Abstract
Background and Purpose
The limits of cerebral blood flow-pressure autoregulation have not been adequately defined for pediatric patients. Mean arterial blood pressure below these limits might contribute to brain injury during cardiac surgery. The purpose of this pilot study was to assess a novel method of determining the lower limits of pressure autoregulation in pediatric patients supported with cardiopulmonary bypass.
Methods
A prospective, observational pilot study was conducted in children (n=54) undergoing cardiac surgery with cardiopulmonary bypass for correction of congenital heart defects. Cerebral oximetry index (COx) was calculated as a moving, linear correlation coefficient between slow waves of arterial blood pressure and cerebral oximetry measured with near-infrared spectroscopy. An autoregulation curve was constructed for each patient with averaged COx values sorted by arterial blood pressure.
Results
Hypotension was associated with increased values of COx (P<0.0001). For 77% of patients, an individual estimate of lower limits of pressure autoregulation could be determined using a threshold COx value of 0.4. The mean lower limits of pressure autoregulation for the cohort using this method was 42±7 mm Hg.
Conclusions
This pilot study of COx monitoring in pediatric patients demonstrates an association between hypotension during cardiopulmonary bypass and impairment of autoregulation. The COx may be useful to identify arterial blood pressure-dependent limits of cerebral autoregulation during cardiopulmonary bypass. Larger trials with neurological outcomes are indicated.
Keywords: cerebrovascular pressure autoregulation, congenital heart disease, near-infrared spectroscopy, pediatrics
Neurological injury occurs during repair of congenital heart disease in 30% to 70% of cases.1–3 Although many of these injuries are not detected, long-term testing of children after congenital heart surgery shows an increase in neurocognitive abnormalities.4,5 High rates of ischemic brain injury persist despite advances in cardiopulmonary bypass (CPB) technology and surgical management.6 Elimination of preventable factors contributing to neurological injury is the modern challenge to the perioperative congenital heart team.7
A significant limitation in caring for pediatric patients is our incomplete understanding of the limits of cerebral blood flow–blood pressure autoregulation (hereafter termed pressure autoregulation). Pressure autoregulation constrains cerebral blood flow (CBF) through vascular constriction and dilatation as mean arterial blood pressure (ABP) fluctuates. A mean ABP below the lower limit of pressure autoregulation (LLA) results in CBF that is passive to changes in ABP, increasing the brain’s vulnerability to ischemia. Identifying the individual LLA for a patient during CPB may guide neuroprotective strategies.
Recently, continuous monitoring of pressure autoregulation has been performed with near-infrared spectroscopy (NIRS) as a surrogate of CBF by using the cerebral oximetry index (COx). The COx is a moving correlation between ABP and cerebral oximetry. When pressure autoregulation is lost, cerebral tissue oxygen saturation becomes passive to changes in ABP and the COx approaches +1. When pressure autoregulation is intact, tissue oxygen saturation is not passive to ABP so the COx fluctuates around zero. The COx has been validated in piglets with hypotension8,9 and correlates with transcranial Doppler-derived measurements of pressure autoregulation in adult patients with sepsis and adult patients during CPB.10,11
The aim of this pilot study was to assess the feasibility of using COx in pediatric patients during cardiac surgery with CPB for detecting the LLA. We hypothesized that the COx would vary across ABP and that the increase in COx during critical hypotension could be used to identify an individual subject’s LLA.
Methods
The Institutional Review Board at The Johns Hopkins Hospital approved this prospective, observational cohort study. Because NIRS is routine for pediatric cardiac surgery in our institution, the need for written informed consent was waived. All subjects received routine care, and caregivers were blinded to the COx. Pediatric patients undergoing CPB were eligible with the exception of patients with significant cyanosis (defined as oxygen saturation <95%) or coarctation of the aorta excluded. These latter subjects were excluded based on the theoretical concerns that: (1) patients with aortic coarctation are at risk for markedly abnormal pressure autoregulation associated with chronically high cerebral perfusion pressure; and (2) patients with cyanosis were excluded, because the COx has not been validated in this saturation range.8,9,12 Therefore, of 91 patients identified for the study, 37 were excluded for cyanosis and/or coarctation, leaving 54 subjects enrolled.
All subjects received standard intraoperative monitoring, invasive ABP monitoring, and reflectance NIRS-based cerebral oximetry (INVOS; Somanetics, Troy, Mich). Nonprotocolized anesthetic management consisted of midazolam, fentanyl, isoflurane, and pancuronium. CPB was carried out with a nonocclusive roller pump, α-stat pH management, and priming with blood products for all patients who weighed <20 kg.
Pressure Autoregulation Monitoring
The analog ABP waveform from the operating room hemodynamic monitor (Marquette Solar 8000; GE Healthcare, Piscataway, NJ) was sampled at 60 Hz to a computer using an analog-to-digital converter (Data Translation, Marlboro, Mass) and ICM+ software (Cambridge University, Cambridge, UK). Right-sided cerebral oximetry was synchronously sampled using the same analog-to-digital converter at 60 Hz, and the refresh rate of the NIRS monitor was 0.25 Hz. Artifactual values, mostly from loss of arterial waveform during blood laboratory sampling, were removed manually. According to our previously described method of calculating the COx, time-integrated 10-second mean values of the ABP and cerebral oximetry waveforms were recorded as a low-pass filtering step to remove pulse and respiratory frequency oscillations. Thirty paired samples of ABP and oximetry, spanning a 300-second epoch, were used in each calculation of the COx, which is a linear (Pearson) coefficient of correlation between ABP and cerebral oximetry.8 Although the COx is a time-domain calculation, it is specific to the frequency of “slow waves”—oscillations and transient waves present in intracranial pressure tracings representing blood volume changes related to autoregulatory activity.12–15 In this study, the COx was updated every 60 seconds in a moving window and recorded with the corresponding ABP from the same 300-second epoch. The time resolution of this method permits reporting of the effect of prolonged state changes such as bypass as well as brief state changes such as ABP fluctuations on autoregulatory function.
Statistical Analysis
A pressure autoregulation curve was constructed for each patient by binning and averaging discrete COx values according to the average ABP (5-mm Hg bins; Figure 1).16 Defining the individual LLA in these histograms in a standardized approach requires the use of a threshold COx value. Such a threshold has not been determined. Based on experiments done in our laboratory with neonatal piglets using similar histograms, we have seen that a COx threshold of 0.35 is 92% sensitive but only 63% specific. A higher threshold COx of 0.45 improves specificity to 75%, but sensitivity decreases to 83% in these limited animal data.8,9 We chose a threshold COx value of 0.4 for this study. The individual LLA was defined for each subject by systematically reviewing individual histograms in the following manner. The mean ABP was determined above which COx values were consistently <0.4 (<1 of 4 contiguous bin averages >0.4) and below which COx values were >0.4 (<1 of 4 contiguous bin averages <0.4). The decision to allow 1 in 4 bins to be ignored was arbitrarily made before data analysis. This method was an attempt to objectively demarcate blood pressures associated with impaired autoregulation from blood pressures associated with intact autoregulation in individual subjects. Historically, these histograms have been subjectively analyzed for similar publications.15 This analysis was also applied to each subject in our adult study. Figure 1 shows examples of this method applied successfully to 4 subjects and unsuccessfully to 2 subjects. A composite pressure autoregulation curve was constructed by summarizing each subject’s COx-MAP histograms into a mean of means for the entire cohort with equal weight assigned to individual subjects. To account for within-subject correlation and inconsistently repeated measures (not every ABP was experienced by every subject), the effect of ABP on COx was determined by a multiple linear regression model with generalized estimating equations and robust variance estimation.17
Figure 1.
LLA determination. For each subject, the average COx is plotted over ABP. A COx value of 0.4 (horizontal dashed line) is the LLA threshold used in this study. Examples A through D demonstrated an LLA. A, A 6-day-old patient with interrupted aortic arch, (LLA at 20 mm Hg); (B) a 7-month-old patient with ventricular septal defect (LLA at 40 mm Hg); (C) a 2-year-old patient with ventricular septal defect (LLA at 50 mm Hg); (D) a 7-year-old patient with anomalous right coronary artery (LLA at 45 mm Hg). Examples E and F did not demonstrate an LLA. E, A 4-month-old patient with truncus arteriosus (LLA indeterminate due to COx values inconsistently >0.4). F, A 14-year-old with cardiomyopathy (LLA indeterminate due to COx values consistently <0.4).
Descriptive statistics were performed to analyze the mean and distribution between all recorded study variables. Univariate and multivariate logistic regression models were built using the method of Hosmer and Lemeshow18 to compare those subjects with an individually estimated LLA (LLA-detected) with subjects for whom the method failed (LLA not detected). A 2-way analysis of variance with post hoc Bonferroni testing was used to determine the significance of differences between the individual LLA detected/LLA not detected groups. Finally, using only the individual LLA-detected group, the relationship between individual LLA and each variable of interest (age, lowest temperature, average COx, diagnosis, average ABP, PaCO2, average glucose, average sodium, average hemoglobin) was modeled using linear regression.
Average ABP, regional cerebral oximetry (rSO2), and COx are reported for the cohort by surgical stage: before, during, and after CPB. Percentage of time during each stage with critical hypotension (cohort mean ABP −2 SDs, or <40 mm Hg) was also compared. Comparisons were made between stages by Kruskal-Wallis analysis of variance and Dunn multiple comparison test. Individual LLA determinations from this pediatric study were compared with similar individual LLA determinations from an adult cohort11 using the Mann–Whitney U test. We reported statistical significance levels at P<0.05. All statistical analysis was performed using STATA (Version 8; Stata Corp, College Station, Texas) and Prism 5 (GraphPad Software Inc, LaJolla, Calif).
Results
Patient demographics are shown in Table 1. The mean subject age was 56.7±65 months (median: 25; range: 0 to 222; 25%, 75% intraquartiles: 6, 89, respectively). Younger subjects (<25 months) had a higher incidence of trisomy 21 diagnosis and of septal defect surgery. Older subjects (>25 months) had a higher incidence of connective tissue disorder diagnosis and of valvular and aortic root surgery. No significant difference in laboratory measurements was found between groups (Table 1).
Table 1.
Subject Characteristics and Physiological Variables
| Parameter | Total (N = 54) |
LLA Detected (N = 43) |
LLA Not Detected (N = 11) |
P |
|---|---|---|---|---|
| Age, months | 56 ± 65 | 58 ± 67 | 53 ± 63 | 0.21* |
| Weight, kg | 20 ± 22 | 21 ± 23 | 16 ± 18 | 0.5294* |
| Sex, no. (% female) | 27 (50%) | 21 (50%) | 6 (54%) | 0.6373‡ |
| Repair, no. (%)§ | ||||
| Isolated septal defect | 20 (37%) | 17 (40%) | 3 (27%) | <0.05 |
| Shunt | 6 (11%) | 2 (5%) | 4 (36%) | <0.05 |
| Obstruction | 10 (19%) | 10 (23%) | 0 (0%) | <0.05 |
| Valvular | 11 (20%) | 9 (21%) | 2 (18%) | NS |
| Transplant | 5 (9%) | 4 (9%) | 1 (9%) | NS |
| Other | 2 (4%) | 1 (2%) | 1 (9%) | NS |
| Syndromes, no. (%) | 15 (28%) | |||
| Connective tissue disorder | 5 (9%) | |||
| Trisomy 21 | 6 (11%) | |||
| 22q11.2 deletion | 1 (2%) | |||
| Other genetic disorder | 3 (6%) | |||
| CPB duration, minutes | 139 ± 54 | 138 ± 55 | 144 ± 50 | 0.7267* |
| Crossclamp duration, minutes | 73 ± 46 | 72 ± 48 | 75 ± 40 | 0.7131* |
| Minimum temperature, °C | 26.9 ± 3.3 | 26.9 ± 3.5 | 26.4 ± 3.1 | 0.6329* |
| Hemoglobin, mg/dL | 9.9 ± 0.2 | 10.1 ± 1.2 | 9.8 ± 0.8 | NS† |
| pH, AU | 7.37 ± 0.01 | 7.38 ± 0.23 | 7.37 ± 0.04 | NS† |
| PaCO2, mmHg | 40 ± 0.62 | 39 ± 3.8 | 40 ± 3.5 | NS† |
| PaO2, mmHg | 274 ± 51 | 280 ± 51 | 265 ± 39 | NS† |
| Sodium, mEq/L | 141 ± 3 | 140 ± 3 | 142 ± 3 | NS† |
| Glucose, mg/dL | 188 ± 42 | 187 ± 44 | 192 ± 34 | NS† |
Values are presented as mean ± SD unless otherwise specified.
P values were calculated with the *Mann–Whitney U test or †analysis of variance for repeated measures except sex and repair, for which the ‡χ2 test was used.
Repair was categorized as: (1) isolated septal defects, atrial or ventricular; (2) shunting lesions, including tetralogy of Fallot, right ventricular outflow tract obstruction, and partial anomalous pulmonary venous return; (3) obstructing lesions, including right and left, subvalvular, supravalvular, and valvular stenosis; (4) valvular lesions, including regurgitant valve disease and aortic root dilatation; (5) cardiac transplantation; and (6) other, including an acyanotic infant with transposition of the great arteries and a 9 year old with an anomalous right coronary artery.
NS indicates nonsignificant.
Univariate logistic regression demonstrated average COx, diagnosis, average glucose, and average sodium to be potentially predictive (P<0.1) of our ability to determine an individual LLA with the COx (data not shown). Multivariate logistic regression of these variables and other factors known to impact cerebrovascular autoregulation found only the average COx to be predictive of our ability to estimate the LLA (Table 2).
Table 2.
Multivariate Analysis of Variables Associated With Ability to Detect the LLA
| Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| Age | 1.00 (0.97–1.02) | 0.73 |
| Lowest temperature | 0.97 (0.68–1.38) | 0.87 |
| Average COx* | 0.00 (0.00–0.95) | 0.05 |
| Diagnosis* | 1.56 (0.74–3.32) | 0.24 |
| Average ABP | 0.98 (0.84–1.13) | 0.75 |
| PaCO2 | 1.11 (0.85–1.45) | 0.44 |
| Glucose* | 1.01 (0.99–1.04) | 0.38 |
| Average sodium* | 1.14 (0.80–1.62) | 0.47 |
| Average hemoglobin | 0.30 (0.07–1.31) | 0.11 |
Those variables with P<0.1 by univariate logistic regression.
Focused analysis of the LLA-detected group showed no significant association between individual LLA and age. There was an association between the estimated individual LLA value and mean ABP. Regression analysis suggests a 0.5-mm Hg increase of the estimated individual LLA per mm Hg increase of mean ABP (95% CI: 0.12 to 0.96) when controlling for the interdependence of age and mean ABP within the LLA-detected group. Sampling bias likely contributes to this result in that patients with higher ABP and low or normal limits of autoregulation were excluded by our inability to detect the individual LLA. Alternative interpretations are that higher ABP management causes a higher individual LLA or that ABP management confounds the COx measurement.
Mean ABP, rSO2, and COx results for the entire cohort, categorized by surgical stage, are shown in Figure 2. Mean ABP and rSO2 were lower during CPB (P<0.01). The COx was higher during CPB, suggesting impaired autoregulation (P<0.001), and remained elevated after CPB (P<0.01). Mean ABP for the cohort was 58±10 mm Hg, 52±9 mm Hg, and 56±10 mm Hg; before, during, and after CPB, respectively. Mean rSO2 for the cohort was 75±12%, 62±9%, and 72±10% before, during, and after CPB, respectively. Mean COx for the cohort was 0.17±0.22, 0.31±0.18, and 0.29±0.20 before, during, and after CPB, respectively.
Figure 2.
Mean ABP, rSO2, and COx during cardiac surgery. A, Average ABP decreased during CPB and normalized after CPB. B, Average rSO2 decreased during CPB and normalized after CPB. C, Average COx values increased during CPB and remained elevated after CPB, indicating impaired autoregulation. (**P<0.01, ***P<0.001 by Kruskal-Wallis analysis of variance and Dunn multiple comparison test).
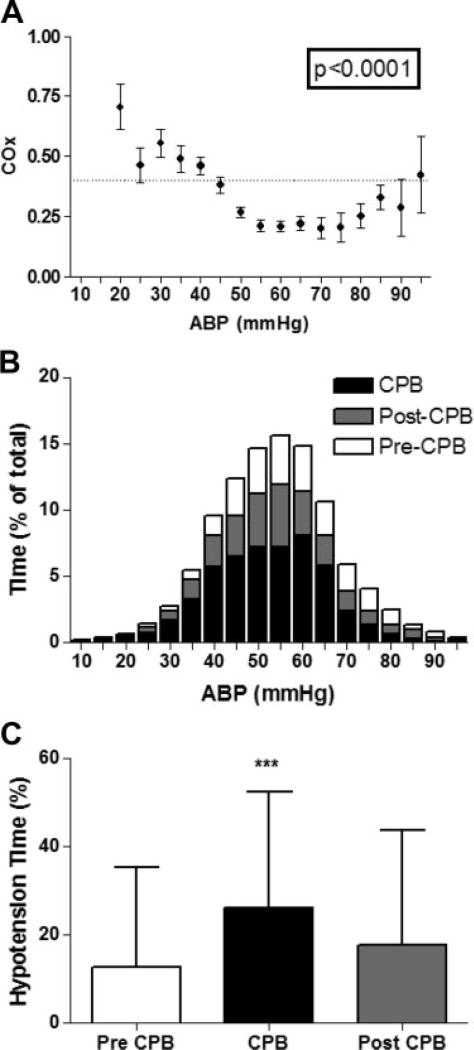
Pressure autoregulation curves for the cohort are shown in Figure 3 along with histograms showing the percentage of time spent at each bin of mean ABP at each stage of surgery. COx variance across mean ABP was significant, and the curves show worsening of pressure autoregulation with hypotension, suggestive of a cohort LLA of approximately 40 mm Hg. This cohort LLA is similar to a previously reported estimation of a cohort LLA in children using transcranial Doppler.19 Critical hypotension (mean ABP <40 mm Hg) was more common during CPB than other stages of surgery. Before CPB, subjects experienced an average of 13±23% of time with critical hypotension compared with 26±26% during and 18±26% after CPB (P<0.001).
Figure 3.
A, The distribution of the COx across binned mean ABP allows visualization of the cohort autoregulation curve. A COx value of 0.4 (horizontal dashed line) is the LLA threshold used in this study. B, The distribution of time (percent of total time) spent at each increment of ABP is sorted by surgical stage. C, The average percentage of time with critical hypotension is shown for each surgical stage. On average, subjects spent 13±23%, 26±26%, and 18±26% of time before, during, and after bypass, respectively, with mean ABP <40 mm Hg (±SD, ***P<0.001 by analysis of variance [ANOVA] by Kruskal-Wallis ANOVA and Dunn multiple comparison test).
Individual estimations of the LLA in this pediatric cohort were compared with estimations of the LLA of a similarly studied adult cohort. The individual LLA estimate was possible using our described method in 77% of pediatric subjects and 62% of adults (Figure 4). This rate is comparable to a subjective method of determining optimal ranges of perfusion pressure in head-injured adults with similar histograms.15 There was a significant difference in pediatric and adult LLA when undergoing CPB (mean individual LLA 42 mm Hg versus 55 mm Hg, respectively; P<0.001).
Figure 4.
The individual LLA could be estimated with the COx for 77% (42 of 54) of pediatric patients and 62% (37 of 60) of adult patients. The average individual LLA for pediatric patients was 42±7 mm Hg compared with 55±14 mm Hg for adults (±SD; P<0.0001 by Mann–Whitney U test).
Discussion
These data demonstrate a method to monitor pressure autoregulation continuously with NIRS and to estimate both individual and cohort LLA in pediatric patients during CPB. The significant relationship between COx and mean ABP (Figure 3A) indicates that hypotension is associated with impairment of pressure autoregulation during cardiac surgery. These findings have implications for monitoring pediatric patients with a goal of individualizing ABP targets.
Management of the pediatric patient undergoing CPB is made difficult by competing hemodynamic goals for the brain, heart, and viscera. The brain is protected from hypo-perfusion by systemic vasoconstriction and pressure autoregulation. In children with cardiac disease, afterload manipulation improves cardiac function and visceral perfusion but encumbers the vasoconstrictive mechanism that normally preserves cerebral perfusion pressure during a drop in cardiac output. Decrements in cerebral perfusion pressure are then compensated by pressure autoregulation. However, extreme hypotension can exceed the dilatory reserve of cerebral resistance vessels, cause CBF to fall, and place the brain at risk. One purpose of autoregulation monitoring is to delineate this limit.
Several methods exist to monitor autoregulation using surrogates for CBF or blood volume.20 Of these methods, transcranial Doppler and NIRS-derived metrics are not intracranially invasive. NIRS-based techniques provide a signal that is more consistent and artifact-free than transcranial Doppler waveforms. Bassan et al21 used NIRS to quantify pressure autoregulation during pediatric CPB by using a frequency-domain analysis. They found that disturbed post-CPB autoregulation was associated with fluctuating ABP and hypercarbia. For our study, we chose the continuous, time-domain analysis method of pressure autoregulation by Czosnyka and colleagues,22 which allows sorting by ABP to determine the individual LLA.16
We found impaired pressure autoregulation during CPB. Does low ABP during CPB engender impaired autoregulation? Autoregulation impairment of the cohort during CPB was accompanied by a greater portion of time spent with an ABP <40 mm Hg, which was 2 SDs less than the mean cohort ABP (Figure 3C). The cohort averaged LLA is also near 40 mm Hg (42 mm Hg, range 20 to 55 mm Hg; Figure 4). It is tempting to conclude that this study validates the use of 40 mm Hg as a universal ABP goal for children during CPB. However, the broad range of individual LLA determinations in this cohort suggests that using a single ABP target for children during CPB is inappropriate. Given these pilot results, it is reasonable to posit that defining and exceeding individual LLA, using pressure autoregulation monitoring, would mitigate CPB-induced autoregulatory disruption. However, coexistence of hypothermia and hypotension during CPB confounds this conclusion. Exposure to hypothermic CPB is known to cause vasomotor dysfunction.23 This disturbance contributes to cerebral vasoconstriction and altered CBF seen in children exposed to hypothermic CPB.3 Our study did not record temperature at a resolution sufficient to address this question.
Taylor et al used transcranial Doppler and fontonometry to study autoregulation in infants during CPB. They created a cohort autoregulation curve by plotting flow velocity as a function of cerebral perfusion pressure. Consistent with our data, they found a transition from impaired to active autoregulation at a mean ABP of 20 to 40 mm Hg during normothermia. However, during hypothermia, they demonstrated only impaired autoregulation. This was confounded by the hypothermic data being obtained at ABP <40 mm Hg.19
An important limitation of our study is the use of a threshold COx value to estimate individual LLA. Most researchers favor finding an “optimal” ABP over defining an LLA threshold.20 For instance, outcome of patients with traumatic brain injury is associated with ABP management that “optimizes” autoregulation.16 However, increasing ABP to optimize autoregulation is likely to impair cardiac function and visceral perfusion in patients with cardiac disease. Therefore, estimating and exceeding the individual LLA is more theoretically suited to cardiac surgical patients. Future animal models using dynamic autoregulation monitoring in the setting of CPB will be helpful to more accurately define the threshold for COx monitoring.
In summary, we have presented a novel application and method to continuously monitor autoregulation. Our data suggest that it is possible to estimate the LLA for individual patients during cardiac surgery using NIRS. CPB was associated with both hypotension and impairment of autoregulation as measured by the COx. Future studies of this modality with neurocognitive outcomes would benefit from the inclusion of high-resolution temperature data and the evaluation of alternative COx thresholds.
Acknowledgments
We thank Claire Levine, BS, MA, for editorial assistance.
Sources of Funding
Supported by the Hartwell Foundation and the Foundation for Anesthesia Education and Research.
Footnotes
Disclosures
ICM+ software is licensed by the University of Cambridge, Cambridge Enterprise Ltd. P.S. and M.C. have a financial interest in a part of licensing fee. Under a licensing agreement with Somanetics, K.B. is entitled to a share of fees and royalty received by The Johns Hopkins University on the monitoring technology described in this article. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL, Chiavacci RM, Wernovsky G, Kurth CD. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–114. [PubMed] [Google Scholar]
- 3.Pua HL, Bissonnette B. Cerebral physiology in paediatric cardiopulmonary bypass. Can J Anaesth. 1998;45:960–978. doi: 10.1007/BF03012304. [DOI] [PubMed] [Google Scholar]
- 4.Wernovsky G, Shillingford AJ, Gaynor JW. Central nervous system outcomes in children with complex congenital heart disease. Curr Opin Cardiol. 2005;20:94–99. doi: 10.1097/01.hco.0000153451.68212.68. [DOI] [PubMed] [Google Scholar]
- 5.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–e767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 6.Newburger JW, Bellinger DC. Brain injury in congenital heart disease. Circulation. 2006;113:183–185. doi: 10.1161/CIRCULATIONAHA.105.594804. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie ED, Andropoulos DB, DiBardino D, Fraser CD., Jr Congenital heart surgery 2005: the brain: it’s the heart of the matter. Am J Surg. 2005;190:289–294. doi: 10.1016/j.amjsurg.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, Koehler RC, Shaffner DH. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke. 2008;39:2531–2537. doi: 10.1161/STROKEAHA.108.514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10:122–128. doi: 10.1007/s12028-008-9140-5. [DOI] [PubMed] [Google Scholar]
- 11.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, Hogue CW., Jr Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass [published online ahead of print July 22, 2010] Stroke. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, Koehler RC, Shaffner DH, Brady KM. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40:1820–1826. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 13.Lemaire JJ, Khalil T, Cervenansky F, Gindre G, Boire JY, Bazin JE, Irthum B, Chazal J. Slow pressure waves in the cranial enclosure. Acta Neurochir (Wien) 2002;144:243–254. doi: 10.1007/s007010200032. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg N, Troupp H, Lorin H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury. A preliminary report. J Neurosurg. 1965;22:581–590. doi: 10.3171/jns.1965.22.6.0581. [DOI] [PubMed] [Google Scholar]
- 15.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–17. doi: 10.1097/00006123-199707000-00005. discussion 17–19. [DOI] [PubMed] [Google Scholar]
- 16.Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. II. New York: Wiley; 2000. [Google Scholar]
- 19.Taylor RH, Burrows FA, Bissonnette B. Cerebral pressure-flow velocity relationship during hypothermic cardiopulmonary bypass in neonates and infants. Anesth Analg. 1992;74:636–642. doi: 10.1213/00000539-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 21.Bassan H, Gauvreau K, Newburger JW, Tsuji M, Limperopoulos C, Soul JS, Walter G, Laussen PC, Jonas RA, du Plessis AJ. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res. 2005;57:35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- 22.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 23.Schmoker JD, Terrien C, III, McPartland KJ, Boyum J, Wellman GC, Trombley L, Kinne J. Cerebrovascular response to continuous cold perfusion and hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2009;137:459–464. doi: 10.1016/j.jtcvs.2008.08.022. [DOI] [PubMed] [Google Scholar]