Abstract
The aim of this study was to research the changes in cytotoxicity and antibacterial properties after silver nanoparticles (AgNPs) were incorporated into the surface coating of dental alloys. AgNPs were attached to cobalt chromium alloys and pure titanium using a hydrothermal method, according to the reaction: AgNO3+NaBH4→ Ag+1/2H2+1/2B2H6+NaNO3. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to evaluate the cytotoxicity of the alloys when in contact with osteogenic precursor cells (MC3T3-E1) from mice and mesenchymal stem cells (BMSC) from rats. The antibacterial properties of dental alloys incorporating three different concentrations (10, 4, and 2 μmol/L) of AgNPs were tested on Staphylococcus aureus (SA) and Streptococcus mutans (MS). High cytotoxicity values were observed for all dental alloys that contained 0% of AgNPs (the control groups). The incorporation of AgNPs reduced cytotoxicity values. No significant difference was observed for antibacterial performance when comparing dental alloys containing AgNPs to the respective control groups. The results demonstrated that the cobalt chromium alloys and pure titanium all had cytotoxicity to MC3T3-E1 and BMSC and that the incorporation of AgNPs could reduce this cytotoxicity. The concentrations of AgNPs adopted in this study were found to have no antibacterial action against SA or MS.
Keywords: Silver nanoparticles (AgNPs), Dental casting, Cytotoxicity, Antibacterial, MC3T3-E1, BMSC
1. Introduction
Implants and removable dentures were widely used in clinical practice to improve patients’ quality of life, but they also resulted in the occurrence of peri-implantitis (Mombelli et al., 2012; Derks and Tomasi, 2015) and denture stomatitis (Ramage et al., 2004; Shulman et al., 2005). Research on surface modifications of dental materials had been done to reduce the failure rate of implants (Morra, 2007; Stanford, 2008). Studies demonstrated that surface modifications using plasma treatments could reduce the adherence of Candida albicans to denture base resins, which reduced the incidence rate of denture stomatitis (Zamperini et al., 2010; Wen et al., 2016).
The antibacterial properties of silver were discovered as early as ancient Greece. With more recent in-depth research, nanosilver was found to have a better antibacterial effect when the particle size was less than 10 nm (Gliga et al., 2014). Methods for preparing silver nanoparticles (AgNPs) are divided into two main categories according to the reaction mechanism: physical synthesis and chemical synthesis. The physical method uses techniques such as mechanical polishing and radiation to generate nanosilver directly from silver (Baker et al., 2005; Gromov et al., 2015). Chemical synthesis uses an oxidation-reduction reaction to reduce silver ions into nanosilver particles, and due to its easy operation and good controllability, this method has been widely used in various fields (Fahmy et al., 2016; Samari and Dorostkar, 2016).
AgNPs are known for demonstrating high antibacterial properties, effectively inhibiting the growth of bacteria, eucaryotic microbes, and viruses (Jung et al., 2009; Wani et al., 2013; Chowdhury et al., 2016). The antibacterial effects of AgNPs are superior to that of other antibacterial materials (Anisha et al., 2013). Due to these properties, medical products containing AgNPs have been widely used, such as in wound dressings, the surface coating of medical devices, surgical sutures, antibacterial excipients, cardiac valves, and target agents (Wright et al., 2002; Huh and Kwon, 2011).
Recently, AgNPs have been used to enhance the antibacterial performance of dental materials (Qin et al., 2015; Vogel et al., 2015). AgNPs were added to composite resins and results demonstrated that, in sufficient concentrations, the presence of nanosilver significantly increased the resins’ antibacterial performance (Fan et al., 2011; Ai et al., 2017). Microwave solidification was applied to the denture base preparation after mixing the AgNPs with polymethyl methacrylate (PMMA) powder, and the results showed that the amount of C. albicans attached to the base surface was significantly reduced (Acosta-Torres et al., 2012). AgNPs have also been added to the surface of titanium implants (Massa et al., 2014; Matsubara et al., 2015; Mishra et al., 2015). This incorporation of AgNPs not only resulted in a significant increase in antibacterial properties (Streptococcus mutans (MS), Porphyromonas gingivalis, and C. albicans), but also significantly increased the expression of osteoblast phenotype genes (Alp, Ocn, RunX2) in cell growth on the Ag-implanted titanium surface (Zheng et al., 2012).
However, safety issues concerning AgNPs have attracted attention. Numerous studies have shown that AgNPs can affect DNA replication and the mitochondrial functions of cells, producing cytotoxicity (Park et al., 2009; Li et al., 2010; You et al., 2011; Chen and Zhang, 2012). AgNPs (15 and 100 nm) can damage the fetal liver cells of mice, thought likely to be mediated by oxidative stress (Hussain et al., 2005), and can also result in long-term inhibition of the cell proliferation (after short-term exposure) of human-derived keratinocytes (Zanette et al., 2011). Many factors are directly related to the biosafety of AgNPs, including size, shape, chemical composition, surface charge, solubility, biological binding site, and metabolic and excretion pathways (Park et al., 2010; Philbrook et al., 2011; Beer et al., 2012). Protective effects have been reported for different compositions (vitamin E, inducible nitric oxide synthase (iNOS), thiolated-2-methacryloyloxyethyl phosphorylcholine (MPC-SH)) regarding AgNPs cytotoxicity (Zhang et al., 2006; Sangsuwan et al., 2016; Zielinska et al., 2016). The method of green synthesis and the characterization of AgNPs have been reported, such as Nigella sativa leaf extract (Gogoi et al., 2015) and the alcoholic flower extract of Nyctanthes arbortristis (Amooaghaie et al., 2015). Until the publication of this article, there has been no complete evaluation method and/or indicator system devised that can determine the biosafety of AgNPs, so great caution should be taken with their use (and indication). The main concern for future research on nanosilver is to figure out how to make AgNPs antibacterial while reducing cytotoxicity to a level acceptable for the human body.
The aim of this study was to investigate whether incorporating AgNPs into the surface coating of different dental-relevant alloys has an effect on the cytotoxicity and antibacterial properties of the alloy. We hoped to find an effective antibacterial concentration of AgNPs that also had a low biological toxicity.
2. Materials and methods
2.1. Sample preparation
Wax discs (Dental Materials Factory of Shanghai Medical Instruments Co., Ltd., Shanghai, China) patterns were prepared with dimensions of 10 mm×10 mm×2 mm (n=96). The lost-wax technique (taking a wax pattern of a dental restoration and converting it into a dental casting alloy) was used in order to obtain six groups of dental castings (Cobalt-chrome: Wirobond C (WC), BEGO, Germany; Keragen (KN), Eisenbacher Dentalwaren ED GmbH, Germany; Ceramill Sintron (CS), Amanngirrbach, Germany; Solibond C plus (SCP), YETI Dentalprodukte GmbH, Germany; QLD-Co, Qianluda Medical Instrument Co., Ltd., China. Pure titanium: QLD-Ti, Qianluda Medical Instrument Co., Ltd., China). For standardization of surface roughness, abrasive SiC (Shanghai Grinding Wheels Plant, Shanghai, China) papers were gradually used (grits 100#, 200#, 400#, and 800#) under water refrigeration. The samples were ultrasonically washed using absolute ethyl alcohol for 15 min, allowed to air-dry for 30 min, then stored in sterile containers at room temperature.
2.2. AgNPs synthesis/incorporation method
AgNPs were prepared using hydrothermal methods according to the reaction: AgNO3+NaBH4→ Ag+1/2H2+1/2B2H6+NaNO3. Twenty milliliters of NaBH4 (Aladdin Reagent, Shanghai, China) at a concentration of 0.004 mol/L was diluted to 500 ml. We added 0.004 mol/L of AgNO3 (Aladdin Reagent, Shanghai, China) dropwise to NaBH4 solution in three separate volumes (2.5, 1.0, and 0.5 ml), with magnetic stirring for 5 min, after which three different concentrations of solutions (with disperse AgNPs) were prepared. Twelve milliliters of each concentration of AgNPs solutions were separately added to a hydrothermal inner tank with the dental casting samples (groups=3×6). The hydrothermal inner tanks were placed into the hydrothermal reactor for 2 h at 130 °C and were annealing for 2 h at 450 °C. The three concentrations of AgNPs were then added to the six groups of dental castings. They were then ultrasonically washed using absolute ethyl alcohol for 15 min, and allowed to air-dry for 30 min. The surface morphology was observed using scanning electron microscopy (SEM) (S-4800, Hitachi, Japan). The elemental composition of the samples was analyzed by energy dispersive spectrometer (EDS) (Oxford Instruments, UK; attached to the field emission scanning electron microscopy (FESEM)).
2.3. Preparation of “leach liquors” from the dental alloy samples
Samples were first marked and ultrasonically washed using absolute ethyl alcohol for 15 min, sterile water-rinsed three times, allowed to air-dry for 30 min and autoclaved at 121 °C for 30 min. Each sample was immersed individually in 2 ml Dulbecco’s modified Eagle’s medium (DMEM, HyClone, LA, USA) containing fetal bovine serum (FBS, HyClone, LA, USA), and allowed to leach at 37 °C (with 5% CO2 concentration), based on the ISO requirement (0.5–0.6 cm2/ml), for 7 d prior to cytotoxicity analysis. Then, the leached liquors went through filtration by using 0.22-μm micro porous membranes and were stored in a refrigerator at 4 °C.
2.4. Cytotoxicity test
Osteogenic precursor cells (MC3T3-E1) from mice (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) and mesenchymal stem cells (BMSC) from rats (Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) were cultured in a DMEM culture medium containing two antibiotics (100 U/ml each of penicillin and streptomycin) and 10% FBS. After the cells grew up to the third generation, or the cell state tended to be stable, the number was counted. Cells were transferred to a 96-hole board according to specific cell density to incubate in the cell incubator (MCO-17AC, SANYO, Japan) for 24 h. The supernatant was removed after the cells had been adherent for 24 h. For positive control, 10 μl of DMEM solution was added to the cell culture, while for negative control, culture solution was added instead of cells, and different leach liquors were used to culture cells separately for Groups 1 to 6. Twenty microliters of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (5 g/L) (Sigma-Aldrich, St. Louis, MO, USA) was added to each hole, after the cells reacted with the leach liquor for 1, 3, and 5 d. The leach liquors were removed after incubating for 4 h, and 150 μl of DMSO was added to each hole and vibrated with the oscillator (Shanghai Precision Instrument Co., Ltd., Shanghai, China) for 10 min. The enzyme-linked immunoassay instrument (SpectraMax i3x, Molecular Devices, SF, USA) was used to determine the absorbance value (A) of 570 nm immediately after the purple crystal had completely dissolved. The relative growth rate (RGR) of cells was calculated according to the formula: RGR=A exp/A neg×100%, where A exp and A neg are the absorbance values of experimental and negative control groups, respectively.
The degree of cytotoxicity of the materials was evaluated based on the RGR value according to the corresponding relationship between cell growth rate and material toxicity level (Table 1).
Table 1.
Cell growth rate and material toxicity level standards
| Level | Growth rate (%) |
| 0 | ≥100 |
| 1 | 75–99 |
| 2 | 50–74 |
| 3 | 25–49 |
| 4 | 1–24 |
| 5 | 0 |
2.5. Antibacterial test
Samples of QLD-Ti and QLD-Co (at three AgNPs concentrations) were distilled, water-rinsed, autoclaved at 121 °C for 30 min, and dried on a laminar flow bench (Suzhou Purification Equipment Company, Suzhou, China). Samples were then immersed in the test tube containing 2 ml of brain heart infusion (BHI) culture medium (Becton Dickenson, Franklin Lakes, NJ, USA) in order to leach (under vibration) for 24 h. The leached liquors were collected into centrifugal tubes. A Staphylococcus aureus (SA) bacterium solution was uniformly coated over a panel containing BHI agar medium. After 5 min, the BHI agar medium was punched by a sterilized puncher and 150 µl of leach liquor was added to the hole. Penicillin was considered a positive control, and the panel was placed in the incubator for 24 h, in order to observe the presence (or not) of an inhibition zone.
Samples were placed in the test tube containing 2 ml of culture medium. Penicillin was considered a positive control. The same amount of bacterial solution was inoculated. Test tubes were placed on a shaking table (HS-111B, Shanghai Hasuc Instrument Manufacture Co., Ltd., Shanghai, China) for 3 h (for SA) or 24 h (for MS). The OD600 (the absorbance of a sample measured at a wavelength of 600 nm) of the bacterial solutions was measured by spectrophotometer (ND-2000, Thermo Scientific, MA, USA) in order to determine the antibacterial effect. Samples were taken from the bacteria solution, sterile water-rinsed and air-dried on a laminar flow bench before being immersed in the test tube containing 5 ml of culture medium, and placed on the shaking table for 24 h. The OD600 was evaluated to observe the number of bacteria adhered to the samples.
2.6. Statistical analysis
Data were expressed as mean±standard deviation (SD) from at least two independent experiments. One-way analysis of variance and Tukey’s multiple-comparison test were used for statistical analysis at a pre-set alpha of 0.05.
3. Results
3.1. Distribution of AgNPs on the surface of samples
SEM images were observed to ensure that the nanoparticles had successfully adhered to the dental castings. As shown in Fig. 1, uniform-grain-size AgNPs were evenly distributed on the surface of dental castings. Spectrum analysis was conducted on a sample of Keragen coated with a low concentration of AgNPs to analyze the metal compositions. Within the detected area as shown in Fig. 1e, four elements (Cr, Co, Ag, and W) were confirmed. We further confirmed that the ions attached to the dental castings surfaces were silver, in a concentration of 1.06% (Table 2).
Fig. 1.
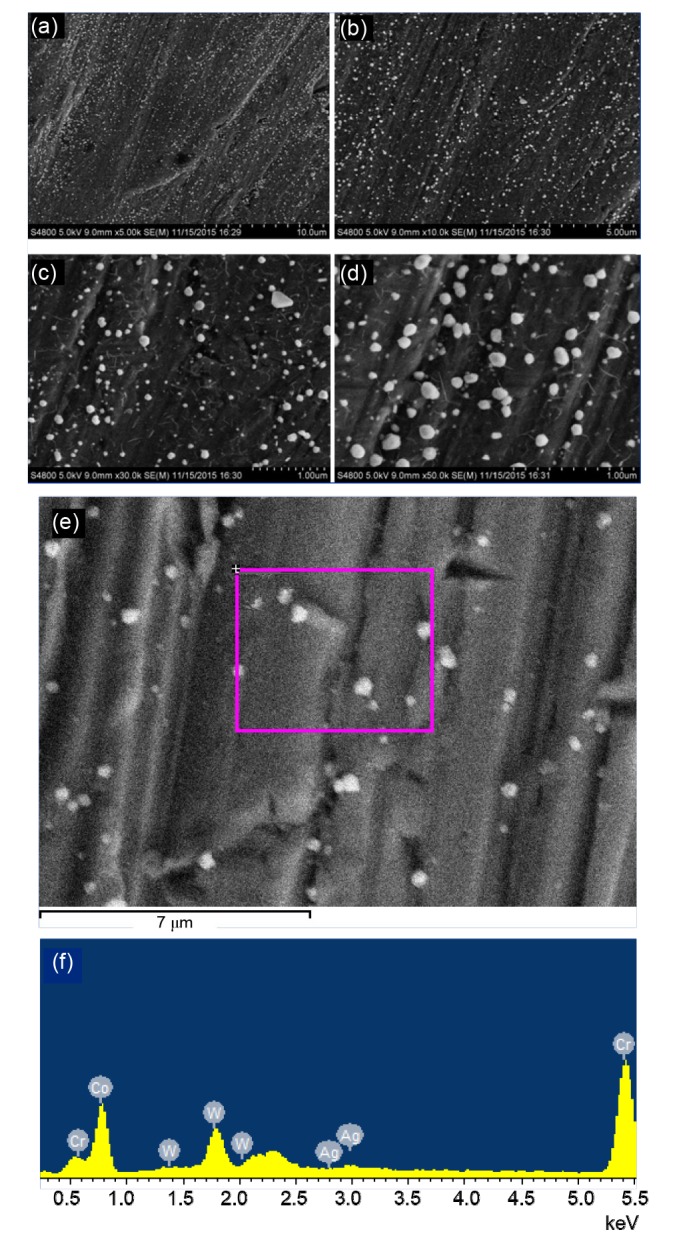
Scanning electron microscopy (SEM) images and spectrum analysis of dental castings (KN) coated with a low concentration of silver nanoparticles (AgNPs)
Image (a) demonstrates a 5000×magnification of the sample surfaces. Images (b), (c), and (d) display details of the nanoparticles at 10 000×, 30 000×, and 50 000×, respectively. Image (e) exhibits the detected area of spectrum analysis. Image (f) demonstrates the metal compositions
Table 2.
Results of the spectrum analysis
| Element | Weight (%) | Atom (%) |
| Cr | 26.32 | 31.88 |
| Co | 58.69 | 62.73 |
| Ag | 1.06 | 0.62 |
| W | 13.94 | 4.78 |
3.2. Cytotoxicity test
The number of MC3T3-E1 cells, cultured with the negative control group (Fig. 2a) leach liquors of dental castings, decreased gradually with time. The cell growth rate was also negative, which indicated strong cytotoxicity of the dental castings. The cytotoxicity in the group with a concentration of 10 μmol/L (Fig. 2b) was reduced after AgNPs were incorporated, but still strong. The cytotoxicity in the groups at the concentrations of 4 μmol/L (Fig. 2c) and 2 μmol/L (Fig. 2d) was strong, and the cells were almost disappeared. No significant differences were observed between the five kinds of cobalt-chrome alloy in the experimental group and the control group, while significant differences were found between the Ti and cobalt-chrome alloys in the control group (P<0.05). Significant differences were observed between the group at the concentration of 10 μmol/L and the control group after the MC3T3-E1 cells had reacted with the leach liquor for 5 d.
Fig. 2.

Effects of silver nanoparticles at three concentrations on cytotoxicity of MC3T3-E1 cells
(a) The negative control group; (b) The AgNPs concentration of 10 μmol/L; (c) The AgNPs concentration of 4 μmol/L; (d) the AgNPs concentration of 2 μmol/L. 1, WC; 2, KN; 3, CS; 4, SCP; 5, QLD-Co; 6, QLD-Ti. Data are expressed as mean±SD (n=4)
Fig. 3a shows the number of BMSC cells cultured with the negative control group leach liquors. The number gradually decreased with time and the cell growth rate was negative, which indicates strong cytotoxicity of the dental castings. The cytotoxicity was reduced after the AgNPs incorporation but still high. No significant differences were found in the cytotoxicity among the three AgNPs concentrations (Figs. 3b–3d).
Fig. 3.

Effects of three concentrations of silver nanoparticles (AgNPs) on the cytotoxicity of BMSC cells
(a) The negative control group; (b) The AgNPs concentration of 10 μmol/L; (c) The AgNPs concentration of 4 μmol/L; (d) The AgNPs concentration of 2 μmol/L. 1, WC; 2, KN; 3, CS; 4, SCP; 5, QLD-Co; 6, QLD-Ti. Data are expressed as mean±SD (n=4)
3.3. Antibacterial test
Fig. 4 demonstrates the inhibition zone of the three different concentrations of AgNPs. The leach liquors had no significant antibacterial effect when compared to the positive control group of penicillin (Fig. 4c-1).
Fig. 4.

SA inhibition zone of silver nanoparticles at three concentrations
(a) The inhibition zone of QLD-Ti: 1, AgNPs concentration of 10 μmol/L; 2, AgNPs concentration of 4 μmol/L; 3, AgNPs concentration of 2 μmol/L. (b) The inhibition zone of QLD-Co: 1, AgNPs concentration of 10 μmol/L; 2, AgNPs concentration of 4 μmol/L; 3, AgNPs concentration of 2 μmol/L. (c) Control group: 1, the positive control group of penicillin; 2, the negative control group of QLD-Ti; 3, the negative control group of QLD-Co
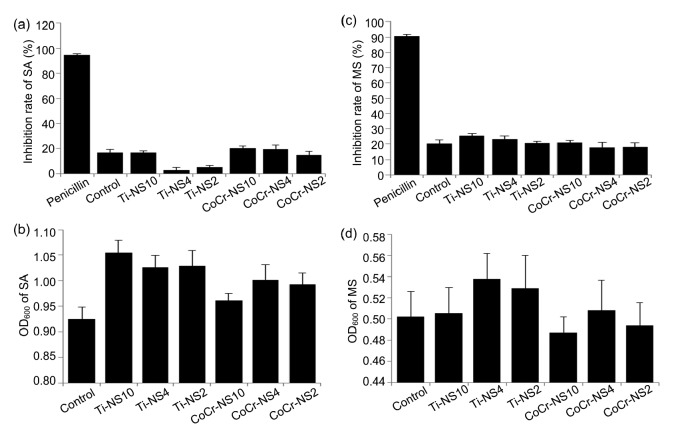
Fig. 5 demonstrates that the inhibition rate of AgNPs samples at different concentrations compared to SA and MS resulted in no significant increase when compared to the negative control group. The amount of bacteria attached to the surface of samples was not statistically reduced when compared to the negative control group.
Fig. 5.
Antibacterial effects of AgNPs samples at three concentrations compared to SA and MS
(a) Inhibition rate of SA; (b) OD600 of SA adhered to the samples; (c) Inhibition rate of MS; (d) OD600 of MS adhered to the samples. Penicillin: the positive control group; Control: the negative control group; Ti-NS10: QLD-Ti with 10 μmol/L of AgNPs; Ti-NS4: QLD-Ti with 4 μmol/L of AgNPs; Ti-NS2: QLD-Ti with 2 μmol/L of AgNPs; CoCr-NS10: QLD-Co with 10 μmol/L of AgNPs; CoCr-NS4: QLD-Co with 4 μmol/L of AgNPs; CoCr-NS2: QLD-Co with 2 μmol/L of AgNPs. Data are expressed as mean±SD (n=4)
4. Discussion
AgNPs have generated a great deal of interest in the field of research on dental materials. Fifty percent of patients who wear complete or partial dentures have problems with stomatitis (Budtz-Jorgensen, 1981). AgNPs were added to composite resins and the antibacterial performance of these resins would significantly increase at appropriate AgNPs concentrations (Fan et al., 2011; Kasraei et al., 2014; Ai et al., 2017). Because of their antimicrobial properties, AgNPs have been added to poly(methyl methacrylate) (PMMA). The results of this study found that PMMA-AgNPs significantly reduced the adherence of C. albicans, while flexural strength of PMMA was decreased (Acosta-Torres et al., 2012; Sodagar et al., 2012). The colonization of the soft lining of denture material by oral fungi could result in infections and stomatitis, and therefore AgNPs were incorporated into composites as antimicrobial agents in order to reduce the microbial colonization of lining materials (Grzegorz et al., 2011; Chladek et al., 2013). Bacterial adhesion to the surface of implants may result in peri-implant diseases and cannot always be avoided by sterilization before implantation. AgNPs coating produced a strong antibacterial effect on the titanium surface by inhibiting the adhesion of bacteria (Massa et al., 2014; Matsubara et al., 2015; Mishra et al., 2015; Qin et al., 2015; Vogel et al., 2015). In our study, AgNPs were incorporated to the surface of dental alloys to improve their antibacterial properties, in order to further reduce the incidence of denture stomatitis and peri-implantitis.
When AgNPs were reduced to sizes in the nanometer range (<100 nm), their biological activity could be changed and their antibacterial properties materialized. Due to their size and surface chemistry, AgNPs could be internalized by endosomal or lysosomal endocytosis (Asharani et al., 2009a; Luther et al., 2011) and then translocate to target organelles such as the nucleus and mitochondria, eliciting a series of biological effects including altered cell morphology, mitochondrial dysfunction, DNA damage, oxidative stress, inflammation, and genotoxicity, and result in cell death by apoptosis or necrosis (Asharani et al., 2012; Yang et al., 2012; Zhang et al., 2014). Oxidative stress and the release of Ag+ ions from AgNPs are two primary mechanisms that mediate AgNPs-associated cytotoxicity (Volker et al., 2013). Intracellularly released Ag+ ions interact with thiol groups of antioxidants such as glutathione, superoxide dismutase, and thioredoxin, leading to increased lipid peroxidation, DNA damage, oxidative stress, and subsequent apoptotic cell death (Arora et al., 2008).
AgNPs toxicity may be attributed to either non-specific interactions, such as size and shape, or specific interactions with cells, such as surface coating, chemical composition and the release of Ag+ ions (Riaz Ahmed et al., 2017). The size of the AgNPs is a main factor in mediating various biological effects such as oxidative stress, DNA damage, cellular uptake, mitochondrial dysfunction, and permeabilization across biological barriers (Gliga et al., 2014; Wang et al., 2014). A study on the effects of three different AgNPs sizes (20, 80, and 110 nm) on RAW 264.7 macrophage viability demonstrated that 20 and 80 nm AgNPs inhibited cell metabolic activity at 7 and 38 μg/ml, respectively. However, no changes in viability were observed in the group of 110 nm AgNPs, which indicates that the smaller-sized AgNPs were more cytotoxic (Park et al., 2011). Another study, however, demonstrated that 100 nm AgNPs caused a higher level of cytotoxicity, while similarly at the same concentrations for both sizes (Hussain et al., 2005). As a result, it was difficult to prove the correlation between cytotoxicity and the size of AgNPs. The shape of AgNPs can also influence the degree of particle toxicity and immunological effects in cells. The effects of silver wires, spherical AgNPs, and silver microparticles on lung epithelial A549 cells were investigated and it was found that silver nanowires had the highest levels of cytotoxicity, while silver nanospheres had minimal cellular effects (Stoehr et al., 2011). As shown in the SEM images of Fig. 1, silver nanowires and spherical AgNPs (<100 nm) were observed, which met the requirements for the size and shape of nanosilver with antibacterial properties.
Studies conducted in vivo have demonstrated that different cell types display different sensitivities to AgNPs (Asharani et al., 2009b). Differential sensitivity to AgNPs has also been discovered among cell lines of the same cell type (Singh and Ramarao, 2012). Fibroblasts are commonly used in toxicological studies as generalized representative cell types since they exist in every organ/tissue. NIH3T3 fibroblast cells (Hsin et al., 2008), normal human lung fibroblasts (IMR-90) (Asharani et al., 2009b), and L929 murine fibroblasts (Park et al., 2011) are often used in in vitro studies. The results of these experiments were directly determined by the sensitivity of cell types, the more sensitive cell lines were more able to reflect the toxicity of materials (Hensten-Pettersen and Helgeland, 1981). A longer period of contact with these dental alloys in the oral environment could lead to higher cytotoxicity to oral fibroblast cells or osteoblasts. In this study, MC3T3-E1 and BMSC cells were selected to replace osteoblasts in order to evaluate the cytotoxicity of dental alloys (Tian et al., 2016).
The results of this study, using the MTT method, determined that the cytotoxicity of dental alloys to MC3T3-E1 and BMSC cells was high, but this same cytotoxicity was reduced (to some extent) when AgNPs, at a certain concentration, were incorporated into the surface coating. The cytotoxicity of AgNPs has been confirmed by numerous in vitro studies (Park et al., 2009; Li et al., 2010; You et al., 2011; Chen and Zhang, 2012), but cytotoxicity has rarely been reported in dental materials. It has been determined that chrome-cobalt alloy has a cytotoxic effect on fibroblast cells (Sabaliauskas et al., 2011). The cytotoxic effects of cobalt-chromium, commercially pure titanium, and palladium-based alloys on L929 cells were evaluated by flow cytometry (FCM) and a real-time quantitative polymerase chain reaction (PCR) assay. The results determined that dental alloys might cause time-dependent early apoptosis via the intrinsic pathway (Pan et al., 2017). It was reported that human cells in culture were less sensitive to coated-AgNPs than mouse cells irrespective of the type of coating used (de Lima et al., 2012). Therefore, the cytotoxicity of dental alloys may be related to the culture cells.
Results from inhibition zone tests showed that AgNPs were ineffective in inhibiting SA and were significantly less effective than the positive control group (penicillin), which was consistent with other findings in the literature (Park et al., 2010). There was no significant difference in the inhibitory rate of SA or MS between the three concentrations of AgNPs samples. The concentrations of AgNPs evaluated for this study had no significant inhibitory effect on SA or MS. The results of in vitro research have demonstrated that AgNPs, though cytotoxic to mammalian cell lines, exhibit no antimicrobial efficacy against bacterial cells such as Escherichia coli and Bacillus subtilis (Suresh et al., 2010). Studies should be conducted in order to determine an effective preparation method which could lead to increased antibacterial properties and reduced cytotoxicity in AgNPs.
5. Conclusions
The dental alloys evaluated by this study demonstrated high cytotoxicity on MC3T3-E1 and BMSC cells. Cytotoxicity was reduced after AgNPs incorporation. No significant difference was observed for cytotoxicity or antibacterial properties among the three AgNPs concentrations. However, the mechanism, by which cytotoxicity was reduced after the incorporation of AgNPs, is still unknown, and studies should be conducted to determine this mechanism.
Footnotes
Project supported by the Public Welfare Projects of Science Technology Department of Zhejiang Province (No. 2013c33139) and the Natural Science Foundation of Zhejiang Province (No. LZ14C200001), China
Compliance with ethics guidelines: Xiao-ting SHEN, Yan-zhen ZHANG, Fang XIAO, Jing ZHU, and Xiao-dong ZHENG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Acosta-Torres LS, Mendieta I, Nuñez-Anita RE, et al. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int J Nanomed. 2012;7:4777–4786. doi: 10.2147/IJN.S32391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai M, Du Z, Zhu S, et al. Composite resin reinforced with silver nanoparticles-laden hydroxyapatite nanowires for dentalapplication. Dent Mater. 2017;33(1):12–22. doi: 10.1016/j.dental.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Amooaghaie R, Saeri MR, Azizi M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticle. Ecotoxicol Environ Saf. 2015;120:400–408. doi: 10.1016/j.ecoenv.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Anisha BS, Biswas R, Chennazhi KP, et al. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int J Biol Macromol. 2013;62:310–320. doi: 10.1016/j.ijbiomac.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Arora S, Jain J, Rajwade JM, et al. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol Lett. 2008;179(2):93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Asharani PV, Hande MP, Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009;10:65. doi: 10.1186/1471-2121-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asharani PV, Low-Kah MG, Hande MP, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3(2):279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 8.Asharani PV, Sethu S, Lim HK, et al. Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome Integr. 2012;3(1):2. doi: 10.1186/2041-9414-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker C, Pradhan A, Pakstis L, et al. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5(2):244–249. doi: 10.1166/jnn.2005.034. [DOI] [PubMed] [Google Scholar]
- 10.Beer C, Foldbjerg R, Hayashi Y, et al. Toxicity of silver nanoparticles-nanoparticle or silver ion. Toxicol Lett. 2012;208(3):286–292. doi: 10.1016/j.toxlet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Budtz-Jorgensen E. Oral mucosal lesions associated with the wearing of removable dentures. J Oral Pathol. 1981;10(2):65–80. doi: 10.1111/j.1600-0714.1981.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen SF, Zhang H. Aggregation kinetics of nano silver in different water conditions. Adv Nat Sci Nanosci Nanotechnol. 2012;3(3):035006. doi: 10.1088/2043-6262/3/3/035006. [DOI] [Google Scholar]
- 13.Chladek G, Kasperski J, Barszczewska-Rybarek I, et al. Sorption, solubility, bond strength and hardness of denture soft lining incorporated with silver nanoparticles. Int J Mol Sci. 2013;14(1):563–574. doi: 10.3390/ijms14010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury NR, MacGregor-Ramiasa M, Zilm P, et al. ‘Chocolate’ silver nanoparticles: synthesis, antibacterial activity and cytotoxicity. J Coll Interf Sci. 2016;482:151–158. doi: 10.1016/j.jcis.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 15.de Lima R, Seabra AB, Duran N. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol. 2012;32(11):867–879. doi: 10.1002/jat.2780. [DOI] [PubMed] [Google Scholar]
- 16.Derks J, Tomasi C. Peri-implant health and disease: a systematic review of current epidemiology. J Clin Periodontol. 2015;42(S16):S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 17.Gromov DG, Lydia MP, Andrey IS, et al. Nucleation and growth of Ag nanoparticles on amorphous carbon surface from vapor phase formed by vacuum evaporation. Appl Phys A. 2015;118(4):1297–1303. doi: 10.1007/s00339-014-8834-0. [DOI] [Google Scholar]
- 18.Fahmy A, Eisa WH, Yosef M, et al. Ultra-thin films of poly(acrylic acid)/silver nanocomposite coatings for antimicrobial applications. J Spectrosc. 2016;2016:7489536. doi: 10.1155/2016/7489536. [DOI] [Google Scholar]
- 19.Fan C, Chu L, Rawls HR, et al. Development of an antimicrobial resin–a pilot study. Dent Mater. 2011;27(4):322–328. doi: 10.1016/j.dental.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Gliga AR, Skoglund S, Wallinder IO, et al. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014;11(1):11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gogoi N, Babu PJ, Mahanta C, et al. Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater Sci Eng C. 2015;46:463469. doi: 10.1016/j.msec.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 22.Grzegorz C, Anna M, Izabela BR, et al. Antifungal activity of denture soft lining material modified by silver nanoparticles–a pilot study. Int J Mol Sci. 2011;12(7):4735–4744. doi: 10.3390/ijms12074735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensten-Pettersen A, Helgeland K. Sensitivity of different human cell line in the biologic evaluation of dental resin-based restorative materials. Scand J Dent Res. 1981;89(1):102–107. doi: 10.1111/j.1600-0722.1981.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsin YH, Chen CF, Huang S, et al. The apoptotic effect of nanosilver is mediated by a ROS-and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179(3):130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156(2):128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Hussain SM, Hess KL, Gearhart JM, et al. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol in Vitro. 2005;19(7):975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 27.Jung R, Kim Y, Kim HS, et al. Antimicrobial properties of hydrated cellulose membranes with silver nanoparticles. J Biomater Sci Polym Ed. 2009;20(3):311–324. doi: 10.1163/156856209X412182. [DOI] [PubMed] [Google Scholar]
- 28.Kasraei S, Sami L, Hendi S, et al. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus . Restor Dent Endod. 2014;39(2):109–114. doi: 10.5395/rde.2014.39.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li WR, Xie XB, Shi QS, et al. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli . Appl Microbiol Biotechnol. 2010;85(4):1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- 30.Luther EM, Koehler Y, Diendorf J, et al. Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology. 2011;22(37):375101. doi: 10.1088/0957-4484/22/37/375101. [DOI] [PubMed] [Google Scholar]
- 31.Massa MA, Covarrubias C, Bittner M, et al. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater Sci Eng C. 2014;45:146–153. doi: 10.1016/j.msec.2014.08.057. [DOI] [PubMed] [Google Scholar]
- 32.Matsubara VH, Igai F, Tamaki R, et al. Use of silver nanoparticles reduces internal contamination of external hexagon implants by Candida albicans . Braz Dent J. 2015;26(5):458–462. doi: 10.1590/0103-644020130087. [DOI] [PubMed] [Google Scholar]
- 33.Mishra SK, Ferreira JM, Kannan S. Mechanically stable antimicrobial chitosan-PVA-silver nanocomposite coatings deposited on titanium implants. Carbohydr Polym. 2015;121:37–48. doi: 10.1016/j.carbpol.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 34.Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23(S6):67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 35.Morra M. Biomolecular modification of implant surfaces. Expert Rev Med Devic. 2007;4(3):361–372. doi: 10.1586/17434440.4.3.361. [DOI] [PubMed] [Google Scholar]
- 36.Pan Y, Jiang L, Lin H, et al. Cell death affected by dental alloys: modes and mechanisms. Dent Mater J. 2017;36(1):82–87. doi: 10.4012/dmj.2016-154. [DOI] [PubMed] [Google Scholar]
- 37.Park EJ, Yi J, Kim Y, et al. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol in Vitro. 2010;24(3):872–878. doi: 10.1016/j.tiv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Park HJ, Kim JY, Kim J, et al. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009;43(4):1027–1032. doi: 10.1016/j.watres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Park MV, Neigh AM, Vermeulen JP, et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011;32(36):9810–9817. doi: 10.1016/j.biomaterials.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 40.Philbrook NA, Winn LM, Afrooz AR, et al. The effect of TiO2 and Ag nanoparticles on reproduction and development of Drosophila melanogaster and CD-1 mice. Toxical Appl Pharmacol. 2011;257(3):429–435. doi: 10.1016/j.taap.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Qin H, Cao H, Zhao Y, et al. Antimicrobial and osteogenic properties of silver-ion-implanted stainless steel. ACS Appl Mater Interf. 2015;7(20):10785–10794. doi: 10.1021/acsami.5b01310. [DOI] [PubMed] [Google Scholar]
- 42.Ramage G, Tomsett K, Wickes BL, et al. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2004;98(1):53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Riaz Ahmed KB, Nagy AM, Brown RP, et al. Silver nanoparticles: significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol in Vitro. 2017;38:179–192. doi: 10.1016/j.tiv.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Sabaliauskas V, Juciute R, Bukelskiene V, et al. In vitro evaluation of cytotoxicity of permanent prosthetic materials. Stomatologija. 2011;13(3):75–80. [PubMed] [Google Scholar]
- 45.Samari F, Dorostkar S. Synthesis of highly stable silver nanoparticles using imidazolium based ionic liquid. Iran Chem Soc. 2016;13(4):689–693. doi: 10.1007/s13738-015-0781-y. [DOI] [Google Scholar]
- 46.Sangsuwan A, Kawasaki H, Iwasaki Y. Thiolated-2-methacryloyloxyethyl phosphorylcholine protected silver nanoparticles as novel photo-induced cell-killing agents. Coll Surf B Biointerf. 2016;140:128–134. doi: 10.1016/j.colsurfb.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 47.Shulman JD, Rivera-Hidalgo F, Beach MM. Risk factors associated with denture stomatitis in the United States. J Oral Pathol Med. 2005;34(6):340–346. doi: 10.1111/j.1600-0714.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 48.Singh RP, Ramarao P. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol Lett. 2012;213(2):249–259. doi: 10.1016/j.toxlet.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Sodagar A, Kassaee MZ, Akhavan A, et al. Effect of silver nano particles on flexural strength of acrylic resins. J Prosthodont Res. 2012;56(2):120–124. doi: 10.1016/j.jpor.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Stanford CM. Surface modifications of dental implants. Aust Dent J. 2008;53(S1):S26–S33. doi: 10.1111/j.1834-7819.2008.00038.x. [DOI] [PubMed] [Google Scholar]
- 51.Stoehr LC, Gonzalez E, Stampfl A, et al. Shape matters: effects of silver nanospheres and wires on human alveolar epithelial cells. Part Fibre Toxicol. 2011;8:36. doi: 10.1186/1743-8977-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suresh AK, Pelletier DA, Wang W, et al. Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on Gram-negative and Gram-positive bacteria. Environ Sci Technol. 2010;44(13):5210–5215. doi: 10.1021/es903684r. [DOI] [PubMed] [Google Scholar]
- 53.Tian B, Chen W, Yu DG, et al. Fabrication of silver nanoparticle-doped hydroxyapatite coatings with oriented block arrays for enhancing bactericidal effect and osteoinductivity. J Mech Behav Biomed Mater. 2016;61:345–359. doi: 10.1016/j.jmbbm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Vogel K, Westphal N, Salz D, et al. Dental implants coated with a durable and antibacterial film. Surf Innov. 2015;3(1):27–38. doi: 10.1680/si.14.00002. [DOI] [Google Scholar]
- 55.Volker C, Oetken M, Oehlmann J. The biological effects and possible modes of action of nanosilver. In: Whitacre DM, editor. Reviews of Environmental Contamination and Toxicology Volume 223. Springer New York, New York; 2013. pp. 81–106. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Ji Z, Chang C, et al. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small. 2014;10(2):385–398. doi: 10.1002/smll.201301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wani IA, Khatoon S, Ganguly A, et al. Structural characterization and antimicrobial properties of silver nanoparticles prepared by inverse micro emulsion method. Coll Surf B Biointerf. 2013;101:243–250. doi: 10.1016/j.colsurfb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Wen JC, Jiang F, Yeh CK, et al. Controlling fungal biofilms with functional drug delivery denture biomaterials. Coll Surf B Biointerf. 2016;140:19–27. doi: 10.1016/j.colsurfb.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright JB, Lam K, Buret AG, et al. Early healing events in a porcine model of contaminated wounds: effects of nanocrystal line silver on matrix metalloproteinases, cell apoptosis and healing. Wound Repair Regen. 2002;10(3):141–151. doi: 10.1046/j.1524-475X.2002.10308.x. [DOI] [PubMed] [Google Scholar]
- 60.Yang EJ, Kim S, Kim JS, et al. Inflammasome formation and IL-1β release by human blood monocytes in response to silver nanoparticles. Biomaterials. 2012;33(28):6858–6867. doi: 10.1016/j.biomaterials.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 61.You J, Zhang Y, Hu Z. Bacteria and bacteriophage inactivation by silver and zinc oxide nanoparticles. Coll Surf B Biointerf. 2011;85(2):161–167. doi: 10.1016/j.colsurfb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Zamperini CA, Machado AL, Vergani CE, et al. Adherence in vitro of Candida albicans to plasma treated acrylic resin. Effect of plasma parameters, surface roughness and salivary pellicle. Arch Oral Biol. 2010;55(10):763–770. doi: 10.1016/j.archoralbio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 63.Zanette C, Pelin M, Crosera M, et al. Silver nanoparticles exert a long-lasting ant proliferative effect on human keratinocyte HaCaT cell line. Toxicol in Vitro. 2011;25(5):1053–1060. doi: 10.1016/j.tiv.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Shen Y, Xie A, et al. One-step synthesis of monodisperse silver nanoparticles beneath vitamin E Langmuir monolayers. J Phys Chem B. 2006;110(13):6615–6620. doi: 10.1021/jp0570216. [DOI] [PubMed] [Google Scholar]
- 65.Zhang T, Wang L, Chen Q, et al. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014;55(2):283–291. doi: 10.3349/ymj.2014.55.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng Y, Li J, Liu X, et al. Antimicrobial and osteogenic effect of Ag-implanted titanium with a nanostructured surface. Int J Nanomed. 2012;7:875–884. doi: 10.2147/IJN.S28450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zielinska E, Tukaj C, Radomski MW, et al. Molecular mechanism of silver nanoparticles-induced human osteoblast cell death: protective effect of inducible nitric oxide synthase inhibitor. PLoS ONE. 2016;11(10):e0164137. doi: 10.1371/journal.pone.0164137. [DOI] [PMC free article] [PubMed] [Google Scholar]