Abstract
Objective: Spontaneous intracranial hypotension (SIH) is recognized far more commonly than ever before. Though usually characterized by low cerebrospinal fluid (CSF) pressure, some patients with SIH are observed to have normal pressure values. In this study, we aimed to confirm the proportion of patients with normal CSF opening pressure (CSF OP) and explore the factors affecting CSF OP in SIH patients. Methods: We retrospectively reviewed 206 consecutive SIH patients and analyzed their clinical and imaging variables (including demographic data, body mass index (BMI), duration of symptoms, and brain imaging findings). Univariate and multivariate analyses were performed to identify the potential factors affecting CSF OP. Results: In a total of 114 (55.3%) cases the CSF OP was ≤60 mmH2O (1 mmH2O=9.806 65 Pa), in 90 (43.7%) cases it was between 60 and 200 mmH2O, and in 2 (1.0%) cases it was >200 mmH2O. Univariate analysis showed that the duration of symptoms (P<0.001), BMI (P<0.001), and age (P=0.024) were positively correlated with CSF OP. However, multivariate analysis suggested that only the duration of symptoms (P<0.001) and BMI (P<0.001) were strongly correlated with CSF OP. A relatively high R 2 of 0.681 was obtained for the multivariate model. Conclusions: Our study indicated that in patients without a low CSF OP, a diagnosis of SIH should not be excluded. BMI and the duration of symptoms can influence CSF OP in SIH patients, and other potential factors need further investigation.
Keywords: Spontaneous intracranial hypotension, Low cerebrospinal fluid opening pressure, Body mass index, Magnetic resonance imaging
1. Introduction
Spontaneous intracranial hypotension (SIH), once regarded as a rare disorder, is becoming far more commonly recognized (Schievink, 2006; 2008; Mokri, 2013). It is characterized by an orthostatic headache, which worsens with standing and improves after lying down. Though commonly believed to be the key feature of this disease, low cerebrospinal fluid (CSF) pressure (≤60 mmH2O; 1 mmH2O=9.806 65 Pa) is not always present. According to some previous reports (Mokri et al., 1998; Schievink, 2006; Mokri, 2013), quite a few SIH patients show normal CSF pressure. This led Mokri (1999) to suggest that CSF hypovolemia rather than intracranial hypotension was the leading cause of the syndrome. The estimated prevalence of a normal CSF pressure in SIH patients is about 25% according to previous studies (Mokri et al., 1998; Mokri, 2013). However, most of these studies related to case series with a limited sample size, and the factors affecting CSF pressure in SIH patients were not fully investigated. Kranz et al. (2015) reviewed 106 patients with SIH and found that only 34% had a CSF opening pressure (CSF OP) of ≤60 mmH2O. They observed that abdominal circumference (P<0.001), symptom duration (P=0.015), and the absence of magnetic resonance imaging (MRI) findings of SIH (P=0.003) were associated with increased CSF pressure, but CSF pressure varied greatly among all patients.
Herein, we present a retrospective analysis of 206 cases in our hospital, to confirm the proportion of patients with normal CSF OP. We analyzed the clinical and radiographic characteristics of the cases to find a possible explanation for the normal CSF OP in some SIH patients and to identify the factors that may affect CSF OP in SIH patients.
2. Methods
2.1. Patients
A total of 251 cases were diagnosed as SIH at our hospital (Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China) from January 2011 to December 2015. Based on the International Classification of Headache Disorders, 2nd Edition (ICHD-2) and modified by Schievink et al. (2011), the inclusion criteria are presented in Table 1.
Table 1.
Diagnostic criteria for spontaneous intracranial hypotension
| No. | Inclusion criteria |
| 1 | Orthostatic headache |
| 2 | The presence of at least one of the following: (1) Low opening pressure (≤60 mmH2O) (2) Sustained improvement of symptoms after epidural blood patching (EBP) (3) Demonstration of an active spinal cerebrospinal fluid leak (4) Cranial magnetic resonance imaging changes of intracranial hypotension (such as brain sagging or pachymeningeal enhancement) |
| 3 | No recent history of dural puncture |
| 4 | Symptoms not attributable to another disorder |
For inclusion, all the cases were required to have an orthostatic headache at some point during the clinical course. The headache occurs upon assuming an upright posture and resolves in recumbency. The latency of its onset or resolution from change in posture can vary greatly among cases (Mokri, 2013). Exclusion criteria included: incomplete medical records (such as absence of CSF OP data), history of epidural blood patch treatment, and history of any diseases affecting CSF OP (Table 2). Patients who recovered well after consultation, with or without prior treatment, were also excluded. Finally, 206 cases met the requirements. Age, gender, body mass index (BMI), and duration of symptoms were recorded. There were 145 females and 61 males, with a mean age of (40.1±9.8) years (range, 21 to 68 years). BMI data were available for 163 (79%) cases. The mean BMI was (21.7±2.9) kg/m2, and ranged from 16.4 to 29.8 kg/m2. The mean duration of symptoms was (37.1±24.8) d, and ranged from 6 to 180 d. This study was approved by the Medical Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. All patients had given written informed consent and written permission for publication of their medical records.
Table 2.
Diseases affecting cerebrospinal fluid (CSF) pressure
| Effect | Disease |
| Increasing CSF pressure | Various diseases that may cause brain edema, such as brain injury, brain surgery, cerebrovascular accident, brain tumor |
| Various diseases that may cause increased cerebral blood flow, such as cerebrovascular expansion after severe chest crush injury | |
| Various diseases that may cause increased hydrocephalus, such as choroid plexus papilloma, tumor compression or inflammation | |
| Intracranial space-occupying lesions, such as tumor, abscess, granuloma | |
| Narrowing of the cranial cavity | |
| Decreasing CSF pressure (secondary) | After epidural or lumbar puncture |
| Head and neck trauma and surgery | |
| Ventricular shunting | |
| Spinal trauma or operation | |
| Decreased generation of CSF caused by dehydration, ketoacidosis, uremia, severe systemic infections, meningoencephalitis |
2.2. Radiographic findings
All included patients received a cranial computed tomography (CT) scan on admission. The scan was regarded as the first choice to diagnose subdural hematoma (SDH), which usually appeares as a crescent-shaped, isodense, or hyperdense hemispheric collection. Thirty-eight (18.4%) cases were accompanied by SDH, of which 34 developed bilateral hematomas and 4 developed unilateral hematomas. Gadolinium (Gd)-enhanced MRI was performed on all patients. Dural enhancement, presented as smooth and diffuse enhancement, was assessed on axial or coronal T1-weighted images. Brain sagging, defined as downward sloping of the third ventricular floor or descent of the mammillary bodies to the level of the dorsum sellae, was evaluated on midsagittal T1-weighted images (Kranz et al., 2016). Venous distension sign (VDS) was judged on sagittal T1-weighted images. Occurrence of one or more abnormalities was deemed as a positive MRI. The total positive rate of MRI was 83.5% (172/206). Diffuse dural enhancement was most prevalent (82% of cases), followed by brain sagging (28%). Of those cases with positive MRI findings, 136 showed more than two kinds of abnormalities, while the remaining 36 showed only diffuse dural enhancement. A total of 34 (16.5%) cases showed no abnormalities on MRI scans, and were considered to be negative. Seventy-one cases received computed tomographic myelography (CTM), while the remaining 135 cases received magnetic resonance myelography (MRM) for detecting leaking sites. One or multiple simultaneous CSF leakage lesions were found in a total of 193 (93.7%) cases. The leakage presented either as a small collection of contrast limited to one single nerve root or widespread bilateral collections in the paraspinal soft tissues (Schievink, 2006; Limaye et al., 2016). All images were reviewed by two board-certified neuroradiologists who each had more than 10 years of experience.
2.3. Lumbar puncture and CSF analysis
Apart from locating leaking sites, CTM/MRM also provided an opportunity to detect CSF OP during dural puncture. The lumbar puncture was conducted by two attending neurologists each with more than five years’ experience. After local anesthesia with 2% lidocaine, a 22-gauge spinal needle was selected and inserted at L3–4 or L4–5 level. The needle was advanced slowly through the spinous ligaments. A “pop” was regarded as signifying penetration through the dura into the subarachnoid space. The stylet could then be removed and CSF could be seen at the hub, flowing out freely. At this point, the CSF OP could be measured, and 2 ml of CSF was collected for further examination. Sometimes, there was a sucking sound from the needle, which indicated a negative CSF pressure. If there was no liquid leakage and no sucking sound, a Stookey test was performed to ensure the correct location. All patients remained in the left lateral decubitus position during the puncture and were asked to extend their legs and relax themselves during the measurement.
CSF was obtained in all except nine cases with so-called “dry taps”. All those with available CSF were subjected to multiple CSF examinations (including routine biochemistry, cytology, and bacteriology). The CSF was typically clear and colorless, but a small number of cases showed blood-tinged fluid. The erythrocyte count was either normal or high, and was quite high in those with blood-tinged CSF. This is thought to be caused by traumatic taps and the dilated epidural venous plexus in SIH patients (Mokri, 2013). The protein concentration was also normal or high, and in two cases was above 1500 mg/L. Normal hydrostatic and oncotic pressure between arachnoid villi and the venous sinus is disrupted by lowered CSF OP, leading to the passage of serum protein into the CSF (Rando and Fishman, 1992). A moderate lymphocytic pleocytosis was found in about half of the cases. Hydrostatic pressure changes are thought to bring about this reactive phenomenon (Rando and Fishman, 1992). The glucose concentration, cytology, and bacteriology of the CSF samples were all normal.
2.4. Statistical analysis
SPSS 20.0 software was employed for statistical analyses. In univariate analysis, two-sample t-tests were used to assess the relationship between categorical variables (gender, MRI findings of SIH, active leakage, and SDH) and CSF OP. Simple linear regression analysis was chosen to evaluate the impact of continuous variables (age, BMI, and duration of symptoms) on CSF OP. Data for the duration of symptoms, which showed a skewed distribution, were log-transformed before analysis. In multivariate analysis, a multivariate linear regression model was used to evaluate the joint effect of the seven univariate factors on CSF OP. P-values of less than 0.05 were considered statistically significant.
3. Results
3.1. Distribution of CSF OP
The mean CSF OP was (65±52) mmH2O (ranging from 0 to 230 mmH2O). A CSF OP of ≤60 mmH2O was found in a total of 114 (55.3%) cases, and was too low to be measured in 23 cases, which we regarded as 0 mmH2O. Among the remaining 92 (44.7%) cases, 90 (43.7%) had a CSF OP between 60 and 200 mmH2O, while 2 (1.0%) had a CSF OP of >200 mmH2O. Fig. 1 shows the distribution of CSF OP in all cases. Fig. 2 shows the radiographic characteristics of a patient who presented with a CSF OP of 220 mmH2O.
Fig. 1.
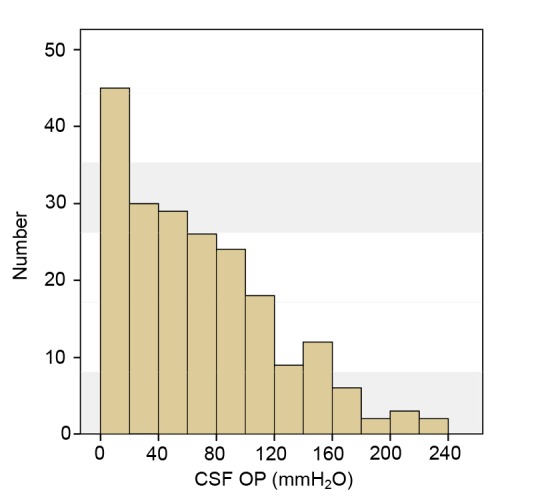
Histogram of CSF opening pressure in SIH patients
Distribution of CSF opening pressure (OP) based on a bin size of 20 mmH2O
Fig. 2.
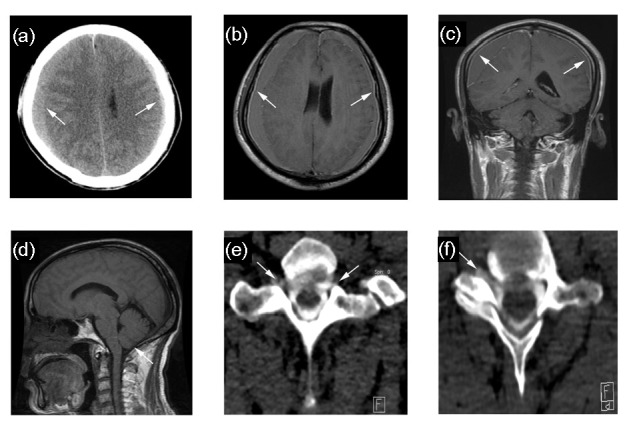
Case of a 60-year-old male, presenting with a CSF opening pressure of 220 mmH2O
(a) Axial CT image shows bilateral subdural hematomas along the frontotemporoparietal area (white arrows) and decrease in size of the ventricles. Axial (b) and coronal (c) T1-weighted MR images with gadolinium enhancement show diffuse dural enhancement (white arrows). (d) Midsagittal T1-weighted MR image show downward displacement of the cerebellar tonsil (white arrows). (e, f) CT myelographs show contrast tracking along bilateral and unilateral nerve roots, respectively (white arrows). Images were taken at segments T1-2 (e) and C6-7 (f)
3.2. Univariate analysis
Univariate analysis was adopted to identify factors that may have been affecting CSF pressure (Figs. 3 and 4). Age (P=0.024), BMI (P<0.001), and duration of symptoms (P<0.001) were positively correlated with CSF OP. The presences of positive MRI findings (P=0.29), active leakage (P=0.324), SDH (P=0.543), as well as gender (P=0.062) were not significantly associated with CSF OP in univariate analysis.
Fig. 3.
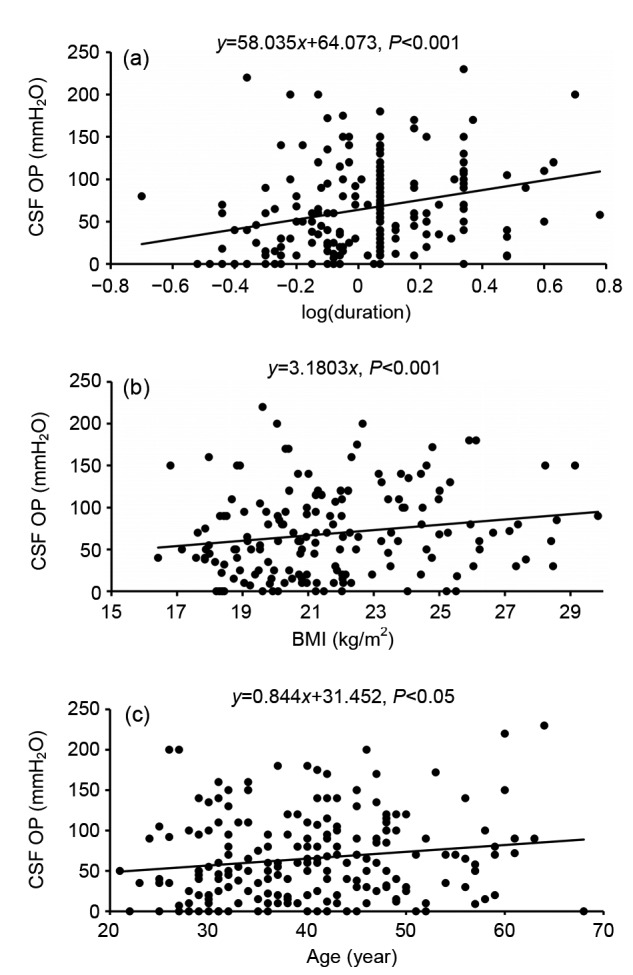
Univariate analysis of continuous variables
Scatterplots present the relationship between CSF opening pressure (OP) and duration of symptoms (a), body mass index (BMI) (b), and age (c)
Fig. 4.
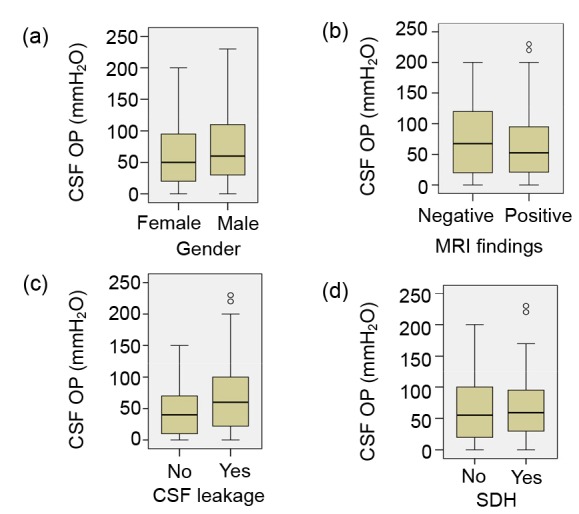
Univariate analysis of categorical variables
Box-and-whisker plots present the relationships between CSF opening pressure (OP) and gender (a), magnetic resonance imaging (MRI) findings (b), active CSF leakage (c), and subdural hematoma (SDH) (d). “ο”: mild outliers
3.3. Multivariate analysis
Multivariate logistic regression was adopted to judge the estimated joint effects of these seven factors. The BMI (P<0.001) and duration of symptoms (P<0.001) were still strongly correlated with CSF OP (Table 3). Although age showed a marginal correlation with CSF OP in univariate analysis, it was not significant in the multivariate model (P=0.29). The remaining factors (MRI findings of SIH, active leakage, SDH, and gender) were still not significant. A relatively high R 2 of 0.681 was obtained for the multivariate model, which reflected low variability (21.9%) in CSF OP.
Table 3.
Positive findings of multivariate logistic regression
| Term | Unstandardized coefficients |
Standardized coefficients |
t | P | |
| B | SE | β | |||
| BMI | 3.111 | 0.175 | 0.793 | 17.822 | 0.000 |
| log(duration) | 60.752 | 15.874 | 0.170 | 3.827 | 0.000 |
4. Discussion
In contrast to previous findings (Mokri et al., 1998; Chung et al., 2000; Wang et al., 2014), only 55.3% of SIH cases in our study showed a low CSF pressure (≤60 mmH2O). Mokri et al. (1998) reviewed a series of 40 patients with orthostatic headaches and diffuse pachymeningeal gadolinium enhancement (DPGE), and found normal CSF pressure in only seven (18%) patients. In our study, among those cases without low CSF pressure, two even showed a pressure more than 200 mmH2O and further myelography showed that they had active CSF leakage. Kranz et al. (2015) reviewed 106 SIH patients and found that 5% of cases had a pressure of >200 mmH2O, and all these cases presented with typical symptoms as well as direct CSF leaks. We concluded that an absence of low CSF pressure cannot exclude the diagnosis of SIH and that high CSF pressure can occur under conditions of active CSF leakage.
To gain a better understanding of the spectrum of CSF pressure in our study, we analyzed several potential influential factors. Variables such as age, gender, body habitus, brain MRI findings, and duration of symptoms had been previously explored by Kranz et al. (2015). They observed that body habitus and duration of symptoms were significantly correlated with CSF OP. This was supported by our results with a larger case series. BMI is a more representative and commonly used index of body habitus than abdominal circumference used by Kranz et al. (2015), and we confirmed significant correlations between BMI and CSF pressure. However, unlike Kranz et al. (2015), we did not find a relationship between brain MRI findings and CSF OP. In addition, recognizing that active leakage and SDH are both typical features of SIH, we were the first to determine whether they are also influential factors for CSF OP in SIH patients.
Similar to the findings of Kranz et al. (2015), our study showed significant correlations between the duration of symptoms and CSF OP, following both univariate and multivariate analyses. The CSF pressure of SIH patients with a long duration of symptoms was higher than of those with a short duration. We found that in several cases, the initial CSF OP was lower than 60 mmH2O, while the follow-up value elevated to within the normal range, which may be explained by compensatory mechanisms (Schievink, 2006; 2008; Mokri, 2013; Kranz et al., 2015; Limaye et al., 2016). In the case of CSF leakage, the volume of CSF decreased as a result. Because of the exponential curve relating pressure to volume, CSF pressure drops initially (Marmarou et al., 1978), but the equilibrium state of the CSF compartment gradually becomes disrupted due to a state of chronic CSF depletion, which activates the compensatory mechanisms. A longer duration leads to persistent compensation by reduced CSF reabsorption, an increase in CSF production, venous engorgement, compliance of brain tissue alteration, and an increase in cerebral blood flow. As a result, CSF pressure may return to within the normal range over time, even though an active CSF leakage exists.
Many previous studies have investigated the relationship between BMI and CSF pressure in normal patients (Whiteley et al., 2006; Berdahl et al., 2012; Markey et al., 2016). Berdahl et al. (2012) reviewed a total of 4235 patients, and found that CSF pressure had a positive linear correlation with BMI (R 2=0.2, P<0.01). In a study involving 242 cases with various neurological complaints and/or conditions that did not affect CSF OP, BMI had a clinically insignificant impact on CSF OP (Whiteley et al., 2006). In this study, we included 163 patients with SIH, and found that BMI was strongly correlated with CSF OP. Normal CSF pressure was found in most cases with a high BMI. Our results were similar to the findings of Kranz et al. (2015), though they used abdominal circumference instead of BMI to represent body habitus. A large number of previous studies, both clinical and experimental, suggested that central obesity raised intracranial pressure (ICP) by increasing intra-abdominal pressure, leading to increased cardiac filling pressure and intracranial venous pressure, hindering cerebral venous drainage (Greer, 1962; Luce et al., 1982; Bloomfield et al., 1995; 1997; Sugerman et al., 1997). Adipose tissue is considered to be a neuroendocrine tissue releasing adipokines, especially leptin. Elevation in leptin might raise ICP by activating Na+/K+ ATPase in epithelial choroid plexus cells to secrete CSF (Markey et al., 2016). Therefore, we postulated that patients with a higher BMI would have a higher premorbid CSF pressure. When a spontaneous leakage develops, it is the relative decrease (still within the normal range) rather than the absolute low value of pressure that may be sufficient to cause the numerous clinical symptoms. Leptin might influence the physiological compensatory mechanisms after CSF leakage in SIH patients, via its direct action on arachnoid granulation or the choroid plexus, or through peripheral mechanisms with secondary central effects (Markey et al., 2016).
SDH is now regarded as an important complication of SIH (Chung et al., 2006; Mokri, 2013). Development of SDH usually elevates the ICP in non-SIH patients. However, whether CSF pressure will increase in SIH patients complicated with SDH is still under debate. Most studies have not shown any relationship between CSF pressure and development of SDH in patients with SIH. Takahashi et al. (2016) reviewed 55 cases of SIH with SDH, and found no cases had elevated pressure. They concluded that SIH with SDH occurred in the case of low CSF pressure. In addition, extended pneumocephalus occurs after hematoma evacuation in SIH patients with SDH, which indicates a negative CSF pressure (Shin et al., 2016). In this study, the presence of SDH was not associated with CSF pressure following either univariate or multivariate analysis. In 38 cases of SIH with SDH, 52.6% (20) of cases showed a low CSF OP, and 2 cases showed 0 mmH2O. However, among those with high pressure, 2 cases showed more than 200 mmH2O CSF OP. Therefore, we suggested that the presence of SDH in SIH patients is not necessarily correlated with CSF OP. However, hematomas differ in size, and there might be a difference between large (>1 cm) and small hematomas. For example, two cases in our series, both with high CSF OP (>200 mmH2O), happened to have a large bilateral SDH (>1 cm) and a hernia of the cerebellar tonsil. Patients with large SDHs might tend to develop a high CSF OP. Further investigation is warranted regarding the effect of hematoma size on CSF OP.
Gd-enhanced MRI is the preferred modality to confirm a diagnosis of SIH. The acronym SEEPS can be used to summarize the typical MRI features: subdural fluid collections, enhancement of pachymeninges, engorgement of venous structures, pituitary enlargement, and sagging of the brain (Schievink, 2006; Spears, 2014; Limaye et al., 2016). The mechanisms of these abnormalities can be well explained by the Monro-Kellie hypothesis (Mokri, 2001; 2004; 2013; Limaye et al., 2016). In the intact rigid skull, the total volume including that of the brain, CSF, and intracranial blood is constant. A change in any one of these components would induce a reciprocal change in the remaining two. Given the nearly constant brain volume, a decrease in CSF volume must be compensated by an increase in intracranial blood volume, leading to diffuse meningeal venous hyperemia, venous sinuses engorgement, and pituitary hyperemia (Mokri, 2001). Subdural fluid collection also acts in volume compensation. In addition, the buoyancy of the brain’s supportive cushion reduces due to the decreased CSF volume, inducing sagging of the brain (Miyazawa et al., 2003). All these changes are a reflection of CSF hypovolemia (Mokri, 1999; Schievink et al., 2005; Kranz et al., 2016). Given the exponential relationship between CSF pressure and volume (Marmarou et al., 1978), the imaging signs of SIH ought to correlate with low CSF pressure. However, several studies have reported no link between pressure and imaging signs in SIH patients (Schoffer et al., 2002; Schievink et al., 2005; Kranz et al., 2016). In this study, we discovered that signs of SIH on brain MRI scans were poorly correlated with CSF OP. This result differs from those of Kranz et al. (2015). We postulated that under the condition of spontaneous CSF leakage, the relationship between CSF volume and pressure was altered by compensatory mechanisms, including dilation of the epidural venous plexus. In addition, other kinds of compensation, undetectable by MRI, might also share the ability to maintain CSF pressure. These compensations include altered compliance of the thecal sac, reduced CSF reabsorption, and increased CSF production. For various durations of symptoms, the degree of compensation varied among patients. Longer durations usually brought about more compensation. We speculated that sufficient compensation would effectively maintain CSF pressure. Note that among those cases with positive MRI findings, some showed only one abnormality while the others presented two to five kinds of abnormalities. Moreover, patients with SIH and a normal MRI were assumed to lack the ability to compensate for loss of CSF volume (Schievink et al., 2005) or to have unseen compensation mechanisms. The number as well as the type of abnormalities on an MRI scan might make a difference and so this possibility will be investigated in detail in our next study. Also, we will explore further the relationships between brain MRI findings, active leakage, duration of symptoms and CSF OP in SIH patients.
Several studies have investigated the relationship between active CSF leakage and CSF OP. Luetmer et al. (2012) reviewed 151 consecutive patients, and failed to find a significant correlation (P=0.30). From a study of 99 cases, Kranz et al. (2016) concluded that myelographic signs of SIH were poorly correlated with CSF pressure. In this study, CTM/MRM was used to detect the exact leaking sites or the evidence of ongoing CSF leakage. We also found no difference in CSF OP between cases with evidence of active CSF leakage and those without. For instance, in those without active leakage, one case showed a CSF pressure of 0 mmH2O, while in the other two cases, CSF pressure was as high as 140 mmH2O. Again, the compensatory mechanisms in SIH, which change the relationship between CSF pressure and volume, were assumed to have played an important role. The rate of CSF leakage can differ. Kranz et al. (2016) observed no difference in CSF OP between patients with a high-flow CSF leakage and those without leakage. High-flow or fast leaks, usually require dynamic CTM to localize since conventional CTM cannot diagnose (Luetmer and Mokri, 2003). In this study, we did not evaluate this difference. The relationship between the rate of CSF leakage, number of leaking sites, and CSF pressure in SIH patients needs further investigation. The remaining factors, including age and gender, were not significantly correlated with CSF OP, which agrees with the findings of Kranz et al. (2015).
The BMI and duration of symptoms were regarded as independent factors influencing CSF OP due to a strong correlation following multivariate analysis. However, a low variability of 21.9% was assumed to have been brought about by several undiscovered factors. In future work, we will try to identify these factors and determine how they affect CSF pressure.
This study had several limitations. First, this was a retrospective analysis and single-center experience. Second, the BMI data were not available in all cases, so in the multivariate analysis, 44 cases had to be excluded. In addition, the inclusion criteria used here were strict to ensure a representative series of SIH patients, and this may have created a selection bias.
5. Conclusions
This study confirmed that normal CSF pressure in SIH patients is not rare, especially in obese patients or those with a long duration of symptoms. Apart from BMI and duration of symptoms, there were still several unidentified factors that interacted with CSF OP.
Acknowledgments
The authors would like to thank Dr. Qiao-wei ZHANG and Dr. Yi YANG at our hospital (Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China) for image re-evaluation and critical review of the manuscript.
Footnotes
Compliance with ethics guidelines: Ling-ling YAO and Xing-yue HU declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Berdahl JP, Fleischman D, Zaydlarova J, et al. Body mass index has a linear relationship with cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2012;53(3):1422–1427. doi: 10.1167/iovs.11-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomfield GL, Dalton JM, Sugerman HJ, et al. Treatment of increasing intracranial pressure secondary to the acute abdominal compartment syndrome in a patient with combined abdominal and head trauma. J Trauma. 1995;39(6):1168–1170. doi: 10.1097/00005373-199512000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield GL, Ridings PC, Blocher CR, et al. A proposed relationship between increased intra-abdominal, intrathoracic, and intracranial pressure. Crit Care Med. 1997;25(3):496–503. doi: 10.1097/00003246-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Chung SJ, Kim JS, Lee MC. Syndrome of cerebral spinal fluid hypovolemia: clinical and imaging features and outcome. Neurology. 2000;55(9):1321–1327. doi: 10.1212/WNL.55.9.1321. [DOI] [PubMed] [Google Scholar]
- 5.Chung SJ, Lee JH, Kim SJ, et al. Subdural hematoma in spontaneous CSF hypovolemia. Neurology. 2006;67(6):1088–1089. doi: 10.1212/01.wnl.0000237338.44702.61. [DOI] [PubMed] [Google Scholar]
- 6.Greer M. Benign intracranial hypertension. I. Mastoiditis and lateral sinus obstruction. Neurology. 1962;12:472–476. doi: 10.1212/wnl.12.7.472. [DOI] [PubMed] [Google Scholar]
- 7.Kranz PG, Tanpitukpongse TP, Choudhury KR, et al. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia. 2015;36(13):1209–1217. doi: 10.1177/0333102415623071. [DOI] [PubMed] [Google Scholar]
- 8.Kranz PG, Tanpitukpongse TP, Choudhury KR, et al. Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. Am J Neuroradiol. 2016;37(7):1374–1378. doi: 10.3174/ajnr.A4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limaye K, Samant R, Lee RW. Spontaneous intracranial hypotension: diagnosis to management. Acta Neurol Belg. 2016;116(2):119–125. doi: 10.1007/s13760-015-0577-y. [DOI] [PubMed] [Google Scholar]
- 10.Luce JM, Huseby JS, Kirk W, et al. Mechanism by which positive end-expiratory pressure increases cerebrospinal fluid pressure in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(1):231–235. doi: 10.1152/jappl.1982.52.1.231. [DOI] [PubMed] [Google Scholar]
- 11.Luetmer PH, Mokri B. Dynamic CT myelography: a technique for localizing high-flow spinal cerebrospinal fluid leaks. Am J Neuroradiol. 2003;24(8):1711–1714. [PMC free article] [PubMed] [Google Scholar]
- 12.Luetmer PH, Schwartz KM, Eckel LJ, et al. When should I do dynamic CT myelography? Predicting fast spinal CSF leaks in patients with spontaneous intracranial hypotension. Am J Neuroradiol. 2012;33(4):690–694. doi: 10.3174/ajnr.A2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markey KA, Uldall M, Botfield H, et al. Idiopathic intracranial hypertension, hormones, and 11β-hydroxysteroid dehydrogenases. J Pain Res. 2016;9:223–232. doi: 10.2147/JPR.S80824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmarou A, Shulman K, Rosende RM. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg. 1978;48(3):332–344. doi: 10.3171/jns.1978.48.3.0332. [DOI] [PubMed] [Google Scholar]
- 15.Miyazawa K, Shiga Y, Hasegawa T, et al. CSF hypovolemia vs intracranial hypotension in “spontaneous intracranial hypotension syndrome”. Neurology. 2003;60(6):941–947. doi: 10.1212/01.WNL.0000049933.51044.81. [DOI] [PubMed] [Google Scholar]
- 16.Mokri B. Spontaneous cerebrospinal fluid leaks: from intracranial hypotension to cerebrospinal fluid hypovolemia–evolution of a concept. Mayo Clin Proc. 1999;74(11):1113–1123. doi: 10.4065/74.11.1113. [DOI] [PubMed] [Google Scholar]
- 17.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56(12):1746–1748. doi: 10.1212/WNL.56.12.1746. [DOI] [PubMed] [Google Scholar]
- 18.Mokri B. Spontaneous low cerebrospinal pressure/volume headaches. Curr Neurol Neurosci Rep. 2004;4(2):117–124. doi: 10.1007/s11910-004-0025-5. [DOI] [PubMed] [Google Scholar]
- 19.Mokri B. Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. Headache. 2013;53(7):1034–1053. doi: 10.1111/head.12149. [DOI] [PubMed] [Google Scholar]
- 20.Mokri B, Hunter SF, Atkinson JL, et al. Orthostatic headaches caused by CSF leak but with normal CSF pressures. Neurology. 1998;51(3):786–790. doi: 10.1212/WNL.51.3.786. [DOI] [PubMed] [Google Scholar]
- 21.Rando TA, Fishman RA. Spontaneous intracranial hypotension: report of two cases and review of the literature. Neurology. 1992;42(3 Pt 1):481–487. doi: 10.1212/WNL.42.3.481. [DOI] [PubMed] [Google Scholar]
- 22.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286–2296. doi: 10.1001/jama.295.19.2286. [DOI] [PubMed] [Google Scholar]
- 23.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks. Cephalalgia. 2008;28(12):1345–1356. doi: 10.1111/j.1468-2982.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 24.Schievink WI, Maya MM, Louy C. Cranial MRI predicts outcome of spontaneous intracranial hypotension. Neurology. 2005;64(7):1282–1284. doi: 10.1212/01.WNL.0000156906.84165.C0. [DOI] [PubMed] [Google Scholar]
- 25.Schievink WI, Dodick DW, Mokri B, et al. Diagnostic criteria for headache due to spontaneous intracranial hypotension: a perspective. Headache. 2011;51(9):1442–1444. doi: 10.1111/j.1526-4610.2011.01911.x. [DOI] [PubMed] [Google Scholar]
- 26.Schoffer KL, Benstead TJ, Grant I. Spontaneous intracranial hypotension in the absence of magnetic resonance imaging abnormalities. Can J Neurol Sci. 2002;29(3):253–257. doi: 10.1017/S0317167100002031. [DOI] [PubMed] [Google Scholar]
- 27.Shin HS, Lee SH, Ko HC, et al. Extended pneumocephalus after drainage of chronic subdural hematoma associated with intracranial hypotension: case report with pathophysiologic consideration. J Korean Neurosurg Soc. 2016;59(1):69–74. doi: 10.3340/jkns.2016.59.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spears RC. Low-pressure/spinal fluid leak headache. Curr Pain Headache Rep. 2014;18(6):425. doi: 10.1007/s11916-014-0425-4. [DOI] [PubMed] [Google Scholar]
- 29.Sugerman H, DeMaria E, Felton WR, et al. Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology. 1997;49(2):507–511. doi: 10.1212/WNL.49.2.507. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Mima T, Akiba Y. Chronic subdural hematoma associated with spontaneous intracranial hypotension: therapeutic strategies and outcomes of 55 cases. Neurol Med Chir (Tokyo) 2016;56(2):69–76. doi: 10.2176/nmc.oa.2015-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Zhang D, Gong X, et al. Rapid resolution of subdural hematoma after targeted epidural blood patch treatment in patients with spontaneous intracranial hypotension. Chin Med J (Engl) 2014;127(11):2063–2066. [PubMed] [Google Scholar]
- 32.Whiteley W, Al-Shahi R, Warlow CP, et al. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690–1691. doi: 10.1212/01.wnl.0000242704.60275.e9. [DOI] [PubMed] [Google Scholar]


