Abstract
Background: Shared Decision-Making (SDM) is a dynamic process by which the health care professional and the patient influence each other in making health-related choices or decisions. SDM is strongly embedded in today’s health care approaches, and is advocated as an ideal model since it renders individuals more control towards the health care they choose to receive, and has been shown to improve patient outcomes.
Objectives: The goal of this systematic review was to investigate the added-value of SDM on clinical health-related outcomes in patients with a variety of musculoskeletal conditions.
Data sources: PubMed and CINAHL.
Study selection: PRISMA guidelines were followed for this review. To be considered for review, the study had to meet all the following criteria: (1) prospective studies that involved treatment decision-making; (2) randomized controlled trial design; (3) involving patients faced with having to make a treatment decision; (4) comparing SDM with a control intervention and (5) including one or more of the following outcome measures: well-being, costs, health-related pain or disability measures, or quality of life.
Study appraisal: A priori, we determined to perform methodological quality assessment using the Cochrane Risk of Bias tool for randomized controlled trials.
Results: We did not find a single study that looked at the true effect of SDM on patient reported outcomes in a population with musculoskeletal pain.
Conclusion: For the management of painful musculoskeletal conditions, in the light of the current evidence (none), we estimate that it would be wise to explore the effectiveness of SDM before forcing its large-scale implementation in rehabilitation.
Keywords: Shared-decision-making, musculoskeletal pain, systematic review, patient-reported outcomes measures
Background
In the recent years, Shared decision-making (SDM) is increasingly advocated as an ideal model of treatment decision-making during the medical encounter, as it has been shown to improve patients outcomes and increase benefits for both clinicians and the health care system [1,2]. SDM is not a new paradigm in health-care, but remains a process that has been rather loosely defined [3]. Work from the late 1990s showed that SDM is characterized as a process involving at least two participants (patient and health care provider), interacting together and sharing information to achieve a decision where both parties agree [4,5]. SDM is also a process whereby health professionals and patients work together to make health care choices and is also seen as fundamental to informed consent and patient-centred care [6]. SDM is advocated as a bridging mechanism to improve physician use and patient adoption of clinical practice guidelines [7]. Others present it as a process where the therapist and the patient influence each other in making health-related choices [8]; a procedure that can implicate compromise from both parties involved. In order to be able to evaluate the merits and limitations of a SDM model in the context of rehabilitation, it is first necessary to be clear about what the model involves.
The constructs of SDM
At present, there are a number of SDM theories that have been developed around physician-patients interactions [4]. The paternalistic model is when the physician is the expert and puts the patient in a passive and dependent role. The information decision-making model is another model of information sharing between physician and patient [1], where the physician educates the patient about his/her condition, but does not necessarily lead to sharing of the treatment decision-making process [1]. In the professional-as–agent model, the physician makes the treatment decision as if he/she has the same preferences as the patient [1], thus the physician’s treatment preferences are not considered. Moreau et al. [9] proposed a model where the physician can provide advice and support the patient in decision-making, which ultimately leads to a more trusting atmosphere. Other authors [1,10] also proposed similar definitions. As recommended by Moumjid et al., [3] we will define our contextual parameters associated with SDM. In the current context, we will use the construct where SDM is defined as a dynamic process by which the health care professional (not limited to the physician) and the patient influence each other in making health-related choices or decisions [8].
Influence of SDM on health-related outcomes
There are a number of systematic reviews that have investigated the direct influence of SDM on health-related outcomes. Early work from Joosten and colleagues [11] found that SDM improved longer term outcomes (rather than results in the short term) in only a few studies that explored both mental and physical health conditions. Positive impacts were observed on patient’s adherence, satisfaction, knowledge and well-being; however, no impact was seen on physical or functional outcomes. Recent work for cancer care [12] and treatment for psychosis [13] found only weak effects related to SDM. A comprehensive review in 2015 of multiple health-related pathologies suggested that outcomes associated with the cognitive-affective domain exhibit stronger effects, whereas non-cognitive health-related outcomes (e.g. function) yield the smallest effects [14]. Moreover, it was found that implementation of SDM could also lead to reduced health disparities [15]. All of these studies looked at the effect of SDM, not its effectiveness. Hence, what remains unclear is SDM’s added value over standard client-centred care practice for the treatment of individuals presenting with musculoskeletal pain complaints.
Embedment of SDM into our health care system
The Agency of Healthcare Research and Quality [16] advocates for a shared approach to care and a five-step process for SDM. The five-step process includes exploring and comparing the benefits, harms and risks of each option through meaningful dialogue about what matters most to the patient. Other initiatives, such as the Patient-Centered Outcomes Research Institute (PCORI), are based on the SDM principle, and tie funding for research to those who have created strong or potentially fruitful relationships between the patient and the care providers. Furthermore, the Affordable Care Act urges the implementation of SDM in health care systems [2].
But, in the context of musculoskeletal rehabilitation, does the implementation of SDM result in superior outcomes than a standard client-centered approach? Although facts show that SDM is strongly embedded in today’s health care reality, the true effects of SDM on patient reported outcomes in the context of musculoskeletal rehabilitation are less known. To our knowledge, to date, there are no systematic reviews that have investigated the influence of SDM on health-related outcomes in the context of painful musculoskeletal conditions. It is well known that painful musculoskeletal conditions, including chronic pain, are one of the leading reasons for lower quality of life scores in the world and represent a major burden [17]. Thus, we feel it is important to appraise the added value of SDM on clinical health-related outcomes in patients with a variety of musculoskeletal conditions. Findings may help drive changes in the clinician–client interaction.
Methods
Study design
The study was a systematic literature review that follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. The authors also worked with a biomedical librarian for the search strategy.
Eligibility criteria
The review included studies that met all the following criteria: (1) studies involving musculoskeletal pain conditions; (2) prospective studies that involved treatment decision-making; (3) randomized controlled trial design; (4) involving patients aged 18 or older faced with having to make a treatment decision; (5) comparing SDM with a control intervention (with SDM as the manipulated variable) and (6) including one or more of the following outcome measures: well-being, costs, health-related pain or disability measures, or quality of life. Excluded studies were those in which treatment decisions based on a choice between alternative treatment options and did not explicitly involve at least one arm of SDM between clinician and patient. Further, studies were excluded if satisfaction with the care was the primary outcome measure or if the process of measurement was the focus of the study.
Information sources
The search was conducted in August 2016 in PubMed and CINAHL. Google Scholar was also used. The search strategy, using PubMed as an example, is outlined in Appendix A. Controlled vocabulary and keywords for each database included terms related to ‘shared decision making (SDM)’ and musculoskeletal conditions.
Study selection
Two authors explored the abstract and full texts of the PubMed, CINAHL and Google Scholar. Both authors (YTL and CEC) were responsible for selection of appropriate studies. When discrepancies were present, the decision to accept or reject was made through consensus.
Data collection process
Two authors (YTL and CEC) were responsible for reading each of the final studies and were responsible for extraction of the relevant data, and tabulation into a Patient, Intervention, Comparator, Outcomes and study Type (PICOT) table.
Data items
This study was particularly interested in the type of SDM elements (philosophy) used as a treatment arm in the study as well as the population, treatment type in addition to SDM, and outcomes measures. As previously stated, we accepted any papers with outcome measures associated with: well-being, costs, health-related pain or disability measures or quality of life.
Risk of bias in individual studies
A priori, we determined to perform methodological quality assessment using the Cochrane Risk of Bias tool for randomized controlled trials [19]. The seven item tool addresses random sequencing (selection bias), allocation concealment (selection bias), blinding of participants (performance bias), blinding of outcomes assessors (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other biases. We determined that studies were to be scored by one author (YTL) and reviewed by another author (CEC).
Risk of bias across studies
Risk of bias across studies was evaluated by examining grey literature, conference proceeding and other environments to identify research on SDM that had not been formally published.
Results
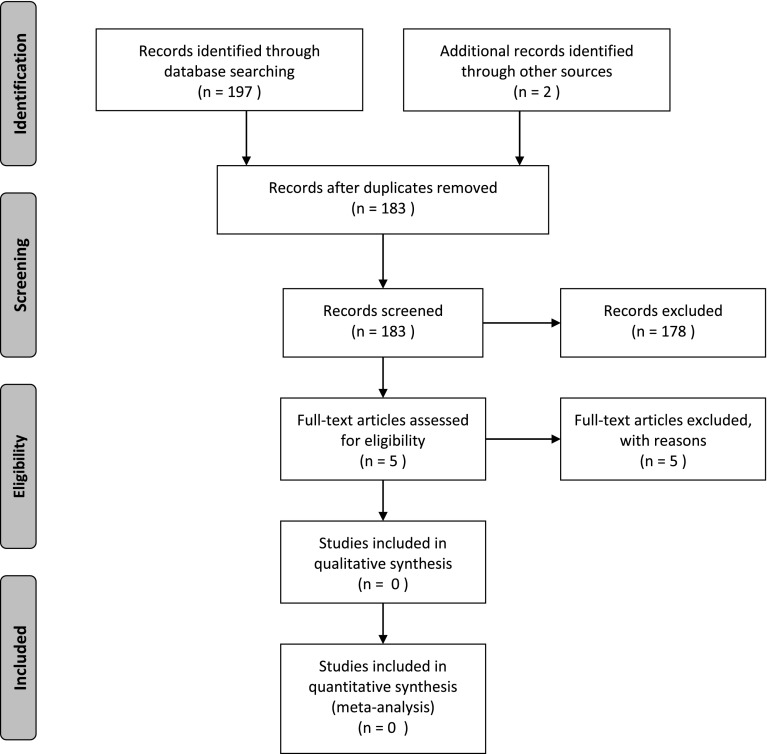
Two authors (YTL and CEC) independently reviewed the abstracts and subsequently selected full text articles. The PubMed search strategy identified 176 potential articles, whereas the other databases yielded fewer potential studies: 21 papers in CINAHL and 2 papers were identified through Google Scholar. After removal of duplicates, 183 unique papers were retained. Only five papers were retained after review of title and abstract and one author selected 1 final paper that qualified, whereas the other author selected none. After discussion of the single study, it was apparent that the paper failed to meet the inclusion criteria (SDM was not manipulated in a randomized fashion) and consensus was met that there were no papers that met the criteria for this systematic review (see Figure 1).
Figure 1.
PRISMA flow diagram.
Discussion
As SDM is strongly advocated by decision-makers, the goal of this systematic review was to shed light on the effectiveness of SDM on clinical health-related outcomes in patients presenting with musculoskeletal conditions. Our results show that there was not a single study that looked at the true effect of SDM on patient reported outcomes in the context of rehabilitation for musculoskeletal pain complaints.
One study by Holland et al. [20] – which was not included in this systematic review since it did not manipulate SDM – described the influence of SDM on analgesic selection for older persons consulting to the emergency department for complaints of acute musculoskeletal pain. They found that patients were satisfied with the decision regarding analgesics, but SDM was not associated with greater pain reduction. This could be explained by the fact that about half of the sample did not want an active role – in fact, 47% of the sample preferred a passive role for such decision.
It is recognized that SDM is at the base of patient-centered care, which is avidly integrated in today’s health care policy worldwide, as it explicitly gives a voice to individuals and renders them more control towards the health care they choose to receive [21]. There is much more literature regarding the use of SDM in non-musculoskeletal populations. The systematic reviews we cited earlier included close to 100 studies [11–15], and although they explored SDM’s effect, not effectiveness, the results suggest that SDM increases patient involvement, thus positively modulate patient compliance and adherence to treatment and have significant positive repercussions on management strategies for these patients. We are far behind in regards to musculoskeletal populations and have almost no evidence to support or reject any implementation decisions. Yet, SDM it is strongly embedded into the decision-making process in many industrialized countries.
We found that SDM has yet to show that it could deliver superior outcomes in the context of musculoskeletal pain and research outside the musculoskeletal population has only investigated its effects. Based on the findings of this study, we would argue that further investigation of this concept is necessary before wholesale implementation and assumption of superiority, especially in chronic pain management (CPM), where there are more questions than answers in terms of treatment effectiveness.
SDM and musculoskeletal pain: considerations for rehabilitation
-
(1)
SDM’s fundamentals relate to potentially harmful treatment options:
SDM is recommended in the context of management of persistent pain related to the musculoskeletal diseases [22]. However, this recommendation made by the American Pain Society mostly concerned the decision-making process between invasive therapies (surgery, or mini-invasive interventions such as Intradiscal Electrothermal Annuloplasty) and interdisciplinary rehabilitation, two approaches with radically different levels of potential harm and risk. In situations where treatments increase the risk of harm, the patient’s values regarding the risk/benefit ratio need to be considered in the clinical decision-making process. However, CPM by manual therapists involves a very low probability of harm compared to surgical or pharmacological options. Hence, rehabilitation specialists rarely have to deal with treatment options that are associated with serious side effects such as increase mortality risk. It is when confronted with such a decision that a full SDM process better serves the patient and the health care system. Furthermore, in CPM of musculoskeletal condition, we are often faced with limited treatment options [23] or many equivocal treatment options to choose from (i.e.: chronic low back pain) [24].
-
(2)
Decision aids are underdeveloped in rehabilitation:
One tactic to implement a SDM approach is by the use of decision aids (educational material) which will stimulate the exchange of information between both parties, and will then drive SDM [2]. However, although decision aids have been shown to be improve quality of medical decisions and reduce costs [25], they remain controversial since other studies have shown that they only decrease the number of patients who remain inactive of passive in the decision process [26,27]. Moreover, a recent study which explored the effectiveness of a decision-aid over usual care on patient satisfaction and physical outcomes in a sample of patients with non-specific low back pain found comparable satisfaction levels, but worse outcomes (pain/physical aspects) and lower cost-effectiveness for the intervention arm! [28] Considering that in musculoskeletal rehabilitation, decision-aids remain scarce and described to be in their developmental phase, they might be seen as premature to implement in clinical practice [29]. Furthermore, they are likely to remain scarce since we don’t fully understand our own intervention effects.
-
(3)
SDM integration into practice requires proper training:
Although the use of SDM is endorsed, data show that rehabilitation professionals are poorly trained at providing it. In a study involving physical therapists in a musculoskeletal practice setting, Dierck et al. [30] assessed the level of SDM using a standardized tool (the OPTION scale). This scale, ‘Observing Patient Involvement in SDM (OPTION)’, is a third-party observer instrument that measures to which extent clinicians engage in SDM with their patients, and has shown good reliability [31]. Dierck et al.’s results showed very low levels of SDM integration (5.2 ± 6.8/100, where 50/100 indicates basic skill level) in experienced therapists (mean: 17.7 years), and reported that physical therapists were more likely to make the clinical decision in the patient’s best interest. In another study, involving occupational therapists, SDM behavior’s in a context of work rehabilitation for persistent pain, the authors found that it took 20 hours of training for experienced OTs (mean 7 years of experience) to reach satisfactory (basic) levels of SDM [32]. These results indicate that musculoskeletal clinicians are well intended, but need sufficient training before reaching basic levels of SDM.
-
(4)
Conundrum of pain science:
Patients and health care professionals are not always on the same page with regard to pain science. If the patient and therapist do not share a common vision in regards to pain science, SDM influence should be questioned. A tenant of SDM involves the assumption that the patient preference will lead to improved outcomes. With chronic pain, patients with maladaptive thoughts or detrimental behaviors may demand care that is detrimental to their progression. For chronic pain, data supporting exercise and active approaches are lauded within the literature but are often at the opposite end of the desirability spectrum by the patient. At present, we simply don’t know the effects of when the best treatment strategies fail to match patient’s values. In such circumstances, SDM could have a negative influence on outcomes. Before thinking of full implementation of SDM in CPM, one of the challenges to overcome is having both the patient and therapist to view pain in a biopsychosocial perspective. Otherwise, both can be off the track and select/offer a non-optimal treatment. We recommend more emphasis on gaining an accurate perspective and common understanding of chronic pain.
Opportunities for SDM with respect to chronic pain and manual therapy
Clinical decisions can be influenced by the patient’s expectation that a treatment will provide benefit or harm. Patients present to physical therapy with intrinsic sets of values, expectations and beliefs about their condition; and positive or negative expectations of beneficial intervention strategies are often developed [33]. Emerging evidence suggests that expectations of benefit (or harm) might influence patient outcomes. For patients with neck pain, when treatment approaches matched the patient’s expectation of benefit, they had better overall short- and long-term outcomes [33]. Similar results were found in a population of patients with low back pain, where matching the intervention to the patient’s preference positively influenced outcomes [34]. As promising as these results are, conflicted findings in the literature exists for patient expectations influence on outcomes. Another study investigating the influence of patient expectations on low back pain and disability found no significant difference in those that were matched, unmatched, or had no preference to their expectation of benefit [35].
Possible explanations for these conflicting results include complexity of the construct, and therefore a lack of a reliable and valid measurement tool [36]. In fact, the categorical representation of expectation of benefit as positive or negative is oversimplified. Another possible explanation resides in the fact that patients might not be able to make accurate predictions of benefit and harm associated with a treatment procedure. A systematic review of the medical literature found that patients typically overestimate benefit and underestimate harm when compared to the research supporting the intervention [37].
The emerging literature associated with the patient expectations influence on outcomes opens up an opportunity to have a dialogue with the patient to discover their values and beliefs about treatments. Ignoring patient expectations of benefit provides a possible disservice to the patient’s role in clinical decision-making. However, clinicians should use caution when leveraging the patient’s expectations when making clinical decisions. Other options besides expectations are also well positioned to enhance outcomes in the context of CPM. Therapeutic alliance [38] and better communication strategies [39] might represent better options at this point, than the integration of SDM in its full extent, since we have no studies to support or reject SDM in musculoskeletal pain.
Conclusion
In this systematic review, we did not find evidence that SDM offered better health-related outcomes for individuals with painful musculoskeletal disorders. In fact, the true effect of this concept has yet to be studied, despite the reality that SDM has been advocated for many years. We recognize the potential benefits of SDM in a patient-centered care approach, as it explicitly gives a voice to individuals and renders them more control towards the health care they choose to receive. Yet, in the light of the current evidence (none), we estimate that it would be wise to study the effectiveness of SDM, and also be prudent before forcing its large-scale implementation.
Notes on contributors
Yannick Tousignant-Laflamme, PT, PhD, is an associate professor at the School of Rehabilitation (Physical Therapy Program) of the Université de Sherbrooke, Qc, Canada. Yannick’s fields of interest include pain science/management, low back pain, and orthopedic physical rehabilitation.
Shefali Christopher, PT, DPT, SCS, LAT, and ATC is a clinician educator in the Doctor of Physical Therapy Department at Duke University and a physical therapist at Duke Health. Christophe’s field of interest includes running rehabilitation and postpartum athletes.
Derek Clewley, PT, DPT, is an assistant professor in the Division of Physical Therapy, Duke University, Durham, United States of America. Clewley’s research interests are the impact of health-seeking behavior on health services utilization, pain science, manual therapy, and dry needling.
Leila Ledbetter, MLIS, is a research and education librarian at the Duke University Medical Center Library & Archives. Ledbetter’s fields of interest include systematic reviews, physical therapy and rehabilitation, and interprofessional education.
Christian Jaeger Cook is a pre-med student at the University of North Carolina. Jaeger has had some exposure to basic science research in biology and has published on medical school admissions and emotional intelligence.
Chade Cook, PT, PhD, is a professor and program director at Duke University. He recently completed a certificate in pain management from McGill University. He has a wide range of research interests including pain, manual therapy effectiveness, and diagnostic accuracy.
Supplemental data
Supplemental data for this article can be accessed https://doi.org/10.1080/10669817.2017.1323607.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
References
- [1].Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters Patient Edu Couns. 2006;60:301–312. 10.1016/j.pec.2005.06.010 [DOI] [PubMed] [Google Scholar]
- [2].Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368:6–8. 10.1056/NEJMp1209500 [DOI] [PubMed] [Google Scholar]
- [3].Moumjid N, Gafni A, Bremond A, et al. Shared decision making in the medical encounter: are we all talking about the same thing? Med Decis Making. 2007;27:539–546. 10.1177/0272989X07306779 [DOI] [PubMed] [Google Scholar]
- [4].Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681–692. 10.1016/S0277-9536(96)00221-3 [DOI] [PubMed] [Google Scholar]
- [5].Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. J Am Med Assoc. 1992;267:2221–2226. 10.1001/jama.1992.03480160079038 [DOI] [PubMed] [Google Scholar]
- [6].Barry MJ, Edgman-Levitan S. Shared decision making–pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. 10.1056/NEJMp1109283 [DOI] [PubMed] [Google Scholar]
- [7].Politi MC, Wolin KY, Légaré F. Implementing clinical practice guidelines about health promotion and disease prevention through shared decision making. J Gen Intern Med. 2013;28:838–844. 10.1007/s11606-012-2321-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Legare F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff. 2013;32:276–284. 10.1377/hlthaff.2012.1078 [DOI] [PubMed] [Google Scholar]
- [9].Moreau A, Carol L, Dedianne MC, et al. What perceptions do patients have of decision making (DM)? Toward an integrative patient-centered care model. A qualitative study using focus-group interviews. Patient Educ Couns. 2012;87:206–211. 10.1016/j.pec.2011.08.010 [DOI] [PubMed] [Google Scholar]
- [10].Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, Cording E, Tomson D, Dodd C, Rollnick S, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367. 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Joosten EAG, DeFuentes-Merillas L, de Weert GH, et al. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219–226. 10.1159/000126073 [DOI] [PubMed] [Google Scholar]
- [12].Kashaf MS, McGill E. Does shared decision making in cancer treatment improve quality of life? A systematic literature review. Med Decis Making. 2015;35:1037–1048. 10.1177/0272989X15598529 [DOI] [PubMed] [Google Scholar]
- [13].Stovell D, Morrison AP, Panayiotou M, et al. Shared treatment decision-making and empowerment-related outcomes in psychosis: systematic review and meta-analysis. Br J Psychiatry. 2016;209:23–28. 10.1192/bjp.bp.114.158931 [DOI] [PubMed] [Google Scholar]
- [14].Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35:114–131. 10.1177/0272989X14551638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS One. 2014;9:e94670. 10.1371/journal.pone.0094670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Agency for Healthcare Research & Quality [Internet] The SHARE Approach. [cited 2017 Jan 11]. Available from: https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html
- [17].Palazzo C, Ravaud J-F, Papelard A, Ravaud P, Poiraudeau S, Vos T, Flaxman A, Naghavi M, Lozano R, Michaud C, et al. The burden of musculoskeletal conditions Chopra A, editor. PLoS One. 2014;9:e90633. 10.1371/journal.pone.0090633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Holland WC, Hunold KM, Mangipudi SA, et al. A prospective evaluation of shared decision-making regarding analgesics selection for older emergency department patients with acute musculoskeletal pain. Acad Emerg Med. 2016;23:306–314. 10.1111/acem.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Härter M. Policy and practice developments in the implementation of shared decision making: an international perspective. Z Evid Fortbild Qual Gesundhwes. 2011;105:229–233. 10.1016/j.zefq.2011.04.018 [DOI] [PubMed] [Google Scholar]
- [22].Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066–1077. 10.1097/BRS.0b013e3181a1390d [DOI] [PubMed] [Google Scholar]
- [23].Harden RN, Oaklander AL, Burton AW, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med. 2013;14:180–229. 10.1111/pme.12033 [DOI] [PubMed] [Google Scholar]
- [24].Powers CM, Bolgla LA, Callaghan MJ, et al. Patellofemoral pain: proximal, distal, and local factors – 2nd international research retreat, August 31–September 2, 2011, Ghent, Belgium. J Orthop Sport Phys Ther. 2012;42:A1–A54. 10.2519/jospt.2012.0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions (Review) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;10(10):Art. No.: CD001431. [Google Scholar]
- [26].Stacey D, Hawker G, Dervin G, et al. Decision aid for patients considering total knee arthroplasty with preference report for surgeons: a pilot randomized controlled trial. BMC Musculoskelet Disord. 2014;15:54. 10.1186/1471-2474-15-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stacey D, Taljaard M, Dervin G, et al. Impact of patient decision aids on appropriate and timely access to hip or knee arthroplasty for osteoarthritis: a randomized controlled trial. Osteoarthritis Cartilage. 2016. Jan;24:99–107. 10.1016/j.joca.2015.07.024 [DOI] [PubMed] [Google Scholar]
- [28].Patel S, Ngunjiri A, Hee SW, et al. Primum non nocere: shared informed decision making in low back pain–a pilot cluster randomised trial. BMC Musculoskelet Disord. 2014;15:282. 10.1186/1471-2474-15-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gross DP, Armijo-Olivo S, Shaw WS, et al. Clinical decision support tools for selecting interventions for patients with disabling musculoskeletal disorders: a scoping review. J Occup Rehabil. 2016. Sep;26:286–318. 10.1007/s10926-015-9614-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dierckx K, Deveugele M, Roosen P, et al. Implementation of shared decision making in physical therapy: observed level of involvement and patient preference. Phys Ther. 2013;93:1321–1330. 10.2522/ptj.20120286 [DOI] [PubMed] [Google Scholar]
- [31].Elwyn G, Hutchings H, Edwards A, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect. 2005;8:34–42. 10.1111/hex.2005.8.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Coutu MF, Légaré F, Stacey D, et al. Occupational therapists’ shared decision-making behaviors with patients having persistent pain in a work rehabilitation context: a cross-sectional study. Patient Educ Couns. 2015;98:864–870. 10.1016/j.pec.2015.03.015 [DOI] [PubMed] [Google Scholar]
- [33].Bishop MD, Mintken PE, Bialosky JE, et al. Patient expectations of benefit from interventions for neck pain and resulting influence on outcomes. J Orthop Sports Phys Ther. 2013;43:457–465. 10.2519/jospt.2013.4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bishop MD, Bialosky JE, Cleland JA. Patient expectations of benefit from common interventions for low back pain and effects on outcome: secondary analysis of a clinical trial of manual therapy interventions. J Man Manip Ther. 2011;19:20–25. 10.1179/106698110X12804993426929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Donaldson M, Learman K, O’Halloran B, et al. The role of patients’ expectation of appropriate initial manual therapy treatment in outcomes for patients with low back pain. J Manipulative Physiol Ther. 2013;36:276–283. 10.1016/j.jmpt.2013.05.016 [DOI] [PubMed] [Google Scholar]
- [36].Bialosky JE, Bishop MD, Cleland JA. Individual expectation: an overlooked, but pertinent, factor in the treatment of individuals experiencing musculoskeletal pain. Phys Ther. 2010;90:1345–1355. 10.2522/ptj.20090306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175:274–286. 10.1001/jamainternmed.2014.6016 [DOI] [PubMed] [Google Scholar]
- [38].Fuentes J, Armijo-Olivo S, Funabashi M, et al. Enhanced therapeutic alliance modulates pain intensity and muscle pain sensitivity in patients with chronic low back pain: an experimental controlled study. Phys Ther. 2014. April;94:477–489. 10.2522/ptj.20130118 [DOI] [PubMed] [Google Scholar]
- [39].Louw A, Puentedura EJ, Zimney K, et al. Know pain, know gain? A perspective on pain neuroscience education in physical therapy. J Orthop Sport Phys Ther. 2016;46:131–134. 10.2519/jospt.2016.0602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.