Abstract
Objective: Low-density granulocytes (LDGs) can form neutrophil extracellular traps (NETs) spontaneously and excessively. When peripheral blood mononuclear cells (PBMCs) are used for studying T lymphocytes, LDGs contained in the PBMCs may decrease the threshold of activating T lymphocytes by forming NETs. This study focused on the profiles of LDGs in common autoimmune diseases and methods for removing LDGs from PBMCs. Methods: The percentages of LDGs in PBMCs from 55 patients with dermatomyositis (DM), 15 with polymyositis (PM), 42 with rheumatoid arthritis (RA), 25 with systemic lupus erythematosus (SLE), and 19 healthy controls were determined by flow cytometry. Three methods of removing LDGs were explored and compared. After removal, PBMCs from six patients with positive T-SPOT.TB were tested again to find out if LDGs contained in the PBMCs could influence T lymphocyte reactions. Results: Significantly higher LDG percentages were found in PBMCs from patients with DM ((8.41±10.87)%, P<0.0001), PM ((8.41±10.39)%, P<0.0001), RA ((4.05±6.97)%, P=0.0249), and SLE ((7.53±11.52)%, P=0.0006), compared with the controls ((1.28±0.73)%). The T-SPOT.TB values significantly decreased after LDGs were removed. Increasing relative centrifugal force (RCF) within a limited range can decrease the LDG percentage from an initial high level, but not markedly increase the LDG clearance rate. Compared with the whole blood sediment method, the PBMC adherence method can significantly remove LDGs yet scarcely influence the T lymphocyte percentage in PBMCs. Conclusions: The LDG percentage in PBMCs is significantly increased in patients with SLE, DM, PM, and RA. The influence of LDGs on T lymphocytes cannot be ignored in PBMC cultures. The adherence method is a simple and easy-to-use method for removing LDGs and purifying T lymphocytes from PBMCs.
Keywords: Low-density granulocyte, Neutrophil extracellular trap, Peripheral blood mononuclear cells, Autoimmune disease, T-SPOT.TB
1. Introduction
Low-density granulocytes (LDGs) have been observed in peripheral blood mononuclear cells (PBMCs) of patients with systemic lupus erythematosus (SLE), a typical autoimmune disease (Denny et al., 2010; Pavón et al., 2012). Similar to mature autologous and healthy control neutrophils, LDGs express highly the neutrophil surface markers CD15, CD10, CD11b, CD11c, and CD16, but unlike neutrophils, their nuclear shape is of an immature phenotype (Denny et al., 2010). LDGs show characteristics of proinflammatory cells. Upon activation, they can injure endothelial cells and release a large quantity of tumor necrosis factors (TNFs) and type I and type II interferons (IFNs) (Denny et al., 2010; Villanueva et al., 2011; Carmona-Rivera and Kaplan, 2013; Carmona-Rivera et al., 2015).
When activated by pathogenic microbes or proinflammatory cytokines, LDGs can release double stranded DNA, histones, antibacterial peptides and enzymes to form neutrophil extracellular traps (NETs) which trap and kill pathogenic microbes (Brinkmann et al., 2004; Fuchs et al., 2007; Villanueva et al., 2011; Carmona-Rivera et al., 2015). Compared to autologous and healthy control neutrophils, LDGs exhibit a stronger capacity to form NETs, and are more likely to do so (Villanueva et al., 2011). In addition to their defensive function, NETs can influence T lymphocyte activation (Tillack et al., 2012).
PBMCs are often used in lymphocyte research and clinical tests (e.g. T-SPOT.TB test). Abnormally increased LDGs may spontaneously form NETs, and this may induce cluster formation, upregulation of the activation markers CD25 and CD69, and phosphorylation of the T-cell receptor (TCR)-associated signaling kinase ZAP70 in CD4+ T cells. T cell priming by NETs lowers the activation threshold (Tillack et al., 2012). Co-culture of T cells, dendritic cells (DCs), and NETting-neutrophils results in T cell activation and IFN-γ release (Tillack et al., 2012).
Therefore, it can reasonably be expected that T lymphocyte function is influenced by LDGs forming NETs in tests using PBMC samples. However, other than the expensive and complex flow cytometer and magnetic bead separation, there is no simple and easy-to-use method of removing LDGs from PBMCs. We focused in this study on finding an ideal method, and studying the influence of LDGs on T lymphocytes in the T-SPOT.TB test. Given the potential pathogenic role of LDGs in autoimmune diseases, it was important to measure the extent of LDG expression in some common autoimmune diseases. In addition to SLE, we included dermatomyositis (DM), polymyositis (PM), and rheumatoid arthritis (RA) to determine whether LDGs were also abnormally increased in their PBMCs.
2. Materials and methods
2.1. Subjects
We recruited 55 patients with DM and 15 with PM diagnosed according to Bohan and Peter’s criteria (Bohan and Peter, 1975a; 1975b) from the Department of Rheumatology at China-Japan Friendship Hospital, Beijing, China (April 2013 to April 2014). Also included were 42 patients with RA fulfilling the 2010 RA classification criteria (Aletaha et al., 2010) and 25 SLE patients fulfilling the 1997 American College of Rheumatology (ACR) SLE classification criteria (Hochberg, 1997); 19 age-and sex-matched healthy Chinese volunteers were selected as controls. General information about each group is shown in Table 1. The study was approved by the Ethics Committee of China-Japan Friendship Hospital. All patients and controls gave written informed consent.
Table 1.
General information about each group
| Group | Number | Age (year) | Sex, M/F | Durance (month) | Disease activity |
| DM | 55 | 44.73±16.11 | 8/47 | 26.4 | 3.23±2.83# |
| PM | 15 | 47.79±15.07 | 3/12 | 44.8 | 3.11±1.84# |
| RA | 42 | 48.42±10.22 | 6/36 | 35.2 | 4.8±1.4* |
| SLE | 25 | 43.45±18.51 | 4/21 | 52.3 | 8.90±4.52∆ |
| Control | 19 | 45.83±11.62 | 4/15 |
MYOACT, myositis disease activity assessment visual analogue scale;
DAS28, disease activity score in 28 joints;
SLEDAI, systemic lupus erythematosus disease activity index. Data of age and disease activity are expressed as mean±SD
2.2. PBMC isolation and LDG measurement
Blood was collected from patients and healthy individuals into an ethylenediaminetetraacetic acid (EDTA) Vacutainer. PBMCs were isolated by density gradient centrifugation over Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions. The mononuclear fraction was resuspended in phosphate buffered saline (PBS) and centrifuged again for 10 min at a relative centrifugal force (RCF) of 300g. The sediment was resuspended in 0.5 ml PBS. Fluorescein isothiocyanate (FITC)-labeled anti-CD15 (Biolegend, Catalog: 301904) and phycoerythrin (PE)-labeled anti-CD14 (Biolegend, Catalog: 325606) antibodies were used for flow cytometry tests. Compensation settings were adjusted using single stained PBMC samples. Isotype-matching labeled antibodies (Biolegend, Catalog: 401605, 400111) were used to calculate the amount of nonspecific staining. Lymphocytes, monocytes, and granulocytes were gated according to their forward and scatter characteristics as previously described (Denny et al., 2010; Pavón et al., 2012). Two color immunofluorescence analyses were performed on a Beckman Coulter Epics XL flow cytometer (Beckman Coulter, Inc., CA, USA).
2.3. PBMC adherence method
When lymphocytes are suspended in cell culture, granulocytes and monocytes easily adhere to the bottom of the cell culture plate. Based on this feature, LDGs should be removed from PBMCs by culturing PBMCs for a suitable time. Herein, separated PBMCs were resuspended in RPMI 1640 (cell concentration 1×105 ml−1) and cultured in a cell culture plate. Cell suspension was added to a 96-well plate (100 µl per well), a 48-well plate (300 µl per well), a 24-well plate (600 µl per well), and a 6-well plate (3 ml per well) according to cell culture routines and our experience. After 1 h of incubation in a CO2 incubator at 37 °C, the supernatant was very carefully collected and centrifuged for 10 min at 300g RCF. The percentage of LDGs remaining in the supernatants was tested by flow cytometry to determine if LDGs were removed from PBMCs by adherence. The PBMCs were ready for further culture after adherence.
2.4. T-SPOT.TB tests
PMBCs from patients with positive T-SPOT.TB results were selected. Their PBMCs were incubated according to the adherence method and then tested by T-SPOT.TB kit (Oxford Immunotec Ltd., Abingdon, Oxfordshire, UK) according to the manufacturer’s instructions to find out if removing LDGs from PBMCs can influence the results of T-SPOT.TB tests.
2.5. Whole blood sedimentation method
Because lymphocytes are usually suspended in cell culture, we investigated whether lymphocytes could be separated and LDGs removed by direct sedimentation of whole EDTA blood. Whole blood was left to stand in a test tube. After 3 h of sedimentation, plasma was carefully collected and centrifuged for 10 min at 300g RCF. The separated cells were resuspended in PBS and the LDG percentages were tested by flow cytometry.
2.6. Influence of RCF in PBMC preparation
PBMCs were isolated according to the manufacturer’s instructions by density gradient centrifugation over Histopaque-1077 for 30 min at 400g RCF, which is considered to be a standard method. To find out whether elevating the RCF can influence the percentage of LDGs in prepared PBMCs, aliquots of the same whole blood samples were centrifuged at RCFs of 600g and 800g for 30 min. The LDG percentages were compared in the PBMCs centrifuged at various RCFs.
2.7. Measurement of T lymphocyte percentages in PBMCs
To find out whether the separation methods could influence the T lymphocyte percentages in PBMCs, FITC-labeled anti-CD15 (Biolegend, Catalog: 301904), allophycocyanin (APC)-labeled anti-CD3 (Biolegend, Catalog: 300312), and PE-labeled anti-CD8 (Biolegend, Catalog: 300908) antibodies were used to quantify the total T lymphocytes, CD8+ T lymphocytes, and LDGs in PBMCs using flow cytometry.
2.8. Statistical analysis
GraphPad Prism 5 was used to perform comparisons between different groups and to draw figures. Calculations were based on a 95% confidence interval. P<0.05 was considered significant. An unpaired t-test was used for continuous variables fulfilling a Gaussian distribution, while a Mann-Whitney test was used for continuous variables that were not normally distributed. A paired t-test was used for the paired variables.
3. Results
3.1. Percentage of LDGs in PBMCs
The monocytes and LDGs could be distinguished based upon expression of the neutrophil marker CD15 and the monocyte marker CD14. Monocytes were CD14+/CD15lo or CD14+/CD15− whereas LDGs had a CD14lo/CD15+ or CD14−/CD15+ profile (Denny et al., 2010). Compared with the healthy control group ((1.28±0.73)% of total PBMCs), the LDG percentage was significantly increased in DM patients ((8.41±10.87)%, P<0.0001), PM patients ((8.41±10.39)%, P<0.0001), RA patients ((4.05±6.97)%, P=0.0249), and SLE patients ((7.53±11.52)%, P=0.0006) (Fig. 1), indicating that an increased percentage of LDGs is common to several systemic connective tissue diseases.
Fig. 1.
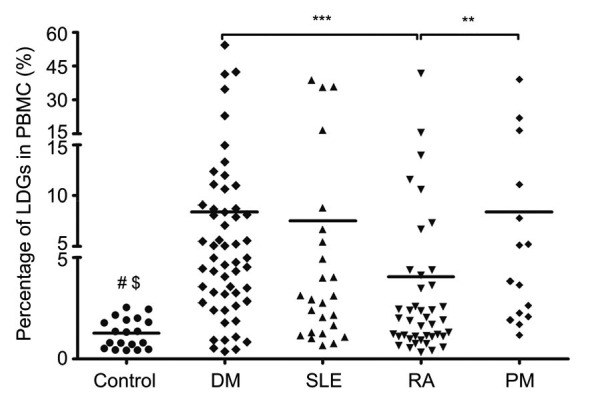
Comparison of LDG percentages in PBMCs of different disease groups
Statistical analyses were performed using an unpaired t-test with Welch’s correction. DM: dermatomyositis; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis; PM: polymyositis. ** P<0.01; *** P<0.001; # DM, PM, and SLE groups vs. control group: P<0.001; $ RA group vs. control group: P<0.05
3.2. Effect of removal of LDGs from PBMCs on T-SPOT.TB results
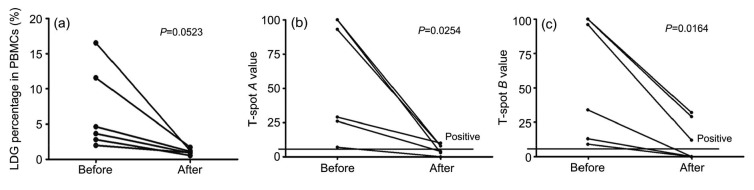
LDGs may decrease the threshold of activation of T lymphocytes by forming NETs when PBMCs are used for studying T lymphocyte function. Therefore, we chose T-SPOT.TB, commonly used in clinical practice to screen for tuberculosis, to test whether removing LDGs from PBMCs can influence the T-SPOT.TB results. Six patients with positive T-SPOT.TB results were selected and their PBMCs were adhered for 1 h before being used for the T-SPOT.TB test. The results indicated that the LDG percentage was markedly decreased after adherence of PBMCs (Fig. 2a). Meanwhile, the T-SPOT.TB A and B values were both significantly decreased compared to the primary results (Figs. 2b and 2c), suggesting that removal of LDGs has an effect on the reaction of T lymphocytes to specific antigens. The possible explanation could be that LDGs decrease the threshold of activation of T lymphocytes and amplify their immune response. Therefore, it was necessary to find a simple and easy-to-use method of removing LDGs from PBMCs.
Fig. 2.
Comparison of T-SPOT.TB values before and after the PBMC adherence method
Six patients with positive T-SPOT.TB results were selected and their PBMCs were adhered for 1 h before being used for the T-SPOT.TB test. (a) The LDG percentages before and after the PBMC adherence method were compared. (b, c) T-SPOT.TB A and B values were compared before and after the PBMC adherence method. Statistical analyses were performed using the paired t-test
3.3. Effect of elevating RCF on percentage of LDGs during PBMC separation
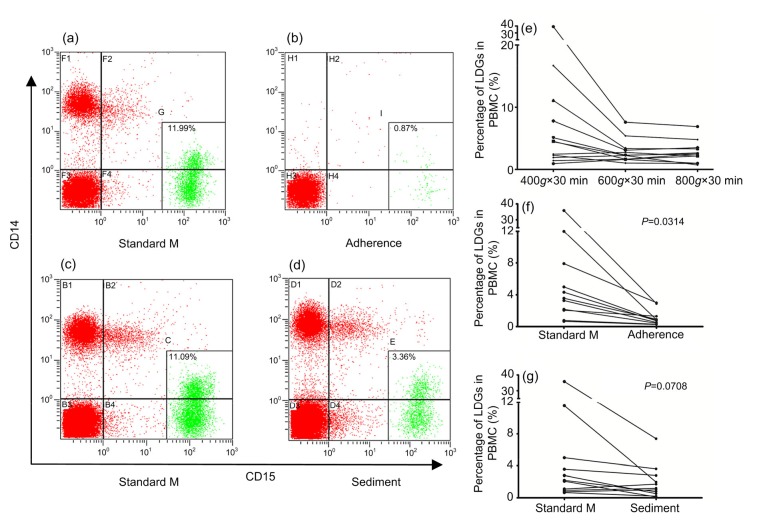
Compared to the standard method, centrifugation with RCFs of 600g and 800g decreased the LDG percentages in the separated PBMCs, especially for samples with higher LDG percentages, but the difference did not reach significance (P=0.0691 and P=0.0671; Fig. 3e). Centrifugation at 800g did not decrease the LDG percentage more than centrifugation at 600g (P=0.4747; Fig. 3e). These results indicate that elevating the RCF within a limited range was conducive to decreasing the LDG percentage in PBMCs, but could not completely remove LDGs.
Fig. 3.
Influence of various methods on the percentage of LDGs in PBMCs
Dot plots of CD15 staining (X-axis) versus CD14 staining (Y-axis) show characteristics of PBMC separated by the PBMC adherence method (a, b) and the whole blood sedimentation method (c, d). (e) PBMCs were isolated by density-gradient centrifugation over Histopaque-1077 for 30 min at 400g, 600g, and 800g RCF. (f) The LDG percentages in PBMCs of the standard method and PBMC adherence method were compared. (g) The LDG percentages in PBMCs of the standard method and whole blood sedimentation method were compared. Adherence: PBMC adherence method; Sediment: whole blood sedimentation method; Standard M: standard method. Statistical analyses were performed using the paired t-test
3.4. Comparison of PBMC adherence method and the whole blood sedimentation method for removing LDGs from PBMCs
Since elevating RCF alone could not completely remove LDGs, the adherence and the whole blood sedimentation methods were developed to further remove LDGs from the separated PBMCs. In Figs. 3a and 3b, the typical dots from the flow cytometer indicate that the PBMC adherence method completely removed not only LDGs but also monocytes. In contrast, the whole blood sedimentation method did not completely remove LDGs, and had no influence on monocytes (Figs. 3c and 3d). Compared to the standard method, the PBMC adherence method could significantly remove LDGs from PBMCs (P=0.0314; Fig. 3f), while the whole blood sedimentation method could also remove LDGs from PBMCs, but not to a significant level (P=0.0780; Fig. 3g). These results mean that T lymphocytes could be purified from PBMCs using the adherence method alone.
3.5. Comparison of LDG clearance rates for different methods
To compare the capacities of the various methods to remove LDGs from PBMCs, the concept of a LDG clearance rate was introduced, defined as the percentage of LDGs separated by the standard method minus the LDG percentage obtained from other methods, the result of which was divided by the percentage obtained using the standard method. We compared the LDG clearance rates for the PBMC adherence method, the whole blood sedimentation method, and centrifugation at RCFs of 600g and 800g. As indicated in Fig. 4, the PBMC adherence method gave a much better LDG clearance rate than centrifugation at 600g (P=0.0074) and at 800g (P=0.0089). The PBMC adherence method also out-performed the whole blood sedimentation method, but the difference did not reach significance (P=0.1927). The other methods exhibited negative LDG clearance rates, suggesting that they had poor stability.
Fig. 4.
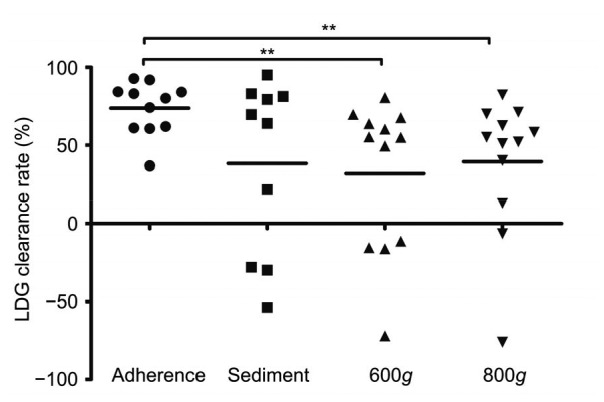
Comparing LDG clearance rates of four methods
LDG clearance rate is defined as the LDG percentage separated from the standard method minus the LDG percentage obtained from other methods, the result of which was divided by the LDG percentage obtained from the standard method. Adherence: PBMC adherence method; Sediment: whole blood sedimentation method; 600g and 800g: PBMCs were isolated by density-gradient centrifugation over Histopaque-1077 for 30 min at 600g and 800g RCF. Statistical analyses were performed using an unpaired t-test with Welch’s correction. ** P<0.001
3.6. Comparison of influences of two methods on T lymphocyte percentages during PBMC separation
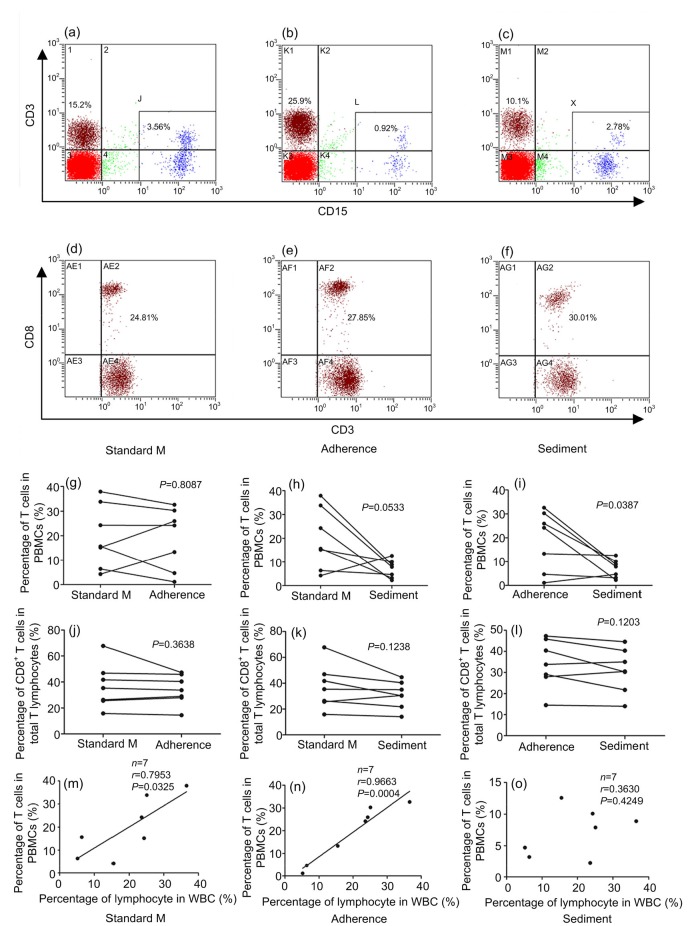
The reason for using various methods to remove LDGs from PBMCs is to enrich T lymphocytes from PBMCs. We compared the T lymphocyte percentages in PBMCs after LDG removal by the whole blood sedimentation and by the PBMC adherence methods. The typical dot plots from the flow cytometer are shown in Figs. 5a–5c and whole data about T lymphocyte percentages in PBMCs are listed in Table 2. The PBMC adherence method had a small influence on the proportion of T lymphocytes in PBMCs (P=0.8087; Fig. 5g), but the whole blood sedimentation method markedly reduced the proportion of T lymphocytes in PBMCs (P=0.0533; Fig. 5h). Compared to the PBMC adherence method, whole blood sedimentation significantly decreased the proportion of T lymphocytes in PBMCs (P=0.0387; Fig. 5i).
Fig. 5.
Influences of three methods on T lymphocyte percentages in PBMCs
Dot plots of CD15 staining (X-axis) versus CD3 staining (Y-axis) show characteristics of T lymphocytes and LDGs of PBMCs separated by the standard method (a), PBMC adherence method (b), and whole blood sedimentation method (c). Dot plots of CD3 staining (X-axis) versus CD8 staining (Y-axis) show characteristics of CD8+ T lymphocytes and CD8− T lymphocytes from PBMCs separated by the standard method (d), PBMC adherence method (e), and whole blood sedimentation method (f). (g–i) Comparison of T cell percentages in PBMCs between the standard method, PBMC adherence method, and whole blood sedimentation method. (j–l) Comparison of CD8+ T cell percentages in total T lymphocytes between the standard method, PBMC adherence method, and whole blood sedimentation method. (m–o) The correction between T cell percentages in PBMCs and lymphocyte percentages in white blood cells (WBCs). Standard M: standard method; Adherence: PBMC adherence method; Sediment: whole blood sedimentation method. Statistical analyses were performed using the paired t-test (g–l) and linear correlation analysis (m–o)
Table 2.
Influences of three methods on LDG and T lymphocyte percentages in PBMCs
| Case | LDGs (%) |
T lymphocyte (%) |
CD4+ T lymphocyte (%) |
CD8+ T lymphocyte (%) |
||||||||
| Standard M | Adherence | Sediment | Standard M | Adherence | Sediment | Standard M | Adherence | Sediment | Standard M | Adherence | Sediment | |
| 1 | 4.98 | 0.78 | 3.60 | 4.25 | 13.30 | 12.57 | 53.14 | 54.18 | 59.65 | 46.86 | 45.82 | 40.35 |
| 2 | 3.56 | 0.92 | 2.78 | 15.20 | 25.94 | 10.08 | 74.40 | 72.05 | 69.52 | 25.60 | 27.95 | 30.48 |
| 3 | 35.82 | 2.89 | 7.38 | 6.37 | 1.10 | 4.70 | 26.63 | 35.71 | 51.23 | 67.68 | 47.26 | 44.58 |
| 4 | 2.17 | 0.43 | 0.78 | 15.64 | 4.69 | 3.20 | 70.50 | 65.98 | 74.20 | 26.29 | 29.01 | 21.76 |
| 5 | 0.77 | 0.30 | 1.00 | 33.85 | 30.29 | 7.88 | 62.97 | 65.62 | 65.00 | 35.38 | 33.75 | 35.00 |
| 6 | 2.06 | 1.30 | 0.10 | 24.27 | 24.22 | 2.26 | 55.96 | 58.51 | 61.79 | 41.67 | 40.39 | 30.27 |
| 7 | 0.66 | 0.26 | 0.20 | 37.91 | 32.61 | 8.88 | 82.45 | 84.75 | 84.94 | 15.82 | 14.53 | 14.02 |
Standard M: standard method; Adherence: PBMC adherence method; Sediment: whole blood sedimentation method
The proportion of CD8+ T cells in PBMCs was compared and the typical dot plots from the flow cytometer are shown in Figs. 5d–5f. The results indicate that the PBMC adherence method had a smaller influence on the percentage of CD8+ T cells in PBMCs than the whole blood sedimentation method, but the difference did not reach statistical significance (P=0.1203; Figs. 5j–5l).
To further validate whether the separation methods influenced the percentage of T lymphocytes in PBMCs, linear correlation analyses were performed between the T lymphocyte percentage in PBMCs and the T lymphocyte percentage in white blood cells (WBCs) of whole blood. The results indicate that the T lymphocyte percentage in PBMCs positively correlated with the T lymphocyte percentage in WBCs of whole blood after using the standard method to separate whole blood (P=0.0325; Fig. 5m). Furthermore, this correlation was enhanced by using the PBMC adherence method to further remove LDGs from PBMCs (P=0.0004; Fig. 5n). However, the whole blood sedimentation method markedly influenced the percentage of T lymphocytes in PBMCs (P=0.4249; Fig. 5o). These results suggest that the PBMC adherence method can not only efficiently remove LDGs from PBMCs, but also make the T cell percentage in PBMCs more stable.
4. Discussion
Similar to SLE patients, LDG percentages were significantly increased in DM, PM, and RA patients relative to healthy controls, suggesting that this increase is universal in common autoimmune diseases. Removing LDGs from PBMCs significantly decreased the T-SPOT.TB results, indicating that LDGs can decrease the threshold of activation of T lymphocytes and amplify their immune response. Therefore, use of a simple method for removing LDGs from PBMCs is necessary. Within a certain range, an increased RCF (600g) can reduce the proportion of LDGs in PBMCs, but the PBMC adherence method can reduce the LDG percentage significantly with a minimal impact on T cell percentages in PBMCs.
As early as 1986, abnormally increased LDGs in PBMCs were reported in a small sample of patients with SLE, RA, and acute rheumatic fever (Hacbarth and Kajdacsy-Balla, 1986). After neutrophils forming NETs were reported in by Brinkmann et al. (2004), LDGs were once more a topic of research until 2010 (Denny et al., 2010). Now the research extends beyond rheumatism to diseases such as human immunodeficiency virus (HIV) infection, severe infections, tumors, and neonatal disorders where a significantly increased number of LDGs in PBMCs have been reported (Morisaki et al., 1992; Rodriguez et al., 2009; Lin et al., 2011; Cloke et al., 2012; 2013; Hoffmann et al., 2013; Ssemaganda et al., 2014). In the classical autoimmune disease of SLE, the LDG percentage in PBMCs was determined for 35 patients and 31 healthy controls, and the results indicated that the average percentage was 15% in SLE patents and 2% in healthy controls (Pavón et al., 2012). In another study, the LDG average percentage was 18% in 65 patients with SLE and 5% in 22 healthy controls (Denny et al., 2010). In our study, the LDG average percentage was 1.28% for healthy controls, 8.41% for DM, 8.41% for PM, 4.05% for RA, and 7.53% for SLE, which were lower than the percentages reported in the literature. The possible reason for this is that different separation methods have an impact on the LDG percentage. This set of data suggests that abnormally increased LDGs in PBMCs are widespread in autoimmune diseases.
LDGs may represent a group of immature neutrophils stimulated by unknown factors and released early from the bone marrow (Carmona-Rivera and Kaplan, 2013). In autoimmune diseases, a variety of proinflammatory cytokines and other materials are abundant, which may promote early release of neutrophil precursor cells from the bone marrow, or interfere with the differentiation ability of neutrophil precursor cells. LDGs can secrete type I IFN, TNF-α, and IFN-γ excessively upon stimulation, which can promote and increase tissue damage (Denny et al., 2010). LDGs can also induce significant endothelial cell cytotoxicity and directly injure endothelial cells by forming NETs (Saffarzadeh et al., 2012; Carmona-Rivera and Kaplan, 2013). Moreover, LDGs show a stronger NET-forming ability and are more likely to form NETs compared to normal density neutrophils (Carmona-Rivera and Kaplan, 2013).
In clinical tests and research on T lymphocytes, PBMCs are commonly used in culture in vitro to substitute for T lymphocytes. If the cultured PBMCs contain abnormally increased numbers of LDGs, the PBMCs will form a co-culture environment of T cells, DCs, and NETting-granulocytes, which may result in T lymphocyte activation and IFN-γ release (Tillack et al., 2012). T-SPOT.TB is widely used to screen for tuberculosis in current clinical practice, in which PBMCs are used to identify the sensitized T lymphocytes that can release IFN-γ upon stimulation of tuberculosis antigens. If the tested PBMCs contain a large number of LDGs, T lymphocytes will be activated by LDG-formed NETs and further release IFN-γ. These will produce a false positive outcome, which could mislead the clinical decision. As exemplified in this study, removing LDGs from PBMCs resulted in a decrease of T-SPOT.TB results. It is therefore clear that the influence on T cells of LDGs forming NETs cannot be ignored when performing PBMC cultivation.
Current methods of obtaining or removing a certain type of cell from a mixture of cells are magnetic bead separation and flow cytometer separation. These two methods use magnetic beads or fluorescent markers tagged with monoclonal antibodies to specifically bind a particular cell line and then separate the cells of interest from the mixed cells. These methods can be divided into positive and negative selections. Positive selection, due to the combination of specific antibodies, may affect cell function, while negative selection uses a variety of antibodies to combine with the cells that are not required and keep the cells of interest (Denny et al., 2010; Carmona-Rivera and Kaplan, 2013), so the separation cost is quite high. Therefore, it is necessary to find a simple and easy-to-use method for removing LDGs from PBMCs.
The influence of RCF on the LDG percentage in PBMCs was verified initially. The results showed that elevating the RCF within a limited range (600g) led to a decrease in the number of LDGs, but elevating RCF further did not remove all LDGs from PBMCs. The PBMC adherence method significantly reduced the proportion of LDGs and showed the highest LDG clearance rate. Whole blood sedimentation could also remove a proportion of the LDGs in some patients, but the results were not stable. Therefore, the PBMC adherence method was the most efficient and stable method of removing LDGs from PBMCs.
A good method should have the highest LDG clearance rate and the lowest impact on lymphocyte percentages in PBMCs. So we compared the PBMC adherence and the whole blood sedimentation methods for their effects on lymphocyte percentages. The PBMC adherence method not only had less influence on the T lymphocyte percentage in PBMCs, but also restored the proportion of T cells in PBMCs to close to the lymphocyte percentage in blood routine analysis.
A good method should be not only efficient and accurate, but also simple and feasible. The PBMC adherence method is very easy for use in research and clinical practice. The separated PBMCs can be seeded on various common culture plates for 1 h, and then the supernatant can be carefully collected for centrifugation again. By this simple procedure, almost all LDGs can be removed from PMBCs.
5. Conclusions
An abnormally increased proportion of LDGs is widespread in SLE, PM, DM, and RA patients. LDGs forming NETs may affect the results of T lymphocytes in PBMC samples from patients with these diseases. The PBMC adherence method can remove LDGs from PBMCs efficiently while affecting the T lymphocyte percentage in PBMCs minimally. When PBMCs are used to perform lymphocyte-related research, it is strongly recommended that cell suspension is reseeded in another culture plate after 1 h to prevent and reduce the effects of LDGs on T cell-related experimental results due to the formation of NETs.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 81560267 and 81401363), the Natural Science Foundation of Gansu Province (No. 1606RJZA213), and the Asia Pacific League of Associations for Rheumatology (APLAR) Research Grant 2015
Compliance with ethics guidelines: Si-gong ZHANG, Yu-xin SONG, Xiao-ming SHU, Hai-li SHEN, Han-bo YANG, Rui-xue DUO, and Guo-chun WANG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 5.Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013;35(4):455–463. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmona-Rivera C, Zhao W, Yalavarthi S, et al. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2015;74(7):1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloke T, Munder M, Taylor G, et al. Characterization of a novel population of low-density granulocytes associated with disease severity in HIV-1 infection. PLoS ONE. 2012;7(11):e48939. doi: 10.1371/journal.pone.0048939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cloke T, Munder M, Bergin P, et al. Phenotypic alteration of neutrophils in the blood of HIV seropositive patients. PLoS ONE. 2013;8(9):e72034. doi: 10.1371/journal.pone.0072034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny MF, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184(6):3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29(11):1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725–1734. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann MH, Bruns H, Bäckdahl L, et al. The cathelicidins LL-37 and rCRAMP are associated with pathogenic events of arthritis in humans and rats. Ann Rheum Dis. 2013;72(7):1239–1248. doi: 10.1136/annrheumdis-2012-202218. [DOI] [PubMed] [Google Scholar]
- 14.Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morisaki T, Goya T, Ishimitsu T, et al. The increase of low density subpopulations and CD10 (CALLA) negative neutrophils in severely infected patients. Surg Today. 1992;22(4):322–327. doi: 10.1007/BF00308740. [DOI] [PubMed] [Google Scholar]
- 16.Pavón EJ, García-Rodríguez S, Zumaquero E, et al. Increased expression and phosphorylation of the two S100A9 isoforms in mononuclear cells from patients with systemic lupus erythematosus: a proteomic signature for circulating low-density granulocytes. J Proteomics. 2012;75(6):1778–1791. doi: 10.1016/j.jprot.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ssemaganda A, Kindinger L, Bergin P, et al. Characterization of neutrophil subsets in healthy human pregnancies. PLoS ONE. 2014;9(2):e85696. doi: 10.1371/journal.pone.0085696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tillack K, Breiden P, Martin R, et al. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188(7):3150–3159. doi: 10.4049/jimmunol.1103414. [DOI] [PubMed] [Google Scholar]
- 21.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]