Abstract
In last decades, knowledge of nociceptive pain mechanisms has expanded rapidly. The use of quantitative sensory testing has provided evidence that peripheral and central sensitization mechanisms play a relevant role in localized and widespread chronic pain syndromes. In fact, almost any patient suffering with a chronic pain condition will demonstrate impairments in the central nervous system. In addition, it is accepted that pain is associated with different types of trigger factors including social, physiological, and psychological. This rational has provoked a change in the understanding of potential mechanisms of manual therapies, changing from a biomechanical/medical viewpoint, to a neurophysiological/nociceptive viewpoint. Therefore, interventions for patients with chronic pain should be applied based on current knowledge of nociceptive mechanisms since determining potential drivers of the sensitization process is critical for effective management. The current paper reviews mechanisms of chronic pain from a clinical and neurophysiological point of view and summarizes key messages for clinicians for proper management of individuals with chronic pain.
Keywords: Pain, sensitization, manual therapy, mechanisms
Introduction
Recent estimates indicate that chronic pain has reached epidemic levels in both the US and Europe. The prevalence of chronic pain in United States (US) adults over the age of 18 has been estimated at 30.7 and 43%, depending on the source [1,2] with similar statistics reported in the United Kingdom [3]. The social and financial ramifications of this epidemic are staggering, particularly in terms of disability and reduced quality of life [4], and the increased risk of hospitalization, institutionalization, and mortality [5].
Several factors may contribute to the high rate of chronic pain, including the fact that our population is getting older. Novak et al. [6] reported that the prevalence of chronic pain increased steadily with age from a low of 14.3% in 18–25 years old to as high as 62% in the over 75 age group. Similarly, the prevalence of disabling musculoskeletal chronic pain increased in the latter years of the twentieth century then stabilized in the early years of the twenty-first century [7]. Thus, the process of aging, including social, psychological, and physiological changes contribute in some manner to chronic pain. Sedentary lifestyle is another major contributor to chronic pain. In modern society, more and more occupations require a significant amount of time sitting in front of a computer. Physical inactivity is a known risk factor for development of chronic pain [8] and central changes in pain processing may occur more readily in persons with a sedentary way of life.
The response to increased levels of chronic pain has been a sharp rise in prescription of opioids. Over the past 15 years, there has been a marked increase in the prescription of almost all types of opioid medications while at the same time opioid misuse and the number of patients seeking rehabilitation for substance abuse has also increased [9]. It is clear that chronic pain is a global problem associated with aging and sedentary lifestyle, and that prescription of opioid medications as a sole solution is unlikely to resolve this current epidemic.
In simplest terms, chronic pain has been defined by its persistence, with the cut point typically defined as greater than three months [10]. However, it is clear that several factors differentiate this condition in terms of disability, contributing factors and underlying mechanisms of pain. One important dimension in the characterization of chronic pain is the concept of pain interference, which has been defined as the extent to which pain limits or interferes with functional activities and daily routines [11]. A second differentiating feature of chronic pain is whether it is widespread, defined as pain hypersensitivity experienced not only at the affected body part but throughout the body. Many chronic pain conditions, including fibromyalgia [12] have varying degrees of diffuse pain and symptoms. Disability is reported to be more severe [13] and quantitative sensory findings more abnormal [14] in patients with widespread rather than regional chronic pain conditions. Other factors have been utilized in the characterization of pain. The Initiative of Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) has developed a consensus of recommendations for improving the design, execution, and interpretation of clinical trials of treatments for pain [15] and factors such as disease, comorbidities, pain duration, and pain intensity, among others, have been suggested for characterization of the pain.
Both medical and rehabilitative management of chronic pain have begun to target various pain mechanisms as one strategy for combatting chronic pain. The purpose of this narrative is to describe the underlying neurophysiologic mechanisms of chronic pain and to propose rehabilitative methods that may address aberrant pain mechanisms.
Contributing factors and risk factors to chronic pain
Several factors have been identified as potentially contributing to the change from acute to chronic pain. These can be characterized into three different but related categories including social, physiological, and psychological factors. A selected number of factors have been listed below.
Social factors
Social factors are the personal characteristics that influence an individual’s personality, attitudes, and lifestyle. In addition to advanced age, several other social influences may contribute to chronic pain. Tenuous housing and employment status have been independently associated with chronic pain [16], as well as low educational levels and low family income [17–19]. Stress over financial or housing insecurity may promote aberrant pain processing. Social isolation and recent divorce, separation, or death of a spouse have also been associated with progression to chronic pain [13,20]. A history of physical abuse and specifically, sexual abuse [21,22] has been identified in the medical history of many subjects with chronic pain. Finally, being a recent immigrant or non-Caucasian may predispose an individual to this disease [13,23].
Physiological factors
In addition to advancing age and low physical activity, another major physiological contributor to chronic pain is poor sleep quality. Studies have demonstrated in both animal models [24] and human studies [25,26] that inadequate or interrupted sleep results in impaired pain inhibitory mechanisms. In addition, a complex relationship exists between menopause and chronic pain due to the fact that menopause occurs with aging, and is associated with increased rates of insomnia and depression [27]. A major consideration with chronic pain is to determine the number of co-morbidities a patient may demonstrate, such as headache and obesity [28].
Psychological factors
Numerous studies have identified depression and anxiety as major contributing factors in chronic pain. A recent meta-analysis found that persons with chronic pain experienced a range of psychological impairments related to anxiety, depression, anger/hostility, self-esteem, and general emotional functioning [29]. Burke et al. [30] reported that depression and chronic pain may co-occur in up to 80% of individuals suffering from those disorders. Clearly, physical therapy management must acknowledge these contributing factors when considering rehabilitative strategies in subjects with chronic pain.
Mechanisms of chronic pain
Interaction between peripheral and central mechanisms for chronic pain
There is significant debate on the role of nociceptive sensitization mechanisms in the etiology and pathology of chronic pain, particularly the interaction of peripheral and central factors. Briefly, peripheral sensitization is defined as an increased responsiveness and reduced threshold of peripheral nociceptors to noxious stimulation of their receptive fields [10]. This occurs in response to a noxious event such as inflammation occurring with tissue injury. Inflammatory mediators cause neuroplastic changes of the nociceptors innervating the damaged tissue. Peripheral sensitization is a protective mechanism and by definition, is limited to nociceptors within the site of the inflammatory milieu and will resolve as tissues heal and inflammation recedes [31]. Central sensitization is defined as the increased response of nociceptive neurons in the central nervous system to noxious stimuli, mediated by amplification of signaling to the central nervous system, potentially at both spinal and supraspinal levels [10]. Two main mechanisms have been recognized as contributing to central sensitization: an increased excitation (i.e. sensitization) by long-lasting peripheral nociceptive stimuli or impaired descending pain inhibition (see review by Woolf [32]). While directly determining central sensitization is not possible in humans, laboratory studies have indirectly determined the former in various patient populations by demonstration of diminished threshold to elicit the nociceptive reflex [33–36] and the latter by examining conditioned pain modulation [37–40]. One mechanism associated with peripheral and central sensitization is neurogenic inflammation. When sensitized peptidergic (e.g. C fibers) are stimulated, neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP) are secreted at the periphery which facilitates vascular changes, plasma extravasation and pain [41], and at the dorsal horn [42], which facilitates central sensitization. Moreover, it has been demonstrated in cutaneous tissues that Aβ fibers, which typically deliver sensory input, such as light touch and vibration, to the spinal cord may undergo a phenotypic change in the presence of inflammation and begin to express C-fiber associated neuropeptides (e.g. substance P) at the periphery and centrally at the dorsal horn [43]. This phenomenon likely occurs in other sensitized tissues as well and would indicate that even non-painful input, such as exercise or manual therapy in the patient with chronic pain, can cause a flare-up of symptoms.
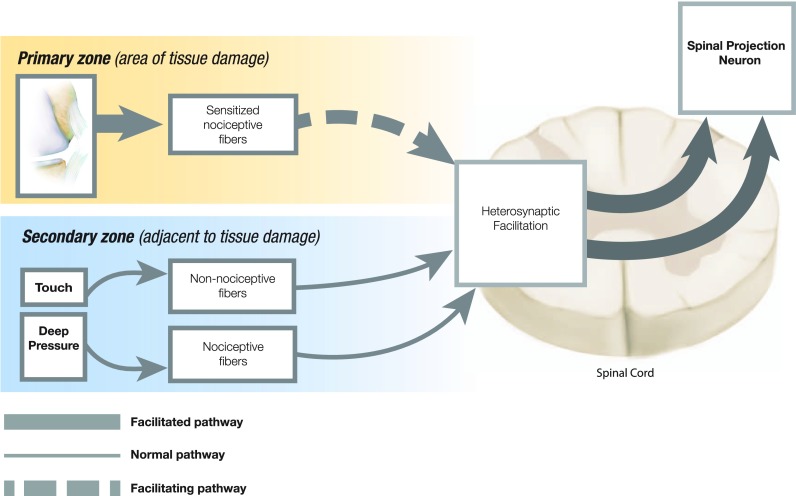
Mechanical hyperalgesia
Distinct clinical findings have been associated with specific mechanisms of central sensitization. Expanded locus of pain is an example of this and may present in various ways such as enlarged distribution of hyperalgesia, increased number of areas of pain including mirror-image pain, regional pain, and even widespread pain. One mechanism that may explain the expansion of pain beyond the area of insult/injury, (i.e. secondary hyperalgesia) is heterosynaptic facilitation (Figure 1) [44]. While repetitive peripheral input may sensitize synaptic connections to dorsal horn neurons (homosynaptic facilitation), secondary hyperalgesia is thought to occur due to interneuronal facilitation of adjacent spinal projection pathways (heterosynaptic facilitation) [45]. Arendt-Nielsen and Graven-Nielsen [46] have described this as an opening of ‘silent’ (ineffective) synapses in the spinal cord by nociceptive input from musculoskeletal tissues, which may also be the genesis of referred pain. Mirror image pain may represent a segmental spread of secondary hyperalgesia, while regional (spreading) hyperalgesia may represent an extra-segmental expansion of secondary hyperalgesia throughout the lower or upper extremities. Heterosynaptic facilitation underlies most major changes in neuron receptive field properties and in pain sensitivity, and is likely responsible for secondary hyperalgesia and allodynia [44,47]. However, impairment of descending inhibitory mechanisms may also be responsible, at least in part, for widespread hyperalgesia [48]. Schliessbach et al. [49] found that generalized (widespread) central hypersensitivity affected 17.5–35.3% of patients with chronic pain, depending on the normative standard used. One measure commonly used to objectively quantify hyperalgesia is pressure pain threshold (PPT), defined as the pressure stimulus of least intensity at which an individual perceives pain [50]. This is typically measured through use of a pressure algometer, with an applicator tip (1 cm2) designed to stimulate deep somatic tissues. Sites for measurement are chosen to identify the extent of pain expansion and comparison is made to the contra-lateral side or to normative values when available. Several studies have used this assessment tool and have clinically identified central sensitization in chronic conditions including tension-type headache [51], carpal tunnel syndrome [52], and low back pain [53]. Similarly, central sensitization has been also demonstrated in non-musculoskeletal widespread pain conditions such as irritable bowel syndrome [54] and migraine [55]. All of these studies support the presence of central sensitization in chronic pain conditions but do not exclude the role of peripheral sensitization mechanisms.
Figure 1.
Heterosynatic facilitation.
Note: Purported mechanism underlying allodynia and secondary hyperalgesia.
Mechanical allodynia
Another clinical finding indicative of central sensitization is allodynia, which is defined as the experience of pain with a normally non-noxious stimulus. It is typically measured dynamically by lightly brushing the skin [56], and thereby only stimulates low threshold non-nociceptive receptors. The presence of allodynia following musculoskeletal injury or chronic condition is generally considered to be centrally mediated, occurring due to extra-synaptic facilitation of spinal projection neurons [44,45]. Specifically, the transient receptor potential cation channel (TRPV1) has been implicated in this mechanism [57]. Tactile allodynia is not uncommon in musculoskeletal conditions or non-musculoskeletal conditions [58] and is often found in the region of most pain [59]. Mapping allodynia can be of value clinically as it may serve as an important outcome measure for reassessment.
Thermal hyperalgesia
In addition, to mechanical hyperalgesia/allodynia, thermal sensitivity is also considered a manifestation of sensitization mechanisms. Thermal quantitative sensory measures, such as heat or cold detection thresholds, or heat pain/cold pain detection thresholds, are used to identify lesions in somatosensory pathways or neuroplastic changes due to chronic pain, and have been commonly used in assessment of neuropathic pain [60]. For example, following peripheral nerve lesions, increased expression of cold-sensing ion channels in dorsal root ganglion cells has been found in an animal model study, which may be the genesis of cold hypersensitivity [61]. It is important to note that pain thresholds are more appropriate than detection thresholds for assessing thermal nociceptive pathways in chronic pain [56]. This may be related to the fact that heat hyperalgesia is thought to be a sign of peripheral nociceptor sensitization [62] and cold hyperalgesia is considered a feature of neuropathic pain as a result of peripheral nerve injury [63]. Nevertheless, changes in thermal sensitivity in uninjured areas are also considered due to central sensitization mechanisms. For instance, bilateral thermal hyperalgesia has been found in individuals with strictly unilateral carpal tunnel syndrome [64]. In addition, thermal pain sensitivity was found to be an important factor in predicting success with management of carpal tunnel syndrome [65]. Cold pain hyperalgesia has been demonstrated in patients with strictly unilateral musculoskeletal pain conditions such as lateral epicondylalgia and associated with higher disability [66], however, other studies have failed to find thermal changes in musculoskeletal disorders without neuropathic involvement [67–69]. Clearly, further research on the relationship between thermal sensitivity and chronic pain is warranted.
Hypoesthesia
Deficits in innocuous sensory modalities such as cutaneous mechanical detection threshold or vibration sense may occur due to a peripheral nerve lesion resulting in sensory deficits. The resulting clinical finding would be hypoesthesia, defined as a decreased sensitivity to stimulation [10]. However, it is possible that pain may cause an inhibition of innocuous input particularly at the site of pain, resulting in hypoesthesia. In fact, a study employing experimentally induced pain demonstrated reduced vibrotactile sensitivity at the site of pain [70]. The authors referred to this phenomenon as a ‘touch gate’ suggesting that pain inhibited sensory afference at a supra-spinal level. Alternatively, other researchers have suggested the mechanism of pain induced hypoesthesia may be spinally mediated [71]. Deficits in mechanical detection (light touch), vibration perception, and proprioception are common in chronic pain conditions, but have mostly been reported in chronic musculoskeletal conditions. The functional significance of pain induced hypoesthesia is mostly unknown, however, diminished vibration detection acuity has been associated with perceived instability during a functional task [59].
Dynamic measures of central sensitization
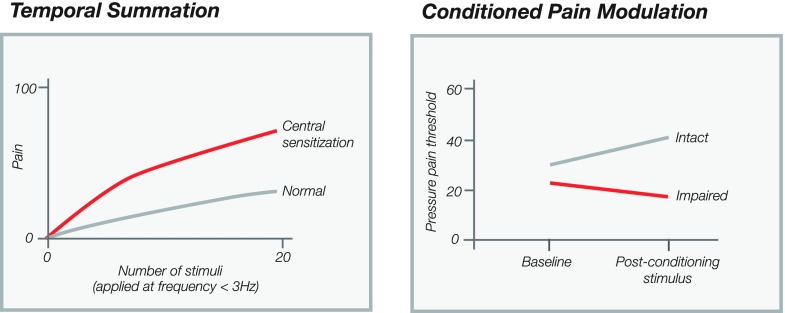
It has been argued that many quantitative sensory measures are static in nature and provide only a snapshot of the neurophysiologic status of the central nervous system. Dynamic measures provoke the system through application of painful stimuli in a specific manner. Two methods commonly used in the laboratory and perhaps less so clinically are temporal summation and conditioned pain modulation. Temporal summation is the clinical correlate of neurophysiological phenomenon of windup, which is defined as the progressively increasing activity in dorsal horn cells following repetitive activation of primary afferent C-fibers (Figure 2) [42]. Temporal summation is produced by repetitive high threshold C- and/or Aδ-fiber stimulation applied at a frequency of less than 3 Hz [72,73] and subjective measures of pain are collected at specific intervals (Figure 2) [74]. A steeper slope of increasing pain is indicative of centrally mediated pain indicating facilitated synaptic efficacy at the dorsal horn [73].
Figure 2.
Dynamic measures of central sensitization. (A) Temporal summation, (B) Conditioned pain modulation.
Conditioned pain modulation examines the ability of descending pain mechanisms to inhibit pain. A test stimulus (baseline measure of pain threshold) is applied before and after application of a conditioning stimulus, such as cold pain or ischemic pain, at a distant site. The conditioning stimulus should activate the diffuse noxious inhibitory control (DNIC) system which is one of the main descending pain inhibition pathways [75]. In a normal response, the test stimulus is perceived as less painful following application of the painful conditioning stimulus (Figure 2). This represents the body’s adaptation of incoming nociceptive information to momentary as well as ongoing needs and requirements [37]. When these mechanisms are impaired, the perception of the test stimulus is unchanged or worsened.
Clinical considerations for pain mechanisms-based management
Clinical and basic science evidence has demonstrated that proper management of patients with chronic pain should be multimodal and approached from a personalized patient’s perspective including proper passive and/or active strategies, active listening, empathy, and consideration of psycho-social issues based on clinical findings during the subjective and objective examination. This is particularly important in patients with chronic pain since it is helpful to encourage patients to choose among various treatment options after proper explanation of the benefits and risks of each therapeutic approach. Asking the patient to participate in decision processes allows them to take responsibility for the management of their condition [76]. Interventions for individuals with chronic pain should be applied based on current knowledge of nociceptive pain mechanisms. The challenge facing clinicians is how to select the proper therapeutic approach for each patient, who is likely to differ in individual presentation [77]. Key indicators of central sensitization may include the presence of regional or widespread hyperalgesia (often measured by PPT), allodynia (in the absence of cutaneous injury), absence of pain modulation with application of painful stimuli (conditioned pain modulation), and steeper elevation in pain response to temporal summation. Findings that may increase suspicion of central sensitization and trigger further testing include the presence of heightened pain either at rest or in response to an activity/intervention, spontaneous pain without trigger, increased number of areas of pain or expanded areas of pain that follow no typical distribution, and/or the presence of hypoesthesia or dysesthesia in the area of most pain that does not follow the distribution of a nerve.
Top-down sensitizers
Determining the drivers of the sensitization process is critical for effective management. For example, objective measures are available to identify psychological contributors, such as depression and anxiety to the pain presentation. These are sometimes referred to as ‘top down’ sensitizers [37]. When appropriate, clinicians may use strategies to address these contributors [78]. Proper referral and communication with other health care providers is critical in the holistic care of the patient with chronic pain.
Bottom-up sensitizers: inflammation
Determining the potential ‘bottom up’ drivers of central sensitization is also important. Inflammation is a potent driver of the sensitization process and thus, must be a target in the rehabilitation plan of care. In a study of over 1100 subjects with knee OA, Neogi et al. [79] found that inflammation, as evidenced by synovitis or effusion, was significantly associated with pain sensitization. In terms of medical interventions, Etoricoxib, (Merck, Kenilworth NJ) a non-steroidal anti-inflammatory (NSAIDS) Cox-II inhibitor medication, has been demonstrated to modulate central sensitization [80]. In comparison to placebo, this medication diminished PPTs, regional sensitization, and temporal summation but had no effect on conditioned pain modulation. More and more, medical practitioners are choosing to target specific pain mechanisms in their prescription of medications [81]. Also in subjects with knee OA, excitability of nociceptive pathways measured via the nociceptive reflex (an indirect indicator of central sensitization) was reduced after joint aspiration and further reduced following intra-articular corticosteroid injection, further demonstrating the importance of controlling inflammation [82]. Medical management has also targeted inflammation in non-musculoskeletal disorders such as inflammatory bowel syndrome [83].
Importance of proper dosage
Accordingly, while manual therapy and therapeutic exercise typically exert hypoalgesia by activating descending inhibitory pain mechanisms [40,84], in subjects with central sensitization the opposite may occur; exercise [85] and potentially manual therapy may induce hyperalgesia if not properly controlled. In fact, aggressive exercise or manual therapy in an early stage of rehabilitation may be detrimental if excessive or forceful movements trigger sensitized peripheral nociceptors and cause increased or prolonged pain. This flare response may occur through mechanisms of neurogenic inflammation where inflammatory mediators such as Substance P and CGRP are released into the periphery and promote pain and chronic inflammation [41]. Further, patients with chronic pain conditions such as fibromyalgia may experience greater exercise induced hypoalgesia with lower intensity exercise. Clinicians must be skilled at discerning and interpreting patient symptoms during rehabilitative programs through serial reassessment. The aim of any intervention is the restoration of the function by limiting the chance of sustained central nervous system facilitation. Directing treatment at aberrant pain processing may have the result of diminishing pain and increasing function (Table 1).
Table 1.
Physical therapy interventions for chronic pain and targeted neurophysiologic mechanisms.
| Physical therapy intervention | Neurophysiologic mechanism |
| Active interventions | |
| Promote quality sleep | Disturbed sleep can result in impaired pain inhibition |
| Aerobic exercise | Promotes descending inhibition of pain |
| Isometric exercise | Systemic and local inhibitory mechanisms |
| 25% MVC* until task failure | |
| Brief MVC* (3 s duration/1 min apart) | |
| Educational – Cognitive interventions | |
| Pain science education | Diminishes psychological (top down) drivers of pain |
| Graded approach to increased functional activity | Promotes pain relief and well-being without triggering inflammatory flare thought to occur via neurogenic inflammation |
| Passive interventions | |
| Manual Therapy | Decreases central sensitization |
| Promotes descending inhibition of pain | |
| TENS | Promotes descending inhibition of pain |
| Noxious electrical stimulation | Promotes descending inhibition of pain |
Note: *MVC: Maximum voluntary contraction.
Sleep quality
An important objective in the rehabilitation of the chronic pain patient is to encourage proper sleep hygiene. Altered/interrupted sleep patterns causes impairment of descending pain mechanisms [25,26], which can promote widespread hyperalgesia and hinder rehabilitative aims. Evaluation of sleep quality may be inconsistent in typical rehabilitative practice settings. A recent systematic review and meta-analysis suggested that the Pittsburgh sleep quality index was a reliable and valid screening tool for sleep dysfunction in non-clinical and clinical samples [86]. Components of sleep quality include sleep efficiency, sleep latency, sleep duration, sleep quality, sleep disturbance, sleep medication use, and daytime dysfunction due to sleepiness [87]. Trouble with falling asleep, staying asleep, and feeling tired were reported as 3 of the main issues for individuals with persistent pain [88]. Clinicians may educate patients on sleep quality and advise them on positions of comfort in the case of pain interrupted sleep.
Aerobic exercise as a pain intervention
Often individuals with chronic pain become extremely deconditioned, due to sedentary lifestyles and activity related painful flare-ups. They become fearful of exercise because they believe that pain equates to tissue damage. The result is a negative cycle of pain and deconditioning. Developing an aerobic exercise routine is valuable as aerobic activity activates descending pain modulation [89]. The clinician may need to progress aerobic routine in a graded manner, gradually increasing time or distance to avoid flare-ups. In the case of regional pain due to central sensitization, the clinician may choose to aerobically exercise the unaffected limbs. For example, in the patient with chronic neck pain, a walking program may be effective while swimming may be beneficial in the chronic visceral pain population. In the lower extremity patient, reduced weight-bearing treadmill walking may be a means to initiate aerobic exercise with the goal of progressing to an independent walking program. Exercise of higher intensity (60–75% V O2max) most consistently produced exercise induced analgesia after aerobic exercise in a low back pain cohort [90] and may be a standard for aerobic exercise in other chronic pain populations.
Muscle contraction as a pain intervention
Proper strength is important for normal balance, gait, and overall function. Recent evidence has suggested that strengthening programs may be effective in the management of chronic pain. Specifically, clinical trials have demonstrated that workplace strength training was valuable in reducing pain and preventing job disruption [91,92]. While strength training has the value of protecting and stabilizing joints and other tissues, it is also known that exercise has analgesic effects, in particular isometric exercise. With isometric contraction, greatest decrease in sensitivity to noxious stimulus occurs after low-intensity contractions (25–50% MVC) held for longer duration [93]. Strength training should be progressed in a graded manner.
Noxious electrical stimulation as a pain intervention
Experimentally, noxious stimulation is employed to induce descending pain modulation when investigating conditioned pain modulation. In the clinical setting, noxious interventions may be used to produce pain relief in a similar manner. Although evidence is limited, it is likely that the treatment effects of interventions such as noxious electrical stimulation, cupping, and even dry needling may be mediated, at least in part, due to this mechanism. Studies in Achilles tendinopathy and knee OA have reported beneficial outcomes with noxious electrical stimulation [94,95].
Conclusion
One of the more significant challenges of chronic pain is the interpretation of the clinical manifestations of peripheral and central sensitization processes. This interpretation should determine treatment parameters, e.g. intensity, amplitude, and frequency of the techniques based on the dominance, peripheral, or central, of each patient. In addition, the potential neurophysiologic and tissue mechanisms underlying the effects (positive and negative) of any intervention should also be considered. Management of individuals with chronic pain should extend beyond local tissue-based pathology to incorporate therapeutic strategies directed at normalizing central nervous system sensitivity. The existence of a wide range of conservative treatments (i.e. medication, electro-physical agents, exercise, cognitive interventions, manual therapies) advocated for chronic pain, is an indication that not one treatment has proven superiority and also that a multi-modal, interdisciplinary approach is warranted.
Contributors
All listed authors had a role in formulation of this clinical commentary. All authors have reviewed and approved the submitted manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Orthopaedic Section of the American Physical Therapy Association [grant number 37-6000511].
References
- [1].Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- [2].IOM Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- [3].Fayaz A, Croft P, Langford RM, et al. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6:2015–010364. doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schäfer I, Kaduszkiewicz H, Wagner HO, et al. Reducing complexity: a visualisation of multimorbidity by combining disease clusters and triads. BMC Public Health. 2014;14:101, 2458-14-1285. doi: 10.1186/1471-2458-14-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Morales-Espinoza EM, Kostov B, Salami DC, et al. Complexity, comorbidity, and health care costs associated with chronic widespread pain in primary care. Pain. 2016;157:818–826. doi: 10.1097/j.pain.0000000000000440. [DOI] [PubMed] [Google Scholar]
- [6].Novak SP, Glasheen C, Roland CL. Prescription pain reliever misuse and levels of pain impairment: 3-year course in a nationally representative outpatient sample of US adults. Subst Abuse Rehabil. 2016;7:87–98. doi: 10.2147/SAR.S102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jimenez-Sanchez S, Jimenez-Garcia R, Hernandez-Barrera V, et al. Has the prevalence of invalidating musculoskeletal pain changed over the last 15 years (1993-2006)? A Spanish population-based survey. J Pain. 2010;11:612–620. doi: 10.1016/j.jpain.2009.09.015. [DOI] [PubMed] [Google Scholar]
- [8].Sluka KA, O’Donnell JM, Danielson J, et al. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol. 1985;2013:725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17:E119–28. [PubMed] [Google Scholar]
- [10].International Association for the Study of Pain Proposed Pain Terminology Available from: http://www.iasp-pain.org/AM/Template.cfm?Section=Pain_Definitions&Template=/CM/HTMLDisplay.cfm&ContentID=1728
- [11].Jensen MP, Tome-Pires C, de la Vega R, et al. What determines whether a pain is rated as mild, moderate, or severe? The importance of pain beliefs and pain interference. Clin J Pain. 2016. [Epub ahead of print]. doi: 10.1097/AJP.0000000000000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–129. doi: 10.1016/j.neuroscience.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25:173–183. doi: 10.1016/j.berh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- [14].Carli G, Suman AL, Biasi G, et al. Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. Pain. 2002;100:259–269. doi:S030439590200297X [pii]. [DOI] [PubMed] [Google Scholar]
- [15].Dworkin RH, Turk DC, Peirce-Sandner S, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149:177–193. doi: 10.1016/j.pain.2010.02.018. [DOI] [PubMed] [Google Scholar]
- [16].Elliott AM, Smith BH, Penny KI, et al. The epidemiology of chronic pain in the community. Lancet. 1999;354:1248–1252. doi:S0140673699030573 [pii]. [DOI] [PubMed] [Google Scholar]
- [17].May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12:455–464. doi: 10.1038/nrneurol.2016.93. [DOI] [PubMed] [Google Scholar]
- [18].Stewart Williams J, Ng N, Peltzer K, et al. Risk factors and disability associated with low back pain in older adults in low- and middle-income countries. results from the WHO study on global ageing and adult health (SAGE). PLos One. 2015;10:e0127880. doi: 10.1371/journal.pone.0127880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abásolo L, Lajas C, León L, et al. Prognostic factors for long-term work disability due to musculoskeletal disorders. Rheumatol Int. 2012;32:3831–3839. doi: 10.1007/s00296-011-2264-5. [DOI] [PubMed] [Google Scholar]
- [20].Hung M, Bounsanga J, Voss MW, et al. The relationship between family support; pain and depression in elderly with arthritis. Psychol Health Med. 2016;22:1–12. doi: 10.1080/13548506.2016.1211293. [DOI] [PubMed] [Google Scholar]
- [21].Spiegel DR, Shaukat AM, Mccroskey AL, et al. Conceptualizing a subtype of patients with chronic pain: the necessity of obtaining a history of sexual abuse. Int J Psychiatry Med. 2016;51:84–103. doi: 10.1177/0091217415621268. [DOI] [PubMed] [Google Scholar]
- [22].Paras ML, Murad MH, Chen LP, et al. Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302:550–561. doi: 10.1001/jama.2009.1091. [DOI] [PubMed] [Google Scholar]
- [23].Kellner U, Halder C, Litschi M, et al. Pain and psychological health status in chronic pain patients with migration background–the Zurich study. Clin Rheumatol. 2013;32:189–197. doi: 10.1007/s10067-012-2099-9. [DOI] [PubMed] [Google Scholar]
- [24].Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94:1659–1663. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- [25].Palermo TM, Wilson AC, Lewandowski AS, et al. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain. 2011;152:89–94. doi: 10.1016/j.pain.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bjurstrom MF, Irwin MR. Polysomnographic characteristics in nonmalignant chronic pain populations: A review of controlled studies. Sleep Med Rev. 2016;26:74–86. doi: 10.1016/j.smrv.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ancoli-Israel S, Soares CN, Gaeta R, et al. Insomnia in special populations: effects of aging, menopause, chronic pain, and depression. Postgrad Med. 2004;116:33–47. doi: 10.3810/pgm.12.2004.suppl38.260. [DOI] [PubMed] [Google Scholar]
- [28].Coronado RA, Alappattu MJ, Hart DL, et al. Total number and severity of comorbidities do not differ based on anatomical region of musculoskeletal pain. J Orthop Sports Phys Ther. 2011;41:477–485. doi: 10.2519/jospt.2011.3686. [DOI] [PubMed] [Google Scholar]
- [29].Burke AL, Mathias JL, Denson LA. Psychological functioning of people living with chronic pain: a meta-analytic review. Br J Clin Psychol. 2015;54:345–360. doi: 10.1111/bjc.12078. [DOI] [PubMed] [Google Scholar]
- [30].Burke NN, Finn DP, Roche M. Neuroinflammatory Mechanisms linking pain and depression. Mod Trends Pharmacopsychiatri. 2015;30:36–50. doi: 10.1159/000435931. [DOI] [PubMed] [Google Scholar]
- [31].Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- [32].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sterling M, Pedler A, Chan C, et al. Cervical lateral glide increases nociceptive flexion reflex threshold but not pressure or thermal pain thresholds in chronic whiplash associated disorders: a pilot randomised controlled trial. Man Ther. 2010;15:149–153. doi: 10.1016/j.math.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [34].Courtney CA, Lewek MD, Witte PO, et al. Heightened flexor withdrawal responses in subjects with knee osteoarthritis. J Pain. 2009;10:1242–1249. doi: 10.1016/j.jpain.2009.05.004. [DOI] [PubMed] [Google Scholar]
- [35].Courtney CA, Durr RK, Emerson-Kavchak AJ, et al. Heightened flexor withdrawal responses following ACL rupture are enhanced by passive tibial translation. Clin Neurophysiol. 2011;122:1005–1010. doi: 10.1016/j.clinph.2010.07.029. [DOI] [PubMed] [Google Scholar]
- [36].Lim EC, Sterling M, Pedler A, et al. Evidence of spinal cord hyperexcitability as measured with nociceptive flexion reflex (NFR) threshold in chronic lateral epicondylalgia with or without a positive neurodynamic test. J Pain. 2012;13:676–684. doi: 10.1016/j.jpain.2012.04.005. [DOI] [PubMed] [Google Scholar]
- [37].Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Perrotta A, Serrao M, Sandrini G, et al. Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia. 2010;30:272–284. doi: 10.1111/j.1468-2982.2009.01914.x. [DOI] [PubMed] [Google Scholar]
- [39].Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- [40].Courtney CA, Steffen AD, Fernandez-de-Las-Penas C, et al. Joint mobilization enhances mechanisms of conditioned pain modulation in individuals with osteoarthritis of the knee. J Orthop Sports Phys Ther. 2016;46:168–176. doi: 10.2519/jospt.2016.6259. [DOI] [PubMed] [Google Scholar]
- [41].Walsh DA, Mapp PI, Kelly S. Calcitonin gene-related peptide in the joint: contributions to pain and inflammation. Br J Clin Pharmacol. 2015;80:965–978. doi: 10.1111/bcp.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ji RR, Kohno T, Moore KA, et al. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. 10.1016/j.tins.2003.09.017 [DOI] [PubMed] [Google Scholar]
- [43].Neumann S, Doubell TP, Leslie T, et al. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- [44].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Treede RD, Magerl W. Multiple mechanisms of secondary hyperalgesia. Prog Brain Res. 2000;129:331–341. doi:S0079-6123(00)29025-0 [pii]. [DOI] [PubMed] [Google Scholar]
- [46].Arendt-Nielsen L, Graven-Nielsen T. Translational musculoskeletal pain research. Best Pract Res Clin Rheumatol. 2011;25:209–226. doi: 10.1016/j.berh.2010.01.013. [DOI] [PubMed] [Google Scholar]
- [47].Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. 10.1038/306686a0 [DOI] [PubMed] [Google Scholar]
- [48].Arendt-Nielsen L, Skou ST, Nielsen TA, et al. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporos Rep. 2015;13:225–234. doi: 10.1007/s11914-015-0276-x. [DOI] [PubMed] [Google Scholar]
- [49].Schliessbach J, Siegenthaler A, Streitberger K, et al. The prevalence of widespread central hypersensitivity in chronic pain patients. Eur J Pain. 2013;17:1502–1510. doi: 10.1002/j.1532-2149.2013.00332.x. [DOI] [PubMed] [Google Scholar]
- [50].Courtney CA, Kavchak AE, Lowry CD, et al. Interpreting joint pain: quantitative sensory testing in musculoskeletal management. J Orthop Sports Phys Ther. 2010;40:818–825. doi: 10.2519/jospt.2010.3314. [DOI] [PubMed] [Google Scholar]
- [51].de Tommaso M, Fernandez-de-Las-Penas C. Tension type headache. Curr Rheumatol Rev. 2016;12:127–139. doi: 10.2174/1573397112666151231113625. [DOI] [PubMed] [Google Scholar]
- [52].Fernandez-de-las-Penas C, de la Llave-Rincon AI, Fernandez-Carnero J, et al. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain. 2009;132:1472–1479. doi: 10.1093/brain/awp050. [DOI] [PubMed] [Google Scholar]
- [53].Sanzarello I, Merlini L, Rosa MA, et al. Central sensitization in chronic low back pain: A narrative review. J Back Musculoskelet Rehabil. 2016;29:625–633. doi: 10.3233/BMR-160685. [DOI] [PubMed] [Google Scholar]
- [54].Stabell N, Stubhaug A, Flægstad T, et al. Widespread hyperalgesia in adolescents with symptoms of irritable bowel syndrome: results from a large population-based study. J Pain. 2014;15:898–906. doi: 10.1016/j.jpain.2014.05.007. [DOI] [PubMed] [Google Scholar]
- [55].Palacios-Ceña M, Lima Florencio L, Natália Ferracini G, et al. Women with chronic and episodic migraine exhibit similar widespread pressure pain sensitivity. Pain Med. 2016;17:2127–2133. doi: 10.1093/pm/pnw056. [DOI] [PubMed] [Google Scholar]
- [56].Rolke R, Baron R, Maier C, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [57].Kim YH, Back SK, Davies AJ, et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron. 2012;74:640–647. doi: 10.1016/j.neuron.2012.02.039. [DOI] [PubMed] [Google Scholar]
- [58].Jarrell J. Demonstration of cutaneous allodynia in association with chronic pelvic pain. J Vis Exp 2009;28 pii: 1232. doi: 10.3791/1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kavchak AJ, Fernández-de-las-Peñas C, Rubin LH, et al. Association between altered somatosensation, pain, and knee stability in patients with severe knee osteoarthrosis. Clin J Pain. 2012;28:589–594. doi: 10.1097/AJP.0b013e31823ae18f. [DOI] [PubMed] [Google Scholar]
- [60].Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain. 2007;129:256–259. doi: 10.1016/j.pain.2007.03.030. [DOI] [PubMed] [Google Scholar]
- [61].Xing H, Chen M, Ling J, et al. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Raja SN, Campbell JN, Meyer RA. Evidence for different mechanisms of primary and secondary hyperalgesia following heat injury to the glabrous skin. Brain. 1984;107:1179–1188. 10.1093/brain/107.4.1179 [DOI] [PubMed] [Google Scholar]
- [63].de Medinaceli L, Hurpeau J, Merle M, et al. Cold and post-traumatic pain: modeling of the peripheral nerve message. Biosystems. 1997;43:145–167. doi:S0303264797016857 [pii]. [DOI] [PubMed] [Google Scholar]
- [64].de la Llave-Rincon AI, Fernandez-de-las-Penas C, Fernandez-Carnero J, et al. Bilateral hand/wrist heat and cold hyperalgesia, but not hypoesthesia, in unilateral carpal tunnel syndrome. Exp Brain Res. 2009;198:455–463. doi: 10.1007/s00221-009-1941-z. [DOI] [PubMed] [Google Scholar]
- [65].Fernandez-de-Las-Penas C, Fernandez-Munoz JJ, Navarro-Pardo E, et al. Identification of subgroups of women with carpal tunnel syndrome with central sensitization. Pain Med 2016;17:1749-1756 doi: 10.1093/pm/pnw054. [DOI] [PubMed] [Google Scholar]
- [66].Coombes BK, Bisset L, Vicenzino B. Thermal hyperalgesia distinguishes those with severe pain and disability in unilateral lateral epicondylalgia. Clin J Pain. 2012;28:595–601. doi: 10.1097/AJP.0b013e31823dd333. [DOI] [PubMed] [Google Scholar]
- [67].Rakel B, Vance C, Zimmerman MB, et al. Mechanical hyperalgesia and reduced quality of life occur in people with mild knee osteoarthritis pain. Clin J Pain. 2015;31:315–322. doi: 10.1097/AJP.0000000000000116. [DOI] [PubMed] [Google Scholar]
- [68].Jensen R, Hystad T, Kvale A, et al. Quantitative sensory testing of patients with long lasting Patellofemoral pain syndrome. Eur J Pain. 2007;11:665–676. doi: 10.1016/j.ejpain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [69].Coronado RA, Kindler LL, Valencia C, et al. Thermal and pressure pain sensitivity in patients with unilateral shoulder pain: comparison of involved and uninvolved sides. J Orthop Sports Phys Ther. 2011;41:165–173. doi: 10.2519/jospt.2011.3416. [DOI] [PubMed] [Google Scholar]
- [70].Apkarian AV, Stea RA, Bolanowski SJ. Heat-induced pain diminishes vibrotactile perception: a touch gate. Somatosens Mot Res. 1994;11:259–267. 10.3109/08990229409051393 [DOI] [PubMed] [Google Scholar]
- [71].Geber C, Magerl W, Fondel R, et al. Numbness in clinical and experimental pain – a cross-sectional study exploring the mechanisms of reduced tactile function. Pain. 2008;139:73–81. doi: 10.1016/j.pain.2008.03.006. [DOI] [PubMed] [Google Scholar]
- [72].Hornby TG, Rymer WZ, Benz EN, et al. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- [73].Arendt-Nielsen L. Central sensitization in humans: assessment and pharmacology. Handb Exp Pharmacol. 2015;227:79–102. doi: 10.1007/978-3-662-46450-2_5. [DOI] [PubMed] [Google Scholar]
- [74].Bialosky JE, Bishop MD, Robinson ME, et al. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther. 2009;89:1292–1303. doi: 10.2522/ptj.20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13:936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- [76].Nijs J, Roussel N, Paul van Wilgen C, et al. Thinking beyond muscles and joints: therapists’ and patients’ attitudes and beliefs regarding chronic musculoskeletal pain are key to applying effective treatment. Man Ther. 2013;18:96–102. doi: 10.1016/j.math.2012.11.001. [DOI] [PubMed] [Google Scholar]
- [77].Nijs J, Van Houdenhove B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: application of pain neurophysiology in manual therapy practice. Man Ther. 2009;14:3–12. doi: 10.1016/j.math.2008.03.001. [DOI] [PubMed] [Google Scholar]
- [78].Archer KR, Devin CJ, Vanston SW, et al. Cognitive-behavioral–based physical therapy for patients with chronic pain undergoing lumbar spine surgery: a randomized controlled trial. J Pain. 2016;17:76–89. doi: 10.1016/j.jpain.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Neogi T, Guermazi A, Roemer F, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol. 2016;68:654–661. doi: 10.1002/art.39488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Arendt-Nielsen L, Egsgaard LL, Petersen KK. Evidence for a central mode of action for etoricoxib (COX-2 inhibitor) in patients with painful knee osteoarthritis. Pain. 2016;157:1634–1644. doi: 10.1097/j.pain.0000000000000562. [DOI] [PubMed] [Google Scholar]
- [81].Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3:263–275. doi: 10.3978/j.issn.2224-5820.2014.10.02. [DOI] [PubMed] [Google Scholar]
- [82].Rice DA, McNair PJ, Lewis GN, et al. The effects of joint aspiration and intra-articular corticosteroid injection on flexion reflex excitability, quadriceps strength and pain in individuals with knee synovitis: a prospective observational study. Arthritis Res Ther. 2015;17:191, 015-0711-5 doi: 10.1186/s13075-015-0711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Vergnolle N. Postinflammatory visceral sensitivity and pain mechanisms. Neurogastroenterol Motil. 2008;20(Suppl 1):73–80. doi: 10.1111/j.1365-2982.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- [84].Koltyn KF, Brellenthin AG, Cook DB, et al. Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15:1294–1304. doi: 10.1016/j.jpain.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Staud R. Is it all central sensitization? role of peripheral tissue nociception in chronic musculoskeletal pain. Curr Rheumatol Rep. 2010;12:448–454. doi: 10.1007/s11926-010-0134-x. [DOI] [PubMed] [Google Scholar]
- [86].Mollayeva T, Thurairajah P, Burton K, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- [87].Nishiyama T, Mizuno T, Kojima M, et al. Criterion validity of the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale for the diagnosis of sleep disorders. Sleep Med. 2014;15:422–429. doi: 10.1016/j.sleep.2013.12.015. [DOI] [PubMed] [Google Scholar]
- [88].Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials: An IMMPACT survey of people with pain. Pain. 2008;137:276–285. doi: 10.1016/j.pain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- [89].Vaegter HB, Handberg G, Graven-Nielsen T. Hypoalgesia after exercise and the cold pressor test is reduced in chronic musculoskeletal pain patients with high pain sensitivity. Clin J Pain. 2016;32:58–69. doi: 10.1097/AJP.0000000000000223 [pii]. [DOI] [PubMed] [Google Scholar]
- [90].Hoffman MD, Shepanski MA, MacKenzie SP, et al. Experimentally induced pain perception is acutely reduced by aerobic exercise in people with chronic low back pain. J Rehabil Res Dev. 2005;42:183–190. 10.1682/JRRD.2004.06.0065 [DOI] [PubMed] [Google Scholar]
- [91].Lidegaard M, Jensen RB, Andersen CH, et al. Effect of brief daily resistance training on occupational neck/shoulder muscle activity in office workers with chronic pain: randomized controlled trial. Biomed Res Int. 2013;2013:262386. doi: 10.1155/2013/262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sundstrup E, Jakobsen MD, Brandt M, et al. Workplace strength training prevents deterioration of work ability among workers with chronic pain and work disability: a randomized controlled trial. Scand J Work Environ Health. 2014;40:244–251. doi: 10.5271/sjweh.3419. [DOI] [PubMed] [Google Scholar]
- [93].Hoeger Bement MK, Dicapo J, Rasiarmos R, et al. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc. 2008;40:1880–1889. doi: 10.1249/MSS.0b013e31817eeecc. [DOI] [PubMed] [Google Scholar]
- [94].Defrin R, Ariel E, Peretz C. Segmental noxious versus innocuous electrical stimulation for chronic pain relief and the effect of fading sensation during treatment. Pain. 2005;115:152–160. doi: 10.1016/j.pain.2005.02.018. [DOI] [PubMed] [Google Scholar]
- [95].Eckenrode BJ, Stackhouse SK. Improved pressure pain thresholds and function following noxious electrical stimulation on a runner with chronic achilles tendinopathy: a case report. Int J Sports Phys Ther. 2015;10:354–362. [PMC free article] [PubMed] [Google Scholar]