Abstract
Objective: We assessed whether or not pain relief could be achieved with a new system that combines 3D augmented reality system (3DARS) and the principles of mirror visual feedback.
Methods: Twenty-two patients between 18 and 75 years of age who suffered of chronic neuropathic pain. Each patient performed five 3DARS sessions treatment of 20 mins spread over a period of one week. The following pain parameters were assessed: (1) visual analogic scale after each treatment session (2) McGill pain scale and DN4 questionnaire were completed before the first session and 24 h after the last session.
Results: The mean improvement of VAS per session was 29% (p < 0.001). There was an immediate session effect demonstrating a systematic improvement in pain between the beginning and the end of each session. We noted that this pain reduction was partially preserved until the next session. If we compare the pain level at baseline and 24 h after the last session, there was a significant decrease (p < 0.001) of pain of 37%. There was a significant decrease (p < 0.001) on the McGill Pain Questionnaire and DN4 questionnaire (p < 0.01).
Conclusion: Our results indicate that 3DARS induced a significant pain decrease for patients who presented chronic neuropathic pain in a unilateral upper extremity. While further research is necessary before definitive conclusions can be drawn, clinicians could implement the approach as a preparatory adjunct for providing temporary pain relief aimed at enhancing chronic pain patients’ tolerance of manual therapy and exercise intervention.
Level of Evidence: 4.
Keywords: Augmented reality, Mirror visual feedback, Physical therapy, Neuropathic pain, Phantom limb pain, CRPS, Mirror therapy
Introduction
Neuropathic symptoms are common in phantom limb pain (PLP),1,2 complex regional pain syndrome (CRPS),3,4 spinal cord injury, and plexopathy. Neuropathic symptoms include pricking, tingling, allodynia, hyperalgesia, and burning pain. This profile usually requires long-term intensive therapy that can often produce various side effects associated with the utilized medications, which can lead to a major impact on the quality of life.
Recent advances in functional imaging technology have revealed that chronic pain conditions are a consequence of neuroplastic changes in the central nervous system. These changes can include cortical reorganization, which has been determined as an origin of PLP and CRPS.4,5 During such reorganization, the cortical areas representing the injured or missing extremity are taken over by the neighboring representational zones in both primary somatosensory and motor cortices. The amount of cortical reorganization has been found to be directly related to the degree of pain and the size of the associated deafferentiated region. This neuronal plasticity results in a maladaptive state between motor output and sensory feedback. To rectify this sensory–motor mismatch, in the case of PLP, authors have proposed the use of mirror therapy for treating the related clinical consequences that are observed in conditions such as CRPS, PLP, and cerebral vascular accident (CVA).1,6–11 While there is reasonable evidence supporting the use of mirror therapy for treating chronic pain, the mechanisms that account for the clinical responses to management are not yet fully understood.
Recent investigations have reported the benefits of virtual reality for pain management.12–15 A general criticism about virtual reality systems is that they are expensive, complex, and likely unusable at home or in the practice settings of with most rehabilitation professionals.16 Another reproach to most commercially available virtual reality systems is that most of them utilize an ‘avatar’ image that is not sufficiently similar to the patient’s human form, potentially creating a perceptual disparity.
The literature that supports virtual reality use in managing chronic pain conditions is limited. Sato et al.17 used virtual reality in the treatment of CRPS during five, once-a-week sessions on five patients. The investigators reported a 50% pain reduction thus opening a new and promising alternative treatment for CRPS. The principles behind this approach are based on an interactive game where a visual tracking device follows the patients’ hand as they complete target-oriented motor response exercises, such as grasping, transferring, and object placement.
Murray et al.18 transposed movements of amputees’ anatomical limbs into movements of a virtual limb in the space occupied by their phantom limb. Their respective setups tracked the motion of the hand using a data glove and movements of the arm or leg using sensors attached to patients’ limbs. The problem with this approach was that it requires the patient to be equipped with a rather complex tracking system, making the entire system cumbersome, and difficult to use in a home setting.
The technological approach common to these studies was built upon the foundational principles associated with mirror therapy. Ramachandran et al.6 used mirror therapy to recreate a coherent body image in the patient’s brain by providing normalized visual feedback of movements that the affected body part is normally challenged to perform, thus modifying the individual’s perceptual constructs associated with the attempted movements. Even if manual therapy can be helpful at the beginning of the treatment, this would seem ineffective once the pain becomes chronic. Presently, no recommendation for manual therapy exists for the treatment of this kind of chronic neuropathic pain.19 Perceptual modification is carried forward and advanced through virtual reality training experiences, offering promise in the management of patients with neuropathic symptoms.
The current study had two purposes. First, to introduce a new virtual reality method that incorporates the mechanisms used in mirror visual feedback through a 3D augmented virtual reality system. Second, to evaluate the efficiency of this new method on a convenience sample of patients presenting with unresolved neuropathic pain (CRPS, PLP, plexopathy, stroke) who were not responding adequately to both pharmaceutical management and traditional mirror therapy.
Methods
Instrumentation
The 3D augmented reality system (3DARS) was designed to increase a person’s immersion experience to as high a level as possible, where immersion represents the individual’s perceptual illusion of movement in the affected body part gathered from visual input. In order to achieve this perceptual response, the system obtains a real-time 3D image of the individual’s moving, non-affected body part (such as the contralateral upper limb) and produces a 3D image of the affected body part moving on the display that the person views. As individuals move the contralateral, non-affected body part, and view the display, they receive visual input that the affected body part is moving in a ‘normal’ fashion without producing pain. The resulting perceptual representation allows patients to feel as though what they are seeing is a reality. The investigators’ use of the 3DARS for testing each patient in the present study avoided the need for an ‘avatar’ and an approximate limb representation. After pilot testing, the perceptual experience of the 3DARS was further improved by adding auditory inputs to enhance the patients’ sense of immersion.
Patients in the study wore 3D glasses and viewed an image of themselves (Fig. 1(A–C)) on a 3D display (Kit NVIDIA 3D Vision®). A 3D camera (Xbox 360 KinectTM Microsoft corporation Model 1414), was used to video-capture the contralateral healthy limb movements in real time.
Figure 1.
(A) 3DARS Virtual Training Procedure 1-Patients raise their non-affected upper extremity and they have the illusion that they move both the affected and the non-affected arms; (B) 3DARS Virtual Training Procedure 2-Patients raise their non-affected upper extremity and they have the illusion that they move the affected upper extremity; (C) Representation of a patient wearing 3D glasses and moving the non-affected upper extremity.
The KinectTM 3D camera produced depth and color images that were synchronized and calibrated. The resolution and frame rate were adjusted to 640 × 480 pixels and 30 frames per second, respectively. The 3D Vision KitTM was used in order to offer active stereovision with a 120 Hz display screen. After video images were captured, the system augmented the 3D reality in two ways: (1) by performing specific image processing, such as lateral flipping or applying a virtual mirror; and (2) by adding to the scene specific virtual objects of different shapes and sizes with which the subject could interact. The 3D images were built thanks to software developed by the department of LISA Laboratory engineering. To construct the 3D images, the depth image, delivered by the Kinect, was used and transformed to a set of points (also called vertices) in 3D space (or point cloud in the literature) using the calibration parameters of the camera. The generated images were realistic and the fact that the patient watched the images in 3D strengthened the perception of quality and realism. A more detailed, technical description of the construction or the 3D images and the application of mirror effect has been reported elsewhere.20
Population
Twenty-two patients (Table 1) were recruited from the local Pain Department Clinic. The inclusion criteria were: (1) age over 18 years; (2) neuropathic pain in the unilateral upper extremity scored by DN4 questionnaire;21 (3) at least 3 months symptom duration following injury; (4) minimum pain of 40 on a visual analog scale (VAS); and (5) a drug treatment regimen that was stable for at least two weeks. Patients were allowed to participate if they presented with any of the following: (1) a CRPS diagnosis, in accordance with the International Association for the Study of Pain criteria, PLP, spinal cord injury, or plexopathy; and (2) a history of failed conventional mirror therapy.
Table 1.
Population demographic data.
| Case | Age | Gender | Pathology | Duration (months) | Cause | Affected limbs | Prescribed medications |
|---|---|---|---|---|---|---|---|
| 1 | 41 | ♀ | Cervical myelopathy | 32 | Discal hernia surgery | L | Clonazepam |
| 2 | 58 | ♂ | CRPS2 | 168 | Plexus brachial c4-t1 | R | Tramadol |
| 3 | 34 | ♀ | CRPS2 | 9 | Carpal tunnel surgery, | R | Medrol |
| 4 | 67 | ♀ | PLP | 16 | Reimplantation | R | Gabapentin, clonazepam |
| 5 | 62 | ♂ | CRPS2 | 6 | Carpel tunnel surgery median | R | Amitriptylin |
| 6 | 51 | ♂ | CRPS1 | 42 | Sauve-Kapandji | L | Paracetamol |
| 7 | 44 | ♀ | Plexopathy | 3 | Acromioplasty | L | Amitriptylin, paracetamol |
| 8 | 38 | ♀ | CRPS1 | 48 | Acromioplasty | L | Oxycodone |
| 9 | 67 | ♂ | Medullopathy | 13 | Tetraparesia | R | None |
| 10 | 48 | ♀ | CRPS1 | 6 | TFCC | L | Clonazepam |
| 11 | 37 | ♂ | CRPS1 | 12 | Tenolysis | R | Tramadol, amitriptylin, paracetamol |
| 12 | 39 | ♂ | CRPS1 | 5 | Suture tendon extensor | L | Calcitonin, clonazepam, chlorhydrate tramadol |
| 13 | 75 | ♂ | CRPS1 | 4 | Distal radius fracture | L | Duloxetin, paracetamol |
| 14 | 63 | ♀ | CRPS1 | 16 | Wrist instability | R | Nothing |
| 15 | 47 | ♀ | CRPS1 | 26 | Trapezectomy | R | Oxycodone, clonazepam, amitriptylin |
| 16 | 37 | ♀ | CRPS1 | 26 | Sauve-Kapandji | R | Pregabalin, prazepam, amitriptylin |
| 17 | 36 | ♂ | PLP | 60 | Amputation | L | Oxycodone |
| 18 | 35 | ♀ | CRPS2 | 24 | Stroke | R | Escitalopram, pregabalin |
| 19 | 59 | ♂ | CRPS2 | 4 | Surgery dupuytren disease | L | Pregabalin |
| 20 | 44 | ♂ | CRPS2 | 12 | Tendinopathy de quervain | R | Pregabalin |
| 21 | 57 | ♀ | CRPS1 | 5 | Wrist fracture | L | Tramadol |
| 22 | 46 | ♀ | CRPS2 | 54 | Median nerve section | R | Pregabalin, clonazepam, fluoxetine |
Legends: ♀: woman, ♂: man, R: right, L: left, CRPS: complex regional pain syndrome.
The exclusion criteria were: (1) bilateral injury, (2) patients with epilepsy, (3) side effects known to 3D (such as nausea, cephalalgia), (4) cognitive disorder, and (5) poor knowledge of the French language.
The experimental protocol was approved by the institutional ethic committee board (ref P2012/112) and all patients gave their approval for the use of their clinical data. This study has been registered on www.clinicaltrials.gov with the reference NCT02582216.
Preparatory procedures
Pain was evaluated by a VAS before and after each treatment session. Patients were asked to report the duration of their pain reduction after each session. The McGill pain scale (French version) and the DN4 questionnaire were completed before the first session and 24 h after the last session.
Intervention
Each patient received five treatments over a period of one week, where each treatment lasted a total of 20 mins. Analgesic medications that had been prescribed at more than two weeks prior to the initiation of the experimental protocol were continued at the same regimen as before. Each treatment period included a 3DARS training session. All 3DARS training sessions were performed in the same quiet room under the supervision of the same single investigator. The 3DARS training session protocol included two virtual training procedures. The first virtual training procedure consisted of applying a vertical virtual mirror to the display screen, where the non-affected side of the patient’s body (such as the right) was symmetrically duplicated by a vertical axis on the affected side (such as the left). When patients moved the non-affected upper extremity (Fig. 1(C)), they had the illusion that they moved both the affected and the non-affected arms (Fig. 1(A)). The second virtual training procedure consisted of flipping the 3D image horizontally along a vertical axis. This allowed patients to observe the reflection of their non-affected upper extremity as if it was the affected one (Fig. 1(B)).
Each treatment was initiated with simple exercises (wrist flexion–extension, forearm pronation–supination, and finger-thumb tip-to-tip movements) period in order to familiarize patients with movements used during the 3DARS training sessions. During that short familiarization experience, they were asked to observe the virtual movements of the affected upper extremity and to maintain their intention to move that extremity.
Following the familiarization exercises, patients were positioned in front of the 3DARS system for both virtual training sessions. During each training session, the 3DARS system implemented a very simple game that consisted of virtual objects of various shapes and sizes popping up in random positions within the field of view. This procedure was aimed at helping patients to focus their attention on the movements of the virtual affected upper extremity. Patients were asked to touch a few targets with the hand or the fingers of the virtual affected upper extremity. After touching the first target it turned red and then disappeared. Then another target would appear at another location in the visual field. The difficulty of that game was progressively increased by asking them to touch the targets first with the palm, then with the fingers and finally with just one finger of the virtual affected upper extremity. Virtual training activities were terminated if patients complained about the development of headache or nausea, which are the most common side effects provoked by the use of stereovision systems similar to the 3DARS system. These symptoms were not present in this population.
Statistical analysis
Descriptive statistics were calculated (means, standard deviation, and 95% confidence intervals) for each data-set. A Friedman one-way analysis of variance (ANOVA) for repeated measurements was used to examine the significant differences between training sessions. The Wilcoxon rank sign test was utilized for post hoc pairwise comparisons in order to identify the location of significant differences. A Spearman coefficient of correlation test was applied to correlate duration of the pathology with changes in pain across the duration of the protocol. In addition, a Cohen’s effect size test was applied on VAS scale evaluating the significant level of the sample. Statistical analysis was performed using Statistica© software with a confidence interval of 95%.
Results
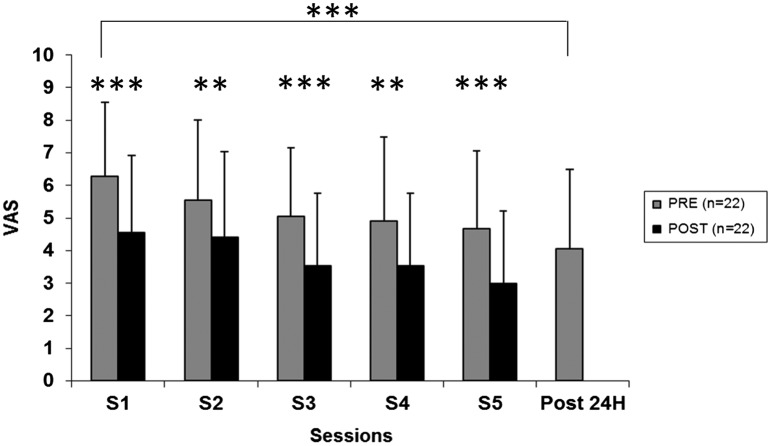
The ANOVA demonstrated a significant 3DARS session main effect (F = 25, 28, df = 5, p < 0.001) with a significant 29% mean VAS improvement per session (Fig. 2).
Figure 2.
Pre, post and 24-h post-pain intensity evaluation for the general population.
Notes: Pain decrease assessed by visual analog scale (VAS) before (Pre) and just after (Post) each session (s1, s2, s3, s4, s5) and 24 h after the last session (Post 24 h). Asteriks denote significant differences (**p < 0.01, ***p < 0.001).
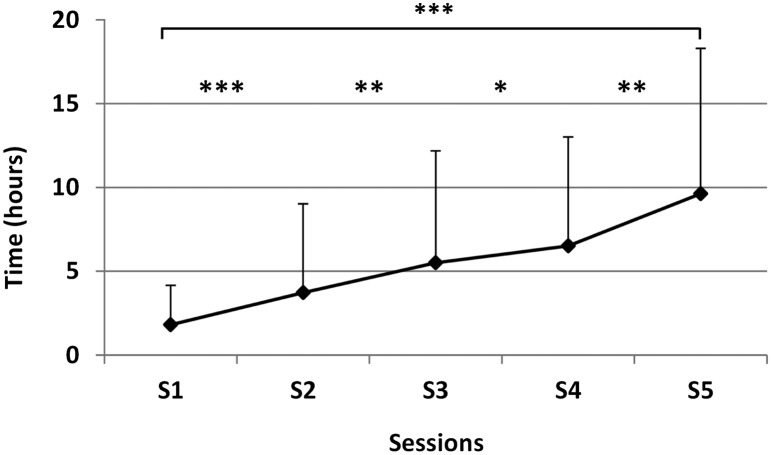
Following post hoc test comparison, the authors noted that the pain reduction was partially preserved until the next session. If we compare the pain level at baseline and 24 h after the last session, there was a significant decrease (p < 0.001) of pain of 37% (±40%). Concerning the duration of pain improvement (Fig. 3), the authors observed a significant increase (p < 0.001) after each session with an average of 1.8 h (±2.3) after the first session and 8 h (±9H) after the last session. From one session to another, pain reappeared progressively without reaching the level of previous session.
Figure 3.
Time effect after the training for the general population.
Notes: Duration (expressed in hours (H)) of pain relief in the general population after each session (s1, s2, s3, s4, s5). Asteriks denote significant differences (*p < 0.05, **p < 0.01, ***p < 0.001).
There was a significant decrease (p < 0.001) of 34% of the McGill’s Pain Questionnaire. The DN4 questionnaire was improved significantly (18%; p < 0.01) (Table 2). No significant or meaningful correlation was observed between the duration of the pathology and pain relief (r:−0.13, r2: 0.02; p < 0.17).
Table 2.
McGill pain and DN4 values (mean ± SD) for pre- and post-24 h after the last session.
| Pre | Post 24 h | p-Value | |
|---|---|---|---|
| McGill pain | 42.2 ± 13.4 | 26.6 ± 19.8 | p = 0.0009 |
| DN4 | 6.7 ± 2.2 | 5.5 ± 2.2 | p = 0.0050 |
The calculated effect size between the first session and 24 h after the last session was 0.97 for the VAS. The mean effect size for all sessions calculated between pre- and post-intervention was 0.65 (±0.13).
Discussion
Chronic pain management is a challenge for healthcare providers. A single treatment is often insufficient for eliminating chronic pain and a multidisciplinary (pharmalogical, physical, and psychological treatment) approach is often recommended. The 3DARS approach may be classified as an augmented reality (AR) system according to the definition of Azuma et al.22 Augmented reality involves projecting a virtual image onto the physical world (rather than immersing the person into a completely artificial environment) and using a screen to provide visual, auditory, and kinesthetic feedback.15
This preliminary study demonstrates that the 3DARS system induced a temporary analgesic effect on patients where mirror therapy had previously failed. It may be a promising alternative treatment of upper limb neuropathic pain. This investigation examined whether a visual illusion, where the patients observed themselves moving the affected arm (through actual movement of the healthy arm), could correct any sensory-mismatch occurring in response to neuropathic changes and, as result, reduce pain.
Such a system, using the healthy arm to create this illusion, is likely based on the same cortical model of modifying pathological pain that has been used to explain the analgesic effect of mirror therapy in patients with PLP and CRPS;1,9,10,23,24 namely, the reorganization of the primary somatosensory cortex (S1). Previous investigations have examined the effects of such a reordering process. For example, Moseley25 used visual illusion to reduce neuropathic pain in patients after spinal cord injury by correcting a mismatch between motor output and sensory feedback.
There have been different hypotheses explaining how this reordering process occurs. Ramachandran and Altschuler1 hypothesized that the vision of the healthy member reflected could activate the mirror neuron system. This system could help to restore the correct information by enhancing a coherent body image in accordance with visual and proprioceptive information.
Another hypothesis suggested that virtual exercise is sufficiently novel and individuals are focused on the task, suggesting that they are simply distracted from the pain. Hoffman et al. 13 reported the efficacy of virtual reality distraction for reducing symptoms associated with burn injury debridement in hydrotherapy setting. Their patients reported significantly less pain when distracted with virtual reality, where pain dropped from 7.6 to 5.1 on a VAS. The six patients who experienced the strongest illusion of going inside the virtual world reported the greatest analgesic effect of virtual reality on worst ratings, decreasing from 7.2 with no virtual reality condition to 3.7 during the virtual reality experience.13
While there is growing evidence demonstrating the effectiveness of virtual reality for managing acute pain, there is a lack of evidence regarding the effect of using virtual reality to manage chronic pain.12–16
Sato et al.17 treated five patients with CRPS using upper limb virtual reality (the gripping hand shown on the screen being guided by the healthy hand, while the forearm was driven by a virtual forearm of the affected side) and hypothesized that the favorable results obtained by this method may be, in part, due to a distractive effect and the reduction of anxiety. McCabe et al.9,23 refuted this assumption for mirror therapy because, in their study, patients who mobilized their limb, which was obscured by an opaque panel, felt no improvement although their attention was focused on the task.
Our patients reported feelings of sleepiness, cold, heaviness, and tingling in the pathological hand both during and immediately after treatment. If a virtual reality experience succeeds in response to distraction and anxiety reduction, the symptoms reported by our patients would be the effect of a cortical reorganization. However, functional MRI studies are required in order to confirm such cortical changes. As reported by McCabe et al.,9 we suspect that distraction cannot be responsible for the results that we observed in the current study. In a study conducted in a large sample of normal subjects, the perception in the mirror of an upper or lower limb movement was a source of subjective sensory experiences in 66% of normal people with discomfort sensations and occasional dysesthesia, slight pain, and changes in perception of temperature or weight. Such changes cannot be ‘simulated’ by the patients and are, as far as we can speculate, the first evidence that objectively measurable physiological changes in a limb can be initiated through visual feedback.
An additional plausible explanation for a positive response to an augmented reality system centers on changing focus and perception. Here, the virtual exercise does not appear to cause any damage and the patients observes their arm moving and touching objects without pain, permitting their perceptual processes to break the vicious circle of pain which leads to kinesiophobia. McCabe et al.9 hypothesized, using mirror therapy for managing a sample of patients with CRPS, that kinesiophobia would be almost always present in patients suffering from this kind of pathology and if they perceive a normal moving limb, the link would be broken between the fear of movement and pain.
Finally, other investigators have suggested that attention plays a heavy role in responding to an approach likened to the 3DARS system, which may evoke an altered attention state with respect to the painful limb. Patients with such a disorder experience extinction of motor function and/or negligence in connection with motor disturbances from somatosensory feedback. In such a disconnection, sensory disturbances disrupt the development of body representations and motion programming.26 Imaging tasks, which include use of a mirror or a virtual reality system, could improve a patient’s sensory awareness of the affected limb. This impression was often mentioned in our patients who felt that their affected extremity was moving or reported that their arm belonged to them once again.
We enhanced the virtual reality experience further by including a game where the patient was required to touch virtual targets positioned randomly in 3D space, allowing the patients to stay focused on the movements of the injured arm throughout the session. This additional task enhanced to the sensorimotor processing, where such activity is designed to force patients to exercise a target-oriented motor control task. Sato et al.17 have suggested that the planning of touching targets magnified the activation in the motor-related area. However, more research (using fMRI or magnetoencephalography) on the effectiveness of 3D augmented reality for managing neuropathic pain is necessary for confirming the mechanism responsible for reduced pain.
Finally, while our 3DARS therapeutic session appeared to provide hours of pain relief and modulated pain levels, the symptoms had reappeared by the phone interview one week later. This observation was expected because our patients only received one treatment session, as opposed to the varying multiple sessions reported by other investigators using similar approaches (mirror therapy, virtual reality …) that resulted in sustained pain relief in their patients.1,8,10,23. It would be unreasonable to expect a dramatic decrease in such a short time and with patients who have already tried a lot of different approaches.
Conclusions
We observed that the 3DARS induced a significant temporally pain decrease for patients who presented to our clinic with chronic neuropathic pain in a unilateral upper extremity. These patients had a history of previous unsuccessful treatment attempts with various treatment strategies. While further research including multiple session treatment through a randomized clinical trial is necessary before definitive conclusions can be drawn regarding the ultimate efficacy of the 3DARS, clinicians could implement the approach as a preparatory adjunct for providing temporary pain relief aimed at enhance chronic pain patients’ tolerance of manual therapy and exercise intervention.
Disclosure statement
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, we did not receive any financial or material support for this research (e.g. NIH or NHS grants). The manuscript submitted does not contain information about medical device(s).
Author’s Contributors
Dominique Mouraux was involved in conceptual design, methods design and development, data collection and analysis, manuscript writing and editing. Eric Brassinne was involved in methods design and development, manuscript writing and editing. Stéphane Sobczak was involved with statistical support, manuscript writing and editing. Stéphane Sobczak was involved with statistical support, manuscript writing and editing. Antoine Nonclercq, Phillip S. Sizer, and Nadine Warzée were involved in the software development, manuscript writing and editing. Turgay Tuna was involved in methods design and development, data collection and analysis, manuscript writing and editing. Benoît Penelle was involved in conceptual design, software development, methods design and development, data collection and analysis, manuscript writing and editing.
References
- 1.Ramachandran VS, Altschuler EL. The use of visual feedback in particular mirror visual feedback in restoring brain function. Brain. 2009;132:1263–710. [DOI] [PubMed] [Google Scholar]
- 2.Subedi B, Grossberg GT. Phantom limb pain: mechanisms and treatment approaches. Pain Res Treat. 2011;2:1–8. 10.1155/2011/864605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harden RN, Bruehl S, Perez RS, Birklein F, Marinus J, Maihofner C, et al. Development of a severity score for CRPS. Pain. 2010;151:870–6. 10.1016/j.pain.2010.09.031 [DOI] [PubMed] [Google Scholar]
- 4.Maihöfner C, Handwerker HO, Neundorfer B, Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61:1707–15. 10.1212/01.WNL.0000098939.02752.8E [DOI] [PubMed] [Google Scholar]
- 5.Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil Neural Repair. 2012;26(6):646–52. 10.1177/1545968311433209 [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263(1369):377–86. 10.1098/rspb.1996.0058 [DOI] [PubMed] [Google Scholar]
- 7.Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353:2035–6. 10.1016/S0140-6736(99)00920-4 [DOI] [PubMed] [Google Scholar]
- 8.McCabe CS. Mirror visual feedback therapy. A practical approach. J Hand Ther. 2011;24:170–9. 10.1016/j.jht.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 9.McCabe CS, Haigh RC, Halligan PW, Blake DR. Simulating sensory motor incongruence in healthy volunteers: implications for a cortical model of pain. Rheumatology (oxford). 2005;44:97–101. [DOI] [PubMed] [Google Scholar]
- 10.Moseley GL. Graded motor imagery for pathologic pain: a randomized controlled trial. Neurology. 2006;67:2129–34. 10.1212/01.wnl.0000249112.56935.32 [DOI] [PubMed] [Google Scholar]
- 11.Rosén B, Lundborg G. Training with a mirror in rehabilitation of the hand. Scand J Plast Reconstr Surg Hand Surg. 2005;39:104–8. 10.1080/02844310510006187 [DOI] [PubMed] [Google Scholar]
- 12.Botella C, Garcia Palacios A, Banos R, Quero S, Breton-Lopez J. Virtual reality in the treatment of pain. J Cyber Ther Rehabil. 2008;1(1):93–100. [Google Scholar]
- 13.Hoffman HG, Patterson DR, Seibel E, Soltani M, Jewett-Leahy L, Sharar SR. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain. 2008; 24:299–304. 10.1097/AJP.0b013e318164d2cc [DOI] [PubMed] [Google Scholar]
- 14.Li A, Montaño Z, Chen VJ, Gold JI. Virtual reality and pain management: current trends and future directions. Pain Manag. 2011; 1(2):147–57. 10.2217/pmt.10.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psychol Rev. 2010;30:1011–8. 10.1016/j.cpr.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Keefe FJ, Huling DA, Coggins MJ, Keefe DF, Rosenthal MZ, Herr NR, et al. Virtual reality for persistent pain: a new direction for behavioral pain management. Pain. 2012;153:2163–6. 10.1016/j.pain.2012.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato K, Fukumori S, Matsusaki T, Maruo T, Ishikawa S, Nishie H, et al. Nonimmersive virtual reality mirror visual feedback therapy and its application for the treatment of complex regional pain syndrome: an open-label pilot study. Pain Med. 2010;11(4):622–9. 10.1111/j.1526-4637.2010.00819.x [DOI] [PubMed] [Google Scholar]
- 18.Murray CD, Pettifer S, Howard T, Patchick E, Caillette F, Kulkarni J, et al. The treatment of phantom limb pain using immersive virtual reality: three case studies. Disabil Rehabil. 2007;29(18):1465–9. 10.1080/09638280601107385 [DOI] [PubMed] [Google Scholar]
- 19.Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med. 2013;14(2):180–229. 10.1111/pme.12033 [DOI] [PubMed] [Google Scholar]
- 20.Penelle B, Mouraux D, Brassinne E, Tuna T, Nonclercq A, Warzée N. 3D augmented reality applied to the treatment of neuropathic pain. Proc. 9th Intl Conf. Disability, Virtual Reality & Associated Technologies Laval, France; 2012. p. 61–8. [Google Scholar]
- 21.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 22.Azuma R, Baillot Y, Behringer R, Feiner S, Julier S, MacIntyre B. Recent advances in augmented reality. IEEE Comput Graph Appl. 2001;21(6):34–47. 10.1109/38.963459 [DOI] [Google Scholar]
- 23.McCabe CS, Haigh RC, Ring EF, Halligan PW, Wall PD, Blake DR. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology (Oxford). 2003; 42:97–101. [DOI] [PubMed] [Google Scholar]
- 24.Moseley GL, Gallace A, Spence C. Bodily illusions in health and disease: physiological and clinical perspectives and the concept of a cortical “body matrix”. Neurosci Biobehav Rev. 2011;3:1–13. [DOI] [PubMed] [Google Scholar]
- 25.Moseley GL. Using visual illusion to reduce at-level neuropathic pain in paraplegia. Pain. 2007;130:294–8. 10.1016/j.pain.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 26.Murray CD, Patchick E, Pettifer S, Caillette F, Howard T. Immersive virtual reality as a rehabilitative technology for phantom limb experience: a protocol. Cyberpsychol Behav. 2006;9(2):167–70. 10.1089/cpb.2006.9.167 [DOI] [PubMed] [Google Scholar]