1, 4, and 5 to assess additive or synergistic effects on reinfection. For each individual, we calculated the sum of the continuous antibody levels for each epitope and then dichotomized based on the median of this calculated value. Combinations of epitopes 4 and 5 and of 1, 4, and 5 were each associated with a significant decrease in parasitemia (16 to 17%; P < 0.05) over the 18-week follow-up period; however, the level of protection seen with these combinations did not exceed that observed with antibody levels to individual epitopes (Fig. 2B). The absence of an additive effect for combinations of antiepitope antibodies is consistent with the high correlation between antibody levels to the individual epitopes (see Table S3).
DISCUSSION
Long-lived, high-affinity antibody responses have proven difficult to obtain for several candidate malaria vaccines in development (4). Recently, we discovered PfSEA-1, a target of antibodies associated with protection from severe malarial disease in children. As expected for a vital parasite target, PfSEA-1 is not highly immunogenic under conditions of natural exposure, with only 6% of 2-year-old children having detectable IgG responses. Immunogens containing tandemly repeated protective PfSEA-1A epitopes would potentially enhance the immunogenicity of PfSEA-1-based vaccines.
B-cell epitope-augmented immunogens have been evaluated for numerous pathogens, including HIV and herpes simplex virus (HSV). In the case of HIV, an epitope-augmented vaccine expressing multiple copies of a single B-cell epitope produced antibodies that neutralized nine of 21 HIV strains tested with a 2-fold higher affinity to the parent protein compared with that of antibodies produced by immunogens expressing a single copy of the epitope, which neutralized none of the tested strains (5). Similarly, an epitope-augmented vaccine for HSV consisting of four tandem repeats of a 13-amino-acid linear neutralizing epitope from the gD protein produced equivalent antibody titers and protection from challenge compared with those of the full-length gD protein. Moreover, while mice vaccinated with either full-length gD or the epitope-augmented gD epitope all survived a lethal HSV challenge, mice vaccinated with either one, two, or three repeats of the gD epitope were observed to have 40%, 20%, or 10% mortality rates, respectively (6). Together, these data support the role of B-cell epitope copy number in determining antibody titer, affinity, and protective efficacy.
Interestingly, the magnitude of protection attributable to the individual PfSEA-1A epitope-specific antibody levels (16 to 17% reduction in parasitemia) does not recapitulate the 50% reduction in parasitemia attributable to antibodies against the larger recombinant PfSEA-1A protein observed in this same cohort (1). These data suggest that either additional, protective linear epitopes await identification or that there are protective conformational epitopes that were not represented in our peptide-based microarray. Another possibility is that PfSEA-1A-vaccinated primates do not recognize the epitope targets of protective anti-PfSEA-1A antibodies in humans. Epitope mapping activities using sera pooled from relatively resistant humans could identify these additional epitopes.
In this study, we identified five unique, immunodominant linear B-cell epitopes using sera from rPfSEA-1A-vaccinated monkeys. We demonstrate that antibodies in naturally exposed humans are recognized by nonhuman primate-identified epitopes and antibody responses to three of these epitopes predict modest protection from parasitemia in a cohort of 12- to 35-year-olds living in a region of western Kenya where infection is holoendemic. These protective PfSEA-1 epitope-specific IgG responses define the epitopes that we will express in multiple copies in second-generation epitope-augmented PfSEA-1A-based immunogens for follow-up preclinical development of PfSEA-1-based vaccines.
MATERIALS AND METHODS
Generation of PfSEA-1A-specific primate antibodies.
We vaccinated Aotus nancymaae monkeys (n = 7) with 50 μg of rPfSEA-1A formulated in LS127 (Infectious Disease Research Institute, Seattle, WA), a liposome-based adjuvant containing the TLR4 agonist glucopyranosyl lipid A and QS-21. Additionally, a control set of monkeys (n = 7) was vaccinated with LS127 only. Animals were vaccinated subcutaneously four times at 4-week intervals, and serum was collected 4 weeks following the final dose. Animal experiments were approved by the Institutional Animal Care and Use Committee at NIH-LMIV.
Microarray screening.
We constructed custom microarrays displaying 15-mer peptides spanning amino acids 810 to 1,023 of PfSEA-1 (PfSEA-1A). Peptides were synthesized with 14-amino acid overlaps and were arrayed to yield a high-density microarray containing 273 different peptides printed in duplicate (543 peptide spots) framed by flag (DYKDDDDKAS) and HA (YPYDVPDYAG) control peptides (78 spots each control). We probed the PfSEA-1A peptide microarray with goat anti-human IgG(H+L) DyLight680 antibody (1:1000) in the presence of the monoclonal anti-HA (12CA5)-DyLight800 control antibody (1:2000). Next, we incubated the microarray with monkey polyclonal serum at a dilution of 1:1000 and detected with a goat anti-human IgG(H+L) DyLight680 antibody in the presence of the monoclonal anti-HA (12CA5)-DyLight800 control antibody. We quantified spot intensities on a LI-COR Odyssey imaging system with a scanning offset of 0.65 mm and a resolution of 21 μm at scanning intensities of 7/7 (red/green), and microarray image analysis was completed with a PepSlide analyzer based on 16-bit gray scale tiff files. Using averaged median foreground intensities, an intensity map was generated (PEPperPrint, Heidelberg, Germany). Microarrays were probed separately for each individual monkey serum sample.
Human cohort.
We performed a secondary analysis with data and serum samples that were collected from a cohort of Kenyan males as part of a treatment-reinfection study (7–9).
Volunteers were residents of subsistence farming villages in western Kenya, north of Lake Victoria, where P. falciparum is endemic. The entomological inoculation rate in this area exceeded 300 infectious bites per person per year (10), and bed nets had not been introduced into the community. Male volunteers (n = 138) aged 12–35 years were entered into the study at the beginning of the high transmission season in April 1997. Detectable parasitemia was eradicated by use of quinine sulfate (10 mg/kg twice daily for 3 days) and doxycycline (100 mg twice daily for 7 days), and individuals were monitored with weekly blood smears for 18 weeks. Serum was collected 2 weeks posttreatment and stored at −80°C. In this age group, clinical or severe malaria is very uncommon.
Determination of anti-PfSEA-1A peptide IgG antibody levels.
To measure anti-PfSEA-1A peptide IgG antibody levels in our cohort, we used a bead-based assay developed from our published method (11). Specifically, 200 μg of each peptide (Thermo Fisher Scientific) or bovine serum albumin (BSA) (Sigma) in 50 mM MES (morpholineethanesulfonic acid), pH 5.0, was amine coupled to 1.25 × 107 microspheres (Luminex). Peptide-coupled and BSA-coupled microspheres were pooled and lyophilized in single-use aliquots. Following reconstitution, peptide-coated beads were incubated with human plasma samples at 1:100 for 3 h at 25°C in assay buffer E (ABE) (phosphate-buffered saline [PBS] [pH 7.4] containing 0.1% BSA, 0.05% Tween 20, and 0.05% sodium azide) in microtiter filter-bottom plates (Millipore). Microspheres were washed three times in ABE by vacuum filtration and incubated for 1 h at 25°C with biotinylated anti-human IgG (Pharmingen) diluted 1:1000 in ABE. Microspheres were washed three times in ABE by vacuum filtration and incubated at 25°C with phycoerythrin-conjugated streptavidin (Pharmingen) diluted 1:1000 in ABE for 15 min. Microspheres were washed a final three times in ABE by vacuum filtration and resuspended in 125 μL ABE. Peptide-specific fluorescence values were quantified on a BioPlex 200 multianalyte analyzer with subtraction of fluorescence values obtained with BSA-conjugated beads.
To measure anti-PfSEA-1A peptide IgG antibody levels in nonhuman primates, we employed the same method with the following modifications. Nonhuman primate serum samples were used at a 1:10,000 dilution and biotinylated anti-human IgG (KPL), which was validated on PfSEA-1A-conjugated beads for use with nonhuman primate sera (data not shown), was used at a 1:1000 dilution.
Statistical analysis.
The goal of the statistical analysis was to assess the relationship between peptide-specific antibody levels and resistance to reinfection. Parasitemia data obtained weekly were analyzed using a generalized estimating equation (GEE) with a Poisson distribution (SAS Institute, Cary, NC, USA). The response variable (parasitemia) was ln transformed. We evaluated the relationship between anti-PfSEA-1A epitope-specific IgG antibodies and parasite density measured on 18 posttreatment blood films. Antibody levels were dichotomized as high (greater than the median value) or low (less than or equal to the median value). We adjusted for several confounders and effect modifiers that had been previously identified as significant predictors of malaria outcomes in this same cohort, including age (12 to 14 years, 15 to 19 years, and 20 to 35 years of age groups), baseline parasitemia, and Tanner score (1 to 2.5, 3 to 4.5, and 5 to 5.5), a physical measure of pubertal development (8). A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank the field staff for their efforts collecting clinical data, sample processing, and interpreting malaria blood smears, as well as the study subjects and their families.
This work was supported by grants from the U.S. National Institutes of Health (grant R01-AI52059) and the Bill & Melinda Gates Foundation (grant 1364) to P.E.D., the Intramural Research Program of the NIAID-NIH, and by grants from the U.S. National Institutes of Health (grant R01-AI076353 and R01 AI110699-01) to J.D.K. Additional support was provided by the High Throughput Immunology Core (J.D.K.) and Biostatistics and Data Management Core (J.F.F.) of NIH/NIGMS COBRE P20GM104317. J.F.F. was supported by the U.S. National Institutes of Health (grant K24-AI112964). C.E.N. was supported by the U.S. National Institutes of Health (grant T32-DA013911).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00068-17.
REFERENCES
- 1.Raj DK, Nixon CP, Nixon CE, Dvorin JD, DiPetrillo CG, Pond-Tor S, Wu H-W, Jolly G, Pischel L, Lu A, Michelow IC, Cheng L, Conteh S, McDonald EA, Absalon S, Holte SE, Friedman JF, Fried M, Duffy PE, Kurtis JD. 2014. Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 344:871–877. doi: 10.1126/science.1254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz L, Brown GV, Genton B, Moorthy VS. 2012. A review of malaria vaccine clinical projects based on the WHO rainbow table. Malar J 11:11. doi: 10.1186/1475-2875-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crompton PD, Pierce SK, Miller LH. 2010. Advances and challenges in malaria vaccine development. J Clin Invest 120:4168–4168. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bejon P, White MT, Olotu A, Bojang K, Lusingu JPA, Salim N, Otsyula NN, Agnandji ST, Asante KP, Owusu-Agyei S, Abdulla S, Ghani AC. 2013. Efficacy of RTS, S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis 13:319–327. doi: 10.1016/S1473-3099(13)70005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Z, Zhu Y, Wang Q, Ye L, Dai Y, Su S, Yu F, Ying T, Yang C, Jiang S, Lu L. 2016. An immunogen containing four tandem 10E8 epitope repeats with exposed key residues induces antibodies that neutralize HIV-1 and activates an ADCC reporter gene. Emerg Microbes Infect 5:e65. doi: 10.1038/emi.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng B, Graham FL, Johnson DC, Hanke T, Mcdermott MR, Prevec L. 1993. Immunogenicity in mice of tandem repeats of an epitope from herpes simplex gD protein when expressed by recombinant adenovirus vectors. Vaccine 11:1191–1198. [DOI] [PubMed] [Google Scholar]
- 7.Kurtis JD, Lanar DE, Opollo M, Duffy PE. 1999. Interleukin-10 responses to liver-stage antigen 1 predict human resistance to Plasmodium falciparum. Infect Immun 67:3424–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtis JD, Mtalib R, Onyango FK, Duffy PE. 2001. Human resistance to Plasmodium falciparum increases during puberty and is predicted by dehydroepiandrosterone sulfate levels. Infect Immun 69:123–128. doi: 10.1128/IAI.69.1.123-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nixon CP, Friedman J, Treanor K, Knopf PM, Duffy PE, Kurtis JD. 2005. Antibodies to rhoptry-associated membrane antigen predict resistance to Plasmodium falciparum. J Infect Dis 192:861–869. doi: 10.1086/432550. [DOI] [PubMed] [Google Scholar]
- 10.Beier JC, Oster CN, Onyango FK, Bales JD, Sherwood JA, Perkins PV, Chumo DK, Koech DV, Whitmire RE, Roberts CR. 1994. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am J Trop Med Hyg 50:529–536. [DOI] [PubMed] [Google Scholar]
- 11.Cham GKK, Turner L, Lusingu J, Vestergaard L, Mmbando BP, Kurtis JD, Jensen ATR, Salanti A, Lavstsen T, Theander TG. 2009. Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J Immunol 183:3356–3363. doi: 10.4049/jimmunol.0901331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.