Abstract
Background: The autonomic nervous system is reported to be involved in the pathogenesis of vasospastic angina (VSA). Studies based on heart rate variability analysis have shown conflicting results with both a reduction and an enhancement of sympathetic nervous system (SNS) activity in patients with Prinzmetal’s variant angina, but direct assessment has never been performed. The aim of our study was to evaluate the SNS activity using microneurography in patients with VSA. Methods and Results: The SNS was evaluated by measuring the muscle sympathetic nerve activity (MSNA) with microneurography in 15 patients with VSA confirmed by positive ergonovine provocation test and 15 controls subjects negative for the provocation test. Over the baseline period, SNS activity was higher in patients with VSA compared with control patients (56.8 ± 5 vs. 49.3 ± 6.3 burst/min, p < 0.001, respectively). During mental stress, SNS activity increased significantly only in patients with VSA, which still presented a higher SNS activity than control patients (66.1 ± 7.2 vs. 53.6 ± 8.7 burst/min; p < 0.001, respectively). Furthermore only VSA patients showed significant hemodynamic modifications with an increase in mean arterial blood pressure (96.2 ± 13.4 vs. 86.6 ± 9.6 mmHg in VSA patients and control subjects, respectively; p < 0.05). Conclusion: Our results provide the first direct evidence of lasting increased sympathetic activity that is worsened by mental stress in patients with VSA. These results suggest that SNS participate to the pathogenesis of VSA by enhancing coronary vascular tone.
Keywords: Coronary artery spasm, vasospastic angina, heart rate variability, muscle sympathetic nerve activity, sympathetic nervous system
Introduction
Coronary artery vasospasm is a recognized cause of angina pectoris that can have various clinical forms such as myocardial infarction, ventricular arrhythmias and sudden cardiac death. In Prinzmetal’s variant angina, attacks usually occur between midnight and early morning and lead to a transient ST segment elevation on electrocardiogram. Its pathogenesis is essentially different from other forms of angina pectoris: myocardial ischemia is a consequence of an excessive contraction of an epicardial coronary artery and not of an occlusive atherothrombotic lesion [1]. But beyond differences in clinical presentation, there is a continuum between atherosclerosis and coronary artery spasm and both affections share common risk factors [2]. Studies using intravascular ultrasound (IVUS) have shown that vasospasm occurs mostly in locations consistent with atherosclerotic lesions even in the absence of significant narrowing on angiographic findings [3]. Coronary artery spasm (CAS) is known to be involved in many ischemic heart diseases and cannot be summarized by only Prinzmetal’s variant angina. The precise mechanism for coronary vasospasm has not been elucidated yet but important findings have been made regarding its pathogenesis [4]. Different systems are involved in the regulation of vascular tone. Among them, endothelium dysfunction plays an important role. It is characterized by an impairment of endothelium-dependent relaxation with reduced eNOS derived nitric oxide bioavailability and appears to be a consequence of atherosclerosis. Hyper contractility of vascular smooth muscle cells is also involved. Recent findings in spastic arteries showed that abnormal contraction of smooth muscle cells was linked to a biochemical disorder with an increased activity of small GTPase, RhoA’s downstream effector that leads to an inhibition of myosin light chain phosphatase [5]. The autonomic nervous system is a key regulator of vascular tone and blood pressure. Because CAS occurs mostly at night or during mild exercise in the early morning, it has long been suspected that changes in autonomic nervous system activity may be related to these circadian variations of ischemic episodes. Using subcutaneous injection of metacholine, Yasue et al first showed that enhanced parasympathetic nervous activity was involved in the initiation of the attack [6]. These results were supported when they further showed that intracoronary injection of acetylcholine, the main neurotransmitter in the parasympathetic nervous system, could trigger a coronary spasm [7]. Using heart rate variability (HRV) analysis on Holter ECG-recordings, some authors have reported acute modifications of the autonomic nervous system activity in patients suffering from vasospastic angina (VSA) with sympatho-vagal imbalance minutes before an attack occurs [8]. Acute sympathetic hyperactivity has been described but contradictory data also suggested a paroxysmal reduction of sympathetic activity [9]. Beside these transient modifications, abnormal circadian variations of autonomic nervous system activity during symptom-free periods have been established [10,11] and long lasting abnormalities of the cardiac regional sympathetic nervous system assessed by scintigraphy are associated with CAS [12,13].
The aim of our study was to investigate lasting changes of sympathetic activity in patients with proven VSA by performing a direct measurement of sympathetic nervous system (SNS) activity using the gold standard method of microneurography.
Methods
Population
Fifteen consecutive patients with VSA confirmed by positive ergonovine provocation test were prospectively included and compared with 15 control patients with negative ergonovine provocation test matched for age, sex and BMI. All patients were admitted for evaluation of spontaneous chest pain at rest with or without ECG modification or troponin elevation, but with no significant stenosis in coronary artery on angiography (> 50%). All patients had standard blood tests and echocardiography. Patients with congestive heart failure, cardiomyopathy, history of ventricular arrhythmia, history of cardiogenic shock and chronic renal failure were excluded of the study.
Provocation protocol
All vasoactive drugs (including ß-adrenergic blockers, calcium channel blockers and other vasodilators) were discontinued 48 hours before angiography. All procedures were performed by transfemoral approach to avoid vasodilator injection. Methylergometrin (0.4 mg) was given intravenously. A positive provocation test result was defined by a transient, total or sub-total occlusion (> 90% stenosis) of a coronary artery with signs and/or symptoms of myocardial ischemia (anginal pain and ischemic ST changes) [14].
Microneurography measurements
Heart rate was continuously measured by an electrocardiogram (ADInstruments, Castle Hill, New South Wales, Australia). Blood pressure was continuously measured by the Finometer system (Finometer; Finapress Medical System BV, Amsterdam, The Netherlands). Oxygen saturation was monitored with a pulse oximeter (AD Instruments). Multiunit postganglionic sympathetic nerve activity was recorded as previously described [15] by the same operator, blinded for the results of ergonovine provocation test. Briefly, a tungsten microelectrode (shaft diameter 200 μm, tapering to an uninsulated tip of 1-5 μm) was inserted selectively into muscle or skin fascicles of the peroneal nerve and a subcutaneous reference electrode was inserted 2-3 cm away from the recording electrode. The neural signal was amplified, filtered, rectified and integrated to obtain a voltage display of sympathetic nerve activity. Sympathetic bursts were identified by inspection of the voltage neurogram and the amplitude of each burst was determined. Sympathetic activity was calculated as bursts per minute. The intralaboratory reproducibility of microneurography has been assessed previously. All recordings were made in the morning at least 24 hours after the angiogram has been performed and in an electrically shielded room. Patients were studied in the supine position under carefully standardized conditions (empty stomach, no mediation taken in the morning and no smoking for at least 48 hours). Baselines of all parameters were recorded for 15 min. After this period, all patients underwent an arithmetic mental stress test. One investigator directed the interventions, which required patients to continuously subtract the number six or seven from a three-digit number. Subjects were verbally encouraged by a second investigator, while the first investigator changed the number to subtract from every 10 seconds. MSNA was then measured during 3 minutes as described by Durocher et al [16].
Statistical analysis
Comparison of quantitative variables for each group was performed with an unpaired Student t test. The chi-square test was used to compare qualitative variables. The p values for differences within a session were obtained by post-hoc tests. Quantitative variables are presented as mean ± SD (standard deviation) and qualitative variables using numbers and percentages. A p value < 0.05 was considered significant. The data were analyzed with PASW statistics version 20.0 (SPSS, Chicago, Illinois).
This study complies with the Declaration of Helsinki and our Institutional Ethics Committee approved the study protocol. Informed consent from all participants was obtained before the initial coronary angiography.
Results
The clinical characteristics of the patients are presented in Table 1. The demographic characteristics, cardiovascular risk factors, standard blood tests results and left ventricular ejection fraction did not differ between the groups. VSA patients presented more frequently with recurring angina and chest pain was more frequently relieved by nitrates (Table 2). During the provocation test, in patients with VSA, the coronary spasm occurred on the left anterior descending artery in 5 (33%) patients, on the right coronary artery in 5 (33%) patients, on the left circumflex artery in 3 (20%) patients, on a diagonal branch in one (7%) patient and on the left main in one (7%) patient (Table 3). During the baseline period, peripheral blood pressure, heart rate, oxygen saturation and respiratory rate did not differ significantly between the groups (Table 4) but baseline MSNA was significantly increased in patients with VSA compared to control subjects (56.8 ± 5 vs. 49.3 ± 6.3 burst/min; p < 0.001, respectively; Figure 1). In control patients, the mental stress test did not produce significant modification in heart rate, oxygen saturation, blood pressure, respiratory rate or MSNA (49.3 ± 6.3 vs. 53.6 ± 8.7 burst/min; p = 0.128, before and after mental stress, respectively). In patients with VSA, mental stress test produced a significant increase in MSNA (56.8 ± 5 vs. 66.1 ± 7.2 burst/min; p < 0.001, before and after mental stress, respectively) and in mean blood pressure (91 ± 13.6 vs. 96.2 ± 13.4 mmHg; p < 0.05, before and after mental stress, respectively). During mental stress, MSNA remained significantly higher in patients with VSA compared to control subjects (66.1 ± 7.2 vs. 53.6 ± 8.7 burst/min, respectively; p < 0.001; Figure 1).
Table 1.
Patients’ clinical characteristics
| Non-VSA (n = 15) | VSA (n = 15) | P | |
|---|---|---|---|
| Male, n (%) | 9 (60) | 9 (60) | 1 |
| BMI (kg/m2) | 24.5 ± 3.9 | 25.3 ± 3.6 | 0.563 |
| Hypertension, n (%) | 5 (33) | 6 (40) | 0.705 |
| Diabetes mellitus, n (%) | 4 (27) | 1 (7) | 0.142 |
| Dyslipidemia, n (%) | 7 (47) | 7 (47) | 1 |
| Current smoking, n (%) | 8 (53) | 6 (40) | 0.464 |
| Past smoking, n (%) | 10 (67) | 14 (93) | 0.068 |
| Serum creatinine (umol/L) | 73.7 ± 16.3 | 74.4 ± 15.2 | 0.916 |
| Total cholesterol (mmol/L) | 4.5 ± 0.7 | 4.6 ± 0.7 | 0.741 |
| LDL-cholesterol (mmol/L) | 2.6 ± 0.8 | 2.6 ± 0.6 | 0.948 |
| HDL-cholesterol (mmol/L) | 1.2 ± 0.2 | 1.1 ± 0.3 | 0.556 |
| Hemoglobin (g/dL) | 13.4 ± 2.1 | 13.5 ± 1 | 0.852 |
| LVEF (%) | 58.5 ± 9.5 | 58.7 ± 6.4 | 0.964 |
Data given as mean ± SD, n (%). VSA: vasospastic angina; BMI: body mass index; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction.
Table 2.
Characteristics of angina
| Non-VSA (n = 15) | VSA (n = 15) | P | |
|---|---|---|---|
| Multiple episodes | 7 (47) | 11 (73) | 0.136 |
| Resting chest pain | 15 (100) | 15 (100) | NS |
| Effort chest pain | 6 (40) | 6 (40) | NS |
| Dynamic ST changes | 6 (40) | 6 (40) | NS |
| STEMI | 4 (27) | 3 (20) | NS |
| T wave inversion | 2 (13) | 3 (20) | NS |
| Troponin elevation | 8 (53) | 5 (33) | 0.431 |
| Relieved by nitrates | 2 (13) | 6 (40) | 0.053 |
Values are n (%). VSA: vasospastic angina; STEMI: ST-elevation myocardial infarction.
Table 3.
Angiographic characteristics of VSA patients
| Site of spasm | n (%) |
|---|---|
| RCA | 5 (36) |
| Diagonal branch | 1 (7) |
| LAD | 5 (36) |
| Left main | 1 (7) |
| LCX | 3 (21) |
| Associated signs | |
| ST elevation | 15 (100) |
| Angina | 15 (100) |
Values are n (%). RCA: right coronary artery; LAD: left anterior descending artery; LCX: left circumflex artery.
Table 4.
Hemodynamic parameters and MSNA during baseline and mental stress
| Non-VSA (n = 15) | VSA (n = 15) | |||
|---|---|---|---|---|
|
| ||||
| Baseline | Mental stress | Baseline | Mental stress | |
| Diastolic blood pressure (mmHg) | 67.7 ± 13.8 | 65.9 ± 8 | 69.3 ± 10.9 | 73.5 ± 10.3* |
| Mean blood pressure (mmHg) | 88.9 ± 16.3 | 89.6 ± 9.6 (+ 0.7%) | 91 ± 13.6 | 96.2 ± 13.4* (+ 5%) |
| Heart rate (beats/min) | 67.1 ± 10.2 | 71.3 ± 10.6 | 66.8 ± 10.5 | 71.8 ± 11.5 |
| Respiratory rate (cycles/min) | 18.1 ± 3.4 | 17.1 ± 4.1 | 18.2 ± 3.8 | 16.2 ± 4.4 |
| Oxygen saturation (%) | 95.5 ± 2.3 | 96.7 ± 2.8 | 96.9 ± 1.3 | 97.7 ± 1.5 |
| MSNA (bursts/min) | 49.3 ± 6.3 | 53.6 ± 8.7 (+ 8.7%) | 56.8 ± 5** | 66.1 ± 7.2**,† (+ 16.4%) |
Values represent average during the recording period. Values are mean ± SD. VSA, vasospastic angina; MSNA, muscle sympathetic nerve activity.
p < 0.05 vs. non-VSA group;
p < 0.001 vs. non-VSA group;
p < 0.001 vs. baseline period.
Figure 1.
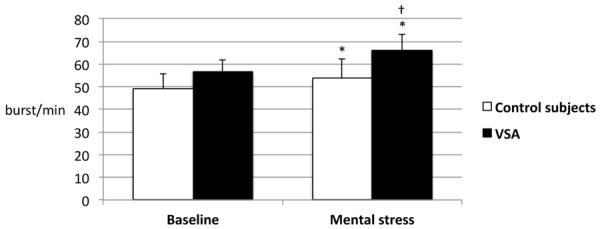
MSNA at baseline and during mental stress in control and VSA patients. MSNA is increased in VSA patients in comparison to control patients at baseline (56.8 ± 5 vs. 49.3 ± 6.3 burst/min, p < 0.001) and during mental stress (66.1 ± 7.2 vs. 53.6 ± 8.7 burst/min, p < 0.001). The increase in SNS activity during mental stress is significant only in VSA patients (p < 0.001). MSNA: muscle sympathetic nerve activity; VSA: vasospastic angina. *p < 0.001 vs. control subjects; †p < 0.001 vs. baseline.
Discussion
Our study provides the first direct evidence of SNS hyperactivity in patients with VSA. MSNA is the gold standard method for measuring the sympathetic nervous tone. HRV has been favored in previous studies because it is an easier and less-invasive technique and allows a dynamic evaluation of the autonomic nervous system activity. However, the sympathetic contribution to HRV is more indicative of the strength of modulation of the autonomic outflow than the intensity of cardiac sympathetic nerve traffic. That is why HRV does not correlate well with other measures of regional or global sympathetic outflow and is highly influenced by hemodynamic conditions and vasoactive drugs routinely used in patients treated for VSA [17,18]. As a consequence, discordant results were obtained in studies using HRV to assess sympathetic nervous system activity. Modulation of the autonomic nervous system is considered to be one of the main mechanisms for VSA [19]. Many triggering stimuli for angina attacks have been proposed and vagal withdrawal can contribute to spontaneous CAS. However HRV is not sufficiently sensitive to detect moderate changes and few studies have tried to evaluate autonomic nervous system activity during the time free of angina attacks. Miwa et al [10] showed a blunted circadian rhythm of sympatho-vagal balance and Watanabe et al [11] further showed that sympathetic nervous activity was enhanced in the nighttime but only in patients with multivessel spasm. So far, persistent sympathetic hyperactivity has been considered only for patients with particularly severe VSA. In the present study, none of the VSA patients had multivessel spasm on provocation testing. Despite this less severe form of VSA, we found a significant increase in SNS activity in all patients. MSNA is the best technique to evaluate the cardiac sympathetic activity as it is directly related to cardiac norepinephrine spillover [20]. By using microneurography we can provide direct proof of lasting sympathetic hyperactivity during daytime in patients with confirmed VSA. Baseline release of neurotransmitters from the sympathetic nervous system has a direct effect on vascular tone. It can lead to platelet activation and release of serotonin, which can act as a potent coronary constrictor [21]. Recently, using optical coherence tomography (OCT), Tanaka et al showed that abnormal medial contraction was already present when the artery was in an asymptomatic state [22]. This increased vascular tone could be a consequence of a cardiac sympathetic nervous hyperactivity. The mental stress has sometimes been reported as a potential inducer of VSA [23]. Thus its sensitivity for VSA diagnosis is poor compared with pharmacological provocation tests such as ergonovine or acethylcholine and is not sufficient to be routinely used in clinical practice [14,24]. Here only VSA patients’ MSNA and blood pressure increased significantly during mental. This could be an indication that patients with VSA are prone to exaggerated an increase of SNS activity when exposed to physiological stress. Two phenomena can be distinguished: acute modifications in the minutes preceding the crisis and lasting changes in autonomic nervous system function. Our results confirmed lasting changes in the autonomic nervous system, favorable to sympathetic hyperactivity. Consequently, our results suggest that the use of drugs that decrease SNS tone could be helpful for the treatment of patients with VSA.
Study limitations
Simultaneous assessment of parasympathetic nervous system activity is not possible with a direct method like MSNA. Vagal activity modifications could also occur and affect global autonomic nervous system balance in patients with VSA.
Conclusions
Our findings show, for the first time, an increase in SNS activity assessed by microneurography in patients with VSA that worsens during mental stress test. Sympathetic hyperactivity could affect cardiovascular regulation and be responsible for smooth muscle cells hypercontraction and increased vascular coronary tone observable in periods free of symptoms. Under specific conditions, a physiological stress could further increase SNS activity and vagal withdrawal could upset the balance of the autonomic nervous system. This could be an important element of pathogenesis by increasing vascular tone and predisposing to CAS.
Disclosure of conflict of interest
None.
Abbreviations
- BMI
Body mass index
- CAS
Coronary artery spasm
- HRV
Heart rate variability
- HDL-C
High-density lipoprotein cholesterol
- IVUS
Intravascular ultrasound
- LAD
Left anterior descending artery
- LCX
Left circumflex artery
- LVEF
Left ventricular ejection fraction
- LDL-C
Low-density lipoprotein cholesterol
- MSNA
Muscle sympathetic nerve activity
- OCT
Optical coherence tomography
- RCA
Right coronary artery
- STEMI
ST-elevation myocardial infarction
- SNS
Sympathetic nervous system
- VSA
Vasospastic angina
References
- 1.Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 2.Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2–17. doi: 10.1016/j.jjcc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Yamagishi M, Miyatake K, Tamai J, Nakatani S, Koyama J, Nissen SE. Intravascular ultrasound detection of atherosclerosis at the site of focal vasospasm in angiographically normal or minimally narrowed coronary segments. J Am Coll Cardiol. 1994;23:352–357. doi: 10.1016/0735-1097(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 4.Yoo SY, Kim JY. Recent insights into the mechanisms of vasospastic angina. Korean Circ J. 2009;39:505–511. doi: 10.4070/kcj.2009.39.12.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsumata N, Shimokawa H, Seto M, Kozai T, Yamawaki T, Kuwata K, Egashira K, Ikegaki I, Asano T, Sasaki Y, Takeshita A. Enhanced myosin light chain phosphorylations as a central mechanism for coronary artery spasm in a swine model with interleukin-1beta. Circulation. 1997;96:4357–4363. doi: 10.1161/01.cir.96.12.4357. [DOI] [PubMed] [Google Scholar]
- 6.Yasue H, Touyama M, Shimamoto M, Kato H, Tanaka S. Role of autonomic nervous system in the pathogenesis of Prinzmetal’s variant form of angina. Circulation. 1974;50:534–539. doi: 10.1161/01.cir.50.3.534. [DOI] [PubMed] [Google Scholar]
- 7.Yasue H, Horio Y, Nakamura N, Fujii H, Imoto N, Sonoda R, Kugiyama K, Obata K, Morikami Y, Kimura T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986;74:955–963. doi: 10.1161/01.cir.74.5.955. [DOI] [PubMed] [Google Scholar]
- 8.Inazumi T, Shimizu H, Mine T, Iwasaki T. Changes in autonomic nervous activity prior to spontaneous coronary spasm in patients with variant angina. Jpn Circ J. 2000;64:197–201. doi: 10.1253/jcj.64.197. [DOI] [PubMed] [Google Scholar]
- 9.Takusagawa M, Komori S, Umetani K, Ishihara T, Sawanobori T, Kohno I, Sano S, Yin D, Ijiri H, Tamura K. Alterations of autonomic nervous activity in recurrence of variant angina. Heart. 1999;82:75–81. doi: 10.1136/hrt.82.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miwa K, Igawa A, Miyagi Y, Nakagawa K, Inoue H. Alterations of autonomic nervous activity preceding nocturnal variant angina: sympathetic augmentation with parasympathetic impairment. Am Heart J. 1998;135:762–771. doi: 10.1016/s0002-8703(98)70034-1. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Kim S, Akishita M, Kario K, Sekiguchi H, Fujikawa H, Mitsuhashi T, Ouchi Y, Shimada K. Circadian variation of autonomic nervous activity in patients with multivessel coronary spasm. Jpn Circ J. 2001;65:593–598. doi: 10.1253/jcj.65.593. [DOI] [PubMed] [Google Scholar]
- 12.Inobe Y, Kugiyama K, Miyagi H, Ohgushi M, Tomiguchi S, Takahashi M, Yasue H. Longlasting abnormalities in cardiac sympathetic nervous system in patients with coronary spastic angina: quantitative analysis with iodine 123 metaiodobenzylguanidine myocardial scintigraphy. Am Heart J. 1997;134:112–118. doi: 10.1016/s0002-8703(97)70114-5. [DOI] [PubMed] [Google Scholar]
- 13.Sakata K, Miura F, Sugino H, Saegusa T, Shirotani M, Yoshida H, Hoshino T, Kurata C. Assessment of regional sympathetic nerve activity in vasospastic angina: analysis of iodine 123-labeled metaiodobenzylguanidine scintigraphy. Am Heart J. 1997;133:484–489. doi: 10.1016/s0002-8703(97)70199-6. [DOI] [PubMed] [Google Scholar]
- 14.JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010;74:1745–1762. doi: 10.1253/circj.cj-10-74-0802. [DOI] [PubMed] [Google Scholar]
- 15.Pathak A, Velez-Roa S, Xhaet O, Najem B, van de Borne P. Dose-dependent effect of dobutamine on chemoreflex activity in healthy volunteers. Br J Clin Pharmacol. 2006;62:272–279. doi: 10.1111/j.1365-2125.2006.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durocher JJ, Schwartz CE, Carter JR. Sympathetic neural responses to mental stress during acute simulated microgravity. J Appl Physiol (1985) 2009;107:518–522. doi: 10.1152/japplphysiol.00284.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notarius CF, Butler GC, Ando S, Pollard MJ, Senn BL, Floras JS. Dissociation between microneurographic and heart rate variability estimates of sympathetic tone in normal subjects and patients with heart failure. Clin Sci (Lond) 1999;96:557–565. [PubMed] [Google Scholar]
- 18.Notarius CF, Floras JS. Limitations of the use of spectral analysis of heart rate variability for the estimation of cardiac sympathetic activity in heart failure. Europace. 2001;3:29–38. doi: 10.1053/eupc.2000.0136. [DOI] [PubMed] [Google Scholar]
- 19.Lanza GA, Pedrotti P, Pasceri V, Lucente M, Crea F, Maseri A. Autonomic changes associated with spontaneous coronary spasm in patients with variant angina. J Am Coll Cardiol. 1996;28:1249–1256. doi: 10.1016/S0735-1097(96)00309-9. [DOI] [PubMed] [Google Scholar]
- 20.Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- 21.Larsson PT, Wallen NH, Hjemdahl P. Norepinephrine-induced human platelet activation in vivo is only partly counteracted by aspirin. Circulation. 1994;89:1951–1957. doi: 10.1161/01.cir.89.5.1951. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka A, Shimada K, Tearney GJ, Kitabata H, Taguchi H, Fukuda S, Kashiwagi M, Kubo T, Takarada S, Hirata K, Mizukoshi M, Yoshikawa J, Bouma BE, Akasaka T. Conformational change in coronary artery structure assessed by optical coherence tomography in patients with vasospastic angina. J Am Coll Cardiol. 2011;58:1608–1613. doi: 10.1016/j.jacc.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida K, Utsunomiya T, Morooka T, Yazawa M, Kido K, Ogawa T, Ryu T, Ogata T, Tsuji S, Tokushima T, Matsuo S. Mental stress test is an effective inducer of vasospastic angina pectoris: comparison with cold pressor, hyperventilation and master two-step exercise test. Int J Cardiol. 1999;70:155–163. doi: 10.1016/s0167-5273(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 24.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]


