Abstract
Polyethylenimine (PEI) is a gold standard polycationic transfectant. However, the highly efficient transfecting activity of PEI and many of its derivatives is accompanied by serious cytotoxic complications and safety concerns at innate immune levels, which impedes the development of therapeutic polycationic nucleic acid carriers in general and their clinical applications. In recent years, the dilemma between transfection efficacy and adverse PEI activities has been addressed from in-depth investigations of cellular processes during transfection and elucidation of molecular mechanisms of PEI-mediated toxicity and translation of these integrated events to chemical engineering of novel PEI derivatives with an improved benefit-to-risk ratio. This review addresses these perspectives and discusses molecular events pertaining to dynamic and multifaceted PEI-mediated cytotoxicity, including membrane destabilization, mitochondrial dysfunction, and perturbations of glycolytic flux and redox homeostasis as well as chemical strategies for the generation of better tolerated polycations. We further examine the effect of PEI and its derivatives on complement activation and interaction with Toll-like receptors. These perspectives are intended to lay the foundation for an improved understanding of interlinked mechanisms controlling transfection and toxicity and their translation for improved engineering of polycation-based transfectants.
Keywords: polyplex, polyethylenimine, cytotoxicity, mitochondrion, complement system
Graphical Abstract
Polycations such as polyethylenimines (PEIs) are widely used as non-viral transfectants, but they often induce cytotoxicity and may trigger immune reactions. Here we examine and discuss molecular events pertaining to dynamic and multifaceted PEI-mediated cytotoxicity and immune system modulation and their translation for improved and safer engineering of polycation-based transfectants.
Main Text
Gene therapy, the transfer of nucleic acids to a target cell, has been endorsed optimistically as an advanced therapeutic treatment/management strategy against otherwise difficult or incurable diseases.1, 2 However, the anionic characteristics of nucleic acids impede cellular uptake through the negatively charged plasma membrane and hinder transfection efficiencies. Accordingly, effective delivery systems are required to successfully transport nucleic acids across the plasma membrane as well as intracellular barriers en route to the nucleus.1, 3, 4 The practical usage of gene therapy in clinical settings, however, is ultimately dependent on the development of safe and efficient delivery systems that are capable of transporting the therapeutic payload to target cells with minimal cytotoxicity. Initially, the application of viral vectors in gene therapy provoked great optimism because of their highly evolved and specific mechanisms for inserting nucleic acids into target cells.5, 6 However, adverse side effects such as severe immunogenicity and fatal incidents during clinical trials greatly compromised the potential of virus-based delivery systems in gene therapy.6, 7 Consequently, these complications placed a greater emphasis on the development of synthetic non-viral vectors to act as a safer option for the delivery of nucleic acid medicines.6 Recent years have witnessed the development of a myriad of synthetic non-viral vectors; however, as a result of various complications, the transfection efficiency of these vectors is commonly lower than those achieved with viral vectors.6, 8, 9, 10, 11, 12 Among the numerous different compounds investigated, cationic polymers have gradually emerged as the most promising non-viral vectors for nucleic acid delivery because of several inherent characteristic aspects. First, most cationic polymers are stable and easily manufactured and can be modified chemically. However, their polydisperse nature is of concern. Second, as a result of their inherent positive charge, polycations have the ability to compact nucleic acids into polyplexes, which effectively protects them against premature degradation and facilitates their delivery across the plasma membrane.
Polyethylenimine (PEI) is among the most intensively investigated polycations for nucleic acid delivery. Because of its highly efficient transfection capabilities, PEI has served as a gold standard transfectant polymer.13 PEI is structured from repeating units of two aliphatic carbon groups and amino nitrogen and is commercially available in both linear and branched morphologies, with the molecular weight ranging between 200 Da and 1,500 kDa.13, 14, 15, 16, 17, 18 Shortly after the discovery of branched PEI as a promising transfecting agent, linear PEI, produced by hydrolysis of poly(2-ethyl-2-oxazoline), was identified as a derivative with more favorable properties.19, 20, 21 The branched PEI contains primary, secondary, and tertiary amines, whereas the linear form has only secondary amines. The protonable amine groups provide PEIs with the unique features of high cationic charge density and buffering capacity at extracellular and endo-lysosomal pH levels.13 This trait makes PEI exceptionally effective in compacting nucleic acids into polyplexes that effectively shield nucleic acids against degradation by nucleases. Additionally, PEI-nucleic acid complexes (PEI polyplexes) with a net positive charge are favorably taken up by mammalian cells through different endocytic mechanisms as well as plasma membrane destabilization.13, 14, 22, 23, 24, 25, 26 Although still debated, PEIs may escape the endo-lysosomal system through a pH-dependent mechanism,27, 28 often called the “proton sponge effect.” Here the protonable amine groups may absorb protons during the natural H+ vacuolar-type proton-ATPase (v-ATPase)-driven acidification process inside endo-lysosomes. This is further associated with an influx of chloride ions and water, resulting in vesicle swelling.29 Initially, these osmotic perturbations were believed to induce vesicle rupture, which releases polyplexes into the cytoplasm. More recently, the pH-triggered increased cationic charge density is considered to contribute to endosomal escape via direct phospholipid membrane destabilization (“needle effect”).8, 13, 29, 30, 31
Regardless of the internalization pathways, the effectiveness of PEI transfection procedures is well known to be both architecture- and molecular weight-dependent and directly correlates with the positive charge of the polyplexes, which, in turn, is associated with cytotoxic complications.19, 24, 32, 33, 34 In particular, higher molecular weight (HMW) PEIs do exhibit superior transfection efficiencies in vitro and in vivo but are, unfortunately, also linked with greater cytotoxicity.13, 19, 20, 21, 35 In contrast, lower molecular weight (LMW) PEIs display very few cytotoxic effects but are also known for their inferior transfection abilities.33, 34 Hence, there exists a fine-tuned intrinsic balance with increasing molecular weight, better transfection efficiency, and higher cytotoxic complications that has to be taken into account when selecting/designing the optimal PEI-based delivery system. Although both linear and branched PEI morphologies have achieved excellent transfection efficiencies in a wide range of clinically relevant cell lines, the structural configuration of PEI appears to influence the transfection abilities.19 Although some studies have promoted the branched morphology, it appears that the linear structure is a superior transfection agent both in vitro and in vivo.19, 20, 36, 37, 38 Indeed, it is possible that these contradictions are related to differences in study design and/or that different PEI morphologies might impose tissue-specific transfection efficiencies based on different cell types and microenvironmental factors. Furthermore, it is still unclear whether these variations in transfection abilities, amid diverse PEI structures in different studies, represent actual beneficial effects from structural differences or whether the superior transfection occurs as a consequence of fewer cytotoxic complications and cell death associated with architecture-dependent cytotoxicity in a cell/tissue-specific manner. Regardless of which structural configuration is superior as a transfection agent, accumulating evidence shows that, although cationic polymers, and PEI in particular, comprise many prominent features required for effective nucleic acid delivery, it remains a great concern that those cationic polymers containing the best transfection abilities are also those generally found to be most cytotoxic.13, 19, 21, 33, 34 To counteract this dilemma, chemical engineering and advanced polymer technologies have, in conjunction with comprehensive cellular and molecular studies, detected important structure-toxicity associations that have aided the design of safer cationic polymer-based vectors for nucleic acid delivery. Accordingly, several strategies have been followed to decrease PEI-mediated cytotoxicity while maintaining the intended efficiency. Important strategies can be classified as follows: control of size and topology, biodegradable cross-linking of LMW PEI, statistical surface modification, synthesis of block co-polymers, and oligoamine segment conjugation. This review aims to provide much needed insight into the principal mechanistic aspects underlying the cytotoxic complications manifested upon exposure to cationic polymers and to address the most promising approaches utilized for chemical engineering of new PEI derivatives that have lowered cytotoxic complications but preserved or even improved transfection capabilities.
PEI-Mediated Cytotoxicity
During the last decade, a number of studies have shown that the PEI-mediated cytotoxic responses are highly interlinked with perturbations in cellular membranes and bioenergetic processes in conjunction with polymer concentration, molecular weight, electric charge, and structure.13, 24, 33, 35, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 However, the polydisperse nature of PEIs has complicated detailed structure-activity relationship studies. Werth et al. demonstrated the possibility to isolate a low molecular weight fraction with a favorable efficiency-toxicity balance by gel permeation chromatography of the commercially available 25-kDa branched PEI.49 Moreover, Godbey et al. proposed the existence of two distinct types of PEI-mediated cell death: one primarily caused by free PEI affecting cells rapidly after treatment, and a second with lower kinetics that was hypothesized to occur after internalization.32 Consistently, the removal of free PEI before transfection was shown to reduce cytotoxicity; however, free PEI also appeared to be responsible for enhanced transfection efficiency.50 More recently, further mechanistic discoveries have been obtained through comprehensive pan-integrated biochemical, cellular, and molecular investigations into the effect of PEI-DNA polyplexes and unmodified free PEIs of different sizes and morphologies on diverse biological systems.40, 41 It has long been known that cationic polymers exert damaging effects on biological membranes, conceivably through nanoscale pore formation and/or decreased membrane integrity, by altering the alignment of the phospholipid composition constituting the lipid bilayer.51, 52, 53 This inherent ability of PEI to destabilize biological membranes is most likely interlinked with the sequential protonation of the repetitive aminoethylene pattern of the polymer and is, in part, considered a valuable feature because it is thought to play a role in facilitating cellular uptake of polyplexes.50 However, excessive exposure can lead to disastrous consequences for cellular health and survival through disruption of the plasma membrane and various other cellular organelles, including endosomes (and, possibly, lysosomes) and mitochondria.44 Specifically, PEI-induced destabilization of the plasma membrane and, to some extent, vesicles constituting the endo-lysosomal system is thought to participate in the cytoplasmic release of PEIs31 but also to contribute to cytotoxicity through activation of lysosome-mediated death pathways.44 The potential consequences of PEI interacting with the endo-lysosomal system are reviewed elsewhere44 and are outside of the scope of this review.
PEI-Mediated Cytotoxicity: Mitochondrial Dysfunction
The ultimate cellular fate (i.e., cell death or survival) following PEI exposure is largely determined by the magnitude of the PEI-induced damage, which, in turn, activates multilayered molecular mechanisms that are associated with apoptotic, necrotic, and programmed necrotic pathways in conjunction with lysosomal damage, autophagy, and mitochondrial dysfunction.40, 41, 42, 43, 44, 47, 48, 54 Accumulating evidence indicates that the extent of the damage exerted by cationic polymers on mitochondrial functionality is among the most important parameter for safety screenings aimed at predicting inherent cytotoxic complications associated with impending novel polycationic vectors intended for transfection purposes.39, 40, 41, 42, 43, 44, 54 Mitochondria are recognized as the most efficient source for cellular energy production; however, their unique structure and functional features make them highly exposed to toxic chemicals interfering with their activities. Mitochondria are comprised of two separate and functionally distinct outer and inner membranes that encapsulate the intermembrane space and the matrix compartment.55, 56, 57, 58 The intactness of the mitochondrial membranes is of great importance for the functional and structural integrity of mitochondria, which, in turn, makes mitochondrial function exceedingly vulnerable to compounds such as cationic polymers with known detergency activity toward lipid bilayers. Malfunctioning mitochondria can pose a serious threat for cellular life through either inadequate energy production, the release of pro-apoptotic proteins, and/or higher emission of free radicals capable of forming reactive oxygen species (ROSs).59, 60
As a consequence of a number of internal or external cellular stresses, alterations in mitochondrial activities can trigger various signaling pathways that influence cell death or survival. Indeed, studies with isolated mitochondrial preparations have found that PEIs are able to promote mitochondrial cytochrome c (cyt c) leakage, allegedly through the ability of the polymers to form nanoscale pores in mitochondrial membranes, resulting in mitochondrial permeability transition, and the subsequent release of cyt c into the cytosol, consequentially activating caspases and cell death processes.42, 43, 54 The execution of PEI-mediated cytotoxicity is known to occur in two distinct successive stages.43 The first phase takes place within the first 30 min after PEI exposure and is characterized by direct binding of PEI to proteoglycans in the plasma membrane,61 resulting in plasma membrane damage and lactate dehydrogenase (LDH) release, both common features of necrosis. The second phase occurs at later time points (24 hr) of PEI exposure and involves mitochondrion-mediated apoptosis characterized by mitochondrial depolarization and caspase-3 activation.43 Subsequent studies demonstrated that PEI exposure also induces autophagy.47, 48 Particularly, the initial autophagy response (3 hr) correlates with lysosomal damage, whereas a secondary autophagy response (24 hr) associates with mitochondrial dysfunction,47 conceivably as a compensatory mechanism in response to bioenergetic crisis.41 More recently, studies have gradually elucidated that unmodified PEI vectors act as potent toxins to mitochondrial bioenergetics in a concentration-, size-, and architecture-dependent manner, with serious consequences for intracellular energy homeostasis, oxidative stress, and cellular survival.40, 41
PEI-Mediated Cytotoxicity: The Mitochondrial Electron Transport System and Bioenergetic Crisis
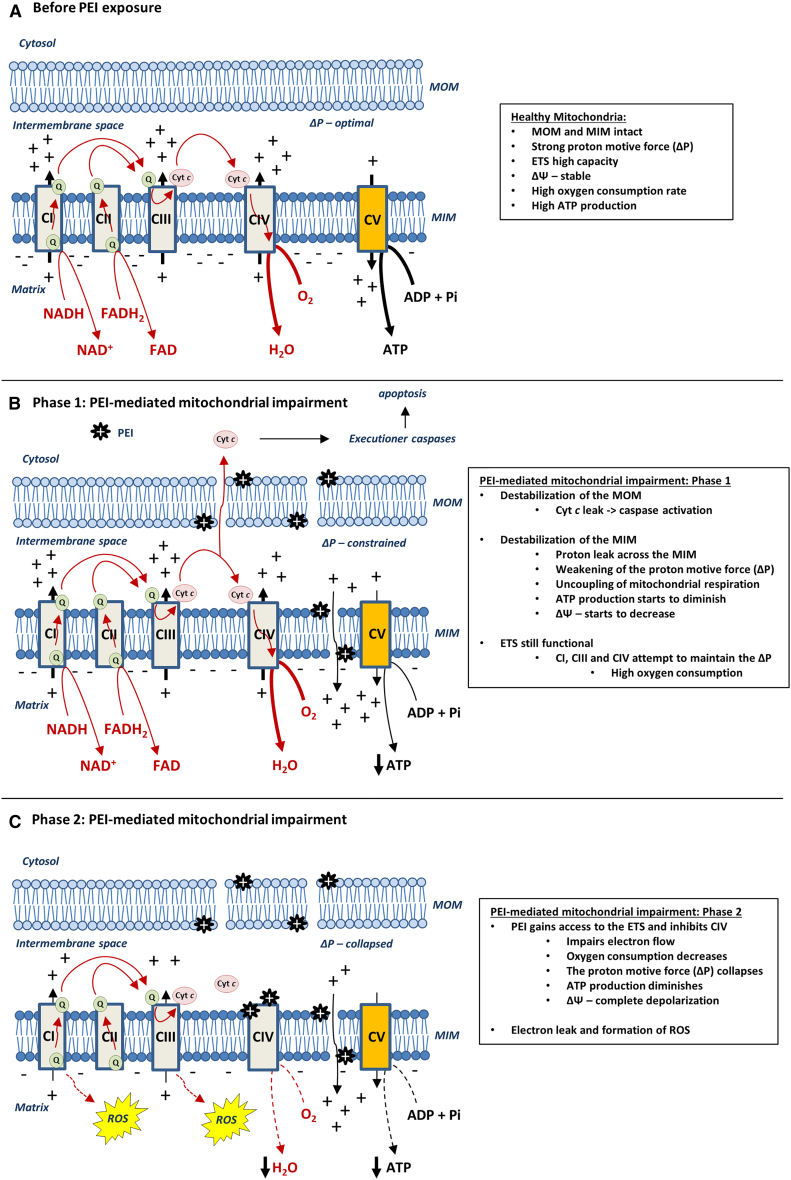
Cells are continuously dependent on large quantities of free energy to drive various essential thermodynamically nonspontaneous biochemical reactions. Generally, cells obtain most of the energy through hydrolysis of ATP, often referred to as intracellular energy currency.62, 63, 64 In most healthy cells, mitochondria are the main source of ATP production through the process of oxidative phosphorylation (OXPHOS).63, 64, 65 Effective energy transduction during OXPHOS is accomplished through tight coupling between the activities of the components of the mitochondrial electron transport system (ETS) and F1F0 ATP synthase.66 Four protein complexes (CI–CIV) comprise the ETS, transferring electrons through a series of redox reactions that conclude with oxygen acting as the final electron acceptor (Figure 1A). Briefly, electrons originating from nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) are transferred to CI and CII, respectively. Thereafter, electrons are further transported from CI and CII to CIII by the quinone pool (Q-pool). Following this, another mobile electron carrier, cyt c, allows for continuous flow of electrons from CIII to CIV, which catalyzes the transfer of electrons from reduced cyt c to oxygen, forming water in the process.66, 67, 68 Essentially, the free energy released during the electron flow through the ETS complexes is interconnected with the ability of CI, CIII, and CIV to translocate protons from the matrix across the impermeable inner membrane into the intermembrane space. This activity generates and maintains the electrochemical proton gradient, establishing the proton motive force (ΔP) and the mitochondrial membrane potential (ΔΨ).69, 70 The accumulation of free energy in the ΔP can be utilized by another inner membrane complex, F1F0 ATP synthase, to generate chemical energy by driving the synthesis of ATP molecules from ADP and inorganic phosphate71 (Figure 1A).
Figure 1.
Schematic of the PEI-Mediated Biphasic Mitochondrial Impairment and Its Consequence on Mitochondrial Functions and Integrity
(A) Representative figure depicting the structural and functional integrity of healthy mitochondria before PEI exposure. The mitochondrial outer membrane (MOM) and mitochondrial inner membrane (MIM) are intact, the proton leak across the MIM is low, the ETS is highly active, the OCR is high, the ΔΨ is stable, the ΔP is strong, and the ATP synthesis is functional. (B) During the first phase of PEI-mediated mitochondrial impairment, PEI destabilizes the MOM, leading to the release of cyt c into the cytosol, which, in turn, can activate caspase-dependent apoptosis. Also, PEI destabilizes the MIM, resulting in increased proton leak across the MIM and the consequential uncoupling of mitochondrial respiration (i.e., fewer protons are used for ATP synthesis). The ΔΨ decreases, and the ΔP is constrained. However, the ETS is still functional, and CI, CIII and CIV enhance proton pumping into the intermembrane space to maintain the ΔP; hence, electron flow and OCR remain high. (C) In the second phase of PEI-mediated mitochondrial impairment, PEI gains access to the ETS and exerts its potent inhibitory effect on CIV. Consequently, electron flow through the ETS is hindered, resulting in decreased proton pumping by CI, CIII, and CIV, which, in turn, leads to the collapse of the ΔP and the ΔΨ and sequential obstruction of the OCR and ATP production. Moreover, as electron flow through the ETS is impaired, build-up of electrons at CI and CIII can lead to electron leakage and the formation of ROSs.
The generation of the ΔΨ results in a large negative transmembrane potential on the matrix side of the inner mitochondrial membrane, rendering mitochondria highly attractive targets for positively charged compounds.72, 73 Furthermore, positively charged chemicals are found to accumulate in mitochondria based on the status of the ΔΨ, where a greater negative charge in mitochondria results in higher accumulation.72, 73 Indeed, PEI and PEI-DNA polyplexes co-localize with the mitochondrial network early after transfection and are therefore known to reach and interact directly with mitochondria.45 Also, cellular susceptibility toward cationic polymer-induced toxicity has been interlinked with mitochondrial physiology because cells with hyperpolarized mitochondria (i.e., a greater negative charge) require lower concentrations of cationic polymers to instigate mitochondrial impairment.39 Recently, the concentration- and time-dependent effect of structurally diverse PEIs on mitochondrial respiratory states was analyzed in intact cells through real-time investigations of the oxygen consumption rate (OCR) with a Seahorse XF Analyzer.41, 74 PEI was found to cause rapid initial acceleration of basal mitochondrial respiration, a phenomenon that occurred in a concentration- and time-dependent manner, both because of an enlarged proton leak across the inner membrane, and increased mitochondrial ATP synthesis.40, 41 Specifically, the rise in OCR was more distinct at early time points (5–20 min) and with lower concentrations (1–2 μg/mL), whereas the increased OCR declined gradually with a longer exposure time (20–90 min), followed by a significant decline in OCR at higher PEI concentrations (4–8 μg/mL). Moreover, PEI was also found to impair the maximum respiratory rate (MRR, indicating the maximum capacity of the ETS) in a concentration- and time-dependent manner. Interestingly, in comparison with its linear counterpart of the same molecular weight, the branched architecture was found to be a more potent inhibitor of mitochondrial respiratory states in intact cells.41 Accordingly, the respiratory control ratio (i.e., the ability of mitochondria to oxidize respiratory substrates) and the coupling efficiency of OXPHOS (i.e., the fraction of protons used for ATP synthesis) diminished in a concentration-dependent manner and to a greater degree in cells exposed to branched PEI compared with the linear architecture.41 Similarly, following controlled plasma membrane permeabilization in cells, the branched PEI structure was more detrimental to mitochondrial OXPHOS compared with the linear form of equal molecular weight. Likewise, compared with the branched form, linear PEI was also less detrimental to OXPHOS activity in freshly isolated mitochondrial preparations. Further experiments in “broken mitochondria” preparations showed that PEI impairs ETS activity by blocking electron flow through both the CI-CIII-CIV and CII-CIII-CIV cascade because of a potent direct inhibitory effect on CIV activity.40, 41 Compared with the linear architecture, branched PEI of equal molecular weight was found to be a more potent inhibitor of CIV activity at low concentrations (0.1–1 μg/mL) in broken mitochondria preparations; however, with increasing concentrations (2–3 μg/mL), the linear architecture exerted a greater inhibitory effect on CIV relative to the branched PEI.41 Taken together, these recent observations show that PEI impairs mitochondrial functions in a biphasic manner (Figure 1), dependent on concentration, molecular weight, electric charge, and the architecture of the polymer.40, 41 In the first phase (Figure 1B), PEI interacts with the mitochondrial membranes and undermines the structural integrity of the lipid bilayer. Subsequently, an increased proton leak across the inner mitochondrial membrane dissipates the ΔP, resulting in uncoupling of mitochondrial respiration, which, in turn, decreases the effectiveness of the F1F0 ATP synthase. During the second phase (Figure 1C), PEI gains access to the ETS and impairs its activity through an exceptionally potent inhibitory effect on CIV (cytochrome c oxidase).40, 41 Accordingly, the electrochemical proton gradient collapses together with obstruction of energy production through OXPHOS and instigation of a bioenergetic crisis. Interestingly, electron flow from cyt c to oxygen is catalyzed by CIV via electrostatic interactions.75, 76, 77 Particularly cyt c contains a net positive charge that enables it to interact with a negatively charged docking site on CIV.75, 76, 77 Because of its inherent positive charge, PEI interferes with the electrostatic interactions between cyt c and CIV through high-affinity binding to the anionic cyt c docking site on CIV. The PEI-induced inhibition of CIV would therefore most likely be competitive toward cyt c, in accordance with previous investigations pertaining to various other types of cationic compounds.78, 79 The observation that structurally diverse PEIs impair mitochondrial bioenergetics differently is directly linked to the higher number of protonable amine groups constituting the branched PEI polymer, which, in turn, correlates with a greater positive charge and more potent mitochondrial toxicity of the branched structure.41 Interestingly, when the accessibility to the mitochondria is increased (i.e., permeabilized cells, isolated mitochondria and broken mitochondria), the damaging effect of the linear architecture approaches the toxicity of the branched structure.41 This strongly suggests that the linear PEI structure has more difficulties in accessing the mitochondrial network and, thus, impairing its activities. It is possible that both architecture and macromolecular size induce cytoplasmic entry of cationic polymers at distinct locations differently and to different degrees. Indeed, PEI exerts different grades of detergency activities on biological membranes based on both structure and molecular weight.41, 51, 52, 53 Compared with the branched PEI, the linear architecture is less detrimental to the plasma membrane integrity and the mitochondrial inner membrane, as measured through LDH release and citrate synthase leak, respectively.41 The branched architecture might form structures of globular shape in accordance with that of higher-generation polyamidoamine dendrimers,52, 80 instigating a greater destabilization of biomembranes in comparison with the alleged cylindrical geometry of the linear PEI. Also, the different geometrical shapes might influence the ability of the polymers to diffuse throughout the gel-like cytoplasm. In particular, the spherical/globular shape of the branched architecture could allow for more rapid diffusion rates than the cylindrical structure of the linear PEI, consequently increasing the probability of the branched PEI to interact with and impair the mitochondrial network. The PEI-induced biphasic destruction of mitochondrial function does result in concentration-dependent intracellular ATP depletion, correlating with the extent of mitochondrial impairment.40, 41 Again, compared with the linear PEI, the branched architecture caused larger intracellular ATP depletion, a phenomenon that was partly attributable to greater plasma membrane damage at higher concentrations (4–8 μg/mL PEI), followed by increased ATP leaking out of cells. Markedly, the extent of the ATP depletion is dependent on polymer architecture, molecular weight, and concentration41 and can conceivably explain the multilayered cell death processes activated upon PEI exposure because mild ATP fluctuations are known to be associated with apoptosis, whereas larger ATP depletions are connected with necrosis.81, 82, 83 The bioenergetic crisis instigated by PEI exposure also results in rapid activation of AMP-activated protein kinase (AMPK),41 a master regulator of cellular metabolism that has the ability to rewire intracellular metabolic processes to reestablish intracellular energy homeostasis.84, 85, 86 For instance, AMPK signaling increases autophagy flux by decreasing the activity of mTOR, a known inhibitor of autophagy.86 Accordingly, the enhanced autophagy flux reported upon exposure to cationic polymers47, 48 is likely regulated through the AMPK-mTOR axis, occurring as a compensatory mechanism in response to bioenergetic perturbations. Therefore, cells exposed to milder bioenergetic crises would be able to restore intracellular homeostasis and survive the insult caused by the cationic polymers, whereas, under conditions of more intense macromolecular damage, the autophagy flux might be overburdened and unable to correct the cellular insult.
PEI Architecture-Dependent Perturbations of Redox Homeostasis
In addition to the abovementioned impairment of mitochondrial bioenergetics, cationic polymers were recently found to perturb intracellular redox homeostasis in a molecular weight and architecture-dependent manner.41 Normally, a certain amount of ROS is generated by OXPHOS as a by-product, and ROS production is frequently enhanced in dysfunctional mitochondria.59, 60, 87, 88 Notably, in conjunction with greater mitochondrial destruction, exposure to the branched PEI architecture was found to result in a greater increase in intracellular ROS levels in comparison with linear PEI.41 These data are in accordance with the more potent ETS inhibition exerted by the branched PEI, directly prompting more rapid production of ROS. However, a large contribution of the branched PEI-mediated increase in ROS production was attributable to PEI-mediated impairment of the redox system and the cellular ability to counteract rising ROS levels.41 To prevent the accumulation of oxidative stress, cells utilize the capability of the endogenous antioxidant glutathione (GSH) to restore intracellular redox homeostasis through ROS scavenging.89, 90 Concurrently, GSH neutralizes excessive ROS formation and is oxidized in the process to GSH disulfide (GSSG), requiring the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent activity of glutathione reductase for converting GSSG back to GSH.90 Overwhelming ROS production or depletion of intracellular NADPH levels can overcome the cellular capability to reduce GSSG to GSH, resulting in accumulation of GSSG and a decreased capacity for antioxidant scavenging.89 The intracellular NADPH pools are mainly maintained by NADP-linked enzymes in the mitochondrial-cytosolic pathway and the pentose phosphate pathway (PPP), indicating the significance of high-quality mitochondria and continuous diversion of glucose flux through the PPP for adequate NADPH production.91, 92, 93
Markedly, the branched PEI structure was also found to cause glycolytic flux to collapse in a concentration-dependent manner,41 decreasing glucose flux through the PPP. This phenomenon is partly related to plasma membrane perturbation events and the subsequent LDH leak across the plasma membrane at cellular exposure to increasing PEI concentrations.41 LDH is a key enzyme in glycolysis, and excessive intracellular LDH depletion will most likely affect the rate of glycolytic flux. The combined mitochondrial damage and glycolytic collapse exerted by the branched PEI structure resulted in depletion of NADPH levels, which, in turn, lowered the capacity of GSH to scavenge ROSs. Addition of N-acetyl-cysteine (NAC), a precursor of GSH, counteracted the PEI-mediated increase in ROSs, partly increased cellular viability, and enhanced the transfection efficiency of branched PEI polyplexes.41 Collectively, these findings demonstrate that the cytotoxicity of branched PEIs is partly caused by increased oxidative stress and that the transfection efficiencies of branched PEI-based polyplexes can be enhanced, to some extent, by co-treating cells with antioxidants such as NAC.
The Effect of PEI on the Immune System: Immune Toxicity versus Immune Stimulation
Intravenously injected nanopharmaceuticals rapidly acquire plasma proteins on their surface. Blood protein adsorption is known to modulate nanoparticle pharmacokinetics and tissue distribution.94 Among these proteins are the opsonic components of the blood that may prime the surface of nanoparticles for binding to blood leucocytes and macrophages of the reticuloendothelial system. Here the complement system, which is the first line of the body’s defense against intruders, plays a major role in nanoparticle recognition by phagocytic cells through opsonization by cleavage products of the third complement protein (C3), such as C3b and iC3b.95 Uncontrolled complement activation, however, is detrimental and can lead to inflammatory reactions and disease progression. For instance, intratumoral complement activation may initiate tumor growth, and, indeed, interstitial accumulation of complement-activating nanoparticles has been shown to promote tumor growth through liberation of C5a, which is chemotactic to immunosuppressive cells.95
Limited studies have focused on complement activation by polycations and polyplexes. PEI, poly(l-lysine), and polyamidoamine dendrimers as well as their corresponding polyplexes have all been shown to incite complement; however, complement activation decreases upon polycation PEGylation.96, 97, 98, 99, 100 The pathways and mechanisms by which polycations and polyplexes trigger complement activation is not clear, but these may be due to the presence of high clusters of surface cationic charge. For instance, surface domains with a high density of cationic charge may attract anionic plasma proteins. Some of these proteins, on binding, may be attacked by nascent C3b and/or C3(H2O) and promote complement activation through the alternative pathway.101 Indeed, this possibility may explain why PEGylated polyplexes incite complement less compared with native polycations. In contrast to this suggestion, direct interaction between the cationic globular head of the complement-sensing and pattern recognition protein C1q with polycations is unlikely (because of charge repulsion) to trigger the classical pathway of the complement system directly; however, C1q may interact with unprotected nucleic acids through electrostatic interactions and/or surface-projected polyethylene glycol (PEG) chains of PEGylated polyplexes through hydrophobic interactions as well as hydrogen bonding.102
There are suggestions that complement activation may induce adverse cardiopulmonary reactions to intravenously administered nanopharmaceuticals.103 Indeed, cardiopulmonary distress upon PEI injection in the porcine model has been demonstrated.99 However, these reactions may not be entirely related to complement activation, as suggested recently.104 The most likely mechanism may be robust polycation and polyplex clearance by intravascular pulmonary macrophages in pigs (which may be independent of complement opsonization), resulting in the release of large quantities of thromboxane A2, prostaglandins, and prostacyclines that correlates with periods of peak vasoconstriction, bronchoconstriction, and pulmonary hypertension.104 Accordingly, future studies should identify responsible macrophage signaling receptors capable of interacting with polycations and polyplexes. To this end, possible interactions between polyplexes and Toll-like receptors (TLRs) are likely, which may further modulate immune reactions in different ways. For example, PEI-coated iron oxide nanoparticles were shown to activate macrophages through TLR-4 signaling and reactive oxygen species production as well as modulating podosome dynamics.105 In a different study, the 25-kDa branched PEI was shown to stimulate interleukin-12 (IL-12) secretion from macrophages through TLR-4 activation.106 Moreover, others have shown activation of TLR-3, 5, and 7 in tumor-associated dendritic cells by linear PEI-small interfering RNA (siRNA) complexes.107 These studies, therefore, attest to the immunostimulatory effect of PEIs and PEI polyplexes through TLR activation, which may be advantageous in vaccination protocols and design as well as for modulating tumor growth (despite its complement activation property). For instance, intratumoral injections of PEI promoted Th1 helper cell and natural killer cell infiltration, decreased tumor angiogenesis, and prolonged the survival of sarcoma-bearing mice but not TLR-4 knockout mice.108
Chemical Engineering of Cationic Polymers: Lowering Cytotoxicity while Preserving Transfection Abilities
The cytotoxic complications associated with cationic polymers have generated a negative trait for their possible application in gene therapy, and serious safety concerns have impeded clinical applications of PEI-based gene therapeutics. In addition, PEI-mediated cytotoxicity can antagonize transgene expression and, therefore, represents a limiting factor for high gene transfer activity. Accordingly, wide-ranging attempts with advanced polymer technologies and chemical engineering have been comprehensively utilized to design safer polymer derivatives, characterized by low inherent cytotoxicity and superior transfection abilities. The following section of this review outlines some of the most promising approaches used to improve transfection performances together with counteracting the toxic complications associated with cationic polymers. We also discuss how these structural-chemical adjustments alter the inherent features of cationic polymers and how they are, presumably, directly accountable for lowering polymer toxicity.
Biodegradable Cross-Linking
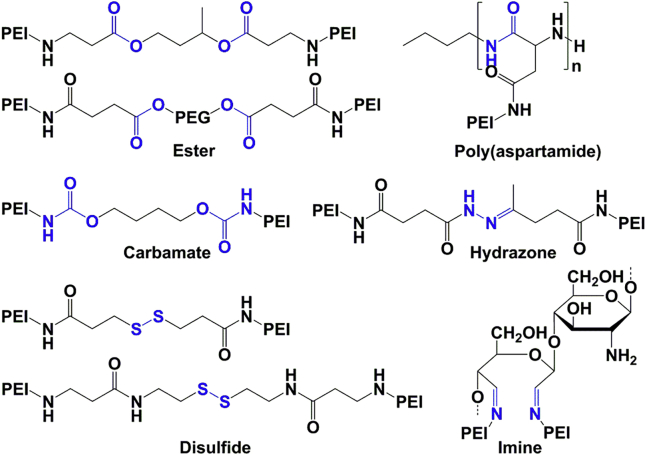
As mentioned above, PEIs of lower molecular weight are less cytotoxic, whereas PEIs of higher molecular weight are more cytotoxic but also more efficient transfection vectors. Therefore, various studies have attempted to design PEI derivatives that are constructed from LMW PEIs that are crosslinked through biodegradable bonds to ensure rapid degradation upon internalization. Depending on the chemical nature of the cross-linkage, biodegradation can occur in different cellular compartments and stages of the nucleic acid delivery pathway. Various approaches have been used to construct biodegradable PEI derivatives from LMW PEIs (Figure 2), including strategies applying ester,35, 109 imine,110 carbamate,111 poly(aspartamide),112 hydrazone,113 or disulfide114, 115, 116 linkage to interconnect the small PEIs constituting the degradable PEI derivative. For instance, ester linkages have been utilized to design biodegradable PEIs prepared with either linear or branched PEI morphology.109, 117, 118 The first biodegradable ester-linked linear PEI constituted PEIs of 800 Da that were produced by using 1,3-butanediol diacrylate as the cross-linking agent.109 Under physiological conditions, the half-life of this PEI derivative is approximately 4 hr because of rapid ester hydrolysis. Importantly, cytotoxicity was found to be lower, and the transfection efficiency was 9-fold higher compared with 25-kDa linear PEI. Additionally, ester linkages have been used to prepare biodegradable branched PEIs; however, the degradability of branched ester-linked PEI derivatives is lower in comparison with the linear form because the branched morphology limits water accessibility to the ester linkages and, hence, the rate of hydrolysis.119 Nonetheless, biodegradable PEI derivatives prepared from repetitive units of 800-Da branched PEIs cross-linked with an ester linkage through a reaction of 1,6-hexanediol diacrylate had up to 16-fold higher transfection efficiency in comparison with branched 25-kDa PEI.109 Disulfide linkages represent another interesting approach for degradable conjugations with unique bioresponsive characteristics.114, 115, 116 The cellular reduction potential with 100- to 1,000-fold higher glutathione concentrations than in the extracellular environment triggers the cleavage of disulfide bridges after reaching the cytosol or nucleus.120 This environmental sensing cannot only be beneficial for polymer degradation but also for a triggered cargo release. Generally, independent of the chemical cross-linking strategy, these investigations showed that the cytotoxicity decreases while the transfection efficiency increases significantly when PEI vectors are prepared by linking a number of low molecular weight PEIs.
Figure 2.
Chemical Strategies for Bioreversible Cross-Linking of LMW PEI
Degradable ester,35, 109 carbamate,111 disulfide,114, 115, 116 poly(aspartamide),112 hydrazone,113 and imine110 linkages are indicated in blue. Cross-linked LMW PEI represents a compromise between highly efficient but toxic HMW PEI and well tolerated LMW PEI. After internalization and accomplished transfection, the PEI derivatives fragment into LMW PEIs with low cationic charge and less cytotoxic complication.
Collectivity, LMW PEIs contain an inferior cationic charge in comparison with HMW PEIs. However, the biodegradable PEI derivative is constructed from a large number of LMW PEIs that jointly form a large PEI vector that contains a suitable inherent cationic charge for efficient condensation of nucleic acids into polyplexes. However, following internalization, the PEI derivative fragments into a larger number of LMW PEIs that, on their own, contain a low cationic charge. Therefore, the LMW PEIs may not have the ability to disrupt biological membranes and may not necessarily modulate the mitochondrial structural integrity, rendering them unable to access and inhibit CIV. Consequently, mitochondrial functionality may be preserved, resulting in well balanced cellular bioenergetic and redox homeostasis, which, in turn, causes few cytotoxic complications.
Statistical Surface Modification
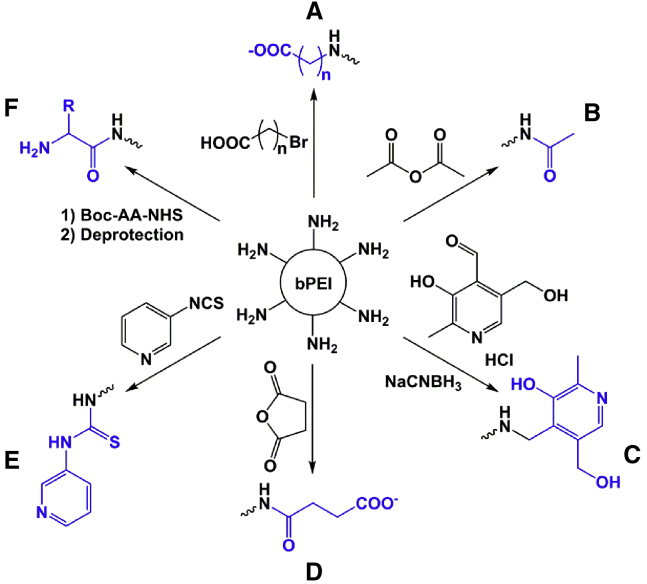
Different chemical strategies have been applied for statistical surface modification of HMW branched PEI (Figure 3). For example, it has been shown that the extent of 25-kDa branched PEI acetylation (and, hence, modification of the surface charge) can reduce cytotoxicity and improve transfection efficiency, although gene transfer can be eliminated in the case of excessive structural modification.121 Several other strategies for statistical modification have been followed. Simple succinylation of 10% PEI amines converted 25-kDa branched PEI in a derivative with up to 10-fold lower polymer toxicity and preserved knockdown efficiency after siRNA transfection.122 The strongly reduced toxicity enabled the usage of higher amounts of polymer with higher knockdown efficiency. Analogously, amine modification by carboxyalkylation below 10%,123, 124 N-acylation with different amino acids,125, 126 pyridylthiourea grafting,127 or conjugation of pyridoxyl groups128 demonstrated favorable properties and improved cellular tolerance toward the branched PEI derivatives. Similar effects of a statistical amine masking and reduced charge density could be found for linear PEI derivatives, which were produced by partial hydrolysis of poly(2-ethyl-2-oxazoline).129 A PEI derivative with 88% hydrolysis ratio, corresponding to 12% masked amines, exhibited remarkably reduced cytotoxicity and distinct DNA transfection efficiency. The lower cytotoxicity observed in these aforementioned studies occurs most likely because the surface modifications decrease the positive charge of the polymers, which, conceivably, lowers the inherent ability of the polymers to disrupt biological membranes. Consequently, this would probably decrease the prospect of the polymers to reach the components of the mitochondrial ETS, which, in turn, reduces their ability to interfere with the electrostatic interactions between cyt c and CIV. Accordingly, mitochondrial integrity and functionality would be preserved, therefore enabling mitochondria to sustain cellular energy demands, potentially preventing toxic complications arising from bioenergetic failures.
Figure 3.
Chemical Strategies for Statistical Surface Modification of HMW Branched PEI with Favorable Effects on Efficiency and Cytotoxicity
(A–F) The final surface groups following (A) carboxyalkylation,123, 124 (B) acetylation,121 (C) conjugation of pyridoxyl groups,128 (D) succinylation,122 (E) pyridylthiourea grafting,127 and (F) N-acylation with amino acids (AA)125, 126 are indicated in blue. A lowered polymer cytotoxicity following chemical surface modification is supposed to be a consequence of the decreased positive charge density and lowered inherent ability for membrane disruption.
Hydrophilic Co-polymers
More complex derivatization, which does not reduce the surface charge based on chemical blockade but, rather, steric shielding, is represented by the synthesis of co-polymers with non-cationic hydrophilic segments, such as PEG, saccharides, or poly[N-(2-hydroxypropyl) methacrylamide] (pHPMA). The resulting hydrophilic co-polymers generally exhibit strongly reduced cytotoxicity35, 97, 130, 131, 132 but, in some cases, also lowered cellular uptake, hampered endosomal escape and overall transfection efficiency,133 resulting from surface charge shielding, and decreased membrane interaction, which is commonly referred to as the “PEG dilemma.” Kataoka and colleagues have used block co-polymers with hydrophilic PEG segments in the context of their polymer systems containing poly(l-lysine) and poly(aspartamide)s to produce better tolerated materials.134, 135, 136, 137, 138 As mentioned above, the low cytotoxicity observed in PEG-based hydrophilic co-polymers is most likely directly related to the shielding of the surface charge by the PEG segment and lower interactions with biological membranes. The PEG segments reduce the inherent ability of the polymers to disrupt lipid bilayers, which, in turn, makes them less likely to irreversibly damage the plasma membrane and other cellular organelles, such as early endosomes or mitochondria. Accordingly, these hydrophilic co-polymers would be less cytotoxic.
Oligoamine Segment Conjugation
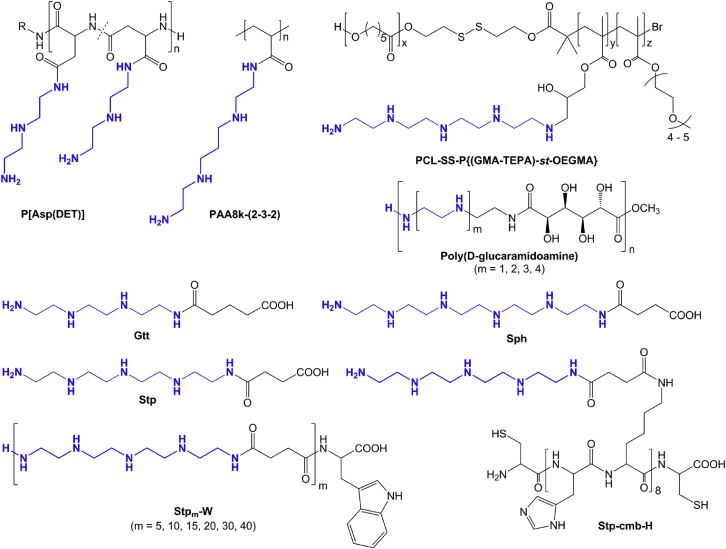
The consequential extension of the approach to cross-link LMW PEIs is the connection of defined oligoamine segments or their site-specific grafting to polymer backbones. By this means, the polydisperse nature of PEI and its continuous macromolecular aminoethylene chain is dissected into PEI-like microdomains with precise size and individual properties. Several chemical strategies have been utilized for defined oligoamine segment conjugation to larger polymer assemblies (Figure 4). Kataoka and collaborators developed poly(aspartamide)s with different defined aminoethylene oligoamines in the side chains and showed high in vitro and in vivo activity with simultaneous low cytotoxicity.139, 140, 141, 142 Pharmacogenomic and bioactivity studies with differential investigation of endogenous and exogenous reporter gene expression revealed differences after DNA transfections with linear PEI or [N-[N-(2-aminoethyl)-2-aminoethyl]aspartamide] (P[Asp(DET)]).143 Despite comparable activity of the exogenous bioluminescence reporter (Renilla luciferase), decreased activity of the endogenous reporter (firefly luciferase), and retarded cell proliferation were observed in PEI-transfected cells. Quantitative determination of the expression of 11 housekeepers by real-time PCR revealed distinct downregulation 72 hr after PEI transfection. The impairment of cellular homeostasis and function also had a negative effect on the induction of osteogenic differentiation by transfer of bioactive genes, which demonstrates the effect of the time-dependent PEI-mediated toxicity on potential therapeutic applications. In contrast, P[Asp(DET)] exhibited minimal toxic effects and successfully induced cell differentiation. The poly(aspartamide) backbone used for grafting of different oligoamine segments, such as diethylentriamine in P[Asp(DET)], showed particularly beneficial properties because it is degradable under physiological conditions.140 This aspect manifests in the more favorable cellular compatibility and less effect on cellular homeostasis and endogenous gene expression compared with the non-degradable polyglutamide analog poly[N-[N-(2-aminoethyl)-2-aminoethyl]glutamide] (P[Glu(DET)]) or linear PEI. In addition, the synthetic control over the precise length of incorporated continuous aminoethylene motifs provided invaluable insights into the sequential protonation of PEI-like polymers and the association with transfection efficiency. Aminoethylene repeats in the side chain with an even number of protonatable nitrogens generally exhibited higher endosomal buffer capacity and DNA transfection efficiency,141 whereas, for mRNA delivery, a compound with an odd number of protonatable amines seemed to be favorable.142 The decisive effect of the particular oligoamine species on bioactivity was also shown recently with a set of different oligoalkylamines grafted to a poly(acrylic acid) scaffold or derivatized to cationic lipids.144 For mRNA transfections, a tetramine with alternating ethyl-propyl-ethyl spacers, differing from the classical diaminoethane motif by a single methylene group, was identified as a superior species regarding protein translation in vitro and in vivo. Pun and colleagues designed ternary block co-polymers comprising tetraethylenepentamine (TEPA) modified glycidyl-methacrylates (GMA) as the cationic proton-sponge constituent.145 The precise polymer architecture with an additional hydrophobic poly(ε-caprolactone) (PCL) block, disulfide linkage, and oligoethylene glycol (OEG) methacrylates represents a dual (pH, redox)-responsive transfecting agent with PEI-like oligoamine units of precise length. The hydrophobic PCL serves as a biodegradable stabilization motif that can combine increased efficiency with suitable cellular tolerance, as has been demonstrated with PEI co-polymers before.131, 132 Reineke and co-workers prepared a set of poly(D-glucaramidoamine)s containing oligoamine segments with systematically varied length, diethylenetriamine, triethylenetetramine, tetraethylenepentamine, or pentaethylenehexamine.146 All compounds exhibited lower cytotoxicity compared with linear PEI but distinct differences in transgene expression depending on the individual oligoamine. The pentaethylenhexamine-containing variant displayed the highest transfection efficiency, equivalent to linear PEI. Although recent polymer chemistry enables the production of well-defined polymer scaffolds with narrow size distribution, a controlled stepwise assembly of building units instead of classical polymerization achieves even higher precision. Convenient and automatable solid-phase synthesis of sequence-defined poly(amidoamine)s composed of sequentially coupled oligo(propanamine)s (3,3′-diamino-N-methyl-dipropylamine or tert-butyloxycarbonyl [Boc]-protected spermine) and a diacid (succinic acid) was established by Hartmann and colleagues.147, 148 This strategy was adapted to the development of artificial fluorenylmethyloxycarbonyl chloride (Fmoc)- and Boc-protected oligo(ethanamino) acids for compatibility with standard Fmoc peptide synthesis.149, 150 The artificial building blocks contain three (glutaryl triethylene tetramine [Gtt]), four (succinyl tetraethylene pentamine [Stp]), or five (succinyl pentaethylene hexamine [Sph]) repeats of the aminoethylene motif and can be assembled precisely into compounds with defined sequence and topology (linear,151, 152, 153 branched,154, 155 comb,156 PEGylated,157, 158 etc.). A library of more than 1,000 compounds has been set up containing several well tolerated members with remarkable siRNA and plasmid DNA (pDNA) transfection efficiency. The high precision of the compounds provides an invaluable possibility for the evaluation of even minor modifications in detailed structure-activity relationship studies. Linear sequences of the oligo(ethanamino) acid Stp, containing three secondary amines per repeat, were assembled to investigate the correlation between the length of the PEI-like oligomers and transfection efficiency or cytotoxicity.153 Oligomers containing ten Stp units (corresponding to 31 protonatable amines) or more efficiently condensed pDNA and showed gene transfer activity. The transition efficiency of the oligomer with 30 Stp units (corresponding to 91 protonatable amines), however, was 6-fold higher than the 22 kDa linear PEI (containing approximately 500 protonatable amines) 6-fold. Although all investigated oligomers exhibited considerably lower cytotoxicity compared with linear PEI, here a correlation between molecular weight, gene transfer activity, and cytotoxicity was also found. Similar to the approaches of cross-linked and stabilized PEI derivatives, one key to highly efficient but well tolerated compounds of this class is the incorporation of cysteines in the sequences of smaller oligomers that form disulfide bridges during polyplex formation or hydrophobic stabilization.150, 151, 152, 159, 160
Figure 4.
Overview of Chemical Strategies for Defined Oligoamine Segment Conjugation to Larger Polymer Assemblies
In the illustrated cationic polymers, the polydisperse nature of PEI is dissected into oligoamine segments (blue) with precise size and individual properties. The novel polycationic systems benefit from innovative synthesis techniques producing well defined compounds, possibility of site-specific modification, investigation of detailed structure-activity relationships, and high degree of flexibility. The poly(aspartamide) P[Asp(DET)] is produced by ring-opening N-carboxyanhydride (NCA) polymerization of β-benzyl-L-aspartate N-carboxyanhydride, followed by aminolysis with diethylenetriamine.139, 140, 141, 142 PAA8k-(2-3-2) is synthesized by carbodiimide-mediated amidation of 8-kDa poly(acrylic acid) with N,N′-bis(2-aminoethyl)-1,3-propanediamine.144 PCL-SS-P{(GMA-TEPA)-st-OEGMA} is produced in multiple steps by ring-opening polymerization of ε-caprolactone, atom transfer radical polymerization of glycidyl methacrylate and oligo(ethylene glycol) monomethyl ether methacrylate, and, finally, attachment of tetraethylene pentamine to the polymer scaffold by ring-opening of the reactive epoxy groups.145 Poly(D-glucaramidoamine)s are synthesized by polycondensation of the esterified D-glucaric acid comonomer with the oligoamine segments.146 Gtt, Stp, and Sph represent the deprotected forms of the building blocks used for solid-phase assisted synthesis of sequence-defined oligo(ethanamino) amides.149 Over 1,000 different oligomers with varying structures and topologies have been generated.150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160 Stpm-W represent linear oligo(ethanamino) amides with precise monodisperse size.153 m indicates 5, 10, 15, 20, 30, or 40 repeats of Stp. Stp-cmb-H contains additional histidines for increased endosomal buffering and cysteines for bioreducible cross-link formation.156
Because the conjugation of defined oligoamine segments represents the logical conceptual continuation of cross-linked LMW PEIs, the abovementioned beneficial properties of crosslinked biodegradable PEIs can also be transferred to biodegradable oligoamine conjugates. Furthermore, the precise chemistry enables more intensive investigation of structure-activity relationships. It appears that the exact structure and number of aminoethylene repeats of the single oligoamine units even have a higher effect than overall nitrogen content in the entire polymer. The type of oligoamine critically determines buffer capacity, nucleic acid binding stability, and transfection efficiency.141, 142, 144, 146, 155, 156 Recent bioenergetic screenings suggested that constitution of oligoamine micro domains could affect cellular impediment (unpublished data). Although generally less toxic than HMW PEIs, assembled oligoamines varying only in single aminoethylene units can mediate significantly dissimilar bioenergetic responses. Such detailed structure-activity relationships with subtle distinction of molecular structures both provides new mechanistic insights into the bioactivity of PEI-like polymers and the possibility for optimization. These aspects together support the rationality of chemical strategies assembling defined oligoamine segments by innovative and precise conjugation techniques.
Conclusions
Since the first discovery of PEI as a transfecting agent, both the biological assessment techniques for the identification of subcellular impairment and the chemical engineering of new synthetic materials have dramatically advanced. The scientific knowledge and methodologies from both perspectives can contribute to the development of next-generation nucleic acid carriers with suitable safety profiles that meet the demands on potential future therapeutics. As a result, new polymers have to be investigated in comprehensive assessments to identify effects on the cellular metabolic situation and detect subtle toxicity. For clinical applications, several other aspects beyond in vitro cytotoxicity also have to be considered to ensure clinical safety: serum interaction, hemolysis, tissue distribution, complement activation, metabolism, excretion, and more affect the suitability of polymers and polyplexes for in vivo usage. Also, here, the identification of underlying mechanisms and establishment of structure-activity relationships is key to the rational design and optimization of materials with intended properties. Finally, a recent study demonstrated that sub-50-nm PEI polyplexes can reach the nuclear membrane and bind exclusively to the nuclear pore complex without causing disruption of the nuclear envelope.161 However, it is still necessary to elucidate whether PEI and DNA dissociate before transcription of DNA or whether transcriptional complexes may bind to DNA while DNA remains bound to PEI and whether PEI in the nucleus can trigger cell death.
Author Contributions
All authors analyzed and discussed the literature. All authors contributed to the writing of the paper and revised the manuscript.
Conflicts of Interest
The authors declare no competing financial interest.
Acknowledgments
A.H. and J.B. acknowledge financial support from the Danish Cancer Society, the Swedish Research Council, the Novo Nordisk Foundation (NNF16584), the Danish Council for Independent Research (13331-00262A), the Lundbeck Foundation, and the Danish National Research Foundation (Project CARD, DNRF 125). E.W. acknowledges financial support from the German Research Foundation (CRC1032 and CRC824), Cluster of Excellence NIM, and SinoGerman Center Grant GZ995. S.M.M. acknowledges financial support from the Danish Agency for Science, Technology, and Innovation (Det Frie Forskningsråd for Teknologi og Produktion, references 274-08-0534and 12-126894, and Det Strategiske Forskningsråd, reference 09-065746/DSF) as well as the International Science and Technology Cooperation of Guangdong Province (reference 2015A050502002) and Guangzhou City (reference 2016201604030050) with RiboBio Co., Ltd.
Contributor Information
Ernst Wagner, Email: ernst.wagner@cup.uni-muenchen.de.
Seyed Moein Moghimi, Email: moein.moghimi@gmail.com.
References
- 1.Al-Dosari M.S., Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlenk F., Grund S., Fischer D. Recent developments and perspectives on gene therapy using synthetic vectors. Ther. Deliv. 2013;4:95–113. doi: 10.4155/tde.12.128. [DOI] [PubMed] [Google Scholar]
- 3.Gao X., Kim K.S., Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;9:E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez-Martínez F.C., Guerra J., Posadas I., Ceña V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm. Res. 2011;28:1843–1858. doi: 10.1007/s11095-010-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayerossadat N., Maedeh T., Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 7.Check E. A tragic setback. Nature. 2002;420:116–118. doi: 10.1038/420116a. [DOI] [PubMed] [Google Scholar]
- 8.Cho Y.W., Kim J.D., Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J. Pharm. Pharmacol. 2003;55:721–734. doi: 10.1211/002235703765951311. [DOI] [PubMed] [Google Scholar]
- 9.Davis M.E. Non-viral gene delivery systems. Curr. Opin. Biotechnol. 2002;13:128–131. doi: 10.1016/s0958-1669(02)00294-x. [DOI] [PubMed] [Google Scholar]
- 10.Lächelt U., Wagner E. Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond) Chem. Rev. 2015;115:11043–11078. doi: 10.1021/cr5006793. [DOI] [PubMed] [Google Scholar]
- 11.Mintzer M.A., Simanek E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 12.Pack D.W., Hoffman A.S., Pun S., Stayton P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 13.Boussif O., Lezoualc’h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neu M., Fischer D., Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med. 2005;7:992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- 15.Bieber T., Meissner W., Kostin S., Niemann A., Elsasser H.P. Intracellular route and transcriptional competence of polyethylenimine-DNA complexes. J. Control. Release. 2002;82:441–454. doi: 10.1016/s0168-3659(02)00129-3. [DOI] [PubMed] [Google Scholar]
- 16.Wagner E. Polymers for siRNA delivery: inspired by viruses to be targeted, dynamic, and precise. Acc. Chem. Res. 2012;45:1005–1013. doi: 10.1021/ar2002232. [DOI] [PubMed] [Google Scholar]
- 17.Zheng M., Librizzi D., Kılıç A., Liu Y., Renz H., Merkel O.M., Kissel T. Enhancing in vivo circulation and siRNA delivery with biodegradable polyethylenimine-graft-polycaprolactone-block-poly(ethylene glycol) copolymers. Biomaterials. 2012;33:6551–6558. doi: 10.1016/j.biomaterials.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 18.Intra J., Salem A.K. Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. J. Control. Release. 2008;130:129–138. doi: 10.1016/j.jconrel.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wightman L., Kircheis R., Rössler V., Carotta S., Ruzicka R., Kursa M., Wagner E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J. Gene Med. 2001;3:362–372. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari S., Moro E., Pettenazzo A., Behr J.P., Zacchello F., Scarpa M. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. Gene Ther. 1997;4:1100–1106. doi: 10.1038/sj.gt.3300503. [DOI] [PubMed] [Google Scholar]
- 21.Coll J.L., Chollet P., Brambilla E., Desplanques D., Behr J.P., Favrot M. In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Hum. Gene Ther. 1999;10:1659–1666. doi: 10.1089/10430349950017662. [DOI] [PubMed] [Google Scholar]
- 22.Godbey W.T., Wu K.K., Mikos A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release. 1999;60:149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 23.Godbey W.T., Wu K.K., Hirasaki G.J., Mikos A.G. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther. 1999;6:1380–1388. doi: 10.1038/sj.gt.3300976. [DOI] [PubMed] [Google Scholar]
- 24.Godbey W.T., Wu K.K., Mikos A.G. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999;45:268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Godbey W.T., Wu K.K., Mikos A.G. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc. Natl. Acad. Sci. USA. 1999;96:5177–5181. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Gersdorff K., Sanders N.N., Vandenbroucke R., De Smedt S.C., Wagner E., Ogris M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol. Ther. 2006;14:745–753. doi: 10.1016/j.ymthe.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Kichler A., Leborgne C., Coeytaux E., Danos O. Polyethylenimine-mediated gene delivery: a mechanistic study. J. Gene Med. 2001;3:135–144. doi: 10.1002/jgm.173. [DOI] [PubMed] [Google Scholar]
- 28.Akinc A., Thomas M., Klibanov A.M., Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 29.Sonawane N.D., Szoka F.C., Jr., Verkman A.S. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 30.Miyata K., Nishiyama N., Kataoka K. Rational design of smart supramolecular assemblies for gene delivery: chemical challenges in the creation of artificial viruses. Chem. Soc. Rev. 2012;41:2562–2574. doi: 10.1039/c1cs15258k. [DOI] [PubMed] [Google Scholar]
- 31.Benjaminsen R.V., Mattebjerg M.A., Henriksen J.R., Moghimi S.M., Andresen T.L. The possible “proton sponge ” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013;21:149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godbey W.T., Wu K.K., Mikos A.G. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 33.Kunath K., von Harpe A., Fischer D., Petersen H., Bickel U., Voigt K., Kissel T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J. Control. Release. 2003;89:113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 34.Fischer D., Li Y., Ahlemeyer B., Krieglstein J., Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 35.Ahn C.H., Chae S.Y., Bae Y.H., Kim S.W. Biodegradable poly(ethylenimine) for plasmid DNA delivery. J. Control. Release. 2002;80:273–282. doi: 10.1016/s0168-3659(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 36.Wiseman J.W., Goddard C.A., McLelland D., Colledge W.H. A comparison of linear and branched polyethylenimine (PEI) with DCChol/DOPE liposomes for gene delivery to epithelial cells in vitro and in vivo. Gene Ther. 2003;10:1654–1662. doi: 10.1038/sj.gt.3302050. [DOI] [PubMed] [Google Scholar]
- 37.Bragonzi A., Boletta A., Biffi A., Muggia A., Sersale G., Cheng S.H., Bordignon C., Assael B.M., Conese M. Comparison between cationic polymers and lipids in mediating systemic gene delivery to the lungs. Gene Ther. 1999;6:1995–2004. doi: 10.1038/sj.gt.3301039. [DOI] [PubMed] [Google Scholar]
- 38.Goula D., Benoist C., Mantero S., Merlo G., Levi G., Demeneix B.A. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Ther. 1998;5:1291–1295. doi: 10.1038/sj.gt.3300717. [DOI] [PubMed] [Google Scholar]
- 39.Hall A., Wu L.P., Parhamifar L., Moghimi S.M. Differential Modulation of Cellular Bioenergetics by Poly(L-lysine)s of Different Molecular Weights. Biomacromolecules. 2015;16:2119–2126. doi: 10.1021/acs.biomac.5b00533. [DOI] [PubMed] [Google Scholar]
- 40.Hall A., Larsen A.K., Parhamifar L., Meyle K.D., Wu L.P., Moghimi S.M. High resolution respirometry analysis of polyethylenimine-mediated mitochondrial energy crisis and cellular stress: Mitochondrial proton leak and inhibition of the electron transport system. Biochim. Biophys. Acta. 2013;1827:1213–1225. doi: 10.1016/j.bbabio.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Hall A., Parhamifar L., Lange M.K., Meyle K.D., Sanderhoff M., Andersen H., Roursgaard M., Larsen A.K., Jensen P.B., Christensen C. Polyethylenimine architecture-dependent metabolic imprints and perturbation of cellular redox homeostasis. Biochim. Biophys. Acta. 2015;1847:328–342. doi: 10.1016/j.bbabio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Hunter A.C. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv. Drug Deliv. Rev. 2006;58:1523–1531. doi: 10.1016/j.addr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Moghimi S.M., Symonds P., Murray J.C., Hunter A.C., Debska G., Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol. Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Parhamifar L., Andersen H., Wu L., Hall A., Hudzech D., Moghimi S.M. Polycation-mediated integrated cell death processes. Adv. Genet. 2014;88:353–398. doi: 10.1016/B978-0-12-800148-6.00012-2. [DOI] [PubMed] [Google Scholar]
- 45.Grandinetti G., Ingle N.P., Reineke T.M. Interaction of poly(ethylenimine)-DNA polyplexes with mitochondria: implications for a mechanism of cytotoxicity. Mol. Pharm. 2011;8:1709–1719. doi: 10.1021/mp200078n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grandinetti G., Smith A.E., Reineke T.M. Membrane and nuclear permeabilization by polymeric pDNA vehicles: efficient method for gene delivery or mechanism of cytotoxicity? Mol. Pharm. 2012;9:523–538. doi: 10.1021/mp200368p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao X., Yao L., Song Q., Zhu L., Xia Z., Xia H., Jiang X., Chen J., Chen H. The association of autophagy with polyethylenimine-induced cytotoxicity in nephritic and hepatic cell lines. Biomaterials. 2011;32:8613–8625. doi: 10.1016/j.biomaterials.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 48.Lin C.W., Jan M.S., Kuo J.H., Hsu L.J., Lin Y.S. Protective role of autophagy in branched polyethylenimine (25K)- and poly(L-lysine) (30-70K)-induced cell death. Eur. J. Pharm. Sci. 2012;47:865–874. doi: 10.1016/j.ejps.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Werth S., Urban-Klein B., Dai L., Höbel S., Grzelinski M., Bakowsky U., Czubayko F., Aigner A. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J. Control. Release. 2006;112:257–270. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Boeckle S., von Gersdorff K., van der Piepen S., Culmsee C., Wagner E., Ogris M. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J. Gene Med. 2004;6:1102–1111. doi: 10.1002/jgm.598. [DOI] [PubMed] [Google Scholar]
- 51.Hong S., Bielinska A.U., Mecke A., Keszler B., Beals J.L., Shi X., Balogh L., Orr B.G., Baker J.R., Jr., Banaszak Holl M.M. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transport. Bioconjug. Chem. 2004;15:774–782. doi: 10.1021/bc049962b. [DOI] [PubMed] [Google Scholar]
- 52.Hong S., Leroueil P.R., Janus E.K., Peters J.L., Kober M.M., Islam M.T., Orr B.G., Baker J.R., Jr., Banaszak Holl M.M. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug. Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 53.Leroueil P.R., Berry S.A., Duthie K., Han G., Rotello V.M., McNerny D.Q., Baker J.R., Jr., Orr B.G., Holl M.M. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett. 2008;8:420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 54.Hunter A.C., Moghimi S.M. Cationic carriers of genetic material and cell death: a mitochondrial tale. Biochim. Biophys. Acta. 2010;1797:1203–1209. doi: 10.1016/j.bbabio.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 55.Frey T.G., Mannella C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000;25:319–324. doi: 10.1016/s0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- 56.Mannella C.A. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta. 2006;1763:542–548. doi: 10.1016/j.bbamcr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reichert A.S., Neupert W. Contact sites between the outer and inner membrane of mitochondria-role in protein transport. Biochim. Biophys. Acta. 2002;1592:41–49. doi: 10.1016/s0167-4889(02)00263-x. [DOI] [PubMed] [Google Scholar]
- 59.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem. J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 60.Halliwell B., Cross C.E. Oxygen-derived species: their relation to human disease and environmental stress. Environ. Health Perspect. 1994;102(Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mislick K.A., Baldeschwieler J.D. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl. Acad. Sci. USA. 1996;93:12349–12354. doi: 10.1073/pnas.93.22.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bar-Even A., Flamholz A., Noor E., Milo R. Thermodynamic constraints shape the structure of carbon fixation pathways. Biochim. Biophys. Acta. 2012;1817:1646–1659. doi: 10.1016/j.bbabio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Pfeiffer T., Schuster S., Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 64.Rich P. Chemiosmotic coupling: The cost of living. Nature. 2003;421:583. doi: 10.1038/421583a. [DOI] [PubMed] [Google Scholar]
- 65.Zheng J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review) Oncol. Lett. 2012;4:1151–1157. doi: 10.3892/ol.2012.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 67.Babcock G.T., Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- 68.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. 1966. Biochim. Biophys. Acta. 2011;1807:1507–1538. doi: 10.1016/j.bbabio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Nicholls D.G. Mitochondrial membrane potential and aging. Aging Cell. 2004;3:35–40. doi: 10.1111/j.1474-9728.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 70.Divakaruni A.S., Brand M.D. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda) 2011;26:192–205. doi: 10.1152/physiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 71.Itoh H., Takahashi A., Adachi K., Noji H., Yasuda R., Yoshida M., Kinosita K. Mechanically driven ATP synthesis by F1-ATPase. Nature. 2004;427:465–468. doi: 10.1038/nature02212. [DOI] [PubMed] [Google Scholar]
- 72.Murphy M.P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 73.Modica-Napolitano J.S., Aprille J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Deliv. Rev. 2001;49:63–70. doi: 10.1016/s0169-409x(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 74.Hall A., Meyle K.D., Lange M.K., Klima M., Sanderhoff M., Dahl C., Abildgaard C., Thorup K., Moghimi S.M., Jensen P.B. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget. 2013;4:584–599. doi: 10.18632/oncotarget.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberts V.A., Pique M.E. Definition of the interaction domain for cytochrome c on cytochrome c oxidase. III. Prediction of the docked complex by a complete, systematic search. J. Biol. Chem. 1999;274:38051–38060. doi: 10.1074/jbc.274.53.38051. [DOI] [PubMed] [Google Scholar]
- 76.Maneg O., Malatesta F., Ludwig B., Drosou V. Interaction of cytochrome c with cytochrome oxidase: two different docking scenarios. Biochim. Biophys. Acta. 2004;1655:274–281. doi: 10.1016/j.bbabio.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt T.R., Wildman D.E., Uddin M., Opazo J.C., Goodman M., Grossman L.I. Rapid electrostatic evolution at the binding site for cytochrome c on cytochrome c oxidase in anthropoid primates. Proc. Natl. Acad. Sci. USA. 2005;102:6379–6384. doi: 10.1073/pnas.0409714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mochan B.S., Elliott W.B., Nicholls P. Patterns of cytochrome oxidase inhibition by polycations. J. Bioenerg. 1973;4:329–345. doi: 10.1007/BF01648976. [DOI] [PubMed] [Google Scholar]
- 79.Hasinoff B.B., Davey J.P. Adriamycin and its iron(III) and copper(II) complexes. Glutathione-induced dissociation; cytochrome c oxidase inactivation and protection; binding to cardiolipin. Biochem. Pharmacol. 1988;37:3663–3669. doi: 10.1016/0006-2952(88)90399-1. [DOI] [PubMed] [Google Scholar]
- 80.Gupta U., Agashe H.B., Asthana A., Jain N.K. A review of in vitro-in vivo investigations on dendrimers: the novel nanoscopic drug carriers. Nanomedicine (Lond.) 2006;2:66–73. doi: 10.1016/j.nano.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Izyumov D.S., Avetisyan A.V., Pletjushkina O.Y., Sakharov D.V., Wirtz K.W., Chernyak B.V., Skulachev V.P. “Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosis. Biochim. Biophys. Acta. 2004;1658:141–147. doi: 10.1016/j.bbabio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Lieberthal W., Menza S.A., Levine J.S. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am. J. Physiol. 1998;274:F315–F327. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- 83.Zong W.X., Thompson C.B. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 84.Oakhill J.S., Chen Z.P., Scott J.W., Steel R., Castelli L.A., Ling N., Macaulay S.L., Kemp B.E. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc. Natl. Acad. Sci. USA. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alexander A., Cai S.L., Kim J., Nanez A., Sahin M., MacLean K.H., Inoki K., Guan K.L., Shen J., Person M.D. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. USA. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 89.Lu S.C. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 90.Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dworkin M.B., Dworkin-Rastl E. Regulation of carbon flux from amino acids into sugar phosphates in Xenopus embryos. Dev. Biol. 1990;138:177–187. doi: 10.1016/0012-1606(90)90187-n. [DOI] [PubMed] [Google Scholar]
- 92.Jo S.H., Son M.K., Koh H.J., Lee S.M., Song I.H., Kim Y.O., Lee Y.S., Jeong K.S., Kim W.B., Park J.W. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 2001;276:16168–16176. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- 93.Veech R.L., Eggleston L.V., Krebs H.A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem. J. 1969;115:609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moghimi S.M., Hunter A.C., Andresen T.L. Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective. Annu. Rev. Pharmacol. Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 95.Moghimi S.M. Cancer nanomedicine and the complement system activation paradigm: anaphylaxis and tumour growth. J. Control. Release. 2014;190:556–562. doi: 10.1016/j.jconrel.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 96.Plank C., Mechtler K., Szoka F.C., Jr., Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum. Gene Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 97.Ogris M., Brunner S., Schüller S., Kircheis R., Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 98.Brus C., Petersen H., Aigner A., Czubayko F., Kissel T. Physicochemical and biological characterization of polyethylenimine-graft-poly(ethylene glycol) block copolymers as a delivery system for oligonucleotides and ribozymes. Bioconjug. Chem. 2004;15:677–684. doi: 10.1021/bc034160m. [DOI] [PubMed] [Google Scholar]
- 99.Merkel O.M., Urbanics R., Bedőcs P., Rozsnyay Z., Rosivall L., Toth M., Kissel T., Szebeni J. In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials. 2011;32:4936–4942. doi: 10.1016/j.biomaterials.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 100.Wu L.-P., Ficker M., Christensen J.B., Trohopoulos P.N., Moghimi S.M. Dendrimers in medicine: therapeutic concepts and pharmaceutical challenges. Bioconjug. Chem. 2015;26:1198–1211. doi: 10.1021/acs.bioconjchem.5b00031. [DOI] [PubMed] [Google Scholar]
- 101.Chen F., Wang G., Griffin J.I., Brenneman B., Banda N.K., Holers V.M., Backos D.S., Wu L., Moghimi S.M., Simberg D. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat. Nanotechnol. 2016 doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moghimi S.M., Hamad I., Andresen T.L., Jørgensen K., Szebeni J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J. 2006;20:2591–2593. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- 103.Moghimi S.M., Farhangrazi Z.S. Nanomedicine and the complement paradigm. Nanomedicine (Lond.) 2013;9:458–460. doi: 10.1016/j.nano.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 104.Moghimi S.M. Complement propriety and conspiracy in nanomedicine: perspective and a hypothesis. Nucleic Acid Ther. 2016;26:67–72. doi: 10.1089/nat.2015.0587. [DOI] [PubMed] [Google Scholar]
- 105.Mulens-Arias V., Rojas J.M., Pérez-Yagüe S., Morales M.P., Barber D.F. Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics. Biomaterials. 2015;52:494–506. doi: 10.1016/j.biomaterials.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 106.Chen H., Li P., Yin Y., Cai X., Huang Z., Chen J., Dong L., Zhang J. The promotion of type 1 T helper cell responses to cationic polymers in vivo via toll-like receptor-4 mediated IL-12 secretion. Biomaterials. 2010;31:8172–8180. doi: 10.1016/j.biomaterials.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 107.Cubillos-Ruiz J.R., Engle X., Scarlett U.K., Martinez D., Barber A., Elgueta R., Wang L., Nesbeth Y., Durant Y., Gewirtz A.T. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J. Clin. Invest. 2009;119:2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang Z., Yang Y., Jiang Y., Shao J., Sun X., Chen J., Dong L., Zhang J. Anti-tumor immune responses of tumor-associated macrophages via toll-like receptor 4 triggered by cationic polymers. Biomaterials. 2013;34:746–755. doi: 10.1016/j.biomaterials.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 109.Forrest M.L., Koerber J.T., Pack D.W. A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjug. Chem. 2003;14:934–940. doi: 10.1021/bc034014g. [DOI] [PubMed] [Google Scholar]
- 110.Jiang H.L., Kim Y.K., Arote R., Nah J.W., Cho M.H., Choi Y.J., Akaike T., Cho C.S. Chitosan-graft-polyethylenimine as a gene carrier. J. Control. Release. 2007;117:273–280. doi: 10.1016/j.jconrel.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 111.Xu S., Chen M., Yao Y., Zhang Z., Jin T., Huang Y., Zhu H. Novel poly(ethylene imine) biscarbamate conjugate as an efficient and nontoxic gene delivery system. J. Control. Release. 2008;130:64–68. doi: 10.1016/j.jconrel.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 112.Xiong M.P., Forrest M.L., Ton G., Zhao A., Davies N.M., Kwon G.S. Poly(aspartate-g-PEI800), a polyethylenimine analogue of low toxicity and high transfection efficiency for gene delivery. Biomaterials. 2007;28:4889–4900. doi: 10.1016/j.biomaterials.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 113.Fang G., Zeng F., Yu C., Wu S. Low molecular weight PEIs modified by hydrazone-based crosslinker and betaine as improved gene carriers. Colloids Surf. B Biointerfaces. 2014;122:472–481. doi: 10.1016/j.colsurfb.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Gosselin M.A., Guo W., Lee R.J. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjug. Chem. 2001;12:989–994. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 115.Breunig M., Lungwitz U., Liebl R., Goepferich A. Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc. Natl. Acad. Sci. USA. 2007;104:14454–14459. doi: 10.1073/pnas.0703882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu H., Russ V., Wagner E. Influence of the molecular weight of bioreducible oligoethylenimine conjugates on the polyplex transfection properties. AAPS J. 2009;11:445–455. doi: 10.1208/s12248-009-9122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park M.R., Han K.O., Han I.K., Cho M.H., Nah J.W., Choi Y.J., Cho C.S. Degradable polyethylenimine-alt-poly(ethylene glycol) copolymers as novel gene carriers. J. Control. Release. 2005;105:367–380. doi: 10.1016/j.jconrel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 118.Kloeckner J., Wagner E., Ogris M. Degradable gene carriers based on oligomerized polyamines. Eur. J. Pharm. Sci. 2006;29:414–425. doi: 10.1016/j.ejps.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 119.Wu D., Liu Y., Jiang X., He C., Goh S.H., Leong K.W. Hyperbranched poly(amino ester)s with different terminal amine groups for DNA delivery. Biomacromolecules. 2006;7:1879–1883. doi: 10.1021/bm0601878. [DOI] [PubMed] [Google Scholar]
- 120.Bauhuber S., Hozsa C., Breunig M., Göpferich A. Delivery of nucleic acids via disulfide-based carrier systems. Adv. Mater. 2009;21:3286–3306. doi: 10.1002/adma.200802453. [DOI] [PubMed] [Google Scholar]
- 121.Gabrielson N.P., Pack D.W. Acetylation of polyethylenimine enhances gene delivery via weakened polymer/DNA interactions. Biomacromolecules. 2006;7:2427–2435. doi: 10.1021/bm060300u. [DOI] [PubMed] [Google Scholar]
- 122.Zintchenko A., Philipp A., Dehshahri A., Wagner E. Simple modifications of branched PEI lead to highly efficient siRNA carriers with low toxicity. Bioconjug. Chem. 2008;19:1448–1455. doi: 10.1021/bc800065f. [DOI] [PubMed] [Google Scholar]
- 123.Oskuee R.K., Dehshahri A., Shier W.T., Ramezani M. Alkylcarboxylate grafting to polyethylenimine: a simple approach to producing a DNA nanocarrier with low toxicity. J. Gene Med. 2009;11:921–932. doi: 10.1002/jgm.1374. [DOI] [PubMed] [Google Scholar]
- 124.Oskuee R.K., Philipp A., Dehshahri A., Wagner E., Ramezani M. The impact of carboxyalkylation of branched polyethylenimine on effectiveness in small interfering RNA delivery. J. Gene Med. 2010;12:729–738. doi: 10.1002/jgm.1490. [DOI] [PubMed] [Google Scholar]
- 125.Thomas M., Klibanov A.M. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. USA. 2002;99:14640–14645. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Creusat G., Rinaldi A.S., Weiss E., Elbaghdadi R., Remy J.S., Mulherkar R., Zuber G. Proton sponge trick for pH-sensitive disassembly of polyethylenimine-based siRNA delivery systems. Bioconjug. Chem. 2010;21:994–1002. doi: 10.1021/bc100010k. [DOI] [PubMed] [Google Scholar]
- 127.Creusat G., Thomann J.S., Maglott A., Pons B., Dontenwill M., Guérin E., Frisch B., Zuber G. Pyridylthiourea-grafted polyethylenimine offers an effective assistance to siRNA-mediated gene silencing in vitro and in vivo. J. Control. Release. 2012;157:418–426. doi: 10.1016/j.jconrel.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 128.Tripathi S.K., Gupta N., Mahato M., Gupta K.C., Kumar P. Selective blocking of primary amines in branched polyethylenimine with biocompatible ligand alleviates cytotoxicity and augments gene delivery efficacy in mammalian cells. Colloids Surf. B Biointerfaces. 2014;115:79–85. doi: 10.1016/j.colsurfb.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 129.Jeong J.H., Song S.H., Lim D.W., Lee H., Park T.G. DNA transfection using linear poly(ethylenimine) prepared by controlled acid hydrolysis of poly(2-ethyl-2-oxazoline) J. Control. Release. 2001;73:391–399. doi: 10.1016/s0168-3659(01)00310-8. [DOI] [PubMed] [Google Scholar]
- 130.Petersen H., Fechner P.M., Martin A.L., Kunath K., Stolnik S., Roberts C.J., Fischer D., Davies M.C., Kissel T. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug. Chem. 2002;13:845–854. doi: 10.1021/bc025529v. [DOI] [PubMed] [Google Scholar]
- 131.Shuai X., Merdan T., Unger F., Wittmar M., Kissel T. Novel Biodegradable Ternary Copolymers hy-PEI-g-PCL-b-PEG: Synthesis, Characterization, and Potential as Efficient Nonviral Gene Delivery Vectors. Macromolecules. 2003;36:5751–5759. [Google Scholar]
- 132.Zheng M., Liu Y., Samsonova O., Endres T., Merkel O., Kissel T. Amphiphilic and biodegradable hy-PEI-g-PCL-b-PEG copolymers efficiently mediate transgene expression depending on their graft density. Int. J. Pharm. 2012;427:80–87. doi: 10.1016/j.ijpharm.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 133.Erbacher P., Bettinger T., Belguise-Valladier P., Zou S., Coll J.L., Behr J.P., Remy J.S. Transfection and physical properties of various saccharide, poly(ethylene glycol), and antibody-derivatized polyethylenimines (PEI) J. Gene Med. 1999;1:210–222. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<210::AID-JGM30>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 134.Fukushima S., Miyata K., Nishiyama N., Kanayama N., Yamasaki Y., Kataoka K. PEGylated polyplex micelles from triblock catiomers with spatially ordered layering of condensed pDNA and buffering units for enhanced intracellular gene delivery. J. Am. Chem. Soc. 2005;127:2810–2811. doi: 10.1021/ja0440506. [DOI] [PubMed] [Google Scholar]
- 135.Kanayama N., Fukushima S., Nishiyama N., Itaka K., Jang W.D., Miyata K., Yamasaki Y., Chung U.I., Kataoka K. A PEG-based biocompatible block catiomer with high buffering capacity for the construction of polyplex micelles showing efficient gene transfer toward primary cells. ChemMedChem. 2006;1:439–444. doi: 10.1002/cmdc.200600008. [DOI] [PubMed] [Google Scholar]
- 136.Miyata K., Fukushima S., Nishiyama N., Yamasaki Y., Kataoka K. PEG-based block catiomers possessing DNA anchoring and endosomal escaping functions to form polyplex micelles with improved stability and high transfection efficacy. J. Control. Release. 2007;122:252–260. doi: 10.1016/j.jconrel.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 137.Han M., Bae Y., Nishiyama N., Miyata K., Oba M., Kataoka K. Transfection study using multicellular tumor spheroids for screening non-viral polymeric gene vectors with low cytotoxicity and high transfection efficiencies. J. Control. Release. 2007;121:38–48. doi: 10.1016/j.jconrel.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 138.Takae S., Miyata K., Oba M., Ishii T., Nishiyama N., Itaka K., Yamasaki Y., Koyama H., Kataoka K. PEG-detachable polyplex micelles based on disulfide-linked block catiomers as bioresponsive nonviral gene vectors. J. Am. Chem. Soc. 2008;130:6001–6009. doi: 10.1021/ja800336v. [DOI] [PubMed] [Google Scholar]
- 139.Miyata K., Oba M., Nakanishi M., Fukushima S., Yamasaki Y., Koyama H., Nishiyama N., Kataoka K. Polyplexes from poly(aspartamide) bearing 1,2-diaminoethane side chains induce pH-selective, endosomal membrane destabilization with amplified transfection and negligible cytotoxicity. J. Am. Chem. Soc. 2008;130:16287–16294. doi: 10.1021/ja804561g. [DOI] [PubMed] [Google Scholar]
- 140.Itaka K., Ishii T., Hasegawa Y., Kataoka K. Biodegradable polyamino acid-based polycations as safe and effective gene carrier minimizing cumulative toxicity. Biomaterials. 2010;31:3707–3714. doi: 10.1016/j.biomaterials.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 141.Uchida H., Miyata K., Oba M., Ishii T., Suma T., Itaka K., Nishiyama N., Kataoka K. Odd-even effect of repeating aminoethylene units in the side chain of N-substituted polyaspartamides on gene transfection profiles. J. Am. Chem. Soc. 2011;133:15524–15532. doi: 10.1021/ja204466y. [DOI] [PubMed] [Google Scholar]
- 142.Uchida H., Itaka K., Nomoto T., Ishii T., Suma T., Ikegami M., Miyata K., Oba M., Nishiyama N., Kataoka K. Modulated protonation of side chain aminoethylene repeats in N-substituted polyaspartamides promotes mRNA transfection. J. Am. Chem. Soc. 2014;136:12396–12405. doi: 10.1021/ja506194z. [DOI] [PubMed] [Google Scholar]
- 143.Masago K., Itaka K., Nishiyama N., Chung U.I., Kataoka K. Gene delivery with biocompatible cationic polymer: pharmacogenomic analysis on cell bioactivity. Biomaterials. 2007;28:5169–5175. doi: 10.1016/j.biomaterials.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 144.Jarzębińska A., Pasewald T., Lambrecht J., Mykhaylyk O., Kümmerling L., Beck P., Hasenpusch G., Rudolph C., Plank C., Dohmen C. A Single Methylene Group in Oligoalkylamine-Based Cationic Polymers and Lipids Promotes Enhanced mRNA Delivery. Angew. Chem. Int. Ed. Engl. 2016;55:9591–9595. doi: 10.1002/anie.201603648. [DOI] [PubMed] [Google Scholar]
- 145.Wei H., Volpatti L.R., Sellers D.L., Maris D.O., Andrews I.W., Hemphill A.S., Chan L.W., Chu D.S., Horner P.J., Pun S.H. Dual responsive, stabilized nanoparticles for efficient in vivo plasmid delivery. Angew. Chem. Int. Ed. Engl. 2013;52:5377–5381. doi: 10.1002/anie.201301896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y., Wenning L., Lynch M., Reineke T.M. New poly(d-glucaramidoamine)s induce DNA nanoparticle formation and efficient gene delivery into mammalian cells. J. Am. Chem. Soc. 2004;126:7422–7423. doi: 10.1021/ja049831l. [DOI] [PubMed] [Google Scholar]
- 147.Hartmann L., Krause E., Antonietti M., Börner H.G. Solid-phase supported polymer synthesis of sequence-defined, multifunctional poly(amidoamines) Biomacromolecules. 2006;7:1239–1244. doi: 10.1021/bm050884k. [DOI] [PubMed] [Google Scholar]
- 148.Hartmann L., Häfele S., Peschka-Süss R., Antonietti M., Börner H.G. Tailor-made poly(amidoamine)s for controlled complexation and condensation of DNA. Chemistry. 2008;14:2025–2033. doi: 10.1002/chem.200701223. [DOI] [PubMed] [Google Scholar]
- 149.Schaffert D., Badgujar N., Wagner E. Novel Fmoc-polyamino acids for solid-phase synthesis of defined polyamidoamines. Org. Lett. 2011;13:1586–1589. doi: 10.1021/ol200381z. [DOI] [PubMed] [Google Scholar]
- 150.Schaffert D., Troiber C., Salcher E.E., Fröhlich T., Martin I., Badgujar N., Dohmen C., Edinger D., Kläger R., Maiwald G. Solid-phase synthesis of sequence-defined T-, i-, and U-shape polymers for pDNA and siRNA delivery. Angew. Chem. Int. Ed. Engl. 2011;50:8986–8989. doi: 10.1002/anie.201102165. [DOI] [PubMed] [Google Scholar]
- 151.Schaffert D., Troiber C., Wagner E. New sequence-defined polyaminoamides with tailored endosomolytic properties for plasmid DNA delivery. Bioconjug. Chem. 2012;23:1157–1165. doi: 10.1021/bc200614x. [DOI] [PubMed] [Google Scholar]
- 152.Fröhlich T., Edinger D., Kläger R., Troiber C., Salcher E., Badgujar N., Martin I., Schaffert D., Cengizeroglu A., Hadwiger P. Structure-activity relationships of siRNA carriers based on sequence-defined oligo (ethane amino) amides. J. Control. Release. 2012;160:532–541. doi: 10.1016/j.jconrel.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 153.Scholz C., Kos P., Leclercq L., Jin X., Cottet H., Wagner E. Correlation of length of linear oligo(ethanamino) amides with gene transfer and cytotoxicity. ChemMedChem. 2014;9:2104–2110. doi: 10.1002/cmdc.201300483. [DOI] [PubMed] [Google Scholar]
- 154.Salcher E.E., Kos P., Fröhlich T., Badgujar N., Scheible M., Wagner E. Sequence-defined four-arm oligo(ethanamino)amides for pDNA and siRNA delivery: Impact of building blocks on efficacy. J. Control. Release. 2012;164:380–386. doi: 10.1016/j.jconrel.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 155.Lächelt U., Kos P., Mickler F.M., Herrmann A., Salcher E.E., Rödl W., Badgujar N., Bräuchle C., Wagner E. Fine-tuning of proton sponges by precise diaminoethanes and histidines in pDNA polyplexes. Nanomedicine (Lond.) 2014;10:35–44. doi: 10.1016/j.nano.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 156.Scholz C., Kos P., Wagner E. Comb-like oligoaminoethane carriers: change in topology improves pDNA delivery. Bioconjug. Chem. 2014;25:251–261. doi: 10.1021/bc400392y. [DOI] [PubMed] [Google Scholar]
- 157.Martin I., Dohmen C., Mas-Moruno C., Troiber C., Kos P., Schaffert D., Lächelt U., Teixidó M., Günther M., Kessler H. Solid-phase-assisted synthesis of targeting peptide-PEG-oligo(ethane amino)amides for receptor-mediated gene delivery. Org. Biomol. Chem. 2012;10:3258–3268. doi: 10.1039/c2ob06907e. [DOI] [PubMed] [Google Scholar]
- 158.Dohmen C., Edinger D., Fröhlich T., Schreiner L., Lächelt U., Troiber C., Rädler J., Hadwiger P., Vornlocher H.P., Wagner E. Nanosized multifunctional polyplexes for receptor-mediated siRNA delivery. ACS Nano. 2012;6:5198–5208. doi: 10.1021/nn300960m. [DOI] [PubMed] [Google Scholar]
- 159.Troiber C., Edinger D., Kos P., Schreiner L., Kläger R., Herrmann A., Wagner E. Stabilizing effect of tyrosine trimers on pDNA and siRNA polyplexes. Biomaterials. 2013;34:1624–1633. doi: 10.1016/j.biomaterials.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 160.Klein P.M., Reinhard S., Lee D.J., Müller K., Ponader D., Hartmann L., Wagner E. Precise redox-sensitive cleavage sites for improved bioactivity of siRNA lipopolyplexes. Nanoscale. 2016;8:18098–18104. doi: 10.1039/c6nr05767e. [DOI] [PubMed] [Google Scholar]
- 161.Andersen H., Parhamifar L., Hunter A.C., Shahin V., Moghimi S.M. AFM visualization of sub-50nm polyplex disposition to the nuclear pore complex without compromising the integrity of the nuclear envelope. J. Control. Release. 2016;244(Pt A):24–29. doi: 10.1016/j.jconrel.2016.11.008. [DOI] [PubMed] [Google Scholar]