Abstract
RNAi-based therapy holds great promise, as it can be utilized for the treatment of multiple conditions in an accurate manner via sequence-specific manipulation of gene expression. To date, RNAi therapeutics have advanced into clinical trials for liver diseases and solid tumors; however, delivery of RNAi to leukocytes in general and to lymphocytes in particular remains a challenge. Lymphocytes are notoriously hard to transduce with RNAi payloads and are disseminated throughout the body, often located in deep tissues; therefore, developing an efficient systemic delivery system directed to lymphocytes is not a trivial task. Successful manipulation of lymphocyte function with RNAi possesses immense therapeutic potential, as it will enable researchers to resolve lymphocyte-implicated diseases such as inflammation, autoimmunity, transplant rejection, viral infections, and blood cancers. This potential has propelled the development of novel targeted delivery systems relying on the accumulating research knowledge from multiple disciplines, including materials science and engineering, immunology, and genetics. Here, we will discuss the recent progress in non-viral delivery strategies of RNAi payloads to lymphocytes. Special emphasis will be made on the challenges and potential opportunities in manipulating lymphocyte function with RNAi. These approaches might ultimately become a novel therapeutic modality to treat leukocyte-related diseases.
Keywords: lymphocytes, RNAi, lipid nanoparticles, drug delivery, aptamer
The development of nanoparticles for efficient systemic delivery of RNAi molecules to lymphocytes is a daunting task. Mizrahy et al. review the challenges and recent opportunities in non-viral delivery strategies of RNAi payloads to lymphocytes and elaborate on the most promising ones.
Main Text
Lymphocytes, the main component of the adaptive immune system, mediate inducible and long-lasting immune responses against pathogens. Lymphocytes include natural killer (NK) cells, T lymphocytes, and B lymphocytes, which are characterized by the expression of different surface molecules and antigen receptors. Lymphocytes are produced in the bone marrow and are the major cell type in the lymphoid system. They recirculate between the blood and lymphatic systems to invade and target damaged tissues and tumor cells upon appropriate signals.1 Lymphocytes can be divided into innate and adaptive immune cells, which differ in both specificity and function. While adaptive lymphocytes can respond to a significantly higher number of antigens in comparison to innate lymphocytes, the response is slower due to its multi-step nature. Long-lasting immunity is achieved by activation of the adaptive B and T lymphocytes and results in expansion of reactive cells that transform into effector plasma cells and activated T lymphocytes. The latter eliminates pathogens and facilitates secondary immune responses upon becoming memory cells.1
Lymphocytes are associated with the pathogenesis of autoimmune diseases, allergies, graft versus host disease, inflammation, lymphotropic viral infection (e.g., HIV), and cancer.2, 3 For example, the entry of HIV is mediated by a specific subset of T lymphocytes. In addition, successful modulation of T lymphocytes is required to overcome the immunosuppressive properties of the tumor microenvironment that utilize the expression of immune checkpoint molecules to escape immune surveillance.4 Therefore, selective gene silencing in lymphocytes using RNAi is not only a great tool for understanding lymphocyte biology, but it could also have attractive therapeutic potential.5, 6
Since the first successful application of RNAi in mammalian cells, RNAi has become an important tool in understanding gene expression and function in many cell types.7 The main advantage of RNAi is that it allows for robust silencing of potentially any gene of interest with high specificity and selectivity. This includes “undruggable” targets, which are uniquely expressed by different types of translocated genes, overexpressed genes, as well as mutated genes.8 Therefore, RNAi-based drugs may represent the future molecular medicine of targeted therapeutics for lymphocyte-implicated diseases.
Hurdles in Directed Gene Silencing of Lymphocytes
Despite its promise, RNAi therapy is not a trivial task, especially when targeting lymphocytes.9, 10 In part, this is due to the nature of RNAi molecules; their large molecular weight, negative charge, and hydrophilicity hinder naked RNAi molecules in crossing the plasma membrane. Additional barriers include the instability of naked RNA molecules in serum and their fast renal clearance. Chemically modified inhibitory RNA molecules have been developed to avoid innate immune recognition and degradation by nucleases.11 However, these modifications are proven to be a “double-edged sword,” as unnatural nucleotides may be immunogenic or toxic. For example, profound hepatotoxicity was reported for locked nucleic acid (LNA)-modified nucleic acids.12
Lymphocytes are highly resistant to transfection using conventional transfection reagents (e.g., cationic lipids or synthetic polymers).13, 14 There is relatively little information available about the cellular mechanism of lymphocyte transfection resistance. In general, lymphocytes differ in morphology compared to other cells; for example, their thinner cell membrane and lower protein content cause them to be more sensitive and hence harder to transfect than adherent cells.15, 16 Transfection of lymphocytes, using conventional transfection reagents, is shown to lead to tumor necrosis factor (TNF)-alpha secretion, apoptosis, and necrosis.17, 18 Another contribution to lymphocyte transfection resistance may be contributed due to the lack of cell surface heparan sulfate (HS) proteoglycans, which is caused mainly due to low expression levels of exostosin-1, a key enzyme in the biosynthesis of HS.19 HS proteoglycans donate a negative charge to the cell surface. Its low expression level on lymphocytes, in comparison to adherent cells, leads to a relatively high positive surface charge. This might increase the sensitivity of lymphocytes to cell lysis by conventional cationic transfection reagents due to excess local positive charge. It should be noted that although electroporation and nucleofection have been successful transfection techniques in lymphocytes,20 they can result in reduced cell viability and are unsuitable for in vivo applications.
Lymphocytes express a variety of Toll-like receptors (TLRs) and other RNA-sensing machineries such as retinoic acid-inducible gene-1 (RIG-1) and double-stranded RNA (dsRNA)-dependent protein kinase (PKR), which can recognize RNAi molecules and induce an inflammatory response.21, 22 Furthermore, it is known that the RNAi machinery in T lymphocytes appears to be inefficient in comparison with other adherent cell lines.23 Moreover, in vivo, lymphocytes are dispersed all over the body and are often located in deep tissues; therefore, it is challenging to reach them. Thus, the key to success in the delivery of RNAi molecules to lymphocytes in vivo is particularly dependent on a suitable delivery system. Delivery of RNAi molecules to lymphocytes needs to be mediated by targeted conjugates or particles that will protect the RNAi molecules from degradation and renal clearance, recognize the target cells specifically, and mediate efficient internalization of RNAi molecules into the lymphocyte cytoplasm with minimal toxicity.
Thus far, RNAi-based therapies have reached clinical trials for the treatment of liver diseases and solid tumors, while applications in lymphocytes lag behind.2 This is because the delivery to lymphocytes, as mentioned above, adds more levels of complexity than delivery to other cell types. Here, we will cover the latest progress in the delivery of RNAi molecules to lymphocytes using non-viral vectors. We will focus on various strategies designed for the delivery to specific subsets of lymphocytes for various applications. Special emphasis will be placed on the multiple challenges and potential opportunities achieved upon successful manipulation of lymphocyte function with RNAi-based therapeutics.
Nanoparticle-Based RNAi Delivery to Lymphocytes
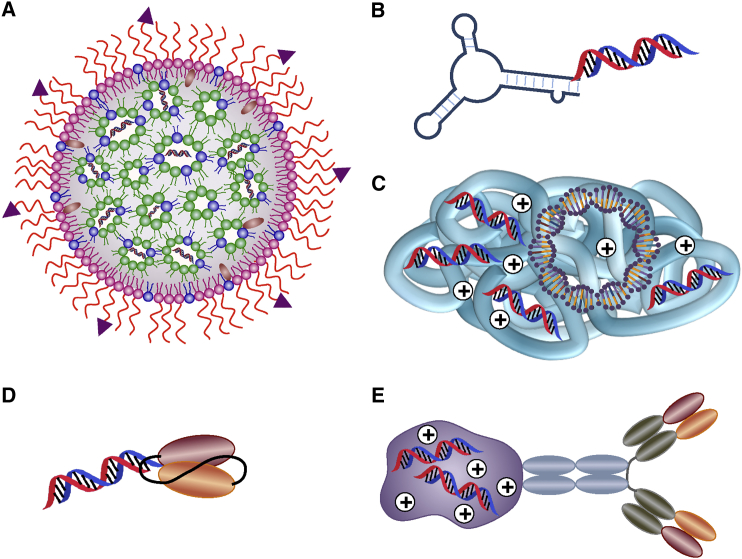
Targeted RNAi delivery systems possess multiple advantages over untargeted systems, as they increase the therapeutic index, reduce toxicity, and improve patient compliance. Targeted delivery is possible by the addition of a specific antibody, ligand, or ligand mimetic to the surface of the nanoparticle that would mediate the targeting and internalization of the RNAi payload into cells.24 Several strategies have been utilized to deliver RNAi molecules into lymphocytes, including lipid nanoparticles (LNPs), aptamer (apt)-small interference RNA (siRNA) conjugates, antibody-siRNA conjugates, and polymer-based nanoparticles (Figure 1).
Figure 1.
RNAi Delivery Strategies Available for Lymphocytes
(A) SNALP coated with specific ligand molecules encapsulated with siRNAs. (B) Apt-siRNA conjugate. (C) Cationic polymer-based nanoparticles encapsulated with plasmid DNA and siRNAs. (D) Single-chain variable fragment (scFv)-siRNA conjugate. (E) Antibody-protamine fusion protein-siRNA conjugate.
The development of more stable, chemically modified oligonucleotides creates an opportunity to deliver naked molecules as conjugates equipped with cell-specific targeting moieties, such as receptor ligands, monoclonal antibodies, or apts.
Apt-siRNA Conjugates
Apts are small-sized (6–30 kDa, ∼2 nm in diameter) synthetic single-stranded DNA or RNA molecules with the ability to fold into three-dimensional structure complexes. Using the SELEX (systematic evolution of ligands by exponential enrichment) combinatorial chemistry process, apts can be selected from a large pool of sequences against various target molecules such as toxins, antibiotics, amino acids, peptides, proteins, or whole living cells. Candidate binding sequences are then subjected to iterative selection rounds to increase the population of high-affinity species, until they eventually dominate in the library.25 Apts not only combine the advantages of monoclonal antibodies, such as high affinity, superior specificity, and low toxicity or immunogenicity, but they are also easy to synthesize, modify, and manipulate. As such, they are usually called chemical antibodies. Apts with high affinity and selectivity to lymphocyte antigens can be linked to inhibitory oligonucleotides to produce multifunctional compounds for targeting lymphocytes and modulating gene expression. A very extensive review on apt-based therapeutics was recently published.25 Several recent studies have demonstrated a promising ability of apt-conjugated siRNA chimeras (AsiCs) to promote in vivo gene silencing of T lymphocytes. Wheeler et al.26 developed an AsiC for targeting in vivo HIV-infected CD4+ T lymphocytes. The authors used a CD4 apt linked to siRNA molecules against chemokine C-C chemokine receptor type 5 (CCR5), which is required for HIV cell entrance and transfection. Prophylactic intravaginal administration of these CD4-AsiCs protected against HIV genital transmission in humanized mouse models without stimulating innate immunity. An alternative strategy for treatment of HIV infection, presented earlier by Neff et al.,27 comprises an RNA apt with high binding affinity to the HIV-1 envelope glycoprotein gp120. This apt was attached to siRNA that triggers degradation of the HIV-1 tat/rev RNAs that encode early regulatory proteins required for HIV replication. This approach significantly improved the overall antiviral effect of the gp120 apt-siRNA chimera in comparison with the apt alone, as tested in a humanized mouse model of HIV infection. A CCR5 apt that was selected by a live cell-based SELEX process was shown to target HIV-susceptible cells (CD4+ T lymphocytes and monocytes) via the CCR5 receptor. Although CCR5 apt-siRNA chimeras (CCR5-AsiCs) protected human CD4 T lymphocytes from HIV infection with a nanomolar inhibitory concentration,28 their efficacy in vivo remains to be determined.
Two recent studies utilized apts targeting either inhibitory or stimulatory receptors on T-cell subsets, in order to augment the efficacy and persistence of anti-tumor immune responses.29, 30 Herrmann et al.30 reported the use of this strategy, in which a cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) apt was conjugated to signal transducer and activator of transcription 3 (STAT3) siRNA (CTLA4-AsiCs). CTLA4-AsiCs successfully delivered STAT3 siRNA to tumor suppressor CD8+ T lymphocytes, T regulatory cells (Tregs), as well as CTLA4-expressing malignant T lymphocytes. Systemic administration of CTLA4-AsiCs robustly inhibited tumor growth and metastasis in various mouse tumor models, including immunodeficient mice bearing human T-cell lymphomas. To specifically target activated CD8+ T lymphocytes, Berezhnoy et al.29 conjugated siRNA targeting the mTORC1 component raptor to an apt that binds 4-1BB, a costimulatory molecule that is expressed on CD8+ T lymphocytes following T-cell receptor (TCR) stimulation. Mechanistic target of rapamycin (mTOR) signaling is known to promote the differentiation of activated CD8+ T lymphocytes into short-lived effectors rather than memory cells, thereby resulting in a potent but only transient immune response. The authors found that systemic administration of 4-1BB-AsiCs to mice downregulated mTORC1 activity in the majority of CD8+ T lymphocytes, leading to the generation of a potent memory response that exhibited cytotoxic effector functions and enhanced vaccine-induced protective immunity in tumor-bearing mice.
Most recently, the same group utilized the 4-1BB-CD25 and 4-1BB-Axin-1 apts for a similar purpose: specific modulation of CD8+ T-lymphocyte differentiation into long-lasting memory CD8+ T lymphocytes.31 The authors attenuated interleukin (IL)-2 signaling in order to achieve anti-tumor immunity. In an in vivo breast cancer model, the authors showed tumor infiltration by CD8+ T lymphocytes and an increase in tumor susceptibility to secondary treatment implemented, in parallel with the apt-siRNA conjugate.32
Apt-short hairpin RNA (shRNA) conjugates were also engineered for lymphocyte targeting. Recently, Soldevilla et al.33 presented a CD40apt-shSMG1 for cancer immunotherapy. The authors utilized HS-SELEX to identify three CD40 therapeutic constructs: a CD40 agonist bivalent apt, a CD40 antagonist apt, and a CD40 agonistic apt-shRNA chimera. While the agonist led to proliferation and activation of B lymphocytes and accelerated recovery of bone marrow aplasia, the antagonist apt showed a direct anti-tumor effect in a B-cell lymphoma model. Upon conjugation to shRNA against SMG1, a serine/threonine kinase essential for nonsense mRNA-mediated decay (NMD), the authors managed to show improved tumor infiltration and survival.34 The highly promising concept of NMD inhibition is relatively new to cancer immunotherapy and was developed by Pastor et al.,34 who showed that NMD inhibition in tumor cells led to the expression of new cancer antigens and therefore increased their immune-mediated recognition and rejection.
Although most siRNA-apt conjugates described in the literature are chimeras, recent advances have been reported that utilize bridge structures in order to improve the utility of apt conjugates. Since chimeras are synthesized as two pieces followed by an annealing step or synthesized as one piece, they require a specific preparation for each siRNA sequence. Systems that utilize molecular platforms or sticky bridge linkers enable non-covalent binding and therefore allow interchange of various siRNAs with the same apt.35 In the sticky bridge systems, the apt and the siRNA are bound to complementary guanine-cytosine (GC)-rich bridge sequences and annealed simply by mixing, which allows Watson-Crick base pairing.25 Such a system was recently utilized by Zhou et al.35 for the delivery of dicer-substrate short interfering RNAs (DsiRNA) cocktails into T lymphocytes, which resulted in potent inhibition of HIV replication. Another alternative presented by Zhou et al.36 for the delivery of siRNA cocktail into peripheral blood mononuclear cells (PBMCs) is an apt chimera consisting of packaging RNA from the bacteriophage φ29 DNA packaging motor; however, this system was not tested in vivo. An additional alternative for covalent conjugation includes apt-streptavidin-siRNA conjugates, although these systems have not yet been tested in lymphocytes.25
Protein-siRNA Conjugates
Antibody-siRNA Conjugates
The first antibody-siRNA conjugates for systematically targeting of immune cells were designed as fusion proteins. These fusion proteins are composed of a cell-target antibody fragment joined to a protamine peptide that binds nucleic acids, such as siRNAs. This approach was used first to deliver siRNAs into tumors implanted in mice that were engineered to express T-cell surface antigens, HIV envelope proteins, or human integrin lymphocyte function-associated antigen-1 (LFA-1) (Figure 1E).37, 38 Kumar et al.39 modified this approach and generated a fusion protein consisting of a scFv against CD7 (a pan T-lymphocyte protein) and the nona-d-arginine (9R) peptide (scFvCD7-9R) for systemic targeting of T lymphocytes (Figure 1D). scFvCD7-9R efficiently delivered CD4 siRNAs to naive T lymphocytes in humanized mice, and it effectively inhibited HIV infection in these mice when combined with siRNAs targeting CCR5, Vif, and Tat. However, antibody-based fusion proteins are expensive to manufacture, are potentially immunogenic, and therefore are probably less suitable for clinical use.
Chemokine-siRNA Conjugates
Biragyn et al.40 provided a novel and simple solution for the use of RNAi in vivo by delivering inhibitory oligonucleotides with chemokines to inactivate a selective subset of immune cells. The authors created chemokine CCL17 (TARC-arp) modified to bind oligonucleotides by linking it with a 15-amino-acid fragment of a single-stranded DNA/RNA-binding portion of the capsid antigen of hepatitis B virus (HBV). The chemokine mediates chemotaxis of the desired cells and internalization of siRNA through binding to chemokine receptors. Biragyn et al. showed that TARC-arp can transduce CCR4-expressing CD4+ T lymphocytes and Tregs with siRNA transiently silencing the genes of interest. TARC-arp-mediated silencing of IL-10 or FoxP3in CCR4+ Tregs is sufficient to block lung metastasis in a breast cancer mouse model.
Supramolecular Nanocarriers
In comparison to antibody- or apt-siRNA conjugates, supramolecular nanocarriers (NCs) provide additional benefits, because they are shown to protect against both rapid renal excretion and nuclease cleavage, reduce unwanted immune response, enable delivery of oligonucleotides in combination with either soluble or insoluble drugs, controlled drug-release mechanisms, and improve intracellular penetration.41 Multiple aspects of NCs can be controlled in order to tailor the optimal NC for a specific condition, including size, shape, and surface chemistry. For instance, formulations of NCs that range between 100 and 200 nm are determined to be optimal for long circulation in the bloodstream, since at this specific size range they can avoid uptake by the reticuloendothelial system (RES), also known as the mononuclear phagocytic system (MPS). In addition, hydrophilic surfaces created by coating NCs with either poly(ethylene glycol) (PEG) or hyaluronan (HA) endow nanoparticles (NPs) with long circulation times.41 NCs can also provide a mechanism for slow release of the therapeutic cargo, such as activated release that breaks the bonds between the drug and NC or leads to particle degradation or efflux of the drug from the NC. All of the above were shown to improve therapeutic efficacy in comparison to non-formulated oligonucleotides, following several parameters such as improved gene silencing and increased therapeutic outcome with minimal adverse effects.42 Although a variety of NCs were developed for delivering therapeutic RNAi molecules to tumors (liposomes, lipid-based particles, polyplexes, lipoplexes, dendrimers, polymeric nanoconjugates, and more), only some are shown to effectively induce gene silencing in lymphocytes upon systemic administration as detailed below.43
Polymer-Based Nanoparticles
Polymer-based RNAi delivery often utilizes cationic polymers such as poly(l-lysine) (PLL) and polyethylenimine (PEI) due to their immense chemical diversity and their potential for functionalization.44 The high positive charge density at reduced pH may enable both nucleic acid condensation and endosomal escape.45 Nevertheless, these polymers have been shown to induce adverse effects such as cytotoxicity and unwanted immune activation.46 Therefore, several chemical modifications have been tested in order achieve improved stability and biocompatibility.42, 47
SNS01-T (NCT01435720) is the most advanced polymer-based system that is now in clinical trials (phase I/II) for the treatment of relapsed or refractory multiple myeloma and B-cell lymphoma. SNS01-Ts are PEI nanoparticles that contain both siRNA and a decoy DNA plasmid (Figure 1C). The siRNA sequence targets eukaryotic translation initiation factor 5A (eIF5A) while the plasmid DNA expresses a non-hypusinable mutant of eIF5A (K50R), which induces apoptosis under a B-cell-specific promoter. Although these positively charged nanoparticles are untargeted, the enhanced tumor cell uptake and relatively low toxicity suggest that SNS01-T preferentially targets malignant cells. Using local and systemic administration methods of the siRNA-DNA chimeric NPs, Francis et al.48 showed significant growth inhibition of multiple myeloma tumors and an increased survival rate in a human myeloma xenograft mouse model.
Dendrimers, previously known as “starburst polymers,” are a class of synthetic, highly branched, and positively charged polymers.49, 50 Dendrimers have been studied extensively for their potential application as carriers for nucleic acids due to their unique characteristics, which include monodispersity, uniformity, and the presence of numerous functionalizable terminal groups.49, 50 As with other positively charged polymers, nucleic acid condensation is mediated by the cationic charge. Carbosilane dendrimers and poly(amidoamine) (PAMAM) dendrimers have been complexed with siRNA for the purpose of suppressing HIV infection.51, 52 Due to the fact that no covalent binding is required for siRNA complexation, dendrimers can be used as a platform for the delivery of different siRNA molecules targeting both viral and cellular transcripts.52 Although not targeted, Zhou et al.52 have shown complete inhibition of HIV-1 titers and protected against viral-induced CD4+ T-cell depletion by the PAMAM dendrimer. Targeted dendrimers have also been reported for the purpose of drug delivery to lymphocytes in vitro and in vivo; however, these formulations have not yet been utilized for nucleic acid delivery.53
LNPs, Liposomes, and SNALPs
LNPs were the first nanoparticle delivery system approved by the US Food and Drug Administration (FDA),54 for many great reasons. LNPs are characterized by their general biocompatibility, biodegradability, isolation of drugs from the surrounding environment, and the ability to entrap both hydrophilic and hydrophobic drugs41 and their production scale-up is cost-effective. In addition, multiple properties of LNPs can be altered via surface chemistry, including their size, charge, and surface functionality, simply by adding new components to the lipid mixture before LNP preparation and/or by varying the preparation methods.41 There are several subclasses of LNPs, including liposomes, micelles, and stabilized nucleic acid lipid particles (SNALPs).
Liposomes are spherical, self-closed structures formed by one or several concentric lipid bilayers with an aqueous phase inside and between different shells of multilayered particles.55 To date, more than 13 liposomal formulations have been approved by the FDA, due to their multiple benefits. Liposomal formulations encapsulating siRNA manage to overcome the limitations of antibody-protamine fusion protein and apt-siRNA fusion systems, as they manage to increase the amount of payloads and simplify the preparation procedures. Liposomal siRNA formulation eliminates the laborious molecular biology techniques of protein engineering and the purification methods required for the formation of each fusion protein.24, 56 Therefore, liposomal formulations manage to achieve robust, targeted gene silencing in leukocytes in vivo.24 This was first demonstrated by utilizing liposomes that were surface modified with an anti-integrin monoclonal antibody, termed integrin-targeted and stabilized nanoparticles (I-tsNPs).57 The siRNA was encapsulated within the I-tsNP upon rehydration of lyophilized particles with deionized water containing protamine-condensed siRNAs. This system is basically a platform technology, as it is possible to target different lymphocytes subsets by simply changing the antibody on the surface of these NPs as detailed below. Upon systemic administration, this system successfully silenced Cyclin D1 in leukocytes and reversed experimentally induced colitis in mice by suppressing leukocyte proliferation and T-helper cell 1 cytokine expression.
In order to explore whether the I-tsNP system can indeed be defined as a platform technology, it was tested with a different surface antibody: the anti-αLβ2 integrin (LFA-1), which is highly expressed on all leukocytes.58 The system was used for systemic delivery of CCR5 siRNA in a humanized murine model.
LFA-1 I-tsNPs were selectively taken up by T lymphocytes and macrophages, the prime targets of HIV, and silencing was sustained for 10 days. Finally, humanized mice challenged with HIV after anti-CCR5 siRNA treatment showed enhanced resistance to infection, as assessed by the reduction in plasma viral load and disease-associated CD4+ T-lymphocyte loss. Therefore, this demonstrates the potential application of the LFA-1 I-tsNP system as anti-HIV prophylaxis. Another liposomal system for the delivery of RNAi, named SMARTICLES, was developed by Marina Biotech. SMARTICLES are composed of a unique combination of lipids having anionic and cationic groups that work together to enable cell uptake and provide serum stability and pH-triggered endosomal escape. This system is currently under evaluation in a phase I dose-escalation clinical trial for delivery of miR34 (MRX34) for patients with advanced cancer with primary liver and hematological malignancies, multiple myeloma, and lymphoma (NCT01829971).
SNALPs are one of the most advanced strategies for siRNA delivery, due to their high siRNA encapsulation efficiency, low immunogenic properties, and potent gene knockdown in humans.59, 60 This new generation of LNPs was originally designed by Pieter Cullis’s laboratory and is extensively reviewed elsewhere.61, 62 SNALPs are composed of ionizable lipids in addition to polyethylene glycol-conjugated (PEGylated) lipids, cholesterol, and nucleic acids (Figure 1A).63 The degree of unsaturation and the ionizable head groups are crucial for efficient transfection and endosomal escape, which is one of the major obstacles upon designing drug delivery systems.64, 65 SNALPs can be produced using microfluidic mixing technology (Figure 2).62, 66, 67 This robust method possesses several advantages over conventional liposomes preparation, including the fact that it is much less laborious, enables higher nucleic acid encapsulation efficiency, and decreases batch-to-batch variability, resulting in consistent production. SNALPs are neutrally charged in the circulation, where they associate with apolipoproteins (in particular, apolipoprotein E3), which mediate their endocytosis, primarily by hepatocytes and monocytes.68, 69 Once inside the endosome, the low pH leads to lipid protonation, which triggers endosomal membrane destabilization and the subsequent cytosolic release of some of its nucleic acid cargo. The first-generation SNALPs were composed of 1,2- dilinoleyloxy-3-dimethylaminopropane (DLinDMA) and potently knocked down genes in the liver of rodents and non-human primates.70 Nevertheless, upon evaluation in clinical trials, this formulation showed limited liver gene knockdown and induced unwanted innate immune activation, such as activation of the complement system. The second-generation SNALPs showed substantially improved siRNA delivery and knockdown effectiveness in the liver. Constructed with the anionic lipid dilinol eylmethyl-4- dimethylaminobutyrate (DLin-MC3-DMA), these NPs mediated potent gene knockdown in humans at reduced doses compared with first-generation SNALPs.59 Upon systemic administration, most SNALPs naturally accumulate in liver cells and were therefore utilized for the treatment of liver viral infections and tumors.60 Using this technology to treat disseminated diseases such as leukemia and lymphoma is even more challenging. Such an effort was recently initiated in a dose-escalation phase I study by Dicerna Pharmaceuticals. Dicerna uses a dicer substrate RNAi-based therapy encapsulated in SNALPs, which was designed to silence the Myc oncogene (DCR-Myc) in liver tumors and selected solid tumors, but also in patients with multiple myeloma and non-Hodgkin’s lymphoma (NCT02110563).
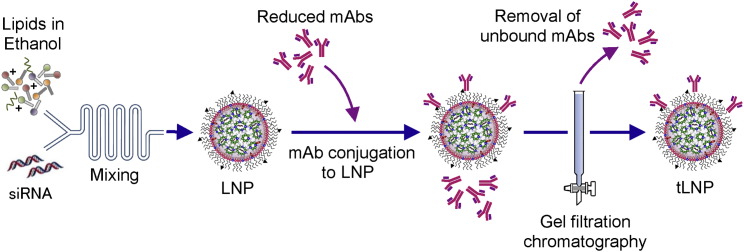
Figure 2.
Schematic Diagram of the Production Process of Targeted LNPs
Lipids including the ionizable lipid and PEG-maleimide dissolved in ethanol and siRNAs in acetate buffer are combined by the microfluidic mixer to produce malamide-functionalized LNPs. Reduced immunoglobulin Gs (IgGs) are conjugated with maleimide-functionalized LNPs to produce targeted LNPs (tLNPs). To remove unconjugated antibody, tLNPs are purified by gel filtration chromatography. mAb, monoclonal antibody.
The use of targeted lipid nanoparticles (tLNPs) is a more promising approach, especially upon targeting specific lymphocyte subsets or treating disseminated diseases (Figure 2). In a recent study, we described a novel strategy to specifically deliver siRNAs into murine CD4+ T lymphocytes using tLNPs. The tLNPs were surface functionalized with anti-CD4 monoclonal antibodies to permit delivery of the siRNAs specifically to CD4+ T lymphocytes. Systemic intravenous administration of these particles led to efficient binding and uptake into CD4+ T lymphocytes, which was followed by CD45 silencing.71 Weinstein et al.72 recently described a similar strategy to specifically deliver siRNA against cyclin D1 to mantle cell lymphoma (MCL) cells in a human MCL-xenograft mouse model. LNPs coated with anti-CD38 monoclonal antibodies and loaded with siRNAs against cyclin D1 induced gene silencing in MCL cells and prolonged survival of tumor-bearing mice with no observed adverse effects. These results present a unique RNAi delivery system that opens new therapeutic opportunities for treating MCL and other B-cell malignancies.
Conclusions
The past 10 years have been very exciting with substantial advances achieved in the field of nanoparticle-based delivery systems and the discovery of RNAi. Several promising delivery systems have been introduced due to the development of new materials and NP preparation techniques such as microfluidics. Utilizing the progress made in materials science and engineering enabled major achievements in the robust, rapid, and reproducible production of drug delivery systems. Other improvements include better NP stability and reduced toxicity and immune activation. Furthermore, the potency of nucleic acids as a therapeutic payload has improved dramatically. Combining this with the advances in the field of genomics and next-generation sequencing enables the possibility to identify new gene targets for precise and possibly personalized RNAi-based therapeutics.
Nevertheless, the development of NPs for efficient systemic RNAi delivery remains a daunting task, particularly in regard to lymphocytes. This is also the major obstacle in translating the knowledge obtained from biomedical research into clinical therapeutics.73, 74
The ability to actively target lymphocytes holds great promise and opens novel therapeutic possibilities in the context of inflammation, autoimmune response, transplant rejections, viral infections such as HIV, and hematologic malignancies.24 The improved delivery of RNAi-based therapeutics to lymphocytes can also hold great promise in cancer immunotherapy, which has been gaining more attention in cancer therapy. To date, upon examining the recent progress achieved in the field of RNAi delivery to lymphocytes, it appears that targeted delivery systems that utilize specific ligands or antibodies are the most promising. As lymphocytes are disseminated throughout the body and are often located in deep tissues, passive targeting and organ localization methods are much less effective. In addition, targeted systems not only improve the therapeutic index and reduce toxic effects in comparison to untargeted systems but also allow for the manipulation of specific lymphocyte subsets, which is required in the example of immune modulation in cancer therapy. The targeting moiety itself should also be carefully chosen, as not every surface receptor is suitable for targeting. An optimal targeting moiety should match the required outcome whether it is subset specific or pan T cell. In addition, utilizing cell surface receptors that undergo continuous endocytosis (e.g., CD4) supports siRNA internalization.9, 26 Recent gene expression analysis has shed more light on appropriate potential cell surface targets.75, 76, 77 Targeting moieties for lymphocytes are not only subset specific, but certain cell surface receptors (e.g., LFA-1) go through activation-dependent modification. LFA-1 is found in its low-affinity non-adhesive form in naive cells and is converted into a high-affinity conformation upon lymphocyte activation.78, 79 This enables specific targeting of activated lymphocytes which is a highly desirable outcome for the therapy of inflammations such as inflammatory bowel disease (IBD). Additional surface molecules that are upregulated in activated lymphocytes include CCR5, CD69, CD25, CD40L, and TNF receptor superfamily member 4 (TNFRSF4).6 In any case, the targeting moiety used would require “humanization” for subsequent clinical translation. Other hurdles in targeted gene silencing in lymphocytes are related to the nature of lymphocytes, which include a different membrane structure and less efficient RNAi machinery.19, 23 This explains why delivery systems for inhibitory RNA molecules designed for solid tumors often fail to target lymphocytes.
Upon examining delivery systems that managed to modify lymphocyte gene expression in vivo (summarized in Table 1), the most abundant systems are apt conjugates. This can be attributable to their small size (∼2 nm in diameter versus 15 nm for antibodies or ∼80 nm for LNPs), which enables deep-tissue extravasation and subsequent nucleic acid delivery to lymphocytes. In comparison to antibodies, the size and flexibility of apts allows them to bind smaller targets and hidden binding domains.25 In addition, apt production is much less lengthy, laborious, and expensive, as the production is performed via a cost-efficient in vitro selection procedure that is characterized by lower batch-to-batch variations.25 Other advantages include lower immunogenicity in comparison to antibodies and a potentially infinite spectrum of target antigens. Nevertheless, apt-based therapeutics still lag behind antibody-based therapeutics, with only one FDA-approved product.25, 80 This can be attributed to the fact that apt-based therapeutics are still in their infancy and their safety profile requires further evaluation.81 In addition, non-conjugated apts are characterized by low circulation time and rapid renal clearance in comparison to antibodies and supramolecular carriers due to their small size. Yet both apts and protein-siRNA conjugates fall short in the amount of nucleic acid payload delivered to cells in comparison to supramolecular carriers. While liposomes can entrap thousands of siRNA molecules (I-tsNPs are characterized by an ∼4,000:1 ratio of siRNAs to NP), antibodies can only bind five to six siRNA molecules after fusion to protamine (and much less without fusion).38, 39, 57, 82 Another advantage of LNPs comes from a manufacturing perspective, as SNALPs prepared using microfluidic mixing technology are highly reproducible and utilize a short one-step preparation procedure. This is a great benefit, particularly for production under current good manufacturing practice (cGMP) standards.
Table 1.
RNAi Delivery Systems that Managed to Silence Lymphocytes In Vivo
| Drug | Vehicle | Target | Targeting Moiety | siRNA Conjugation Technique | Route | Condition | Reference |
|---|---|---|---|---|---|---|---|
| CD4-AsiCs | apt-siRNA conjugate | CCR5 | CD4 | covalent linkage | IVAG | HIV | 26 |
| Ch A-1 | apt-siRNA conjugate | HIV-1 tat/rev RNAs | gp120 | covalent linkage | i.v. | HIV | 27 |
| 4-1BB-AsiCs | apt-siRNA conjugate | mTORC1 | 4-1BB | covalent linkage | i.v. | solid tumors | 29 |
| CTLA4-AsiCs | apt-siRNA conjugate | STAT3 | CTLA4 | covalent linkage | i.v. | T cell lymphoma | 30 |
| 4-1BB apt-CD25 siRNA | apt-siRNA conjugate | CD25, Axin-1 | 4-1BB | covalent linkage | i.v. | solid tumors | 31 |
| CD40Apt-SMG1-shRNA | apt-shRNA conjugate | SMG1 | CD40 | covalent linkage | i.v. | B cell lymphoma | 33 |
| gp120 A-DsiRNA | apt-siRNA conjugate | HIV-1 tat/rev, CD4 and TNPO3 | gp120 | 3′ 7-carbon linker | i.v. | HIV | 35 |
| LFA-1 protamine | antibody-siRNA conjugate | Ku70 | LFA-1 | protamine condensation | i.v. | activated leukocyte populations | 6 |
| scFvCD7-9R | antibody-siRNA conjugate | CD4, CCR5, Vif, and Tat | CD7 | oligo-9-arginine peptide | i.v. | HIV | 39 |
| TARC-arp | CCL17-siRNA conjugate | IL-10, FoxP3 | CCR4 | DNA/RNA-binding portion of the capsid antigen of HBV | i.v. | lung cancer | 40 |
| SNS01-T | PEI-based nanoparticle | siRNA eIF5A, and plasmid NH mutant of eIF5A | untargeted | polyelectrolyte complexation | i.v. | B cell malignancies | 48 |
| PAMAM dendrimer | cationic PAMAM dendrimers | HIV-1 tat/rev, CD4, and TNPO3 | untargeted | ionic crosslinking of siRNA to PAMAM | i.v. | HIV | 52 |
| I-tsNP | targeted HA-coated liposomes | CCND1 | LFA-1 | protamine condensation | i.v. | colitis | 57 |
| I-tsNP | targeted HA-coated liposomes | CCR5 | LFA-1 | protamine condensation | i.v. | HIV | 58 |
| tLNPs | LNPs | CD45 | CD4 | complexation with ionizable lipids | i.v. | healthy mice | 71 |
| tLNPs | LNPs | CCND1 | CD38 | complexation with ionizable lipids | i.v. | MCL | 72 |
apt, aptamer; AsiC, aptamer-conjugated siRNA chimera; DsiRNA, dicer-substrate short interfering RNA; HA, hyaluronan; HBV, hepatitis B virus; I-tsNP, integrin-targeted and stabilized nanoparticle; IL, interleukin; i.v., intravenous; IVAG, intravaginal; LNP, lipid nanoparticle; MCL, mantle cell lymphoma; PAMAM, poly(amidoamine); PEI, polyethylenimine; shRNA, short hairpin RNA; siRNA, small interference RNA; tLNP, targeted lipid nanoparticle.
An additional consideration is the conjugation of siRNA to the carrier; non-covalent attachment of siRNA appears to be the preferred option, as it facilitates the interchange of various siRNAs with the same carrier and can therefore be utilized as a platform.35, 52 Such platforms will be easier to translate to the clinic, as they are more robust and cost-effective. Similarly, cationic polymers and lipids also do not require chemical conjugation (as the attachment is charge based); however, they may lead to toxicity and unwanted immune activation.83, 84, 85 Thus, ionizable polymers and lipids may be the best choice for polyelectrolyte complexation with nucleic acids.
Modulation of gene expression in lymphocytes possesses additional challenges. As these cells are immunostimulatory by nature, harmful outcomes such as unwanted immune activation and induction of autoimmunity should be avoided. One methodology is utilizing monovalent as opposed to multivalent targeting moieties, as monovalent targeting (although it possesses lower affinity) is much less likely to lead to lymphocyte activation. This has been shown in both apts and antibodies.58, 86
Additional barriers to the clinic are not lymphocyte specific but are related to the delivery of nucleic acids in general. These barriers and routes to overcome them have been extensively reviewed elsewhere.74
As our understanding regarding lymphocyte biology at different settings deepens, so too do the potential therapies. Recent developments in nanoparticle and conjugate formulation, fueled by this immense therapeutic potential, have led to successful systemic delivery of RNA molecules with reduced toxicity. Eventually, a deeper understanding of lymphocyte biology, better selection of targeting moieties, and improved structure-function relationships of nanoparticle delivery systems could provide great benefit in the delivery of RNAi-based therapeutics to lymphocytes. These advances will later pave the way for the delivery of mRNA and gene editing methods such as the CRISPR-Cas system, in which the challenges for effective delivery are even greater.
Acknowledgments
The authors thank Ms. Varda Wexler for help with the graphics and illustrations. This work was supported in part by grants from the NIH (5R01CA139444-10), Dotan Hematology Center at Tel Aviv University, Lewis Family Trust, William Cohen Trust for Chronic Lymphocytic Leukemia (CLL), Focal Technology Area (FTA): Nanomedicines for Personalized Theranostics of the Israeli National Nanotechnology Initiative, Leona M. and Harry B. Helmsley Nanotechnology Research Fund, Kenneth Rainin Foundation, and European Research Council (ERC) (LeukoTheranostics grant 647410 awarded to D.P.).
References
- 1.Hwang, S.-A. and Actor, J.K. (2014). Lymphocytes. eLS. http://dx.doi.org/10.1002/9780470015902.a0001190.pub2.
- 2.Ramishetti S., Landesman-Milo D., Peer D. Advances in RNAi therapeutic delivery to leukocytes using lipid nanoparticles. J. Drug Target. 2016;24:780–786. doi: 10.3109/1061186X.2016.1172587. [DOI] [PubMed] [Google Scholar]
- 3.Dykxhoorn D.M., Lieberman J. The silent revolution: RNA interference as basic biology, research tool, and therapeutic. Annu. Rev. Med. 2005;56:401–423. doi: 10.1146/annurev.med.56.082103.104606. [DOI] [PubMed] [Google Scholar]
- 4.Lee L., Gupta M., Sahasranaman S. Immune checkpoint inhibitors: an introduction to the next-generation cancer immunotherapy. J. Clin. Pharmacol. 2016;56:157–169. doi: 10.1002/jcph.591. [DOI] [PubMed] [Google Scholar]
- 5.Behlke M.A. Progress towards in vivo use of siRNAs. Mol. Ther. 2006;13:644–670. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peer D., Zhu P., Carman C.V., Lieberman J., Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc. Natl. Acad. Sci. USA. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y. Mammalian RNAi for the masses. Trends Genet. 2003;19:9–12. doi: 10.1016/s0168-9525(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 9.Freeley M., Long A. Advances in siRNA delivery to T-cells: potential clinical applications for inflammatory disease, cancer and infection. Biochem. J. 2013;455:133–147. doi: 10.1042/BJ20130950. [DOI] [PubMed] [Google Scholar]
- 10.Peer D., Lieberman J. Special delivery: targeted therapy with small RNAs. Gene Ther. 2011;18:1127–1133. doi: 10.1038/gt.2011.56. [DOI] [PubMed] [Google Scholar]
- 11.Behlke M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18:305–319. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- 12.Swayze E.E., Siwkowski A.M., Wancewicz E.V., Migawa M.T., Wyrzykiewicz T.K., Hung G., Monia B.P., Bennett C.F. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35:687–700. doi: 10.1093/nar/gkl1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floch V., Le Bolc’h G., Audrézet M.P., Yaouanc J.J., Clément J.C., des Abbayes H., Mercier B., Abgrall J.F., Férec C. Cationic phosphonolipids as non viral vectors for DNA transfection in hematopoietic cell lines and CD34+ cells. Blood Cells Mol. Dis. 1997;23:69–87. doi: 10.1006/bcmd.1997.0123. [DOI] [PubMed] [Google Scholar]
- 14.Harrison G.S., Wang Y., Tomczak J., Hogan C., Shpall E.J., Curiel T.J., Felgner P.L. Optimization of gene transfer using cationic lipids in cell lines and primary human CD4+ and CD34+ hematopoietic cells. Biotechniques. 1995;19:816–823. [PubMed] [Google Scholar]
- 15.Polevaya Y., Ermolina I., Schlesinger M., Ginzburg B.Z., Feldman Y. Time domain dielectric spectroscopy study of human cells. II. Normal and malignant white blood cells. Biochim. Biophys. Acta. 1999;1419:257–271. doi: 10.1016/s0005-2736(99)00072-3. [DOI] [PubMed] [Google Scholar]
- 16.Chiba H., Osanai M., Murata M., Kojima T., Sawada N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta. 2008;1778:588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Ebert O., Finke S., Salahi A., Herrmann M., Trojaneck B., Lefterova P., Wagner E., Kircheis R., Huhn D., Schriever F., Schmidt-Wolf I.G. Lymphocyte apoptosis: induction by gene transfer techniques. Gene Ther. 1997;4:296–302. doi: 10.1038/sj.gt.3300394. [DOI] [PubMed] [Google Scholar]
- 18.Ebert O., Röpke G., Märten A., Lefterova P., Micka B., Buttgereit P., Niemitz S., Trojaneck B., Schmidt-Wolf G., Huhn D. TNF-alpha secretion and apoptosis of lymphocytes mediated by gene transfer. Cytokines Cell. Mol. Ther. 1999;5:165–173. [PubMed] [Google Scholar]
- 19.Jarousse N., Chandran B., Coscoy L. Lack of heparan sulfate expression in B-cell lines: implications for Kaposi’s sarcoma-associated herpesvirus and murine gammaherpesvirus 68 infections. J. Virol. 2008;82:12591–12597. doi: 10.1128/JVI.01167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstein S., Emmanuel R., Jacobi A.M., Abraham A., Behlke M.A., Sprague A.G., Novobrantseva T.I., Nagler A., Peer D. RNA inhibition highlights cyclin D1 as a potential therapeutic target for mantle cell lymphoma. PLoS ONE. 2012;7:e43343. doi: 10.1371/journal.pone.0043343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Petterson T., Månsson A., Riesbeck K., Cardell L.O. Nucleotide-binding and oligomerization domain-like receptors and retinoic acid inducible gene-like receptors in human tonsillar T lymphocytes. Immunology. 2011;133:84–93. doi: 10.1111/j.1365-2567.2011.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberdoerffer P., Kanellopoulou C., Heissmeyer V., Paeper C., Borowski C., Aifantis I., Rao A., Rajewsky K. Efficiency of RNA interference in the mouse hematopoietic system varies between cell types and developmental stages. Mol. Cell. Biol. 2005;25:3896–3905. doi: 10.1128/MCB.25.10.3896-3905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peer D. A daunting task: manipulating leukocyte function with RNAi. Immunol. Rev. 2013;253:185–197. doi: 10.1111/imr.12044. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J., Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov. 2017;16:181–202. doi: 10.1038/nrd.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler L.A., Trifonova R., Vrbanac V., Basar E., McKernan S., Xu Z., Seung E., Deruaz M., Dudek T., Einarsson J.I. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J. Clin. Invest. 2011;121:2401–2412. doi: 10.1172/JCI45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neff C.P., Zhou J., Remling L., Kuruvilla J., Zhang J., Li H., Smith D.D., Swiderski P., Rossi J.J., Akkina R. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Sci. Transl. Med. 2011;3:66ra6. doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J., Satheesan S., Li H., Weinberg M.S., Morris K.V., Burnett J.C., Rossi J.J. Cell-specific RNA aptamer against human CCR5 specifically targets HIV-1 susceptible cells and inhibits HIV-1 infectivity. Chem. Biol. 2015;22:379–390. doi: 10.1016/j.chembiol.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berezhnoy A., Castro I., Levay A., Malek T.R., Gilboa E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J. Clin. Invest. 2014;124:188–197. doi: 10.1172/JCI69856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrmann A., Priceman S.J., Swiderski P., Kujawski M., Xin H., Cherryholmes G.A., Zhang W., Zhang C., Lahtz C., Kowolik C. CTLA4 aptamer delivers STAT3 siRNA to tumor-associated and malignant T cells. J. Clin. Invest. 2014;124:2977–2987. doi: 10.1172/JCI73174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajagopalan A., Berezhnoy A., Schrand B., Puplampu-Dove Y., Gilboa E. Aptamer-targeted attenuation of IL-2 signaling in CD8+ T cells enhances antitumor immunity. Mol. Ther. 2017;25:54–61. doi: 10.1016/j.ymthe.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng B.D., Peer D. Orchestrating a symphony on a single conjugate: aptamer targeting, gene silencing, and immunomodulation to enhance antitumor response. Mol. Ther. 2017;25:5–7. doi: 10.1016/j.ymthe.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soldevilla M.M., Villanueva H., Bendandi M., Inoges S., López-Díaz de Cerio A., Pastor F. 2-fluoro-RNA oligonucleotide CD40 targeted aptamers for the control of B lymphoma and bone-marrow aplasia. Biomaterials. 2015;67:274–285. doi: 10.1016/j.biomaterials.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Pastor F., Kolonias D., Giangrande P.H., Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465:227–230. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J., Neff C.P., Swiderski P., Li H., Smith D.D., Aboellail T., Remling-Mulder L., Akkina R., Rossi J.J. Functional in vivo delivery of multiplexed anti-HIV-1 siRNAs via a chemically synthesized aptamer with a sticky bridge. Mol. Ther. 2013;21:192–200. doi: 10.1038/mt.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J., Shu Y., Guo P., Smith D.D., Rossi J.J. Dual functional RNA nanoparticles containing phi29 motor pRNA and anti-gp120 aptamer for cell-type specific delivery and HIV-1 inhibition. Methods. 2011;54:284–294. doi: 10.1016/j.ymeth.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peer D., Shimaoka M. Systemic siRNA delivery to leukocyte-implicated diseases. Cell Cycle. 2009;8:853–859. doi: 10.4161/cc.8.6.7936. [DOI] [PubMed] [Google Scholar]
- 38.Song E., Zhu P., Lee S.K., Chowdhury D., Kussman S., Dykxhoorn D.M., Feng Y., Palliser D., Weiner D.B., Shankar P. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 39.Kumar P., Ban H.S., Kim S.S., Wu H., Pearson T., Greiner D.L., Laouar A., Yao J., Haridas V., Habiro K. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biragyn A., Bodogai M., Olkhanud P.B., Denny-Brown S.R., Puri N., Ayukawa K., Kanegasaki S., Hogaboam C.M., Wejksza K., Lee-Chang C. Inhibition of lung metastasis by chemokine CCL17-mediated in vivo silencing of genes in CCR4+ Tregs. J. Immunother. 2013;36:258–267. doi: 10.1097/CJI.0b013e318294357c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 42.Petersen H., Fechner P.M., Martin A.L., Kunath K., Stolnik S., Roberts C.J., Fischer D., Davies M.C., Kissel T. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug. Chem. 2002;13:845–854. doi: 10.1021/bc025529v. [DOI] [PubMed] [Google Scholar]
- 43.Landesman-Milo D., Peer D. Transforming nanomedicines from lab scale production to novel clinical modality. Bioconjug. Chem. 2016;27:855–862. doi: 10.1021/acs.bioconjchem.5b00607. [DOI] [PubMed] [Google Scholar]
- 44.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 45.Lungwitz U., Breunig M., Blunk T., Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Lv H., Zhang S., Wang B., Cui S., Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Breunig M., Lungwitz U., Liebl R., Goepferich A. Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc. Natl. Acad. Sci. USA. 2007;104:14454–14459. doi: 10.1073/pnas.0703882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francis S.M., Taylor C.A., Tang T., Liu Z., Zheng Q., Dondero R., Thompson J.E. SNS01-T modulation of eIF5A inhibits B-cell cancer progression and synergizes with bortezomib and lenalidomide. Mol. Ther. 2014;22:1643–1652. doi: 10.1038/mt.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Peng L. Dendrimer nanovectors for siRNA delivery. Methods Mol. Biol. 2016;1364:127–142. doi: 10.1007/978-1-4939-3112-5_11. [DOI] [PubMed] [Google Scholar]
- 50.Biswas S., Torchilin V.P. Dendrimers for siRNA Delivery. Pharmaceuticals (Basel) 2013;6:161–183. doi: 10.3390/ph6020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perisé-Barrios A.J., Jimenez J.L., Dominguez-Soto A., de la Mata F.J., Corbi A.L., Gomez R., Muñoz-Fernandez M.Á. Carbosilane dendrimers as gene delivery agents for the treatment of HIV infection. J. Control Release. 2014;184:51–57. doi: 10.1016/j.jconrel.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J., Neff C.P., Liu X., Zhang J., Li H., Smith D.D., Swiderski P., Aboellail T., Huang Y., Du Q. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 2011;19:2228–2238. doi: 10.1038/mt.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah N.D., Parekh H.S., Steptoe R.J. Asymmetric peptide dendrimers are effective linkers for antibody-mediated delivery of diverse payloads to b cells in vitro and in vivo. Pharm. Res. 2014;31:3150–3160. doi: 10.1007/s11095-014-1408-1. [DOI] [PubMed] [Google Scholar]
- 54.Barenholz Y. Doxil® –the first FDA-approved nano-drug: lessons learned. J. Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Hazan-Halevy I., Landesman-Milo D., Rosenblum D., Mizrahy S., Ng B.D., Peer D. Immunomodulation of hematological malignancies using oligonucleotides based-nanomedicines. J. Control Release. 2016;244:149–156. doi: 10.1016/j.jconrel.2016.07.052. [DOI] [PubMed] [Google Scholar]
- 56.Freeley M., Long A. The two hit hypothesis: an improved method for siRNA-mediated gene silencing in stimulated primary human T cells. J. Immunol. Methods. 2013;396:116–127. doi: 10.1016/j.jim.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Peer D., Park E.J., Morishita Y., Carman C.V., Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S.S., Peer D., Kumar P., Subramanya S., Wu H., Asthana D., Habiro K., Yang Y.G., Manjunath N., Shimaoka M. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol. Ther. 2010;18:370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coelho T., Adams D., Silva A., Lozeron P., Hawkins P.N., Mant T., Perez J., Chiesa J., Warrington S., Tranter E. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 60.Tabernero J., Shapiro G.I., LoRusso P.M., Cervantes A., Schwartz G.K., Weiss G.J., Paz-Ares L., Cho D.C., Infante J.R., Alsina M. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 61.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 62.Leung A.K., Tam Y.Y., Chen S., Hafez I.M., Cullis P.R. Microfluidic mixing: a general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B. 2015;119:8698–8706. doi: 10.1021/acs.jpcb.5b02891. [DOI] [PubMed] [Google Scholar]
- 63.Morrissey D.V., Lockridge J.A., Shaw L., Blanchard K., Jensen K., Breen W., Hartsough K., Machemer L., Radka S., Jadhav V. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 64.Heyes J., Palmer L., Bremner K., MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Control Release. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 65.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., Sah D.W., Stebbing D., Crosley E.J., Yaworski E. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 66.Belliveau N.M., Huft J., Lin P.J., Chen S., Leung A.K., Leaver T.J., Wild A.W., Lee J.B., Taylor R.J., Tam Y.K. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids. 2012;1:e37. doi: 10.1038/mtna.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhigaltsev I.V., Belliveau N., Hafez I., Leung A.K., Huft J., Hansen C., Cullis P.R. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir. 2012;28:3633–3640. doi: 10.1021/la204833h. [DOI] [PubMed] [Google Scholar]
- 68.Akinc A., Querbes W., De S., Qin J., Frank-Kamenetsky M., Jayaprakash K.N., Jayaraman M., Rajeev K.G., Cantley W.L., Dorkin J.R. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Novobrantseva T.I., Borodovsky A., Wong J., Klebanov B., Zafari M., Yucius K., Querbes W., Ge P., Ruda V.M., Milstein S. Systemic RNAi-mediated gene silencing in nonhuman primate and rodent myeloid cells. Mol. Ther. Nucleic Acids. 2012;1:e4. doi: 10.1038/mtna.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmermann T.S., Lee A.C., Akinc A., Bramlage B., Bumcrot D., Fedoruk M.N., Harborth J., Heyes J.A., Jeffs L.B., John M. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 71.Ramishetti S., Kedmi R., Goldsmith M., Leonard F., Sprague A.G., Godin B., Gozin M., Cullis P.R., Dykxhoorn D.M., Peer D. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano. 2015;9:6706–6716. doi: 10.1021/acsnano.5b02796. [DOI] [PubMed] [Google Scholar]
- 72.Weinstein S., Toker I.A., Emmanuel R., Ramishetti S., Hazan-Halevy I., Rosenblum D., Goldsmith M., Abraham A., Benjamini O., Bairey O. Harnessing RNAi-based nanomedicines for therapeutic gene silencing in B-cell malignancies. Proc. Natl. Acad. Sci. USA. 2016;113:E16–E22. doi: 10.1073/pnas.1519273113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwon I.K., Lee S.C., Han B., Park K. Analysis on the current status of targeted drug delivery to tumors. J. Control Release. 2012;164:108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wittrup A., Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet. 2015;16:543–552. doi: 10.1038/nrg3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKinney E.F., Lee J.C., Jayne D.R., Lyons P.A., Smith K.G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McHugh R.S., Whitters M.J., Piccirillo C.A., Young D.A., Shevach E.M., Collins M., Byrne M.C. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 77.Pfoertner S., Jeron A., Probst-Kepper M., Guzman C.A., Hansen W., Westendorf A.M., Toepfer T., Schrader A.J., Franzke A., Buer J., Geffers R. Signatures of human regulatory T cells: an encounter with old friends and new players. Genome Biol. 2006;7:R54. doi: 10.1186/gb-2006-7-7-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hogg N., Patzak I., Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat. Rev. Immunol. 2011;11:416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 79.Alon R., Shulman Z. Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp. Cell Res. 2011;317:632–641. doi: 10.1016/j.yexcr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Ng E.W., Shima D.T., Calias P., Cunningham E.T., Jr., Guyer D.R., Adamis A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 81.Lincoff A.M., Mehran R., Povsic T.J., Zelenkofske S.L., Huang Z., Armstrong P.W., Steg P.G., Bode C., Cohen M.G., Buller C., REGULATE-PCI Investigators Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): a randomised clinical trial. Lancet. 2016;387:349–356. doi: 10.1016/S0140-6736(15)00515-2. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y.K., Kim K.S., Kim J.S., Baek J.E., Park S.I., Jeong H.Y., Yoon S.S., Jung K.C., Song H.G., Park Y.S. Leukemia-specific siRNA delivery by immunonanoplexes consisting of anti-JL1 minibody conjugated to oligo-9 Arg-peptides. Mol. Cells. 2010;29:457–462. doi: 10.1007/s10059-010-0056-5. [DOI] [PubMed] [Google Scholar]
- 83.Knudsen K.B., Northeved H., Kumar P.E., Permin A., Gjetting T., Andresen T.L., Larsen S., Wegener K.M., Lykkesfeldt J., Jantzen K. In vivo toxicity of cationic micelles and liposomes. Nanomedicine (Lond.) 2015;11:467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Elsabahy M., Wooley K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013;42:5552–5576. doi: 10.1039/c3cs60064e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kedmi R., Ben-Arie N., Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31:6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 86.McNamara J.O., Kolonias D., Pastor F., Mittler R.S., Chen L., Giangrande P.H., Sullenger B., Gilboa E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]