Abstract
This study investigated the protective effect of the compatibility of hypaconitine (HA) and glycyrrhetinic acid (GA) on H9c2 cells under oxygen and glucose deprivation (OGD)-induced injury, and the possible mechanisms. We found that HA+GA significantly improved pathology and morphology of the nucleus and ultrastructure of H9c2 cells under OGD as determined by Hoechst 33342 staining and transmission electron microscopy (TEM) tests. It also reduced the releases of lactate dehydrogenase (LDH), creatine kinase-myocardial band isoenzyme (CK-MB), and aspartate transaminase (AST) from the cultured supernatant of H9c2 cells, which were tested by enzyme-linked immune sorbent assay (ELISA) kits. In addition, it lessened the apoptotic rate as determined by a fluorescein isothiocyanate-annexin V/propidium iodide (FITC-AV/PI) double staining assay. It was also found that HA+GA might regulate the protein expression associated with the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. Overall, the study demonstrated that HA+GA protected H9c2 cells against OGD-induced injury, and the signaling mechanism might be related to the PI3K/Akt signaling pathway.
Keywords: Hypaconitine (HA), Glycyrrhetinic acid (GA), H9c2 cells, Apoptosis, PI3K/Akt
1. Introduction
Cardiovascular diseases (CVDs) are disorders of heart and blood vessels, including ischemic heart disease, with high morbidity and mortality around the world (Menezes et al., 2011; Nabel and Braunwald, 2012). It is generally accepted that ischemic heart injury is a pathological process, which will cause extensive death of cells (Yucel et al., 2011). Furthermore, ischemic heart diseases can produce apoptosis, and the apoptosis might be related to both extrinsic and intrinsic apoptotic pathways. However, preconditioning may reduce apoptosis via regulation of the expression of Bcl-2 and Bax (Lai et al., 2015). There are also many risk factors. For example, inflammatory reactions have been proved to participate in CVD progression (Munk and Larsen, 2009). Thus it is important to investigate possible therapeutic and prevention strategies for improving CVD patients’ quality of life. Drug treatment is still an effective method for enhancing both the quality and length of life (van der Hoeven et al., 2012). It has been reported that the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway is an amenable pharmacological target for CVD protection, and it achieves its effect mainly through regulating caspase activation, Bcl-2 family activity, and so on (Wang J. et al., 2015). In the past few years, many traditional Chinese medicines have been claimed to be useful for controlling CVD, particularly for the injury induced by ischemia (Quan et al., 2014; Zheng et al., 2014). Therefore, searching for anti-necrosis and anti-apoptotic compounds with minimal side effects from traditional Chinese medicines probably is an ideal way for CVD patients, and to help create a much safer and more effective treatment.
Aconitum carmichaelii Debeaux is a well-known traditional Chinese herb, including diester diterpenoid alkaloids, which are responsible for not only biological activity but also toxicity. It is widely applied in treatments for the cardiovascular system, such as heart failure, hypertension, and arrhythmia (Zhao et al., 2012; Yu B. et al., 2015), and for anti-inflammation and analgesic action, such as in rheumatism and painful joints (Cai et al., 2013). Then there are other applications, including for anti-aging, protecting the kidney, as an anti-diabetic and for lipid-lowering, helping the immune system, and so on (Zhou et al., 2015). Hypaconitine (HA) (Fig. 1) is one of the major active alkaloids of Aconitum carmichaelii Debeaux, which has both therapeutic and high toxic activity, with cardiotoxity and evident side-effect (Xie et al., 2015). It is frequently prescribed with other ordinary herbal medicines, such as the Glycyrrhiza uralensis Fisch. It is largely used in clinics as an adjuvant, and in some cases for reducing toxicity. Glycyrrhizic acid (GL) and glycyrrhetinic acid (GA) (Fig. 2) have aglycone saponins. It has been proved that GA can ameliorate ventricular arrhythmia (Wu et al., 2015) and GL transforms to GA in the absorption process (Fan et al., 2016). HA is considered to be effective on CVD, and its heat stability in water is better than that of other alkaloids (Yue et al., 2008; Zhang et al., 2015). Studies have also found that HA and GA are the major compounds after oral administration of Fuzi-Gancao decoction (Gao et al., 2004; Zhang et al., 2013; Yang et al., 2014). It is known that oxygen and glucose deprivation (OGD) is a generally applicable issue in CVD studies, since OGD treatment can cause necrosis and apoptosis of cells. Though the effects of HA and GA have been elucidated separately in H9c2 cells, their combination effects and mechanisms are not so well understood. Hence we chose to use the compatibility of HA and GA (HA+GA) to interpret the effects and mechanisms on H9c2 cells.
Fig. 1.
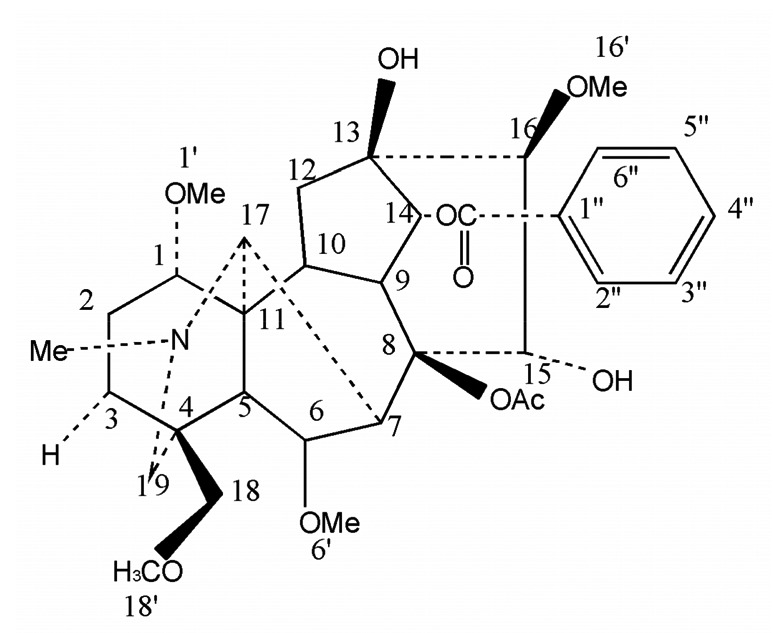
Structure of hypaconitine (HA)
Fig. 2.
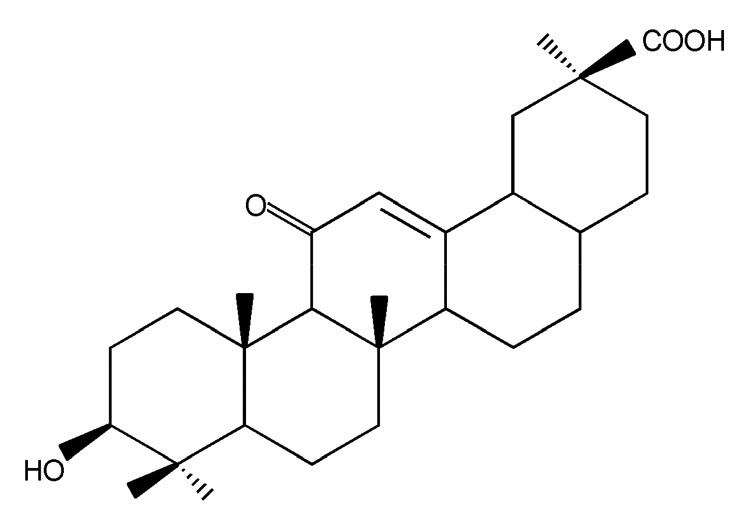
Structure of glycyrrhetinic acid (GA)
2. Materials and methods
2.1. Materials
HA (molecular formula: C33H45NO10; molecular weight: 615.71; purity: ≥98%) and GA (molecular formula: C30H46O4; molecular weight: 470.64; purity: ≥98%) were purchased from Baoji Chenguang Biotechnology Co., Ltd. (Xi’an, China). LY-294002 (molecular formula: C19H17NO3; molecular weight: 307.34; purity: ≥98%) was obtained from Calbiochem Novabiochem Co. (San Diego, USA). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from GIBCO Co. (Grand Island, NY, USA). Fetal bovine serum (FBS) was provided by the Shanghai Shengnabao Biological Technology Development Co., Ltd. (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) were purchased from Beijing dingguochangsheng Biotechnology Co., Ltd. (Beijing, China). Phosphate buffered saline (PBS) was obtained from Wuhan Boster Bio-Engineering Co., Ltd. (Wuhan, China). The kits for determination of lactate dehydrogenase (LDH), creatine kinase-myocardial band isoenzyme (CK-MB), and aspartate transaminase (AST) were obtained from Jiancheng Bioengineering Institute (Nanjing, China). Anti-phospho-Akt (anti-p-Akt), anti-Akt, anti-Bax, anti-Bcl-2, anti-caspase-9, and anti-β-actin antibodies were obtained from Santa Cruz Biotechnology Co. (CA, USA). The fluorescent kit for Hoechst 33342 was obtained from Beyotime Institute of Biotechnology Co., Ltd. (Hangzhou, China). The fluorescein isothiocyanate-annexin V/propidium iodide (FITC-AV/PI) apoptosis detection kit was provided by BD Pharmingen (San Diego, USA).
2.2. Cell culture
The H9c2 cells were obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (IBCB, CAS). The H9c2 cells were maintained in DMEM supplemented with 10% FBS and penicillin plus streptomycin (both 100 μg/ml), incubated at 37.0 °C in a 5% CO2 atmosphere. The medium was replaced every 2–3 d, and cells were subcultured or subjected to experimental procedure when developed into 80%–90% density.
2.3. OGD model building and experimental grouping
OGD has been used as a cellular technique of ischemic heart damage in cultured myocardial cells (Marambio et al., 2010). According to a described protocol (Rizvi et al., 2010), the OGD injury was produced by incubating with a glucose-free balanced salt solution (116 mmol/L NaCl, 5.4 mmol/L KCl, 0.8 mmol/L MgSO4, 1.0 mmol/L NaH2PO4, 1.8 mmol/L CaCl2, 26.2 mmol/L NaHCO3, 0.025 mmol/L phenol red, and 20 mmol/L sucrose) and exposing to a hypoxic environment of 5% CO2 and 95% N2 in air tight gas chambers at 37 °C for 4 h. These basic experimental conditions had been confirmed in our previous work. After 4 h culture, H9c2 cells were used in the experiment. H9c2 cells were randomly divided into seven groups: (1) control group, without any treatment; (2) OGD group, being developed under OGD for 4 h with no treatment; (3) OGD+HA group, being pretreated with HA (120 μmol/L) before being cultured under OGD for 4 h; (4) OGD+GA group, being preconditioned with GA (120 μmol/L) before being incubated under OGD for 4 h; (5) OGD+HA+GA group, being pretreated with HA+GA (120 μmol/L, separately) before being cultivated under OGD for 4 h; (6) OGD+LY group, being incubated under OGD for 4 h with LY-294002 (10 μmol/L) pretreatment 1 h before OGD; (7) OGD+HA+GA+LY-294002 group, being preprocessed with LY-294002 (10 μmol/L) 1 h before being developed with HA+GA (120 μmol/L, separately) under OGD for 4 h.
2.4. Cell viability assay
H9c2 cell viability was measured by MTT assay as described by Chen et al. (2013). The optical density (OD) values were detected at 490 nm by a Molecular Devices SpectraMAX plus-384 microplate reader (MD, USA), and the survival ratio of H9c2 cells was obtained as a percentage of the control. On the basis of results of previous experiments (Liu et al., 2013), both the concentrations of HA and GA were selected at 120 μmol/L for the further investigation.
2.5. Hoechst 33342 staining assay
H9c2 cells were washed in ice-cold PBS, fixed in 10% neutral buffered formalin for 10 min at room temperature, and washed again with ice-cold PBS. Then the H9c2 cells were exposed to Hoechst 33342 (2 μg/ml in PBS) and incubated for 20 min at room temperature. The H9c2 cells were then washed three times with pre-cooled PBS, and lastly the stained cells were observed in an appropriate filter of the fluorescence microscope.
2.6. Transmission electron microscopy
The ultrastructure presences of H9c2 cells were surveyed by transmission electron microscopy (TEM), and cells were gathered from 6-well plates, and washed with PBS prior to fixing in a fixative buffer. Then cells were centrifuged at 3000 r/min for 10 min, suspended and incubated for 2 h in 2.5% glutaraldehyde. Cell samples were processed with 2% osmium tetroxide in a 0.1 mol/L sodium cacodylate buffer, dehydrated through a graded series of acetone, and embedded in resin. Then the cell samples were sliced into 65-nm sections, and ultrastructures were observed via TEM.
2.7. LDH, CK-MB, and AST activity assays
To be confident of the level of cell necrosis in the cultured supernatant of H9c2 cells, the activity of LDH, CK-MB, and AST was measured using enzyme-linked immune sorbent assay (ELISA) kits. The procedures were conducted according to the manufacturers’ instructions.
2.8. FITC-AV/PI assay
FITC-AV/PI doubles staining assay was used to examine the apoptosis of H9c2 cells. H9c2 cells were collected, washed with calcium-free PBS, resuspended with binding buffer, and incubated with annexin V at room temperature in the dark for 15 min. Then the H9c2 cells were centrifuged and resuspended with binding buffer. PI was added to the resuspended cells before they were analyzed by flow cytometry.
2.9. Western blot analysis
The relevant protein 50 μg was resolved on a 15% sodium dodecyl sulfate (SDS)-polyacrylamide gel. The fractionated proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membranes and probed with rabbit anti-rat polyclone anti-p-Akt, anti-Akt, anti-Bcl, anti-Bax, and anti-caspase-9 at 4 °C overnight, and then incubated with the peroxidase-conjugated secondary antibodies at room temperature for 2 h. The blots were visualized with ECL (enhanced chemiluminescent) Plus reagent (GE Healthcare, Piscataway, NJ, USA). In all these experiments, the blots were reprobed with an anti-β-actin antibody to control the protein loading.
2.10. Statistical analysis
Routine statistical analysis was completed using IBM SPSS 19.0. Data were presented as the mean±standard deviation (SD). One-way analysis of variance (ANOVA) was applied to evaluate between group differences, followed by Tukey’s test. In this study, values of P<0.05 were considered statistically significant.
3. Results
3.1. Morphological characteristics of apoptosis by Hoechst 33342 staining
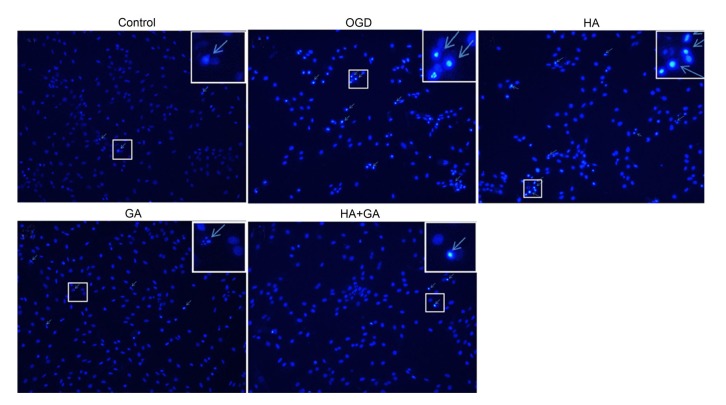
Nucleus morphologies of H9c2 cells in the first five groups were performed by Hoechst 33342, which is a permeable membrane dye. As shown in Fig. 3, the nuclei of cells in the control group appeared with normal contours and were elliptical or round in shape with few bright spots, which represented the apoptotic cells. However, in the OGD group, cells were revealed with many more bright spots, together with condensed chromatin and smaller cell nuclei. The other three pretreatment groups had ameliorated morphological characteristics and reduced number of apoptotic cells, but the HA group had slight differences from the OGD group. The HA+GA and GA groups had apparent changes, and the HA+GA group emerged with a better appearance.
Fig. 3.
Effects of HA+GA on histochemical characterizations of H9c2 cells in OGD injury
Arrowheads in the pictures indicate the nuclei of apoptotic cells (magnification ×100, enlarged part ×200)
3.2. Ultrastructure changes of H9c2 cells by TEM observation
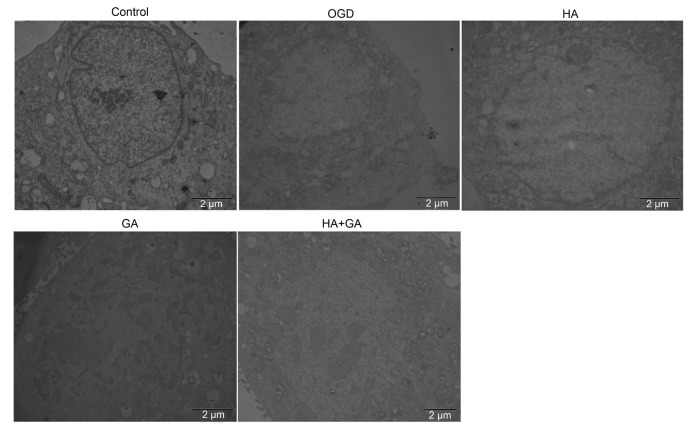
To observe the ultrastructure changes of OGD-induced injury on H9c2 cells by the pretreatments of HA+GA, TEM was used. As presented in Fig. 4, the control group had a generally normal cell ultrastructure and shape, the cell nuclei membrane was intact and clear, and most of the mitochondria were in a nearly clear and integrated structure with compact cristae. Compared with the control group, the OGD group had some different and damaged forms of cell apoptosis; for example, the mitochondria were swollen with disrupted and lysed cristae, even many of hypervacuolization, with cell nuclei chromatin condensed and migrated to the cell edge. Meantime, the HA group revealed a similar morphological change with the OGD group, but the pretreatment groups with GA and HA+GA displayed apparent morphological improvements, with a small amount of swollen mitochondria, which meant GA and HA+GA could help protect the H9c2 cells from OGD-induced injury.
Fig. 4.
Effects of HA+GA on ultrastructure changes of H9c2 cells in OGD injury
3.3. Protective activity of HA+GA against OGD-induced LDH release
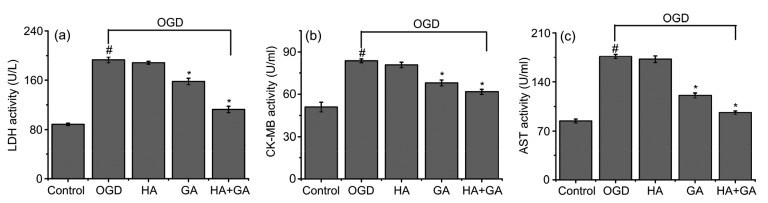
To address the protective activity of HA+GA on cultured H9c2 cells from OGD injury, LDH assay was performed to assess whether HA+GA can secure H9c2 cells against OGD-induced membrane damage. As demonstrated in Fig. 5a, a larger increase of LDH was observed in the OGD group than in other groups, and the OGD group had a significant difference with the control group (P<0.05). In contrast, GA and HA+GA pretreatment groups revealed markedly inhibited LDH releases, and the value of the LDH release in the HA+GA group was lower than that of the GA group. Compared with the OGD group, the HA group had no significant difference (P>0.05), yet the HA+GA group had a significant difference (P<0.05).
Fig. 5.
Effects of HA+GA on releases of LDH (a), CK-MB (b), and AST (c) of H9c2 cells in OGD injury
Values were expressed as mean±SD (n=6). # P<0.05 compared with the control group; * P<0.05 compared with the OGD group
3.4. Protective activity of HA+GA against OGD-induced CK-MB and AST releases
CK-MB and AST are enzymes that can assess the range and degree of myocardial injury, and a release of them was performed to reveal the OGD-induced damage or necrosis of H9c2 cells. As shown in Figs. 5b and 5c, marked improvements of CK-MB and AST were discovered in the OGD group compared with the other groups, and most of all had a highly significant difference compared with the control group (P<0.05). GA and HA+GA pretreatment groups showed tremendous inhibition of CK-MB and AST releases, and the HA+GA group revealed slightly better results than the GA group. Compared with the OGD group, the HA group had no significant difference (P>0.05), but the GA and HA+GA groups had a significant difference (P<0.05).
3.5. Anti-apoptotic effects of HA+GA on H9c2 cells with OGD injury
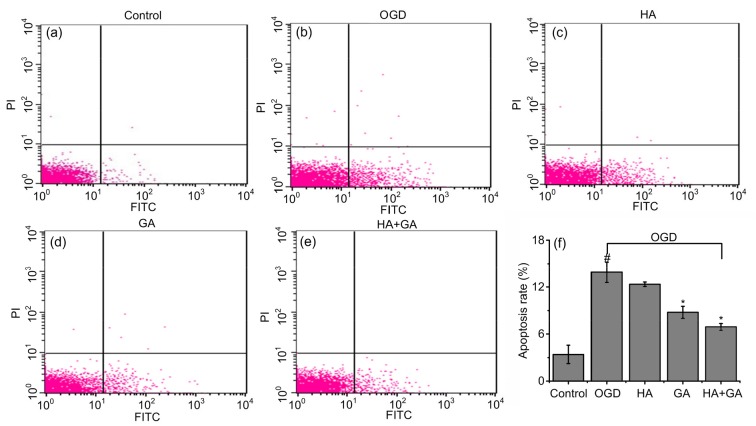
To analyze the possible anti-apoptotic capability of HA+GA under OGD conditions, the apoptotic rate was detected by flow cytometry using FITC-AV/PI double staining methods. As shown in Fig. 6, a small number of apoptotic cells were found in the control group. Compared to that, the apoptotic rate of H9c2 cells was significantly increased in the OGD group (P<0.05). However, the number of apoptotic cells was decreased by the pretreatments of HA+GA, GA, and HA; particularly, the HA+GA and GA groups had a significant difference (P<0.05), the HA group had no significant difference (P>0.05), and the HA+GA group was better than the GA group.
Fig. 6.
Effects of HA+GA on apoptosis rates of H9c2 cells in OGD injury
(a–e) FITC-AV/PI double staining of the control, OGD, HA, GA, and HA+GA groups. (f) Apoptotic rates of different groups. Values were expressed as mean±SD (n=6). # P<0.05 compared with the control group; * P<0.05 compared with the OGD group
3.6. Intracellular mechanisms of the anti-apoptosis by HA+GA
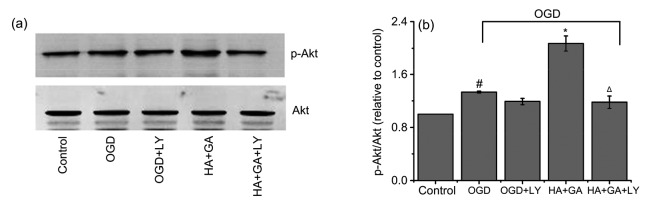
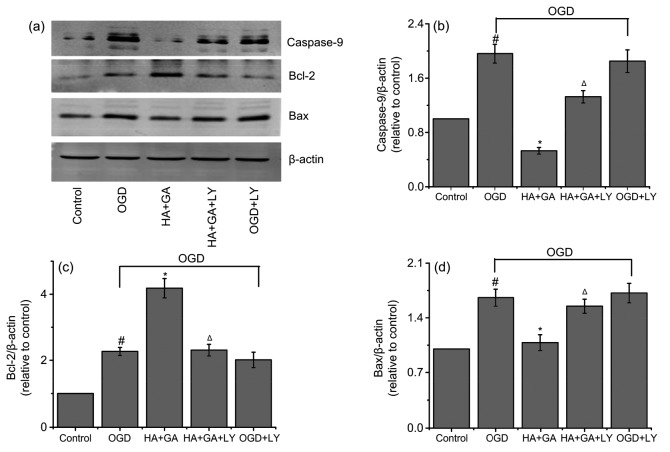
To further explore mechanisms underlying the protection effects of HA+GA, we examined the expression of the apoptosis-related proteins, such as Akt, p-Akt, caspase-9, Bax, and Bcl-2. As shown in Fig. 7, the ratio of p-Akt/Akt of the OGD group was markedly higher than that of the control group (P<0.05). However, the group pretreated with HA+GA before OGD significantly increased p-Akt level compared with the OGD group (P<0.05). When pretreated with LY-294002 for 1 h before HA+GA and OGD, the level of p-Akt was greatly decreased compared with the HA+GA group (P<0.05), but the OGD and OGD+LY-294002 groups had no significant difference (P>0.05). Furthermore, as shown in Fig. 8, compared with the control group, the protein level of caspase-9 was enhanced remarkably in the OGD group (P<0.05), whereas, in contrast with the OGD group, the groups pretreated with HA+GA declined significantly (P<0.05). The HA+GA+LY-294002 group increased the protein levels of caspase-9 substantially when compared with the HA+GA group at a remarkable level of difference (P<0.05). The OGD group increased Bcl-2 protein expression and promoted Bax expression in cells compared with the control group. However, the HA+GA group dramatically changed the effects of OGD on Bcl-2 and Bax expression, but the HA+GA+LY-294002 group notably reversed the expression of Bcl-2 and Bax of the HA+GA group. At the same time, the OGD and OGD+LY-294002 groups had no significant difference (P>0.05).
Fig. 7.
Changes of p-Akt after the use of LY-294002
(a) Band diagrams; (b) p-Akt/Akt. HA+GA activates PI3K and phosphorylation of Akt. Values were expressed as mean±SD (n=6). # P<0.05 compared with the control group; * P<0.05 compared with the OGD group; Δ P<0.05 compared with the HA+GA group
Fig. 8.
Regulation of proteins caspase-9, Bcl-2, and Bax associated with apoptosis by HA+GA
(a) Band diagrams; (b) Caspase-9/β-actin; (c) Bcl-2/β-actin; (d) Bax/β-actin. Values were expressed as mean±SD (n=6). # P<0.05 compared with the control group; * P<0.05 compared with the OGD group; Δ P<0.05 compared with the HA+GA group
4. Discussion
There has been some research illustrating the benefits of HA+GA treatment in CVD and pointing out the possibility of multiple targets where HA+GA may have protective effects. However, the direct effects of HA+GA on the H9c2 cells under OGD conditions were not clearly stated, and the relevant pathway and potential mechanisms were also not well expounded. In this study, the observations were displayed by fluorescence microscope and TEM, and the H9c2 cells and nucleus demonstrated prominent morphological injuries under OGD. Yet, these morphological changes and damages were mitigated in the group pretreated with HA+GA. Therefore, the results from morphological analysis suggested that preconditioning with HA+GA exerted a protective effect on H9c2 cells against OGD injury.
In previous research, the releases of LDH, CK-MB, and AST were assessed to investigate whether myocardial injury could be inhibited (Shi et al., 2007; Wakayama et al., 2012). Accumulating evidence indicates that necrosis is one of the main forms of cardiomyocyte death in CVD (Wang J.X. et al., 2015). As we know, under a normal physiological state, LDH, CK-MB, and AST cannot cross cytoplasmic membranes. Nevertheless, when a cell is damaged or dead, they are released out of the cell (Qiao et al., 2016). In this present study, LDH, CK-MB, and AST releases were downregulated, which proved that HA+GA protected H9c2 cells from OGD-induced cell damage.
It is said that a wide variety of regulatory stimuli can influence apoptosis (a programmed cell death) in a cell, displaying a crucial role in normal development of cells, tissue remodeling, immune response, and the stability of an organism. When the cell apoptosis process is disordered, it will become the pathogenesis of many human diseases, including cancer, viral infections, autoimmune diseases, and so on (Thompson, 1995). Meanwhile, apoptosis has an important effect on many CVD (Freude et al., 2000). The apoptosis rates collected by flow cytometry in this paper confirmed that HA+GA might exert a protective effect against OGD-induced apoptosis. Estimates of apoptosis rates of H9c2 cells widely make use of FITC-AV/PI double staining analyzed by flow cytometry (Shang et al., 2013). The annexin V assay is sensitive and supplies the possibility of detecting early phases of apoptosis before the loss of cell membrane integrity, and annexin V in polyethylenimine film can hold its high affinity following with phosphatidylserine altered from the inner to the outer cell membrane of the apoptotic cells in in vitro experiments (Vermes et al., 1995; Liu et al., 2009). In this study, the cardiomyocyte protection of HA+GA may be associated with the suppression of the cell apoptosis through the decreased apoptotic rates.
On the basis of results on the protective activity of HA+GA against OGD, further studies focused on the mechanisms of the anti-apoptotic effects of HA+GA. The PI3K/Akt signaling pathway regulates many processes in mammals, such as modulating cell survival, hypertrophy, contractility, and metabolism, inhibiting cell apoptosis, and controlling cell proliferation (Oudit and Penninger, 2009). It has been proved that the PI3K/Akt signaling pathway was critical in ischemic defense and pharmacologic preconditioning of cardioprotection (Qiao et al., 2016). In order to be cardioprotective and prevent the development of heart failure, the damage of myocardial cells could be lowered via inhibiting the apoptosis process (Lee and Gustafsson, 2009). Studies also revealed that part of the mechanisms of protecting cardiomyocytes relied on the activity of Akt, which can activate the downstream spot targets, improve energy generation of mitochondria, and then decrease the pro-apoptotic factors (Tong et al., 2000). Researchers have found PI3K/Akt to be a powerful survival pathway in numerous systems; cells can accelerate apoptosis by inhibiting PI3K and decelerate apoptosis by activating Akt (Hemmings and Restuccia, 2012). It was found that a decrease of caspase-9 activation could indicate the cardioprotective effects against diabetic cardiomyopathy by inhibiting cardiomyocyte apoptosis (Sun et al., 2014; Yu H.T. et al., 2015). Meanwhile, studies have demonstrated that an increased expression of pro-apoptotic Bax family proteins and a decreased expression of anti-apoptotic Bcl-2 family proteins were connected with the process of cell apoptosis (He et al., 2010; Yu et al., 2010). In the whole pathway system, Bax and Bcl-2 are the relevant downstream indexes of PI3K/Akt signaling pathway (Gao et al., 2010) and their regulation can induce the activity of caspase-9, so we evaluated the protein expression of Akt, p-Akt, caspase-9, Bax, and Bcl-2 to elucidate the possible mechanism pathway. Our data showed that HA+GA inhibited the cell apoptosis through suppressing Bax and caspase-9 expression and increasing Bcl-2 synthesis. In addition, to dissect the role of HA+GA in protecting cells against OGD-induced injury, the LY-294002, which is the specific inhibitor of PI3K/Akt pathway, was used. The data demonstrated that LY-294002 prevented HA+GA to generate cardioprotection by inhibiting the p-Akt expression and decreasing the amount of caspase-9, together with increasing the ratio of p-Akt/Akt. So, these results suggested that HA+GA might protect H9c2 cells from OGD-induced injury through the PI3K/Akt signaling pathway.
5. Conclusions
The results indicated that GA could reduce the toxicity of HA through the anti-necrotic and anti-apoptotic ways, and that the mechanism of this herb-pair’s effect might be correlated with the PI3K/Akt signaling pathway. These results also provided strong evidence for clinical use of Aconitum carmichaelii and Radix Glycyrrhizae. Furthermore, this study method might be a guide for researching mechanisms of other compatibility medicines.
Contributors
Jie-hong YANG and Hai-tong WAN guided the experiment progress, Li-qin WANG implemented the experiment and wrote the paper, Yu HE participated in the pharmacodynamics experiment and the modification of this paper, Hao-fang WAN performed the cell culture work, and Hui-fen ZHOU performed the fluorescence microscope and flow cytometry work.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 81473412, 81573868, and 81630105), the Zhejiang Provincial Natural Science Foundation of China (No. LZ17H270001), and the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, China
Compliance with ethics guidelines: Li-qin WANG, Yu HE, Hao-fang WAN, Hui-fen ZHOU, Jie-hong YANG, and Hai-tong WAN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Cai Y, Gao Y, Tan G, et al. Myocardial lipidomics profiling delineate the toxicity of traditional Chinese medicine Aconiti Lateralis radix praeparata. J Ethnopharmacol. 2013;147(2):349–356. doi: 10.1016/j.jep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Shen X, Shen F, et al. TAK1 activates AMPK-dependent cell death pathway in hydrogen peroxide-treated cardiomyocytes, inhibited by heat shock protein-70. Mol Cell Biochem. 2013;377(1-2):35–44. doi: 10.1007/s11010-013-1568-z. [DOI] [PubMed] [Google Scholar]
- 3.Fan R, Li N, Xu H, et al. The mechanism of hydrothermal hydrolysis for glycyrrhizic acid into glycyrrhetinic acid and glycyrrhetinic acid 3-O-mono-β-D-glucuronide in subcritical water. Food Chem. 2016;190:912–921. doi: 10.1016/j.foodchem.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 4.Freude B, Masters TN, Robicsek F, et al. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol. 2000;32(2):197–208. doi: 10.1006/jmcc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 5.Gao F, Hu XY, Xie XJ, et al. Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(8):608–617. doi: 10.1631/jzus.B1001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao QT, Chen XH, Bi KS. Comparative pharmacokinetic behavior of glycyrrhetic acid after oral administration of glycyrrhizic acid and Gancao-Fuzi-Tang. Biol Pharm Bull. 2004;27(2):226–228. doi: 10.1248/bpb.27.226. [DOI] [PubMed] [Google Scholar]
- 7.He W, Zhang MF, Ye J, et al. Cordycepin induces apoptosis by enhancing JNK and p38 kinase activity and increasing the protein expression of Bcl-2 pro-apoptotic molecules. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(9):654–660. doi: 10.1631/jzus.B1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9):a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai CC, Tang CY, Chiang SC, et al. Ischemic preconditioning activates prosurvival kinases and reduces myocardial apoptosis. J Chin Med Assoc. 2015;78(8):460–468. doi: 10.1016/j.jcma.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14(4):536–548. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu QY, Zhang YY, Wan HT, et al. Detoxicated effect of compatibility of hypaconitine and liquiritin, glycyrrhetinic acid. China J Tradit Chin Med Pharm. 2013;28(9):2601–2604. [Google Scholar]
- 12.Liu T, Zhu W, Yang X, et al. Detection of apoptosis based on the interaction between annexin V and phosphatidylserine. Anal Chem. 2009;81(6):2410–2413. doi: 10.1021/ac801267s. [DOI] [PubMed] [Google Scholar]
- 13.Marambio P, Toro B, Sanhueza C, et al. Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim Biophys Acta. 2010;1802(6):509–518. doi: 10.1016/j.bbadis.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Menezes AR, Lavie CJ, Milani RV, et al. Psychological risk factors and cardiovascular disease: is it all in your head? Postgrad Med. 2011;123(5):165–176. doi: 10.3810/pgm.2011.09.2472. [DOI] [PubMed] [Google Scholar]
- 15.Munk PS, Larsen AI. Inflammation and C-reactive protein in cardiovascular disease. Tidsskr Nor Laegeforen. 2009;129(12):1221–1224. doi: 10.4045/tidsskr.08.0011. (in Norwegian) [DOI] [PubMed] [Google Scholar]
- 16.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366(1):54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 17.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82(2):250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 18.Qiao S, Mao X, Wang Y, et al. Remifentanil preconditioning reduces postischemic myocardial infarction and improves left ventricular performance via activation of the Janus activated kinase-2/signal transducers and activators of transcription-3 signal pathway and subsequent inhibition of glycogen synthase kinase-3β in rats. Crit Care Med. 2016;44(3):e131–e145. doi: 10.1097/ccm.0000000000001350. [DOI] [PubMed] [Google Scholar]
- 19.Quan W, Wu B, Bai Y, et al. Magnesium lithospermate B improves myocardial function and prevents simulated ischemia/reperfusion injury-induced H9c2 cardiomyocytes apoptosis through Akt-dependent pathway. J Ethnopharmacol. 2014;151(1):714–721. doi: 10.1016/j.jep.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Rizvi M, Jawad N, Li Y, et al. Effect of noble gases on oxygen and glucose deprived injury in human tubular kidney cells. Exp Biol Med (Maywood) 2010;235(7):886–891. doi: 10.1258/ebm.2010.009366. [DOI] [PubMed] [Google Scholar]
- 21.Shang M, Zhang Q, Zhang MX, et al. Effects of endothelial microvesicles induced by A23187 on H9c2 cardiomyocytes. Chin J Appl Physiol. 2013;29(6):559–564. [PubMed] [Google Scholar]
- 22.Shi R, Liu L, Huo Y, et al. Study on protective effects of Panax notoginseng saponins on doxorubicin-induced myocardial damage. China J Chin Mat Med. 2007;32(24):2632–2635. (in Chinese) [PubMed] [Google Scholar]
- 23.Sun X, Chen RC, Yang ZH, et al. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 25.Tong H, Chen W, Steenbergen C, et al. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87(4):309–315. doi: 10.1161/01.RES.87.4.309. [DOI] [PubMed] [Google Scholar]
- 26.van der Hoeven BL, Schalij MJ, Delgado V. Multimodality imaging in interventional cardiology. Nat Rev Cardiol. 2012;9(6):333–346. doi: 10.1038/nrcardio.2012.14. [DOI] [PubMed] [Google Scholar]
- 27.Vermes I, Haanen C, Steffens-Nakken H, et al. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 28.Wakayama K, Fukai M, Yamashita K, et al. Successful transplantation of rat hearts subjected to extended cold preservation with a novel preservation solution. Transpl Int. 2012;25(6):696–706. doi: 10.1111/j.1432-2277.2012.01469.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Ji SY, Liu SZ, et al. Cardioprotective effect of breviscapine: inhibition of apoptosis in H9c2 cardiomyocytes via the PI3K/Akt/eNOS pathway following simulated ischemia/reperfusion injury. Pharmazie. 2015;70(9):593–597. [PubMed] [Google Scholar]
- 30.Wang JX, Zhang XJ, Li Q, et al. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res. 2015;117(4):352–363. doi: 10.1161/circresaha.117.305781. [DOI] [PubMed] [Google Scholar]
- 31.Wu HJ, Yang JY, Jin M, et al. Glycyrrhetinic acid protects the heart from ischemia/reperfusion injury by attenuating the susceptibility and incidence of fatal ventricular arrhythmia during the reperfusion period in the rat hearts. Cell Physiol Biochem. 2015;36(2):741–752. doi: 10.1159/000430134. [DOI] [PubMed] [Google Scholar]
- 32.Xie S, Jia Y, Liu A, et al. Hypaconitine-induced QT prolongation mediated through inhibition of KCNH2 (hERG) potassium channels in conscious dogs. J Ethnopharmacol. 2015;166:375–379. doi: 10.1016/j.jep.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Yin XJ, Guo HM, et al. Identification and comparative analysis of the major chemical constituents in the extracts of single Fuzi herb and Fuzi-Gancao herb-pair by UFLC-IT-TOF/MS. Chin J Nat Med. 2014;12(7):542–553. doi: 10.1016/s1875-5364(14)60084-4. [DOI] [PubMed] [Google Scholar]
- 34.Yu B, Cao Y, Xiong YK. Pharmacokinetics of aconitine-type alkaloids after oral administration of Fuzi (Aconiti Lateralis Radix Praeparata) in rats with chronic heart failure by microdialysis and ultra-high performance liquid chromatography-tandem mass spectrometry. J Ethnopharmacol. 2015;165:173–179. doi: 10.1016/j.jep.2015.01.057. [DOI] [PubMed] [Google Scholar]
- 35.Yu HT, Zhen J, Pang B, et al. Ginsenoside Rg1 ameliorates oxidative stress and myocardial apoptosis in streptozotocin-induced diabetic rats. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(5):344–354. doi: 10.1631/jzus.B1400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu LN, Yu J, Zhang FJ, et al. Sevoflurane postconditioning reduces myocardial reperfusion injury in rat isolated hearts via activation of PI3K/Akt signaling and modulation of Bcl-2 family proteins. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(9):661–672. doi: 10.1631/jzus.B1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yucel AF, Kanter M, Pergel A, et al. The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol. 2011;42(6):579–587. doi: 10.1007/s10735-011-9364-0. [DOI] [PubMed] [Google Scholar]
- 38.Yue H, Pi ZF, Li HL, et al. Studies on the stability of diester-diterpenoid alkaloids from the genus Aconitum L. by high performance liquid chromatography combined with electrospray ionisation tandem mass spectrometry (HPLC/ESI/MSn) Phytochem Anal. 2008;19(2):141–147. doi: 10.1002/pca.1027. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JM, Liao W, He YX, et al. Study on intestinal absorption and pharmacokinetic characterization of diester diterpenoid alkaloids in precipitation derived from Fuzi-Gancao herb-pair decoction for its potential interaction mechanism investigation. J Ethnopharmacol. 2013;147(1):128–135. doi: 10.1016/j.jep.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Zhang H, Sun S, et al. Comparative pharmacokinetics of hypaconitine after oral administration of pure hypaconitine, Aconitum carmichaelii extract and Sini Decoction to rats. Molecules. 2015;20(1):1560–1570. doi: 10.3390/molecules20011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao D, Wang J, Cui Y, et al. Pharmacological effects of Chinese herb aconite (Fuzi) on cardiovascular system. J Tradit Chin Med. 2012;32(3):308–313. doi: 10.1016/S0254-6272(13)60030-8. [DOI] [PubMed] [Google Scholar]
- 42.Zheng K, Sheng Z, Li Y, et al. Salidroside inhibits oxygen glucose deprivation (OGD)/re-oxygenation-induced H9c2 cell necrosis through activating of Akt-Nrf2 signaling. Biochem Biophys Res Commun. 2014;451(1):79–85. doi: 10.1016/j.bbrc.2014.07.072. [DOI] [PubMed] [Google Scholar]
- 43.Zhou G, Tang L, Zhou X, et al. A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux. J Ethnopharmacol. 2015;160:173–193. doi: 10.1016/j.jep.2014.11.043. [DOI] [PubMed] [Google Scholar]