Abstract
Marsdeniae tenacissimae extract (MTE) has been used as an adjuvant medicine for cancer therapy for a long time. Although massive studies demonstrated its considerable anti-cancer effect, there is no research on its influence on erythrocytes, which are firstly interacted with MTE in the circulation. To investigate the influence of MTE on erythrocytes, we used a flow cytometer to detect the MTE-treated alternations of morphology, calcium concentration, and reactive oxygen species (ROS) level in erythrocytes. We used hemolysis under different osmotic solutions to evaluate the fragility of erythrocytes. Data showed that MTE treatment dose-dependently increased the ratio of erythrocyte fragmentation (P<0.001) and shrinking, and elevated the forward scatter (FSC) value (P<0.001) and calcium accumulation (P<0.001). MTE induced ROS production of erythrocytes under the high glucose condition (P<0.01) and consequently caused a rise in fragility (P<0.05). These results suggest that MTE induces cytotoxicity and aging in erythrocytes in a dose-dependent manner, and presents the possibility of impairment on cancer patients’ circulating erythrocytes when MTE is used as an anti-cancer adjuvant medicine.
Keywords: Marsdeniae tenacissimae extract, Erythrocyte, Calcium, Reactive oxygen species (ROS), Fragility
1. Introduction
In recent decades, traditional herbal medicine has been increasingly used in anti-tumor therapy as an enhancement of radiation therapy and chemotherapy. The plant Marsdeniae tenacissimae, belonging to the section asclepiadaceous, has been used as a folk medicine for thousands of years in China. In clinical terms, it plays an immunoregulation role in asthma, trachitis, tonsillitis, pharyngitis, pneumonia, and rheumatism (Ye et al., 2014a; Zhu et al., 2014; Fan et al., 2015). Additionally, M. tenacissimae extract (MTE) has long been used as an adjuvant medicine for cancer therapy (Han et al., 2012; 2014). Using the water extract of M. tenacissimae, Xiao-Ai-Ping injection has been approved by China Food and Drug Administration (CFDA) for antitumor therapy (Huang et al., 2013a). A number of recent studies suggested that MTE treatment alone presented a distinctive effect against various cancers, including gastric cancer, esophageal cancer, lung cancer, and hepatocellular carcinoma, through suppressing proliferation and promoting apoptosis of cancer cells (Huang et al., 2013a; 2013b; Li et al., 2015). Previous research in our lab showed that the inhibition of MTE on tumor angiogenesis depended on the attenuation of vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR2) interactions and the protein kinase C pathway in vein endothelial cells (Chen et al., 2016).
Currently, most studies on MTE focus on its anti-tumor effect, but no information is available about its influence on blood cells, which are firstly interacted with MTE in the circulation after its injection. Erythrocytes are the largest in the number of blood cells with the principal function of delivering oxygen to the body tissues. Mature erythrocytes are flexible and oval biconcave disks, without a cell nucleus, mitochondria, or most organelles, in order to accommodate maximum space for hemoglobin (Ellsworth et al., 2009; Pretorius et al., 2016). Some traditional medicine-induced apoptosis and suicidal erythrocyte death have been reported (Lupescu et al., 2012; Zelenak et al., 2012; Bouguerra et al., 2015). However, other compounds, such as salidroside, protected erythrocytes against oxidative stress and apoptosis (Qian et al., 2011; 2012; Shaik et al., 2012). Because of the cytotoxicity of MTE on tumor cells, we supposed MTE might also induce biochemical and biophysical alterations in erythrocytes.
In the current study, we address the influence of MTE on human erythrocytes and the underlying mechanism. To achieve this goal, we carried out comparative studies on erythrocyte size, integrality, reactive oxygen species (ROS), calcium content and hemolysis (fragility) after MTE treatment.
2. Materials and methods
2.1. MTE and erythrocytes
MTE was extracted from the stem of M. tenacissimae as follows: the powder of M. tenacissimae stem was gained by extracting with water three times, followed by filtering and concentrating. The water extract was resuspended with 85% ethanol and centrifuged at 4 °C three times. Finally the ethanol extract was lyophilized and resuspended at 500 μg/ml in DMSO (Sigma, St. Louis, MO, USA).
Fresh leukocyte-reduced erythrocytes (within 5 d after donation) were kindly provided by the Blood Transfusion Department of Zhejiang Provincial People’s Hospital, Hangzhou, China. Erythrocytes, before use, were washed three times in saline to remove the preservative. Then the erythrocytes were suspended and incubated in RPMI 1640 media (Invitrogen, Carlsbad, CA, USA) at a hematocrit 0.5% with indicated glucose and MTE concentrations at 37 °C for 24 h.
2.2. Forward scatter and side scatter analysis
After incubation in RPMI 1640 media containing 0, 64, 128, and 256 μg/ml MTE for 24 h, 200 μl erythrocyte solutions were suspended in fresh media and analyzed for forward scatter (FSC) and side scatter (SSC) using a flow cytometer (FACS, ACEA NovoCyte, San Diego, CA, USA). Fresh washed erythrocytes were used to determine the integral ranges of FSC and SSC for calculating the integrity rate.
2.3. ROS analysis
Erythrocytes were incubated in RPMI 1640 media with different levels of glucose (2.8, 5.6, and 11.1 mmol/L) and MTE (0, 64, 128, and 256 μg/ml) for 24 h, and then immediately loaded with 2 mmol/L ROS fluorescent dye 2',7'-dichlorofluorescin diacetate (DCFH-DA; Beyotime, Peking, China) for 20 min at 37 °C. The fluorescence intensity of the erythrocytes was measured in the FACS device with excitation 488 nm and emission 530 nm.
2.4. Calcium analysis
The erythrocytes were treated with different levels of MTE (0, 64, 128, and 256 μg/ml) for 24 h, and then immediately loaded with 1 μmol/L calcium fluorescent dye Fluo-AM (Molecular Probes, Eugene, OR, USA) for 20 min at 37 °C. The fluorescence intensity of the erythrocytes was measured by the FACS device with excitation 488 nm and emission 530 nm.
2.5. Erythrocyte osmotic fragility
Erythrocyte fragility was examined in different amounts of NaCl solution (pH 7.4) from 0.0% to 0.9% (1%=0.01 g/ml). Briefly, after 24 h treatment with MTE, erythrocytes were carefully suspended in various concentrations of NaCl and incubated for 2 h at 4 °C. Subsequently, the test tubes were centrifuged at 600g, and the supernatant was collected for hemolysis measurement via 540 nm absorbance using a microplate reader (Tecan, Männedorf, Switzerland). The percentage of hemolysis was normalized by pure water hemolysis supernatant.
2.6. Statistics
All data are presented as mean±standard deviation (SD) and were analyzed using Student unpaired two-tailed t-test and one-way analysis of variance (ANOVA) with Tukey post-hoc test (GraphPad Prism 6 software). P<0.05 was considered significant.
3. Results
3.1. MTE treatment decreased the normal integrated erythrocyte ratio
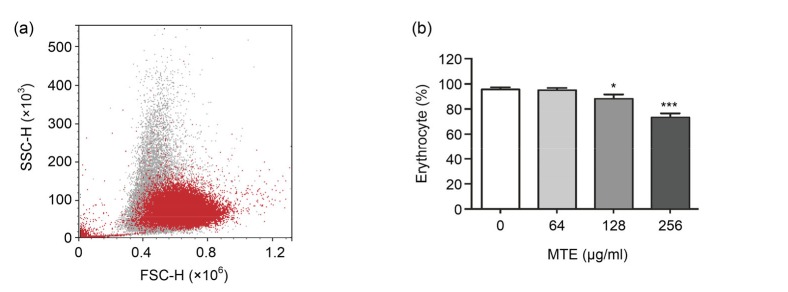
To investigate the influence of MTE on erythrocytes, we first tested the number alternation of erythrocytes after MTE treatment. MTE treatment showed a dose-dependent reduction on the ratio of integrated erythrocytes, which were defined by fresh erythrocytes (Fig. 1). Meanwhile small fragments of erythrocyte were much increased after 256 µg/ml MTE treatment, and a typical result is shown in Fig. 1a.
Fig. 1.
Impact of MTE on the integrated erythrocyte ratio
(a) The decreased ratio of normal erythrocyte after 256 μg/ml MTE treatment (red) compared with untreated (gray). (b) Various concentrations of MTE decreased the integrated erythrocyte ratio gradually. Data are expressed as mean±SD (n=3). * P<0.05, *** P<0.001 vs. control group (no MTE) (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
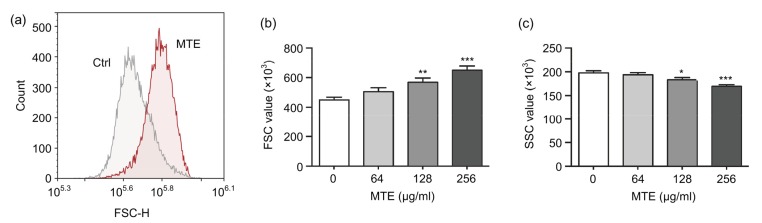
3.2. MTE elevated FSC but reduced SSC of erythrocytes
To explore the effects of MTE on the size and granularity of erythrocytes, the FSC and SSC of erythrocytes were detected by a flow cytometer. Incubation in 256 µg/ml MTE for 24 h significantly increased the FSC level as shown in Fig. 2a. A more detailed experiment showed MTE dose-dependently elevated erythrocyte FSC level, in which 256 and 128 µg/ml but not 64 µg/ml MTE have a significant increase (Fig. 2b). Meanwhile, the SSC level of erythrocytes gradually decreased with increasing MTE concentration (Fig. 2c). Under the microscope, normal erythrocytes appeared as an oval biconcave disk (Fig. 3a). However, low concentration of MTE (64 and 128 µg/ml) treatment induced much more bending of the cell (Figs. 3b and 3c). High dosage of MTE resulted in cell shrinking and the presence of irregular, or even absence of, concave characteristic (Fig. 3d).
Fig. 2.
Effects of MTE on FSC and SSC values of erythrocytes
(a) 256 μg/ml MTE induced a higher FSC level of erythrocytes. (b) Various concentrations of MTE increased the FSC level of erythrocyte gradually. (c) Various concentrations of MTE decreased the SSC level of erythrocyte. Data are expressed as mean±SD (n=3). * P<0.05, ** P<0.01, *** P<0.001 vs. control (Ctrl) group (no MTE)
Fig. 3.
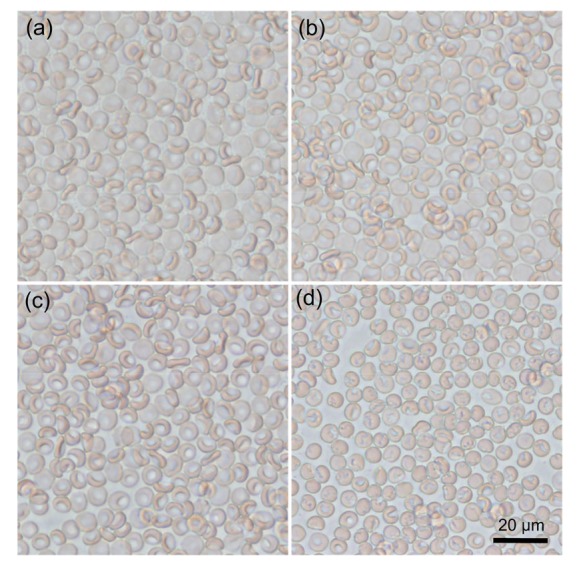
Typical light field photos of erythrocyte after various concentrations of MTE treatment
(a) Control; (b) 64 μg/ml MTE; (c) 128 μg/ml MTE; (d) 256 μg/ml MTE. Bar=20 μm
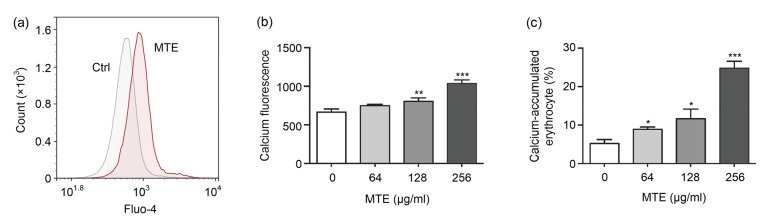
3.3. MTE treatment induced erythrocytes’ calcium accumulation
To investigate the underlying mechanism of erythrocytes’ shape alternation and eryptosis, their calcium concentrations were measured by Fluo-4 fluorescence. Erythrocytes’ calcium influx is an important trigger of eryptosis, and is dramatically induced by 256 µg/ml MTE (Fig. 4a). Further experiments revealed that the higher the concentration of MTE treated, the more calcium accumulated in erythrocytes (Fig. 4b). Counting the ratio of erythrocytes with high calcium content (fluorescence value >1000) also showed the same result, i.e. that the calcium cell ratio was markedly increased after MTE treatment (Fig. 4c).
Fig. 4.
Effect of MTE on calcium content in erythrocytes
(a) 256 μg/ml MTE stimulated calcium accumulation in erythrocytes. (b) Various concentrations of MTE increased the calcium level of erythrocytes gradually. (c) Otherwise various concentrations of MTE induced a high ratio of calcium-accumulated erythrocytes (calcium fluorescence >1000). Data are expressed as mean±SD (n=3). * P<0.05, ** P<0.01, *** P<0.001 vs. control (Ctrl) group (no MTE)
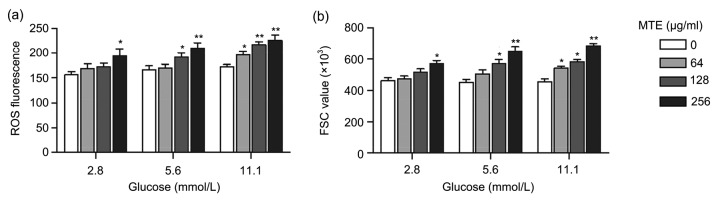
3.4. MTE treatment stimulated erythrocyte ROS production in high glucose condition
To reveal the relation between MTE and erythrocyte ROS production, the erythrocytes were loaded with DCFH-DA after MTE incubation. Under a normal glucose condition (5.6 mmol/L), 128 and 256 µg/ml MTE markedly stimulated ROS production (Fig. 5a), and 2.8 mmol/L glucose attenuated the increasing effect of 128 µg/ml MTE. However, 11.1 mmol/L glucose enhanced the effect of 64 µg/ml MTE, leading to evident ROS elevation (Fig. 5a). ROS is one of the byproducts of aerobic metabolism, and would damage erythrocytes. Therefore, we examined erythrocyte FSC under different glucose conditions. The data showed that the FSC level has the same results as ROS (Fig. 5b), which suggested the influence of MTE on erythrocyte ROS and FSC depended on a high glucose metabolism.
Fig. 5.
Effects of MTE on erythrocyte ROS content and FSC under different glucose concentrations
(a) ROS in erythrocytes was more induced by MTE under high glucose (11.1 mmol/L) environment than under low glucose (2.8 mmol/L). (b) FSC levels of erythrocytes were much more stimulated by MTE under high glucose environment than under low glucose. Data are expressed as mean±SD (n=3). * P<0.05, ** P<0.01 vs. control group (no MTE) for each glucose concentration
3.5. MTE induced high erythrocytes’ fragility
To investigate the erythrocytes’ fragility, we tested the hemolysis under different osmotic solutions. Fig. 6 shows that low osmotic solution induced severe erythrocyte hemolysis, and under 0.40%‒0.45% NaCl concentrations MTE-treated erythrocytes presented a high hemolysis ratio, which implicated that MTE induced erythrocyte impairment and a greater sensitivity to decreasing osmosis.
Fig. 6.
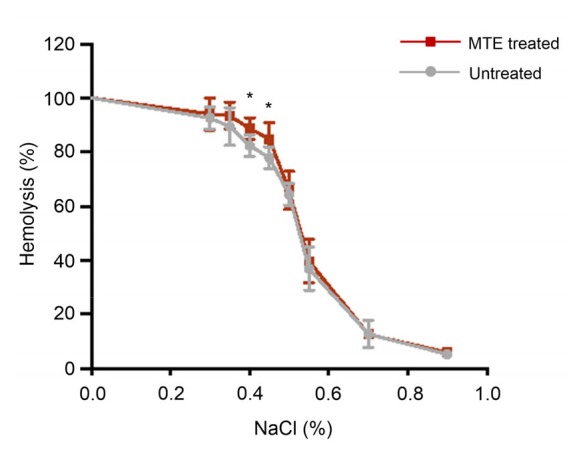
Erythrocytes fragility test under a serial solution containing different NaCl concentrations
MTE treatment induced a high hemolysis ratio under 0.40%‒ 0.45% NaCl solutions (1%=0.01 g/ml). Data are expressed as mean±SD (n=3). * P<0.05 vs. untreated group
4. Discussion
In China, M. tenacissimae has already been used in asthma, trachitis, tonsillitis, pharyngitis, pneumonia, and rheumatism as an immunoregulator (Ye et al., 2014a; Zhu et al., 2014; Fan et al., 2015). In recent decades, M. tenacissimae water extract Xiao-Ai-Ping has been approved by CFDA and used as an adjuvant medicine for cancer therapy in China (Huang et al., 2013a). Studies have reported that MTE could decrease the proliferation and increase the apoptosis of hematologic neoplasm tumor cells both in vitro and in vivo (Ye et al., 2014a). At the same time MTE showed an obvious enhancement on gefitinib-induced anti-cancer efficacy via prompting cell cycle arrest and apoptosis and suppression of the activation of epidermal growth factor receptor (EGFR) and c-Met in both EGFR mutant and wild-type non-small cell lung cancer cells (Han et al., 2012; 2014). Our lab previously revealed the strong anti-proliferation effect of MTE on human vein endothelial cells due to the attenuated VEGF/VEGFR2 interactions and promoted apoptosis through p53-dependent mitochondrial pathway (Chen et al., 2016).
Because of the marked cytotoxicity of MTE, we speculated that it might have the same or even more serious impairment on erythrocytes, which are firstly interacted with MTE in the circulation. However, there was no report about the influence of MTE on erythrocytes. Erythrocyte is the unique cell for delivering oxygen to the body tissues. Its damage may cause a series of symptoms of tissue hypoxia (Tziakas et al., 2012). It has been shown that other adjuvant anti-cancer medicines, such as gambogic acid and tanshinone IIA, impaired erythrocytes via shrinking, reduction of ATP, and promotion of apoptosis (Lupescu et al., 2012; Zelenak et al., 2012). Salidroside, naringin, and cisplatin, on the other hand, have a protective effect through inhibiting ROS production and calcium influx (Qian et al., 2011; 2012; Shaik et al., 2012; Yang et al., 2015). In this study, we elucidated the impairment of MTE on erythrocytes’ quantity and appearance (Figs. 1‒3). After MTE treatment, the ratio of integral erythrocyte was reduced while increased FSC suggested the alternation of erythrocyte in shape (Fig. 2). Observation under microscope confirmed that change of erythrocytes that lost the oval biconcave disks after exposure to MTE (Fig. 3). Consequently, the MTE-induced change of erythrocytes in shape led to a greater sensitivity and higher fragility to decreasing osmosis (Fig. 6).
To investigate the underlying mechanism of MTE on eryptosis, we further examined calcium accumulation and ROS production of erythrocytes after MTE treatment. Due to the lack of mitochondria and nuclei, eryptosis is unregulated by mitochondria dysfunction and gene expression. Indeed, calcium influx activates Ca2+-sensitive K+ channels, cell shrinkage, and membrane scrambling, then triggers erythrocyte aging and eryptosis (Maher and Kuchel, 2003; Bogdanova et al., 2013). MTE-treated erythrocytes showed a high average calcium concentration (Fig. 4b) and an increasing ratio of high-calcium cells (Fig. 4c), and these may contribute to the acute erythrocyte decline (Fig. 1) and chronic erythrocyte aging as demonstrated by high erythrocyte fragility (Fig. 6). ROS is another important injury factor. In erythrocytes, because of a deficiency of mitochondria, ROS is mainly produced by cytosolic aerobic oxidation and auto-oxidation during oxygen delivering (Abed et al., 2014; Lang et al., 2014). A high level of ROS will interrupt the cell membrane lipid and cell cytoskeleton, and consequently impair the normal erythrocyte shape (Ziobro et al., 2013). Our data showed that 24 h MTE incubation induced high ROS production (Fig. 5) in erythrocytes. Experiments of different glucose levels revealed that erythrocyte ROS and FSC were higher as the glucose concentration level increased (Fig. 5), which suggested that metabolism-dependent ROS production may be the major reason for the rise in FSC and shape alteration.
MTE, in our experiments, was a crude extract containing abundant C21 steroids with anti-cancer activity, which have been previously extracted from several kinds of herbal medicine and have presented cytotoxicity in various cancer cell lines (Li et al., 2016; Yin et al., 2016). From M. tenacissimae, more than 40 kinds of C-21 steroidal glycosides have been isolated (Zhang et al., 2010). Tenacissoside C, a natural bioactive compound of C-21 steroidal saponins, was purified from M. tenacissimae and exhibited a marked anti-tumor effect on the myelogenous leukemia cell line (Ye et al., 2014b). In contrast, another important component of Xiao-Ai-Ping injection, such as 17β-tenacigenin B, had no significant anti-tumor effect in vitro (Wang et al., 2015). Therefore, we have strong hopes to reveal the possible compounds causing the erythrocyte impairment and try to attenuate this adverse effect in future research.
5. Conclusions
In this study, we found MTE-induced dose-dependent cytotoxicity and aging in erythrocytes. MTE treatment increases the ratio of erythrocyte fragmentation and shrinking, elevates the calcium and ROS levels, and consequently causes increased fragility. These findings suggest a possible impairment on cancer patients’ circulating erythrocytes when the Xiao-Ai-Ping injection is used as an anti-cancer adjuvant medicine.
Contributors
Ke HAO and Bing-yu CHEN equally contributed to the experimental research, data analysis, and writing and editing of the manuscript. Kai-qiang LI and Yu ZHANG contributed to the experimental research. Cai-xia LI and Ying WANG contributed to the writing and editing of the manuscript. Lu-xi JIANG and Jiang SHEN contributed to the fragility test. Xiang-chai GUO and Wei ZHANG contributed to the study design and data analysis. Meng-hua ZHU and Zhen WANG contributed to the study design, data analysis, and writing and editing of the manuscript. Meng-hua ZHU and Zhen WANG are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation (Nos. LQ16H070003, LY15H280010, and LY15C090004), the Key Project of Chinese Medicine in Zhejiang Province (No. 2015ZZ001), the Traditional Chinese Medicine Scientific Research Foundation of Zhejiang Province (No. 2014ZB007), the Traditional Chinese Medicine Outstanding Young Talent Foundation of Zhejiang Province (No. 2014ZQ005), and the Medicine and Health Research Foundation of Zhejiang Province (No. 2015ZDA002), China
Compliance with ethics guidelines: Ke HAO, Bing-yu CHEN, Kai-qiang LI, Yu ZHANG, Cai-xia LI, Ying WANG, Lu-xi JIANG, Jiang SHEN, Xiang-chai GUO, Wei ZHANG, Meng-hua ZHU, and Zhen WANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Abed M, Artunc F, Alzoubi K, et al. Suicidal erythrocyte death in end-stage renal disease. J Mol Med. 2014;92(8):871–879. doi: 10.1007/s00109-014-1151-4. [DOI] [PubMed] [Google Scholar]
- 2.Bogdanova A, Makhro A, Wang J, et al. Calcium in red blood cells–a perilous balance. Int J Mol Sci. 2013;14(5):9848–9872. doi: 10.3390/ijms14059848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouguerra G, Aljanadi O, Bissinger R, et al. Embelin-induced phosphatidylserine translocation in the erythrocyte cell membrane. Cell Physiol Biochem. 2015;37(4):1629–1640. doi: 10.1159/000438529. [DOI] [PubMed] [Google Scholar]
- 4.Chen BY, Chen D, Lyu JX, et al. Marsdeniae tenacissimae extract (MTE) suppresses cell proliferation by attenuating VEGF/VEGFR2 interactions and promotes apoptosis through regulating PKC pathway in human umbilical vein endothelial cells. Chin J Nat Med. 2016;14(12):922–930. doi: 10.1016/S1875-5364(17)30017-1. [DOI] [PubMed] [Google Scholar]
- 5.Ellsworth ML, Ellis CG, Goldman D, et al. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology. 2009;24(2):107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan W, Sun L, Zhou JQ, et al. Marsdenia tenacissima extract induces G0/G1 cell cycle arrest in human esophageal carcinoma cells by inhibiting mitogen-activated protein kinase (MAPK) signaling pathway. Chin J Nat Med. 2015;13(6):428–437. doi: 10.1016/S1875-5364(15)30036-4. [DOI] [PubMed] [Google Scholar]
- 7.Han SY, Zhao MB, Zhuang GB, et al. Marsdenia tenacissima extract restored gefitinib sensitivity in resistant non-small cell lung cancer cells. Lung Cancer. 2012;75(1):30–37. doi: 10.1016/j.lungcan.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Han SY, Ding HR, Zhao W, et al. Enhancement of gefitinib-induced growth inhibition by Marsdenia tenacissima extract in non-small cell lung cancer cells expressing wild or mutant EGFR. BMC Complement Altern Med. 2014;14:165. doi: 10.1186/1472-6882-14-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z, Wang Y, Chen J, et al. Effect of Xiaoaiping injection on advanced hepatocellular carcinoma in patients. J Tradit Chin Med. 2013;33(1):34–38. doi: 10.1016/S0254-6272(13)60097-7. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Lin H, Wang Y, et al. Studies on the anti-angiogenic effect of Marsdenia tenacissima extract in vitro and in vivo. Oncol Lett. 2013;5(3):917–922. doi: 10.3892/ol.2013.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang F, Abed M, Lang E, et al. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal. 2014;21(1):138–153. doi: 10.1089/ars.2013.5747. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Li C, Song Y, et al. Marsdenia tenacssima extract and its functional components inhibits proliferation and induces apoptosis of human Burkitt leukemia/lymphoma cells in vitro and in vivo . Leuk Lymphoma. 2015;57(2):419–428. doi: 10.3109/10428194.2015.1043546. [DOI] [PubMed] [Google Scholar]
- 13.Li JL, Gao ZB, Zhao WM. Identification and evaluation of antiepileptic activity of C21 steroidal glycosides from the roots of Cynanchum wilfordii . J Nat Prod. 2016;79(1):89–97. doi: 10.1021/acs.jnatprod.5b00766. [DOI] [PubMed] [Google Scholar]
- 14.Lupescu A, Jilani K, Zelenak C, et al. Induction of programmed erythrocyte death by gambogic acid. Cell Physiol Biochem. 2012;30(2):428–438. doi: 10.1159/000339036. [DOI] [PubMed] [Google Scholar]
- 15.Maher AD, Kuchel PW. The Gárdos channel: a review of the Ca2+-activated K+ channel in human erythrocytes. Int J Biochem Cell Biol. 2003;35(8):1182–1197. doi: 10.1016/S1357-2725(02)00310-2. [DOI] [PubMed] [Google Scholar]
- 16.Pretorius E, Olumuyiwa-Akeredolu OO, Mbotwe S, et al. Erythrocytes and their role as health indicator: using structure in a patient-orientated precision medicine approach. Blood Rev. 2016;30(4):263–274. doi: 10.1016/j.blre.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Qian EW, Ge DT, Kong SK. Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin. J Ethnopharmacol. 2011;133(2):308–314. doi: 10.1016/j.jep.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Qian EW, Ge DT, Kong SK. Salidroside protects human erythrocytes against hydrogen peroxide-induced apoptosis. J Nat Prod. 2012;75(4):531–537. doi: 10.1021/np200555s. [DOI] [PubMed] [Google Scholar]
- 19.Shaik N, Zbidah M, Lang F. Inhibition of Ca2+ entry and suicidal erythrocyte death by naringin. Cell Physiol Biochem. 2012;30(3):678–686. doi: 10.1159/000341448. [DOI] [PubMed] [Google Scholar]
- 20.Tziakas D, Chalikias G, Grapsa A, et al. Red blood cell distribution width–a strong prognostic marker in cardiovascular disease–is associated with cholesterol content of erythrocyte membrane. Clin Hemorheol Microcirc. 2012;51(4):243–254. doi: 10.3233/CH-2012-1530. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Cao J, Wang P, et al. Antitumor effect of C21 steroidal glycosides on adenoid cystic carcinoma cell line SACC83. Clin Lab. 2015;61(10):1553–1560. doi: 10.7754/clin.lab.2015.141218. [DOI] [PubMed] [Google Scholar]
- 22.Yang JT, Tang LH, Liu YQ, et al. Cisplatin combined with hyperthermia kills HepG2 cells in intraoperative blood salvage but preserves the function of erythrocytes. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(5):395–403. doi: 10.1631/jzus.B1400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye B, Li J, Li Z, et al. Anti-tumor activity and relative mechanism of ethanolic extract of Marsdenia tenacissima (Asclepiadaceae) against human hematologic neoplasm in vitro and in vivo . J Ethnopharmacol. 2014;153(1):258–267. doi: 10.1016/j.jep.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Ye B, Yang J, Li J, et al. In vitro and in vivo antitumor activities of tenacissoside C from Marsdenia tenacissima . Planta Med. 2014;80(1):29–38. doi: 10.1055/s-0033-1360128. [DOI] [PubMed] [Google Scholar]
- 25.Yin ZQ, Yu SL, Wei YJ, et al. C21 steroidal glycosides from Cynanchum stauntonii induce apoptosis in HepG2 cells. Steroids. 2016;106:55–61. doi: 10.1016/j.steroids.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Zelenak C, Pasham V, Jilani K, et al. Tanshinone IIA stimulates erythrocyte phosphatidylserine exposure. Cell Physiol Biochem. 2012;30(1):282–294. doi: 10.1159/000339064. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Tan AM, Zhang AY, et al. Five new C21 steroidal glycosides from the stems of Marsdenia tenacissima . Steroids. 2010;75(2):176–183. doi: 10.1016/j.steroids.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhu RJ, Shen XL, Dai LL, et al. Total aglycones from Marsdenia tenacissima increases antitumor efficacy of paclitaxel in nude mice. Molecules. 2014;9(9):13965–13975. doi: 10.3390/molecules190913965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziobro A, Duchnowicz P, Mulik A, et al. Oxidative damages in erythrocytes of patients with metabolic syndrome. Mol Cell Biochem. 2013;378(1-2):267–273. doi: 10.1007/s11010-013-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]