Abstract
Over the last 15 years, a plethora of materials and different formulations have been proposed for the realization of nanomedicines. Yet drug-loading efficiency, sequestration by phagocytic cells, and tumor accumulation are sub-optimal. This would imply that radically new design approaches are needed to propel the clinical integration of nanomedicines, overcoming well-accepted clichés. This work briefly reviews the use of deformable discoidal nanoconstructs as a novel delivery strategy for therapeutic and imaging agents. Inspired by blood cell behavior, these nanoconstructs are designed to efficiently navigate the circulatory system, minimize sequestration by phagocytic cells, and recognize the tortuous angiogenic microvasculature of neoplastic masses. This article discusses the notion of nanoparticle margination and vascular adhesion, as well as advantages associated with deformable particles. Finally, details on the synthesis, physico-chemical properties, and in vivo characterization of discoidal polymeric nanoconstructs are provided, with particular emphasis on their ability to independently control size, shape, surface properties, and mechanical stiffness. These nanoconstructs could help in gaining a deeper understanding of the mechanisms regulating the behavior of nanomedicines and identifying optimal delivery strategies for patient-specific therapeutic interventions.
Keywords: cancer, nanomedicine, rational design, vascular targeting
The size, shape, surface properties, and mechanical stiffness of discoidal polymeric nanoconstructs can be independently controlled during the synthesis process to enhance tumor accumulation and blood longevity while minimizing unspecific sequestration from the mononuclear phagocyte system.
Main Text
Nanoparticles for the systemic delivery of therapeutic and imaging agents have been demonstrated in pre-clinical studies for the early detection and treatment of cancer1 and cardiovascular,2 neurodegenerative,3 and chronic inflammatory diseases.4 A few successful clinical applications are also starting to appear.5, 6 Compared to freely administered molecules, nanoparticle-loaded agents (nanomedicines) provide improved bioavailability and blood longevity, protection from enzymatic degradation, higher accumulation, and controlled release at the biological target. Furthermore, nanoparticles can carry multiple and different agents, enabling de facto what is known as combination therapy (the controlled release of different therapeutic agents synergistically contributing to cell death or repair7, 8), multi-modal imaging (the interrogation of tissue morphological and biological features using two or more imaging modalities9, 10), and theranostics (the co-delivery of therapeutic and imaging agents for disease treatment and follow-up11, 12). In addition, multiple targeting moieties can be conjugated on the nanoparticle surface and used to enhance the specific recognition of vascular and extravascular biological targets.13 Finally, the selection of proper materials for nanoparticle synthesis can facilitate biodegradation and support the triggered release of therapeutic molecules via both endogenous and exogenous stimuli.14 Despite all of these advantages, nanomedicines are yet to be fully integrated into clinical settings.
In the process of improving the performance of systemically injected nanoparticles, three major challenges should be addressed: increasing loading efficiency and controlled release of therapeutic molecules, mitigating recognition by the mononuclear phagocytic system (MPS), and enhancing tissue-specific accumulation. Over the last 15 years, a large variety of materials have been proposed for the realization of nanomedicines. Yet amounts of encapsulated drugs are still a minor portion of the overall nanoparticle mass, leading to inadequate loading efficiencies. In most lipid or polymeric-based delivery systems, only up to 10% of the nanoparticle mass is constituted by active therapeutic agents, whereas the remaining portion is therapeutically inert.15, 16 The loading efficiency reduces even more in the case of non-organic nanoparticles where a small number of therapeutic molecules are conjugated or adsorbed over the surface of a metallic or carbon-based core.17 Indeed, these inadequate loading efficiencies are of concern, in that large masses of inert materials would need to be administered in order to reach therapeutically effective doses within the diseased tissue. These materials would have to be disposed and could lead to systemic or local toxicities, particularly in the case of multiple and chronic treatments.
The second challenge is related to modulating the interaction of nanoparticles with the MPS. Nanoparticles are seen as foreign objects and they tend to be rapidly coated by small blood proteins from the complement system that facilitate their recognition and sequestration by professional phagocytic cells, mostly located within the liver (Kupffer cells) and the spleen (splenic macrophages).18, 19 Results from biodistribution studies indicated that over 50% of the injected dose of nanoparticles is trapped into filtering organs (liver and spleen).20, 21, 22, 23, 24, 25 Although this feature could be exploited for the treatment of systemic inflammatory diseases or hepatic and splenic abnormalities, it is definitely an undesirable effect when biological targets reside in different tissues and vascular districts.
Non-specific MPS sequestration is closely intertwined with the third challenge, which is related to enhancing the accumulation of systemically injected nanoparticles at the biological target. Importantly, a recent retrospective study, conducted on data published over the past years and dealing with the accumulation of spherical nanoparticles in cancer, documented that, on average, only 0.7% of the injected nanoparticles actually reach the neoplastic mass.26 Although this is an average number and could be affected by multiple factors (including the type and stage of neoplasia, the animal model, dosing, and so on), it clearly emphasizes that much more should be done in order to boost the percentage of nanoparticles stably accumulating within the tumor tissue. Importantly, these data strongly suggest that investigators should explore new delivery strategies and move away from conventional paradigms.
The Current Paradigm in Cancer Nanomedicine Design
In the early 1990s, Maeda et al.27, 28 observed in pre-clinical animal models that the rapidly growing tumor vasculature is characterized by a discontinuous endothelium and exhibit irregular openings (fenestrations) ranging in size from several tens up to a few hundreds of nanometers. As a consequence, sufficiently small blood-borne nanoparticles could passively permeate through these fenestrations and be retained within the diseased tissue, following a process named the enhanced permeability and retention (EPR) effect. Furthermore, passive tumor accumulation would be enhanced by longer circulation half-lives, in that this would increase the number of passages through the diseased vasculature of blood-borne nanoparticles. Inspired by the EPR effect and the need for long circulation times, nanoparticles for cancer treatment and imaging are synthesized with a spherical shape, an average diameter of 100 nm, and a surface mostly decorated with polyethylene glycol (PEG) chains. Currently, this is the major paradigm for cancer nanomedicine design.
In the late 1990s and early 2000s, the US Food and Drug Administration approved the first liposomal chemotherapeutic drug (liposomal doxorubicin29) and the US federal government launched a vast funding initiative on nanotechnology. This has stimulated pharmaceutical and material scientists, chemists, and biomedical engineers to develop more nano-based delivery systems. Relying on self-assembly and colloidal interactions, a plethora of nanoparticles satisfying the above paradigm have been documented with varying material compositions, sizes, and surface properties. In particular, organic materials have been used, including lipids, polymers, block copolymers, and combinations thereof, as well as non-organic materials, such as iron oxide, gold, silver, and carbon-based materials.30, 31, 32, 33, 34, 35, 36, 37 The nanoparticle surface has been modified using different coatings, including lipid and polymeric chains of different types and molecular weights, and a large variety of moieties for specific molecular targeting. Importantly, this plethora of nanoparticles, accounting for over 10,000 scientific papers published since the late 1990s, are mostly “alternate takes” of the above-cited paradigm: nanoparticles with a spherical shape, about 100 nm in diameter, and covered by a stealth coating for enhancing blood longevity. Despite such an incredibly large number of formulations, tumor deposition is about 1% on average of the injected dose.26 Although this level of tumor accumulation has been clearly sufficient to eradicate or modulate disease progression in immunocompromised mouse models, similar successes have not been replicated in other species, including canine and porcine models and humans. This again would imply that radically new design approaches are needed to propel the clinical integration of nanomedicines.
Biological Inspiration in Cancer Nanomedicine Design
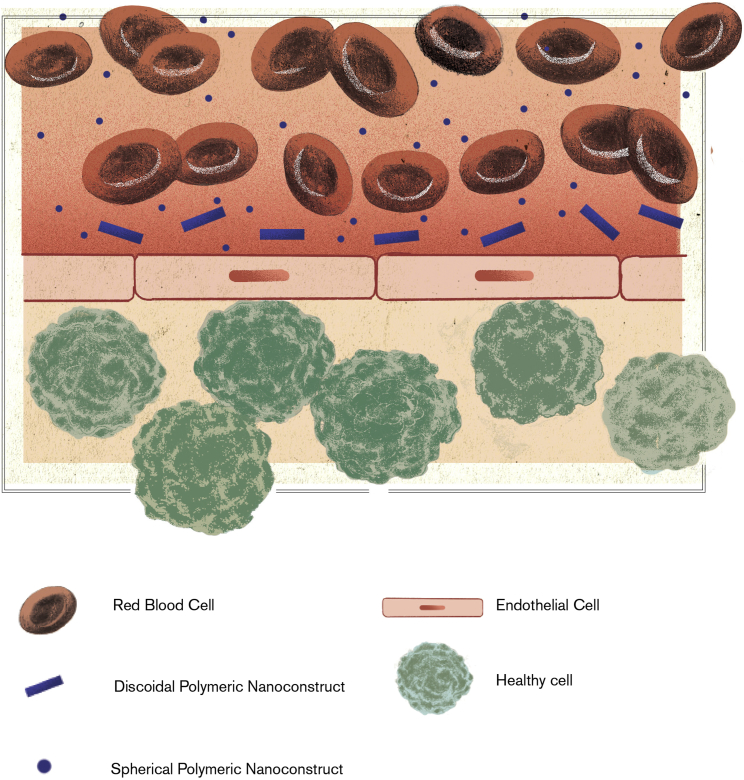
Upon injection within the blood stream, nanoparticles are transported away by hemodynamic forces and can virtually reach any vascular district within an organism. In this journey, nanoparticles are not alone; rather, they are surrounded by a multitude of cells and molecules, including the abundant red blood cells (RBCs) occupying from 40% to 50% of the vascular volume. Under flow, deformable RBCs tend to accumulate within the vessel core, leaving a cell-depleted area (cell-free layer) next to the walls (Figure 1).38 Interestingly, the far less abundant leukocytes and platelets are pushed laterally, toward the vessel walls, by the fast-moving RBCs. This specific behavior facilitates the lateral margination, wall adhesion, and extravasation of leukocytes and platelets. This is particularly relevant at sites of inflammation, where leukocytes abandon the vascular district and invade the tissue seeking the inflammatory source (extravasation), and at sites of vascular injury, where platelets synergistically accumulate to resolve vessel damage (adhesion).39, 40, 41
Figure 1.
Vascular Distribution of Red Blood Cells and Nanoparticles
Deformable red blood cells (RBCs) tend to accumulate within the vessel core, leaving a cell-depleted area (cell-free layer) next to the wall. Sub-micrometric and micrometric nanoconstructs, which are comparable in size to RBCs, tend to be pushed laterally and accumulate within the cell-free layer moving next to the vessel walls. In contrast, small nanoparticles can find their way within the shoal of RBCs and tend to be distributed quite uniformly across the blood vessel.42
Inspired by these natural processes, marginating nanoparticles can be designed to drift laterally across the stream lines, navigate within the cell-free layer, and efficiently seek vascular abnormalities, such as the presence of fenestrations and expression of specific endothelial receptors. Note that since RBCs accumulate within the core of the blood vessels forming a relatively compact shoal, only sufficiently large nanoparticles would be pushed laterally and preferentially kept within the cell-free layer in close proximity to the vessel walls. Small nanoparticles, presenting a characteristic size in the tens up to a few hundreds of nanometers, would move quite comfortably in the shoal of RBCs and accumulate less efficiently within the cell-free layer (Figure 1).42 Moreover, non-spherical particles, such as discoidal or cylindrical particles, would drift across the stream lines, thus further increasing the likelihood of escaping the RBC core.43 Therefore, lateral margination is boosted by designing nanoparticles comparable in size to platelets and characterized by a non-spherical shape.
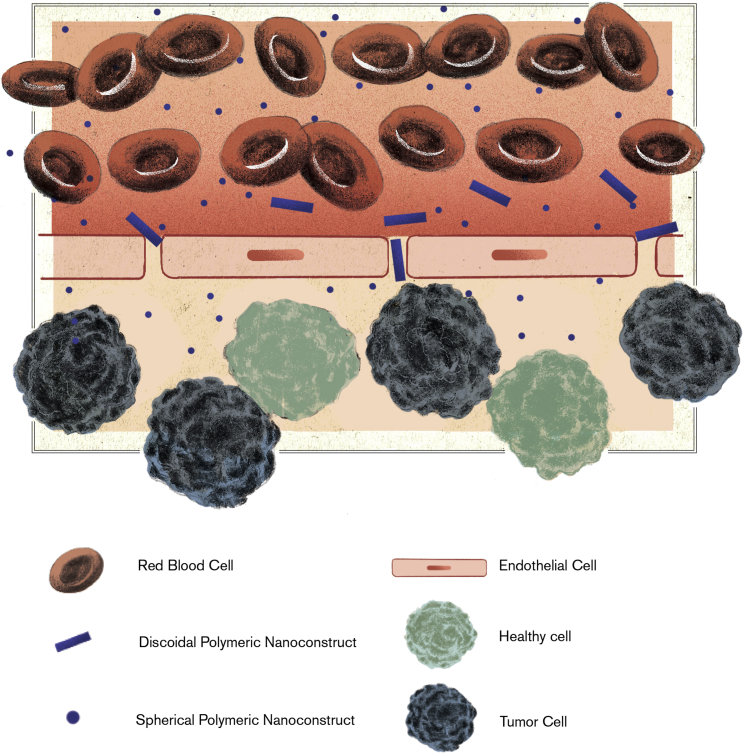
The second step is the realization of adhering nanoparticles. Importantly, the tortuous tumor microvasculature has significantly different biophysical features, as compared to healthy capillary networks. First, the mean blood velocities in tumors are up to one order of magnitude lower because of the overall higher hydraulic resistance and lower perfusion, as compared to normal vascular networks.44 Second, tumor capillaries expose specific and more abundant endothelial receptors, as compared to healthy capillaries.45 The proper combination of size and shape is crucial in facilitating nanoparticle adhesion to the tumor microvasculature. For a given volume, discoidal or cylindrical nanoparticles would expose a larger surface of adhesion to vessel walls and simultaneously establish multiple molecular bonds and strong colloidal interactions with endothelial cells, as compared to spherical nanoparticles. Also, adhering discoidal and cylindrical nanoparticles would feel lower hemodynamic dislodging forces. Therefore, for a given nanoparticle volume, the non-spherical shape is pivotal in maximizing vascular adhesive interactions while minimizing dislodging hemodynamic forces, (Figure 2).43, 46 Furthermore, it should be emphasized here that small discoidal or cylindrical nanoparticles would feel small dislodging hemodynamic forces even in healthy capillaries, where they could readily deposit, leading to undesired, non-specific accumulation. On the other hand, large discoidal or cylindrical nanoparticles would feel large dislodging forces even within the tumor microvasculature, where they would less likely deposit. Consequently, preferential deposition within the tortuous tumor microvasculature is favored for discoidal or cylindrical nanoparticles exhibiting a sub-micrometric to micrometric characteristic size.
Figure 2.
Nanoparticle Accumulation within the Tumor Parenchyma
Small nanoparticles passively cross the endothelial fenestrations and accumulate in a perivascular position. Sub-micrometric and micrometric nanoconstructs, which are sufficiently deformable, adhere to the vessel walls and could squeeze in through fenestrations. Therefore, both small nanoparticles and deformable particles would expose their therapeutic cargo to the tumor parenchyma from a perivascular position.
In recent years, this evidence has led to the development of non-spherical micrometric and sub-micrometric particles for the delivery of drug molecules and contrast agents. Table 1 summarizes some of the features of these nanoparticles. For instance, P.D. has contributed to the design and pre-clinical testing of Ferrari’s discoidal mesoporous silicon particles that tend to preferentially deposit within the tumor microvasculature because of a favorable balance between adhesion and margination dynamics.46, 47, 48, 49 Muro et al.50 also documented the advantages of using micrometric particles for targeting the lung microvasculature. More recently, Merkel et al.51, 52 introduced the notion of mechanobiological mimicry in the fabrication of long-circulating micrometric particles as artificial RBCs. Indeed, the systemic administration of sub-micrometric and micrometric particles raises concerns mostly on the MPS sequestration, occlusion of small capillary beds, difficulty in crossing the fenestrated tumor endothelium, and limited blood longevity. However, these concerns could be addressed by employing deformable particles.
Table 1.
Anti-cancer Non-spherical Nanoparticles: Materials, Size, Shape, Stiffness, Tumor Accumulation, and Circulation Half-Life of Non-Spherical Nanoparticles That Have Been Documented to Have an Effect in Cancer Therapy and Imaging
| Reference | Material | Size (μm) | Shape | Young’s Modulus (kPa) | Tumor (% ID/g) | t1/2 (hr) |
|---|---|---|---|---|---|---|
| Key et al.53 | polymeric | 1 | discoidal | 1–15 | ∼20 | 24 |
| van de Ven et al.47 | silicon | 0.6–1 | discoidal | rigid | 5–10 | NA |
| Merkel et al.51 | polymeric | 1–10 | discoidal | 6.5 | no tumor | 30–90 |
| Merkel et al.52 | polymeric | 5–6 | discoidal | 8–65 | no tumor | 3–90 |
| Anselmo and Mitragotri57 | polymeric | 0.2 | spherical | 0.1–3,000 | no tumor | 1–4 |
| Smith et al.55 | SWNT | 0.1–0.3 | cylindrical | rigid | 10–15 | NA |
ID, injected dose; SWNT, single-walled nanotube; t1/2, half-life.
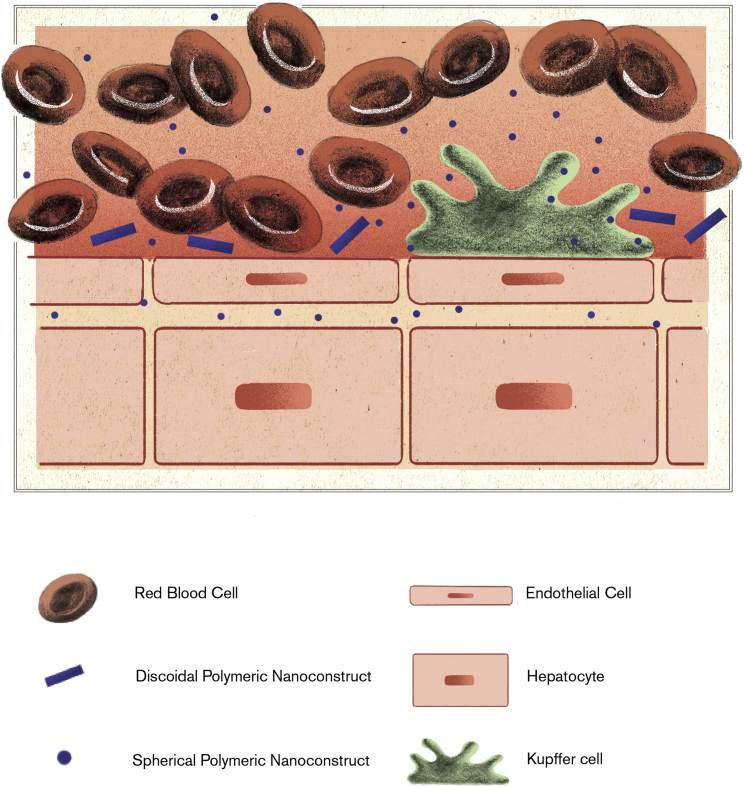
Therefore, the final piece in the puzzle is related to the design of deformable nanoparticles. Like RBCs, deformable particles would drift away from the walls toward the core of the blood vessels. However, particles in the sub-micron to micron size range would still be pushed laterally by the far abundant RBCs and confined to move within the cell-free layer, bouncing between the endothelial wall and the RBC shoal (Figure 1). Also, deformable particles would squeeze and more efficiently navigate through the smallest vessels in the brain, pulmonary, and splenic microcirculation, resulting in enhanced blood longevity.51, 52 Moreover, the intrinsic resistance of deformable particles to macrophage uptake would limit their sequestration by Kupffer cells (Figure 3).53 In contrast, small nanoparticles can be rapidly uptaken by Kupffer cells and passively permeate the discontinuous liver endothelium ending up in the space of Disse. These features would allow deformable particles to simultaneously accomplish two crucial objectives: (1) sensing the vessel walls for abnormalities, like marginating nanoparticles; and (2) circulating in the blood for a long time, like RBCs and platelets.
Figure 3.
Liver Sequestration of Blood-Borne Nanoparticles
The sub-micrometric size and deformability of discoidal polymeric nanoconstructs minimizes permeation across the liver endothelium and the non-specific sequestration by hepatic Kupffer cells. In contrast, small nanoparticles are rapidly taken up by phagocytic cells and can permeate within the space of Disse of the liver sinusoids. Note that the discontinuous endothelium of the liver sinusoids features regular openings of approximatively 100 nm, which are often comparable to that of tumor fenestrations.
Deformable Discoidal Polymeric Nanoconstructs
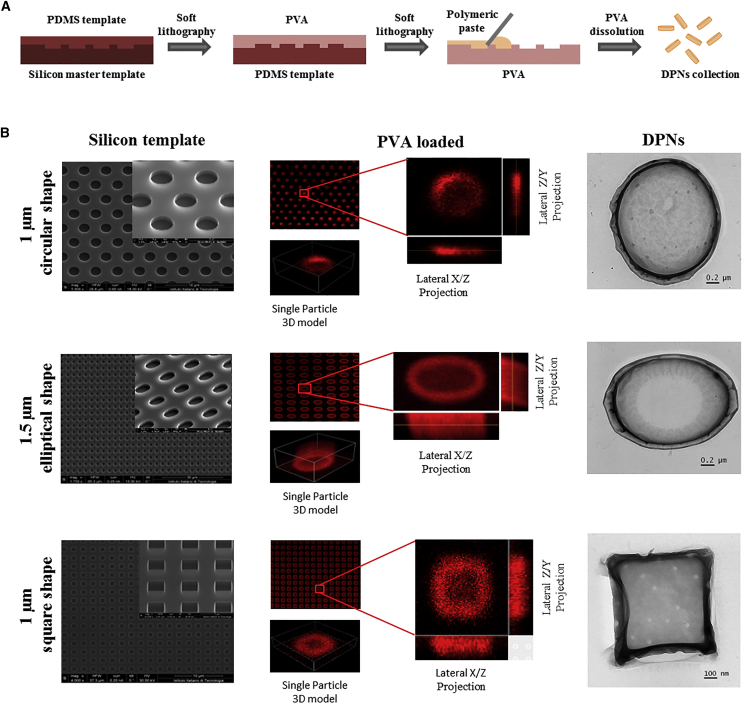
Over the past few years, the authors have been developing discoidal polymeric nanoconstructs (DPNs) to put into practice the above-described notions of marginating, adhering, and deformable nanoparticles. A hybrid fabrication strategy, combining nanoscale lithographic techniques, wet etching, and polymer chemistry, was adopted to tailor the size, shape, surface properties, and mechanical stiffness of nanoconstructs.53, 54 Specifically, the nanoconstruct geometry (size and shape) is imposed with a master silicon template, which is realized via electron beam lithography for small sizes (<500 nm), direct laser writing for sub-micron to micron sizes (500 nm to 10 μm), and optical lithography for large sizes (>10 μm). Circular, rectangular, elliptical, hexagonal, square, triangular, and other shapes can be readily realized. Nanoconstruct thickness can also be modulated by properly adjusting the etching of the silicon wafers. Nanoconstructs with an aspect ratio lower than unity (disks), larger than unity (rods), and comparable to unity (cubes) can be realized. Note that cubical nanoconstructs exposed to aqueous solutions would turn into a quasi-spherical shape. Representative images for circular, elliptical, and square DPNs are presented in Figure 4.
Figure 4.
Discoidal Polymeric Nanoconstructs
(A) Schematic representation of the process for the synthesis of DPNs. (B) Scanning electron microscopy images of the silicon master templates for circular, elliptical, and square DPNs (left column); optical images of PVA sacrificial templates for circular, elliptical, and square DPNs (central column), filled with a fluorescent polymeric paste; TEM images of circular, elliptical and square DPNs (right column). Red fluorescent is obtained by dispersing lipid-Rhodamine B complexes within the nanoconstruct polymeric matrix.53 DPN, discoidal polymeric nanoconstruct; PDMS, polydimethylsiloxane; PVA, poly(vinyl alcohol); TEM, transmission electron microscopy.
The nanoconstructs are obtained by polymerizing a mixture of poly(lactic-co-glycolic acid) (PLGA) and PEG chains in a sacrificial template, which is then dissolved in water under gentle agitation (Figure 4). Polymer concentrations affect the hydrophobicity of the resulting matrix, thus influencing water content and deformability. Low PEG concentrations lead to more hydrophobic matrices, which are characterized by reduced swelling and lower deformability, whereas high PEG concentrations lead to more hydrophilic matrices, which are associated with increased swelling and higher deformability. Atomic force microscopy can be conveniently used for testing the mechanical properties of these nanoconstructs, whose Young’s modulus can vary from a cell-like value (Young’s modulus of ∼10 kPa) to that of more rigid blocks (Young’s modulus on the order of 10 MPa). Furthermore, surface properties can be readily modified after releasing nanoconstructs and activating PLGA terminations for chemical conjugation with antibodies, peptides, aptamers, and various other ligands. These nanoconstructs are constituted by polymer matrices comprising both hydrophobic and hydrophilic micro-domains that serve as pockets for a variety of therapeutic and imaging molecules. Also, lipid- and polymer-drug conjugates and contrast agents can be readily incorporated within these polymeric matrices, leading to the development of truly theranostic agents.
In pre-clinical studies involving mice bearing melanoma and brain tumors, Key et al.53 showed that 1-μm soft, discoidal nanoconstructs can circulate over 48 hr and accumulate in the neoplastic tissues up to 20% of the injected dose per gram tissue. Importantly, these results also demonstrate that deformable particles can circulate for long times and avoid rapid sequestration by the MPS, which were major concerns associated with the systemic administration of sub-micron and micron-sized particles.53 These findings also demonstrate that the efficient navigation of the vascular network and the ability to identify abnormal blood vessels are key features in favoring tumor deposition of nanoparticles. Not surprisingly, other authors have shown that tumor accumulation of nanoparticles can also be enhanced by exploiting long-circulating RBCs and macrophages as vascular carriers.55, 56 Furthermore, it is important to recall that 1-μm deformable nanoconstructs were shown to pass through 400-nm filters and recover their size and shape distribution afterward.53 This reversible, morphing ability of DPNs would, on one hand, limit the risk of entrapment in the pulmonary microcirculation and occurrence of splenic infarction; on the other hand, it could favor firm entrapment in sufficiently large endothelial fenestrations, providing a new mechanism for tumor accumulation (Figure 3). Although this is still to be observed in vivo, such a likely scenario could definitely facilitate the release of therapeutic agents from DPNs directly in the tumor parenchyma and surrounding endothelial cells.
Additional work is still required in enhancing the encapsulation and stability of payloads as well as in scaling up the production of DPNs. In the first case, the traditional approach based on direct loading, where therapeutic and imaging agents are dispersed within the polymeric paste and directly loaded into the wells of the template, is often inefficient, leading to low encapsulation values. Per the stability of payloads, Key et al. demonstrated in previous works that radioactive (64Cu(DOTA))53 and fluorescent (Cy5.5 and Rhodamine B)54 molecules can be readily dispersed and retained within the polymeric matrix for several days. Unpublished data confirm similar trends also for Gd(DOTA) molecules and therapeutic agents. Nonetheless, further efforts are needed to identify different loading strategies for achieving encapsulation efficiencies well above 50% and triggering the release of therapeutic agents via endogenous and exogenous stimuli. Finally, the DPN fabrication process can be integrated with roll-to-roll and roll-to-plate industrial technologies, which allow scaling up in concert with good manufacturing practices.
Conclusions and Future Perspectives
Although a variety of material compositions and formulations have been tested for nanomedicines, the fundamental design paradigm has not yet changed and is still firmly grounded in the notion of the EPR effect. As a consequence, the majority of nanomedicines are still spherical with an average diameter ranging from tens to a few hundreds of nanometers. Using a mathematical metaphor, nanomedicines appear to be trapped in a local optimum, which does not necessarily coincide with the global optimum. As in mathematics, the sole way to seek other optima, and eventually reach the absolute optimum, is to change the initial conditions or, in other words, to reject well-received clichés.
Following biological inspiration, deformable nanoparticles with a sub-micrometric, or even micrometric, characteristic size have been demonstrated to resist sequestration by professional phagocytic cells, circulate for long times, and efficiently deposit within the tortuous tumor microvasculature. Moreover, template-based microfabrication strategies allow the development of polymeric nanoconstructs whose size, shape, surface properties, mechanical stiffness, and payloads can be systematically and independently changed during the synthesis process. This supports the realization of a broad spectrum of polymeric nanoconstructs.
As combinatorial chemistry allows the synthesis, development, and testing of millions of compounds for a variety of applications, these deformable DPNs could open the path toward a deeper understanding of the mechanisms regulating the behavior of nanomedicines and the identification of optimal therapeutic strategies for patient-specific drug delivery.
Author Contributions
A.L.P. and I.F.R. synthesized and characterized the physico-chemical properties of DPNs; M.F. synthesized and characterized the magnetic properties of DPNs; R.P. characterized the biological properties of DPNs; P.D. conceived the idea, analyzed the data, and wrote the manuscript. All authors analyzed and discussed the results.
Conflicts of Interest
The authors report no conflicts of interest.
Acknowledgments
This project was partially supported by the European Research Council (European Union Seventh Framework Programme [FP7/2007-2013]/ERC grant agreement no. 616695) and the Italian Association for Cancer Research (AIRC) (individual investigator grant no. 17664).
References
- 1.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 2.Mulder W.J., Jaffer F.A., Fayad Z.A., Nahrendorf M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci. Transl. Med. 2014;6:239sr1. doi: 10.1126/scitranslmed.3005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunes A., Al-Jamal K.T., Kostarelos K. Therapeutics, imaging and toxicity of nanomaterials in the central nervous system. J. Control. Release. 2012;161:290–306. doi: 10.1016/j.jconrel.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Crielaard B.J., Lammers T., Schiffelers R.M., Storm G. Drug targeting systems for inflammatory disease: one for all, all for one. J. Control. Release. 2012;161:225–234. doi: 10.1016/j.jconrel.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Min Y., Caster J.M., Eblan M.J., Wang A.Z. Clinical translation of nanomedicine. Chem. Rev. 2015;115:11147–11190. doi: 10.1021/acs.chemrev.5b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sainz V., Conniot J., Matos A.I., Peres C., Zupancic E., Moura L., Silva L.C., Florindo H.F., Gaspar R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015;468:504–510. doi: 10.1016/j.bbrc.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Lee A., Di Mascolo D., Francardi M., Piccardi F., Bandiera T., Decuzzi P. Spherical polymeric nanoconstructs for combined chemotherapeutic and anti-inflammatory therapies. Nanomedicine (Lond.) 2016;12:2139–2147. doi: 10.1016/j.nano.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Kolishetti N., Dhar S., Valencia P.M., Lin L.Q., Karnik R., Lippard S.J., Langer R., Farokhzad O.C. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. USA. 2010;107:17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee D.E., Koo H., Sun I.C., Ryu J.H., Kim K., Kwon I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012;41:2656–2672. doi: 10.1039/c2cs15261d. [DOI] [PubMed] [Google Scholar]
- 10.Kircher M.F., de la Zerda A., Jokerst J.V., Zavaleta C.L., Kempen P.J., Mittra E., Pitter K., Huang R., Campos C., Habte F. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao G., Mitragotri S., Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013;15:253–282. doi: 10.1146/annurev-bioeng-071812-152409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthu M.S., Leong D.T., Mei L., Feng S.S. Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4:660–677. doi: 10.7150/thno.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poon Z., Chen S., Engler A.C., Lee H.I., Atas E., von Maltzahn G., Bhatia S.N., Hammond P.T. Ligand-clustered “patchy” nanoparticles for modulated cellular uptake and in vivo tumor targeting. Angew. Chem. Int. Ed. Engl. 2010;49:7266–7270. doi: 10.1002/anie.201003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 15.Puri A., Loomis K., Smith B., Lee J.H., Yavlovich A., Heldman E., Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaucher G., Marchessault R.H., Leroux J.C. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J. Control. Release. 2010;143:2–12. doi: 10.1016/j.jconrel.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Gaihre B., Khil M.S., Lee D.R., Kim H.Y. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: drug loading and in vitro drug release study. Int. J. Pharm. 2009;365:180–189. doi: 10.1016/j.ijpharm.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Monopoli M.P., Walczyk D., Campbell A., Elia G., Lynch I., Bombelli F.B., Dawson K.A. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011;133:2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 19.Moghimi S.M., Hunter A.C., Murray J.C. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 20.Sadekar S., Ray A., Janàt-Amsbury M., Peterson C.M., Ghandehari H. Comparative biodistribution of PAMAM dendrimers and HPMA copolymers in ovarian-tumor-bearing mice. Biomacromolecules. 2011;12:88–96. doi: 10.1021/bm101046d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frellsen A.F., Hansen A.E., Jølck R.I., Kempen P.J., Severin G.W., Rasmussen P.H., Kjær A., Jensen A.T., Andresen T.L. Mouse positron emission tomography study of the biodistribution of gold nanoparticles with different surface coatings using embedded copper-64. ACS Nano. 2016;10:9887–9898. doi: 10.1021/acsnano.6b03144. [DOI] [PubMed] [Google Scholar]
- 22.Schipper M.L., Iyer G., Koh A.L., Cheng Z., Ebenstein Y., Aharoni A., Keren S., Bentolila L.A., Li J., Rao J. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schipper M.L., Cheng Z., Lee S.W., Bentolila L.A., Iyer G., Rao J., Chen X., Wu A.M., Weiss S., Gambhir S.S. microPET-based biodistribution of quantum dots in living mice. J. Nucl. Med. 2007;48:1511–1518. doi: 10.2967/jnumed.107.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morton S.W., Poon Z., Hammond P.T. The architecture and biological performance of drug-loaded LbL nanoparticles. Biomaterials. 2013;34:5328–5335. doi: 10.1016/j.biomaterials.2013.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chrastina A., Schnitzer J.E. Iodine-125 radiolabeling of silver nanoparticles for in vivo SPECT imaging. Int. J. Nanomedicine. 2010;5:653–659. doi: 10.2147/IJN.S11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. [Google Scholar]
- 27.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 28.Fang J., Nakamura H., Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Gabizon A., Shmeeda H., Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 30.Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 31.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009;71:431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan J.M., Zhang L., Yuet K.P., Liao G., Rhee J.W., Langer R., Farokhzad O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30:1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 34.Kataoka K., Harada A., Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 35.Svenson S., Tomalia D.A. Dendrimers in biomedical applications--reflections on the field. Adv. Drug Deliv. Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch L.R., Stafford R.J., Bankson J.A., Sershen S.R., Rivera B., Price R.E., Hazle J.D., Halas N.J., West J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee N., Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012;41:2575–2589. doi: 10.1039/c1cs15248c. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J., Johnson P.C., Popel A.S. Effects of erythrocyte deformability and aggregation on the cell free layer and apparent viscosity of microscopic blood flows. Microvasc. Res. 2009;77:265–272. doi: 10.1016/j.mvr.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Springer T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 40.Pappu V., Bagchi P. Hydrodynamic interaction between erythrocytes and leukocytes affects rheology of blood in microvessels. Biorheology. 2007;44:191–215. [PubMed] [Google Scholar]
- 41.Andrews R.K., López J.A., Berndt M.C. Molecular mechanisms of platelet adhesion and activation. Int. J. Biochem. Cell Biol. 1997;29:91–105. doi: 10.1016/s1357-2725(96)00122-7. [DOI] [PubMed] [Google Scholar]
- 42.Lee T.R., Choi M., Kopacz A.M., Yun S.H., Liu W.K., Decuzzi P. On the near-wall accumulation of injectable particles in the microcirculation: smaller is not better. Sci. Rep. 2013;3:2079. doi: 10.1038/srep02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adriani G., de Tullio M.D., Ferrari M., Hussain F., Pascazio G., Liu X., Decuzzi P. The preferential targeting of the diseased microvasculature by disk-like particles. Biomaterials. 2012;33:5504–5513. doi: 10.1016/j.biomaterials.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arap W., Kolonin M.G., Trepel M., Lahdenranta J., Cardó-Vila M., Giordano R.J., Mintz P.J., Ardelt P.U., Yao V.J., Vidal C.I. Steps toward mapping the human vasculature by phage display. Nat. Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 46.Decuzzi P., Pasqualini R., Arap W., Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm. Res. 2009;26:235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 47.van de Ven A.L., Kim P., Haley O., Fakhoury J.R., Adriani G., Schmulen J., Moloney P., Hussain F., Ferrari M., Liu X. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J. Control. Release. 2012;158:148–155. doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Decuzzi P., Godin B., Tanaka T., Lee S.Y., Chiappini C., Liu X., Ferrari M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release. 2010;141:320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 49.Xu R., Zhang G., Mai J., Deng X., Segura-Ibarra V., Wu S., Shen J., Liu H., Hu Z., Chen L. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat. Biotechnol. 2016;34:414–418. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muro S., Garnacho C., Champion J.A., Leferovich J., Gajewski C., Schuchman E.H., Mitragotri S., Muzykantov V.R. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merkel T.J., Chen K., Jones S.W., Pandya A.A., Tian S., Napier M.E., Zamboni W.E., DeSimone J.M. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J. Control. Release. 2012;162:37–44. doi: 10.1016/j.jconrel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merkel T.J., Jones S.W., Herlihy K.P., Kersey F.R., Shields A.R., Napier M., Luft J.C., Wu H., Zamboni W.C., Wang A.Z. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA. 2011;108:586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Key J., Palange A.L., Gentile F., Aryal S., Stigliano C., Di Mascolo D., De Rosa E., Cho M., Lee Y., Singh J., Decuzzi P. Soft discoidal polymeric nanoconstructs resist macrophage uptake and enhance vascular targeting in tumors. ACS Nano. 2015;9:11628–11641. doi: 10.1021/acsnano.5b04866. [DOI] [PubMed] [Google Scholar]
- 54.Key J., Aryal S., Gentile F., Ananta J.S., Zhong M., Landis M.D., Decuzzi P. Engineering discoidal polymeric nanoconstructs with enhanced magneto-optical properties for tumor imaging. Biomaterials. 2013;34:5402–5410. doi: 10.1016/j.biomaterials.2013.03.078. [DOI] [PubMed] [Google Scholar]
- 55.Smith B.R., Ghosn E.E., Rallapalli H., Prescher J.A., Larson T., Herzenberg L.A., Gambhir S.S. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 2014;9:481–487. doi: 10.1038/nnano.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villa C.H., Anselmo A.C., Mitragotri S., Muzykantov V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016;106(Pt A):88–103. doi: 10.1016/j.addr.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anselmo A.C., Mitragotri S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Deliv. Rev. 2017;108:51–67. doi: 10.1016/j.addr.2016.01.007. [DOI] [PubMed] [Google Scholar]