Abstract
Objective: To evaluate the possible photoprotection mechanisms of cyclic and linear electron flux (CEF and LEF) under specific high temperature and high light (HH) stress. Methods: Six-leaf-stage tomato seedlings (“Liaoyuanduoli”, n=160) were divided into four parts: Part 1, served as control under 25 °C, 500 μmol/(m2·s); Part 2, spayed with distilled water (H2O) under 35 °C, 1000 μmol/(m2·s) (HH); Part 3, spayed with 100 μmol/L diuron (DCMU, CEF inhibitor) under HH; Part 4, spayed with 60 μmol/L methyl viologen (MV, LEF inhibitor) under HH. Energy conversion, photosystem I (PSI), and PSII activity, and trans-thylakoid membrane proton motive force were monitored during the treatment of 5 d and of the recovering 10 d. Results: HH decreased photochemical reaction dissipation (P) and the maximal photochemical efficiency of PSII (F v/F m), and increased the excitation energy distribution coefficient of PSII (β); DCMU and MV aggravated the partition imbalance of the excitation energy (γ) and the photoinhibition degree. With prolonged DCMU treatment time, electron transport rate and quantum efficiency of PSI (ETRI and Y I) significantly decreased whereas acceptor and donor side limitation of PSI (Y NA and Y ND) increased. MV led to a significant decline and accession of yield of regulated and non-regulated energy Y NPQ and Y NO, respectively. Membrane integrity and ATPase activity were reduced by HH stress, and DCMU and MV enhanced inhibitory actions. Conclusions: The protective effects of CEF and LEF were mediated to a certain degree by meliorations in energy absorption and distribution as well as by maintenance of thylakoid membrane integrity and ATPase activity.
Keywords: Photoinhibition, Photoprotection, Thylakoid membrane, Tomato
1. Introduction
When photosynthetic organisms are exposed to all kinds of environmental stresses like high or low temperature, water or nutrient stress, and light intensity exceeds the leaf’s ability to use it, this can lead to an imbalance between the capability for absorbing light energy and consuming this excess light energy through metabolic mechanisms, resulting in the generation of reactive oxygen species (ROS) which are injurious to photosystems (Chen et al., 2012; Yamori et al., 2016). Extensive research has shown that many sites in the photosynthetic membrane are susceptible to high temperature and high light intensity (HH), in particular photosystem II (PSII) (Pospisil, 2012; Deng et al., 2013). It can be inhibited either on the acceptor side or on the donor side disordering their functions as a result. For instance, on the acceptor side it can act by suppressing closed reaction center’s fraction rise and the electron transport from primary quinone electron acceptor of PSII (QA) to secondary quinone electron accepter of PSII (QB) (Zhou et al., 2011); on the donor side through impairing the oxygen evolving complex (de Filippis and Ziegler, 1993). Except for PSII, diverse environmental stress conditions can also result in photoinhibition impairment of photosystem I (PSI) (Ivanov et al., 2015). For example, the reaction center of PSI can be impaired and the activity of PSI can be lessened under HH (Miyake et al., 2004). In addition, photosynthetic pigment synthesis, photosynthetic electron transport, energy distribution in photosystems, and cell membrane function integrity are negatively affected by HH (Camejo et al., 2005; Bose et al., 2011). Some studies demonstrated that PSI was less impacted than PSII under HH, and suggested that PSI was not a limiting factor in overall photosynthetic activity (de Filippis et al., 1981). Consequently, the inhibition effects of HH on the regulation and functional mechanisms of PSII and PSI need further study.
To reduce the probability of damage to photosynthetic apparatus resulting from HH, many distinctive adaption mechanisms have been evolved in plants, including chloroplast and leaf movement, conduction and convection of heat flux, absorbed energy thermal dissipation of PSII (Takahashi and Badger, 2011; de la Rosa-Manzano et al., 2015), carotenoid production, protein complexes’ organization and/or abundance changes (Zivcak et al., 2014; Agrawal et al., 2016) and so on. Foyer et al. (2012) supported the idea that to cope with high irradiance, the thylakoid membranes were more flexible and in dynamic change. Arabidopsis thaliana plants grown in HH showed an increased amount of PSII units and a typically lower ratio of chlorophyll a/chlorophyll b (Bailey et al., 2004; Zivcak et al., 2014). Under high temperature conditions, wheat protected other chloroplast and cell components against damage by inactivating PSI (Brestic et al., 2016). In spite of these findings, the major photoprotection mechanism that plays an important role for de-excitation of excess light energy in green plants is the non-photochemical quenching (NPQ) mechanism, containing the xanthophyll cycle and the protonation of residues on key LHCII proteins (Kanazawa and Kramer, 2002; Ivanov et al., 2015). Aside from NPQ, the photosynthetic electron transportation regulation in chloroplasts of the thylakoid membrane is cardinally important for plant development (Partelli et al., 2011). There are two types of electron flux on the thylakoid membrane, linear electron flux (LEF) and cyclic electron flux (CEF). LEF goes through the Cytochrome b 6/f (Cyt b 6/f) complex and generates adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) used in the Calvin-Benson cycle and other assimilatory reactions subsequently. CEF recycles from the ferredoxin (Fd) to photochemical quenching (PQ) pool, also producing proton gradient (ΔpH) and ATP in consequence, but there is no NADPH accumulation in chloroplasts (Shikanai, 2014). The role of CEF is thought to be essential for balancing the production ratio of ATP/NADPH and/or for defending photosystems from impairment by over-reduction of stroma. Under HH conditions, extra ΔpH comes from CEF across the thylakoid membrane to preserve the PSII reaction center (Shikanai, 2014; Liu et al., 2015). In addition, plants effectively dissipated over-excitation by the transition of LEF to CEF, which relieves the excitation pressure in photosystems and diminishes ROS generation (Heber et al., 1995; Johnson, 2005; Tu et al., 2015). Supplementary/alternative electron transportation pathways and the NPQ mechanism can dispel PSII reaction center-accumulated excessive excitation pressure and do not occasion injurious effects. Compared to PSII, there are two defending mechanisms of PSI photoinhibition: one is CEF around PSI and the other is photoinhibition of PSII (Zhang et al., 2014). CEF can oxidize pigment molecule of PSI reaction center (P700) into oxidized pigment molecule of PSI reaction center (P700+) and dispel superfluous NADPH reducing power. Simultaneously, the electrons brought to PSI can be decreased by the PSII reaction center closure (Munekage et al., 2002; Shikanai, 2007; 2014). Up to 30% of photosynthetic electrons can be consumed by CEF in higher plants, and in green algae, especially in light stress conditions the utilization proportion is larger (Tu et al., 2015). Since abiotic stresses limit plant growth, comprehending the physiological actions alleviating impairment and the plant tolerance mechanisms is of tremendous significance to agriculture. However, the physiological significance of CEF remains to be clarified and the mechanisms of photoinhibition and photoprotection need to be fully appreciated.
In the process of photosynthesis, exergonic reaction releases free energy, and it is stored in the proton motive force (PMF), a form of transmembrane electrochemical proton gradient. The PMF is comprised of the transmembrane electrical field (membrane potential, Δψ) and the transmembrane proton concentration (proton gradient, ΔpH), and it is coupled to the synthesis of ATP (Cruz et al., 2001; Deng et al., 2013). PMF generation depends upon both LEF and CEF, whereas ATPase relaxes PMF (Bailleul et al., 2010). ΔpH and Δψ carry cardinal information of the coupled electron and proton transport rate. ΔpH impacts turnover rate of critical photosynthesis enzymes and motivates downstream regulatory processes. The H+ depending on light separates the oxidization rate of PQ managed by stroma and thylakoid at Cyt b 6/f. Divalent cations modulate the activity of enzyme, and Δψ actuates flow of that in stroma and influences PSI, PSII, and Cyt b 6/f electrogenic reactions (Bilger et al., 1989; Deng et al., 2013; Johnson and Ruban, 2014). Electrochromic shift (ECS) signal decay kinetics responding to a dark pulse that is implemented for photosynthesis steady state under light is often used to detect the activity of ATPase (Kramer et al., 2004). Additionally, ECS analysis indicates that the contributions of ΔpH and Δψ to PMF are almost equal (Cruz et al., 2001). However, down-regulation of Cyt b 6/f complex and pH-dependent energy dissipation (qE) induction depend exclusively on ΔpH (Johnson and Ruban, 2014). Moreover, as the primary product of CEF is ΔpH, the ΔpH amplitude can be used to indirectly calculate the electron transport rate. Prior research has noted that under high temperature (42 °C) stress in mutant tobacco, the build-up of ΔpH was significantly suppressed (Wang et al., 2006). Without considering their important roles in the photosynthesis, several fundamental questions remain. For instance, how are the two components of PMF regulated in chloroplasts under different environmental stresses? How is the ATPase protonic conductivity regulated under high light intensity?
In the light of these significant and versatile functions in the photoprotection of the photosynthetic apparatus, the effect was estimated in a tomato plant (Solanum lycopersicum L.). In this paper, the effects of exogenous electron transport inhibitor (diuron (DCMU) and methyl viologen (MV)) treatment on excitation energy distribution, PSI and PSII activity and electron transportation, thylakoid membrane integrity, ATPase activity, and thylakoid transmembrane PMF components of tomato seedlings under sub-high temperature and high light intensity stress are researched. These outcomes furnish a perspective on the potential roles of LEF-PSII and CEF-PSI in photoinhibition and photoprotection mechanisms of tomato leaves under HH stress.
2. Materials and methods
2.1. Plant materials and study design
“Liaoyuanduoli” of tomato (Solanum lycopersicum L.) were germinated and cultivated in 12 cm×12 cm nutrition pots within a glasshouse (average day/night temperatures, 25 °C/15 °C). The relative air humidity was 60%. Tomato seedlings were separated into four growth rooms (Qiushi Environment, Zhejiang, China) at the six-leaf stage from the greenhouse, with a total of 40 pots per room. In each growth room, metal halide lamps (400 W, Osram, Germany) were used as the light source. To block CEF and LEF, before measurement the electron transfer inhibitors DCMU and MV were used. DCMU impedes electron transport after QA which is the PSII primary acceptor (Munekage et al., 2002; Gao et al., 2013). MV suppresses Fd-dependent CEF around PSI by accepting electrons from PSI (Shikanai, 2007; Setif, 2015). The data for the different treatments are given in Table 1. After being treated for 5 d, plants were shifted to the control room and spraying solution was discontinued, and then they were allowed to recuperate for 10 d. From Day 0, the treatments were initiated.
Table 1.
Different treatments in the growth rooms
| Treatment | Temperature (°C, day/night) | Illumination (μmol/(m2·s)) | Solution (μmol/L) | RH (%) | CO2 (μmol/mol) |
| Control | 25/15 | 500 (±50) | 60 (±10) | 600 | |
| H2O+HH | 35/15 | 1000 (±50) | 60 (±10) | 600 | |
| DCMU+HH | 35/15 | 1000 (±50) | 100 | 60 (±10) | 600 |
| MV+HH | 35/15 | 1000 (±50) | 60 | 60 (±10) | 600 |
The solutions were sprayed in the early morning every day. RH, relative humidity
2.2. Measurement of fluorescence
To acquire the P700 redox state and chlorophyll fluorescence synchronously, Dual-PAM-100 measuring system (Heinz Walz, Effeltrich, Germany) was used following Yamori et al. (2011) with small-scale modifications. The dark-and light-adapted initial fluorescence (F 0 and F 0') were determined through switching on less than 0.1 μmol/(m2·s) photosynthetic photon flux density (PPFD)-modulated irradiation to the leaf. The dark-and light-adapted maximal fluorescences (F m and F m') were obtained by 10 000 μmol/(m2·s) saturating light of 300 ms pulse. Reduced state of P700 (P700 red) was measured with help of a saturation pulse in a given state. P m and P m' are analogous to F m and F m', respectively. They were given by the same means as the former fluorescence parameters. The leaf was illuminated with far-red light and then a saturation pulse was applied. The formulae of other fluorescence parameters are shown in Table 2. A recent study reported that Y I and Y II (effective photochemical quantum yields of PSI and PSII, respectively) may represent information from different leaf tissues’ sections due to a P700 and P680 (PSII reaction center) signal coming from the whole issue and mesophyll nearby leaf surface differently (Huang et al., 2010a).
Table 2.
Formulae used to calculate chlorophyll fluorescence parameters
| Parameter | Description | Formula |
| F v/F m | The maximum photochemistry efficiency of PSII | F v/F m=(F m−F 0)/F m |
| F v'/F m' | The efficiency of excitation energy capture by open PSII reaction center | F v'/F m'=(F m'−F 0')/F m' |
| qP | The photochemical quenching coefficient | qP=(F m'−F s)/(F m'−F 0') |
| NPQ | The non-photochemical quenching coefficient | NPQ=(F m−F m')/F m' |
| ETRII | The electron transport rate of PSII | ETRII=Y II×PPFD×0.84×0.5 |
| Y II | The quantum efficiency of PSII photochemistry | Y II=(F m'−F s)/F m' |
| Y NO | The quantum yield of non-regulated energy of PSII | Y NO=F s/F m |
| Y NPQ | The quantum yield of regulated energy of PSII | Y NPQ=1−Y II−Y NO |
| ETRI | The electron transport rate of PSI | ETRI=Y I×PPFD×0.84×0.5 |
| Y I | The effective photochemical quantum yield of PSI | Y I=1−Y ND−Y NA |
| Y ND | The donor side limitation of PSI | Y ND=1−P700 red |
| Y NA | The acceptor side limitation of PSI | Y NA=(P m−P m')/P m |
F v and F v', variable fluorescences which yield in the dark-and light-adapted states; F m and F m', dark-and light adapted maximal fluorescences
Plants were allowed for dark adaptation for 20 min and then sent for measurement. All determinations were carried out on the fourth operational leaves adopting at least three seedlings from each treatment and the actinic light for measurements of chlorophyll fluorescence was 531 μmol/(m2·s).
2.3. Calculation of distribution of absorbed light energy
The distribution of absorbed light energy in leaf and the excitation energy distribution coefficient among two photosystems was calculated as previously described (Braun and Malkin, 1990; Hendrickson et al., 2004; Zhou et al., 2007). The formulae are as follow: (1) P=(F v'/F m')×qP, D=1−F v'/F m', E=(F v'/F m')×(1−qP); P is the photochemical reaction energy, D is the antenna heat dissipation energy, E is the excess energy, F v' is the variable fluorescence which yields in the light-adapted state, and qP is the photochemical quenching coefficient. (2) f=(F m−F s)/(F m−F 0), α=f/(1+f), β=1/(1+f), γ=β/α−1; f is the excitation energy distribution coefficient of photosystem, F s is the steady-state fluorescence, α and β represent the excitation energy distribution coefficient of PSI and PSII respectively, and γ is the partition imbalance of the excitation energy between PSI and PSII.
2.4. Measurement of P515/535
As also carried out by Schreiber and Klughammer, (2008), we monitored the dual-beam 550 nm to 515 nm different signals simultaneously with the Dual-PAM-100 P515/535 module. To evaluate thylakoid membrane integrity, seedlings were placed for 1 h in dark adaptation, and then P515 induction changes were recorded by saturated single turnover flashes. To estimate ATPase activity, plants were pre-illuminated at 531 μmol/(m2·s) for 10 min before 4 min of dark adaptation, then P515 induction changes were recorded by saturated single turnover flashes.
Changes in both ESC and content of zeaxanthin can be affirmed in the slow phase curve of dark-light-dark-induced transients in 550 to 515 nm signals. At least 1 h of dark adaptation was necessary before determining these transients. Actinic light (AL) was turned on (531 μmol/(m2·s)) after 30 s and off after 330 s. After that, two components of PMF (Δψ and ΔpH) were measured by analyzing the dark-light-dark induction transients.
2.5. Statistical analyses
The figures were drawn with Origin 9 Software (OriginLab, Northampton, MA, USA). The data were analyzed using SPSS 20 Software (IBM SPSS Statistics, USA) by analysis of variance (ANOVA). Statistically significant difference was set at a probability level of 0.05 (or 0.01).
3. Results and discussion
3.1. Effects of DCMU and MV on distribution of absorbed light energy in leaves and excitation energy in photosystems under HH stress
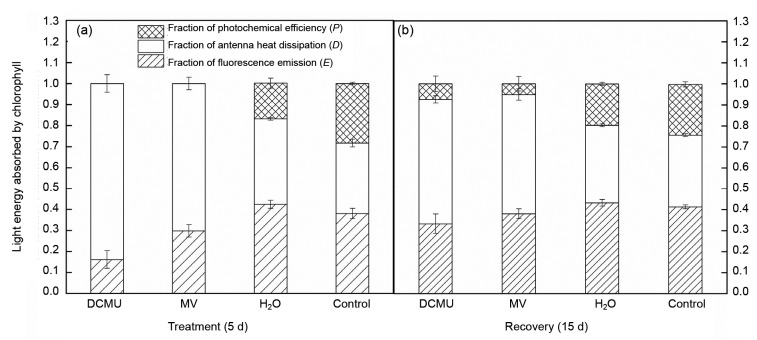
We divided absorbed light energy into three parts by the fluorescence parameter (Liu et al., 2013) to find the light energy utilization ability of tomato under HH. Symbols are as follows: P, the photochemical reaction energy; D, the antenna heat dissipation energy, and E, the dissipation of non-photochemical reaction energy. The results showed that HH resulted in a decline of P and an increase of D; E of DCMU and MV treatment was decreased; P was not present in DCMU and MV treatment plants (Fig. 1a). However, during recovery, P and E of DCMU and MV treatment were recovered to some degree (Fig. 1b). These changes suggested an adaptation ability of the plants to adversity. Plants avoided the over-reduction of PSII and electron transport chain by increasing D which was linked to the running status of the xanthophyll cycle and decreasing content of chlorophyll a (a reaction center pigment). It was also a protection mechanism to prevent photosynthetic photodamage by excess energy (Zhou et al., 2004).
Fig. 1.
Effects of DCMU and MV on distribution of absorbed light energy in tomato leaves during treatment (5 d) (a) and recovery (15 d) (b) under sub-high temperature and high-light intensity stress (HH)
Data are expressed as mean±SD (n=6)
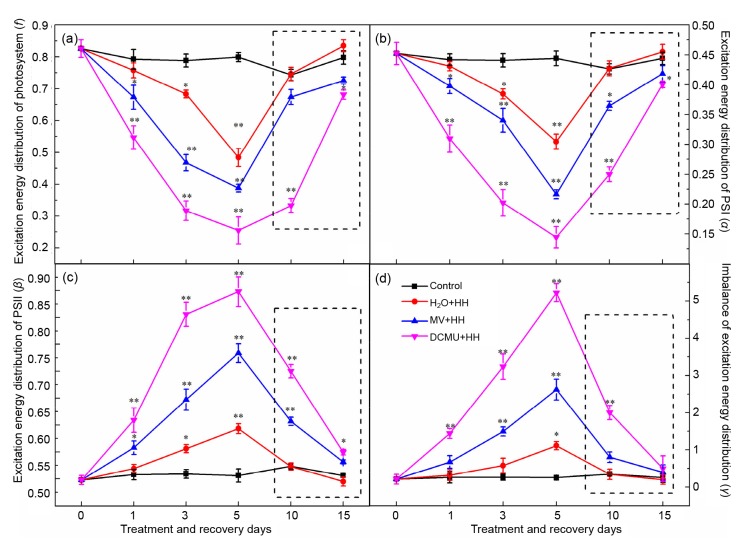
At the same time, f and α were considerably decreased and β was significantly increased under HH. The changing extent was aggravated with prolonged treatment time (Figs. 2a–2c), resulting in the excitation energy distribution coefficient γ increasing gradually (Fig. 2d). DCMU and MV would dramatically intensify the imbalance of excitation energy distribution between PSI and PSII. When either electronic transport of PSII or PSI was inhibited, the excitation energy distribution portion of PSI was reduced opposite to that of PSII. These findings indicated that HH stress would lead to an excitation energy distribution which was out of balance, resulting in tomato seedlings being unable to get rid of excess excitation energy and thus leading to photodamage to photosystems especially PSII. Results consistent with these can be found in cucumber, cotton, and mulberry seedlings (Guissé et al., 1995; Zhou et al., 2004; Yin and Tian, 2013). In addition, CEF-PSI can alleviate the distribution of excitation energy imbalance to some extent.
Fig. 2.
Effects of DCMU and MV on allocation of excitation energy in tomato photosystems under HH
Excitation energy distribution coefficients f (a), α (b), β (c), and the partition imbalance of the excitation energy between PSI and PSII γ (d). The inside of the dotted line is the treatment of recovery. Data are means of six repetitions with standard errors demonstrated by vertical bars. * and ** represent significant and highly significant differences (P≤0.05 and P≤0.01), respectively
3.2. Effects of DCMU and MV on PSII activity and energy conversion of PSI and PSII in tomato leaves under HH stress
3.2.1 Effects of DCMU and MV on PSII activity
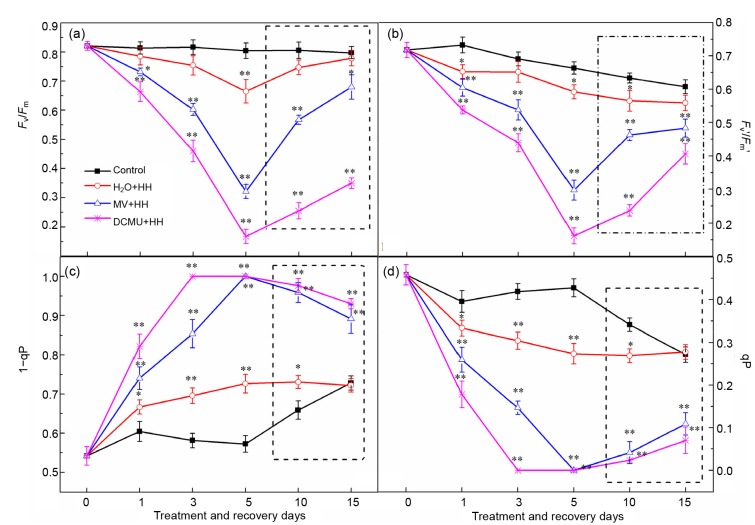
In line with recent studies (Ivanov et al., 2015; Yamori et al., 2016), F v/F m and F v'/F m' measured as the PSII photochemical efficiency and the antenna efficiency, respectively, in tomato plants under HH were both inhibited in an identical manner to those in peas and rice (Figs. 3a and 3b), where F v is the variable fluorescence which yields in the dark state. Additionally, the PSII-excited energy pressure (1−qP) was significantly increased in the HH plants (Fig. 3c). In parallel, the value of photochemical quenching (qP) was significantly affected (Fig. 3d) compared to control plants. However, tomato plants treated with DCMU and MV exhibited a much higher sensitivity to HH stress. Furthermore, decreased qP corresponded to higher 1−qP, i.e. for a given irradiance, a higher reduction state of QA changed the energy redistribution between PSII and PSI, and the ratio of functionally active PSIIα to PSIIβ centers, as well as the oxygen burst decay kinetics (Apostolova et al., 2006). F v'/F m' of DCMU and MV plants showed a declining tendency during the treatment, suggesting that the decrease of primary electron transfer efficiency might be on account of the acceptor and donor impairment of PSII and decrease of photosynthetic activity, which would lead to reduction of carbon fixation and assimilation with the surge of excess energy (Guissé et al., 1995). Also, the damage degree was aggravated by DCMU and MV.
Fig. 3.
Effects of DCMU and MV on F v/F m (a), F v'/F m' (b), 1−qP (c), and qP (d) of tomato leaves under HH
The inside of the dotted line is the treatment of recovery. Data are means of six repetitions with standard errors demonstrated by vertical bars. * and ** represent significant and highly significant differences (P≤0.05 and P≤0.01), respectively
3.2.2 Effects of DCUM and MV on energy conversion of PSI and PSII
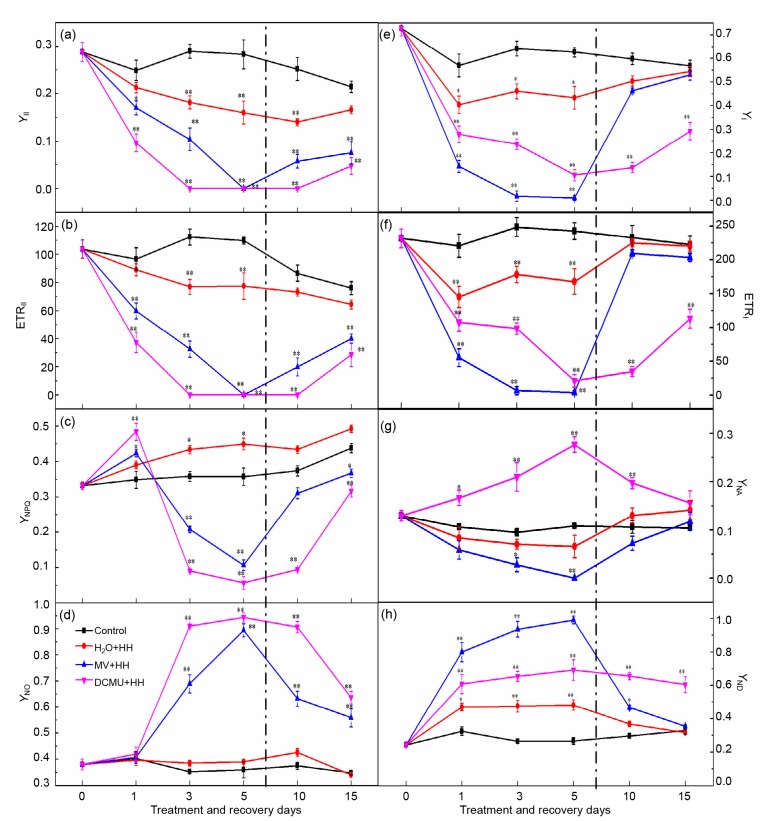
On the photosynthetic membrane, there are plenty of sites where LEF and CEF exert their functions. Energy conversion of PSII and PSI was substantially affected after 5 d of DCMU and MV treatment under HH (Figs. 4a–4h). As shown in Fig. 4c, the decline of the quantum yield of regulated energy of PSII (Y NPQ) indicated that DCMU and MV treatment resulted in detrimental excess excitation energy which could not be dissipated in a timely fashion, causing PSII activity reduction (for instance, the decline of F v/F m in Fig. 3a). PSII is the most susceptible photosynthetic organ including the acceptor and donor side (Pagliano et al., 2006; Deng et al., 2013), and inhibition of PSII has been attributed to the PsbA protein of PSII (D1 protein) loss and pigment degradation. On the thylakoid membrane, since the abundance and the stability of PSI are lesser and greater than those of PSII, respectively, so PSI is more strongly resistant to adversity (Jiang and Qiu, 2010). In this research, DCMU suppressed PSII to a much greater extent than PSI. In comparison with the control, DCMU and MV not only caused PSII inhibition but also led to a decrease of Y NPQ and an increase of the quantum yield of non-regulated energy of PSII (Y NO) (Figs. 4c and 4d). Y NO represents excess excitation energy which should be consumed by a non-regulated process of PSII. In MV and DCMU-treated plants, the increase of Y NO reflected the rise of the closed reaction center fraction in accordance with the decline of qP (Fig. 3d) and the inability of the PSII center to deal with superfluous radiation (Jin et al., 2009). Y NPQ symbolizes superfluous excitation energy and could be consumed by regulated approaches in respect of carotenoids and the xanthophyll cycle. The decrease of Y NPQ in DCMU-and MV-treated seedlings suggests that inordinate excited energy could not be efficiently dispelled into harmless heat and that the regulatory mechanism did not work well (Klughammer and Schreiber, 2008; Deng et al., 2013). Our outcomes also demonstrated that Y II was significantly inhibited by DCMU and MV compared to the control under HH (Fig. 4a). This may be on account of electron transportation inhibition between PSII and P700+ by HH (Figs. 4b and 4f), while electron transport inhibitors (DCMU and MV) exacerbated the suppression effect. As functional PQ pools were substantially over-reduced by HH compared to the control, we regarded the decline in PQ as the dominant factor obstructing electron transfer. In addition, PQ pool reduction might lead to thylakoid membrane protein phosphorylation, status 2 transition activation, CEF and PMF promotion, and ATP deficit alleviation. Accordingly the antenna of PSII would be down-regulated by the qE mechanism (Yi et al., 2005; Zhang et al., 2014).
Fig. 4.
Effects of DCMU and MV treatment and recovery on energy distribution and electron transport rate in photosystems of tomato leaves under HH
(a) Quantum efficiency of PSII photochemistry (Y II); (b) Electron transport rate of PSII (ETRII); (c) The quantum yield of regulated energy of PSII (Y NPQ); (d) Quantum yield of non-regulated energy of PSII (Y NO); (e) The effective photochemical quantum yield of PSI (Y I); (f) Electron transport rate of PSI (ETRI); (g) Acceptor side limitation of PSI (Y NA); (h) Donor side limitation of PSI (Y ND). The left side of the dotted line is different treatments and the other side is the treatments of recovery. Data are means of six repetitions with standard errors demonstrated by vertical bars. * and ** represent significant and highly significant differences (P≤0.05 and P≤0.01), respectively
HH stress significantly inhibited PSI activity. DCMU and MV treatment led to PSI being more fragile and open to abiotic stress. The synthesis of ATP needs a trans-thylakoid H+ gradient (ΔpH) which is generated from CEF, and PSI is important to CEF. The Y I diminution implies a substantial impediment to effective and functional photosynthesis (Bailey et al., 2008). The concurrent decline of Y I and Y II indicated that HH stress suppressed the electronic transfer of PSI and PSII (Figs. 4a and 4e). This inhibitory effect can be confirmed by both the decline of the electron transport rate of PSI (ETRI) and the electron transport rate of PSII (ETRII) and the increment of the donor side limitation of PSI (Y ND) (Figs. 4b, 4f, and 4h). Since ETRI was greatly suppressed (Fig. 4f) in all treatments, the treated plants were weak in the generation of ΔpH across the thylakoid membrane. Furthermore, DCUM and MV resulted in a stepwise growth of PQ pool redox state (high 1−qP) in tomato leaves (Fig. 3c). This increase indicated that extra reducing power was accumulated in DCMU and MV plants by the electron transportation system, which was incapable of dispersing it as heat during the period of HH stress. The significant decrease of the acceptor side limitation of PSI (Y NA) and increase of Y ND implied that the decline of Y I was chiefly owing to the limitation of the PSI donor side in H2O-treated plants. Y ND is the oxidized P700 fraction due to PSI electron deprivation or donors shortage. PSI photochemical efficiency can be inhibited at the donor side by a PSII small antenna size assimilating light inefficiently (Huang et al., 2010b). As well as the donor side limitation, at the PSI acceptor side, as signaled by Y NA, DCMU would bring about a substantial reducing power as HH stress accumulated in the whole electron transportation system reduction, finally resulting in photoinhibition of PSI. In consequence, through the ETRII and ETRI plants declining rate of CO2 assimilation, subsequently their development was decelerated. For the 5th day, PSI became precarious and no dependable data of Y I, ETRI, Y ND or Y NA were collected in MV treatment plants, implying that MV damaged the function of PSI.
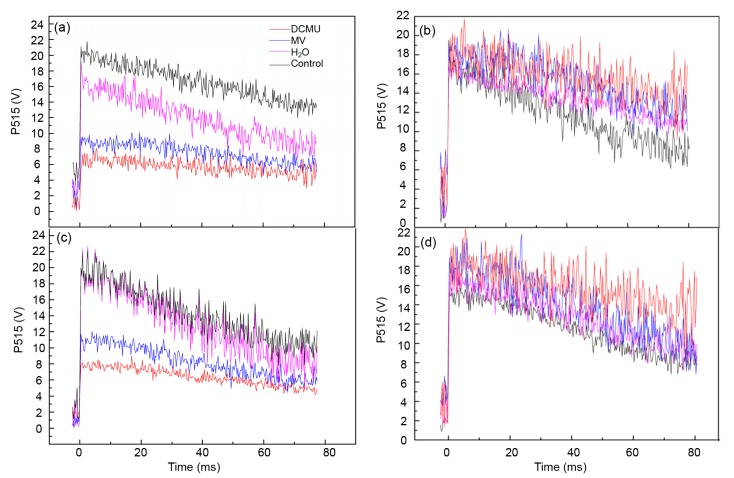
3.3. Effects of DCMU and MV on P515 signal of tomato leaves under HH stress
3.3.1 Effects of DCMU and MV on thylakoid membrane integrity and ATPase activity
Pursuant to a chemiosmotic hypothesis, in the mitochondrial matrix across the inner mitochondrial membrane, the intermediate couples the electron flux through the respiratory chain with ATP synthesis by ATPase (Deng et al., 2013). The activity of ATPase and the integrity of the membrane can be determined by using the ECS signal decay kinetics. The decay of the P515 signal gives an index to the flash relaxation of H+ efflux via the H+ channel of ATPase, and by electrogenic Q-cycle electron transfer in the Cyt b 6/f complex and charge separation in the two photosystems, the flash may induce an electric field. Slow decay after adaptation to darkness (high membrane integrity) and fast decay after irradiation to active light (high ATPase activity) are always used as indicators of a functionally undamaged photosynthetic apparatus (Zhang et al., 2014). In our research, a faster decay after adaptation to darkness and a slower decay after irradiation to active light in HH stress plants implied that the thylakoid membrane integrity was impaired as well as the fact that ATPase activity was inhibited. Furthermore, exogenous DCMU and MV treatment severely enhanced the damage degree (Figs. 5a and 5b). Once HH stress was removed for recovery treatment, thylakoid membrane integrity and ATPase activity of DCMU and MV continued to be at a low level compared with the control and even H2O-treated plants (Figs. 5c and 5d), suggesting that the inhibitors caused severe damage to the thylakoid membrane and ATPase.
Fig. 5.
Effects of DCMU and MV treatment and recovery on fast P515 signals in tomato leaves under HH
Effects of DCMU and MV on thylakoid membranes integrity (a) and ATPase activity (b) by 5-d treatment, and thylakoid membrane integrity (c) and ATPase activity (d) by 10-d recovery
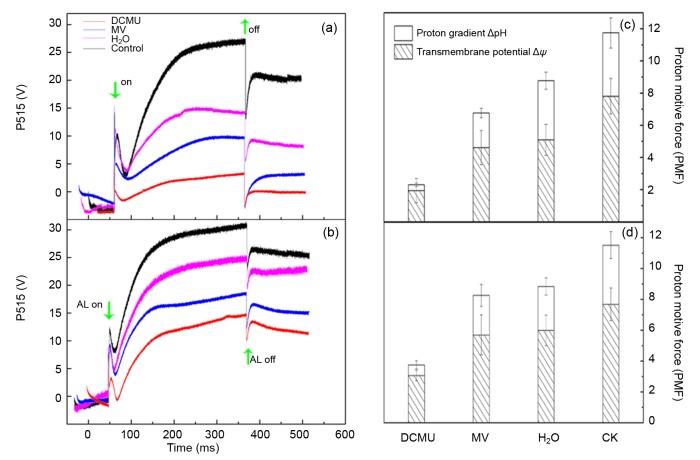
3.3.2 Effects of DCMU and MV on the changes of Δψ and ΔpH
Through dark-light-dark induction kinetics, the P515 signal rise reflects the increment of ECS. After light-off, the “dark baseline” improvement manifestly assesses the formation of zeaxanthin. Compared to the control, the increase of “dark baseline” was significantly lower under HH stress, indicating the decrease of zeaxanthin content. Additionally, MV and DCMU further inhibited the formation of zeaxanthin (Figs. 6a and 6b).
Fig. 6.
Effects of DCMU and MV treatment and recovery on slow P515 signals and proton motive force (PMF) in tomato leaves under HH
Slow P515 signals by 5-d treatment (a) and 10-d recovery (b) and PMF by 5-d treatment (c) and 10-d recovery (d)
In higher plant photosynthesis, PMF has been detected in thylakoids and in intact leaves. It is proposed that PMF plays two major functions by being parsed into Δψ and ΔpH. Firstly, Δψ motivates the carrier of ATP/ADP, while ΔpH drives the carrier of phosphate (Dzbek and Korzeniewski, 2008). Secondly, NPQ is principally connected with ΔpH and the xanthophyll cycle, and ΔpH down-regulates the light capture efficiency of photosynthesis antennae with the aid of the qE mechanism (one component of NPQ) dissipating the superfluous energy as heat (Ioannidis et al., 2012). Moreover, the partition of PMF is impacted by three potential factors: the thylakoid membrane capacitance, the stroma and lumen ionic composition, and the lumen proton-buffering capability (Ioannidis and Kotzabasis, 2014). In our study, ΔpH and Δψ components of tomato plants decreased under HH, suggesting the inhibition of zeaxanthin formation and the reduction of H+ outflow from lumen to stroma through the thylakoid ATPase (Figs. 6a and 6c, Fig. 5b). Furthermore, in the presence of DCMU and MV treatment, ΔpH was significantly inhibited in parallel with the impairment of NPQ mechanism. The decrease of Δψ and ΔpH was also found in some other environmental stresses (Bilger and Schreiber, 1990; Wang et al., 2006; Deng et al., 2013). The present study demonstrated that ΔpH of DCMU and MV decreased more than Δψ under HH stress, indicating that NPQ was seriously inhibited compared to the ATP driver.
4. Conclusions
In summary, sub-high temperature and high-light intensity stress induced photoinhibition of PSI and PSII, which is attributed to an imbalance of excess excitation energy between them. DCMU and MV treatment further intensified the degree of photoinhibiton and imbalance. Furthermore, once linear or cyclic electron flux was blocked, the thylakoid membrane integrity, ATPase synthesis, and the transmembrane proton motive force suffered serious photodamage and suppression.
Footnotes
Project supported by the China Agriculture Research System (No. CARS-25), the National Natural Science Foundation of China (No. 31301813), and the National Key Technologies R & D Program of China (No. 2015103003)
Compliance with ethics guidelines: Tao LU, Jie-wei SHI, Zhou-ping SUN, Ming-fang QI, Yu-feng LIU, and Tian-lai LI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Agrawal D, Allakhverdiev SI, Jajoo A. Cyclic electron flow plays an important role in protection of spinach leaves under high temperature stress. Russ J Plant Physiol. 2016;63(2):210–215. doi: 10.1134/S1021443716020023. [DOI] [Google Scholar]
- 2.Apostolova EL, Dobrikova AG, Ivanova PI, et al. Relationship between the organization of the PSII super complex and the functions of the photosynthetic apparatus. J Photochem Photobiol B. 2006;83(2):114–122. doi: 10.1016/j.jphotobiol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Bailey S, Horton P, Walters RG. Acclimation of Arabidopsis thaliana to the light environment: the relationship between photosynthetic function and chloroplast composition. Planta. 2004;218(5):793–802. doi: 10.1007/s00425-003-1158-5. [DOI] [PubMed] [Google Scholar]
- 4.Bailey S, Melis A, Mackey KR, et al. Alternative photosynthetic electron flow to oxygen in marine Synechococcus . Biochim Biophys Acta. 2008;1777(3):269–276. doi: 10.1016/j.bbabio.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Bailleul B, Cardol P, Breyton C, et al. Electrochromism: a useful probe to study algal photosynthesis. Photosynth Res. 2010;106(1-2):179–189. doi: 10.1007/s11120-010-9579-z. [DOI] [PubMed] [Google Scholar]
- 6.Bilger W, Schreiber U. Chlorophyll luminescence as an indicator of stress-induced damage to the photosynthetic apparatus. Effects of heat-stress in isolated chloroplasts. Photosynth Res. 1990;25(3):161–171. doi: 10.1007/BF00033158. [DOI] [PubMed] [Google Scholar]
- 7.Bilger W, Bjorkman O, Thayer SS. Light-induced spectral absorbance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Plant Physiol. 1989;91(2):542–551. doi: 10.1104/pp.91.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bose S, Kuila T, Nguyen , et al. Polymer membranes for high temperature proton exchange membrane fuel cell: recent advances and challenges. Prog Polym Sci. 2011;36(6):813–843. doi: 10.1016/j.progpolymsci.2011.01.003. [DOI] [Google Scholar]
- 9.Braun G, Malkin S. Regulation of the imbalance in light excitation between photosystem II and photosystem I by cations and by the energized state of the thylakoid membrane. Biochim Biophys Acta. 1990;1017(1):79–90. doi: 10.1016/0005-2728(90)90181-3. [DOI] [Google Scholar]
- 10.Brestic M, Zivcak M, Kunderlikova K, et al. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth Res. 2016;62(3):281–283. doi: 10.1007/s11120-016-0249-7. [DOI] [PubMed] [Google Scholar]
- 11.Camejo D, Rodriguez P, Morales MA, et al. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol. 2005;162(3):281–289. doi: 10.1016/j.jplph.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Jia H, Tian Q, et al. Protecting effect of phosphorylation on oxidative damage of D1 protein by down-regulating the production of superoxide anion in photosystem II membranes under high light. Photosynth Res. 2012;112(2):141–148. doi: 10.1007/s11120-012-9750-9. [DOI] [PubMed] [Google Scholar]
- 13.Cruz JA, Sacksteder CA, Kanazawa A, et al. Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (PMF) in vitro and in vivo. control of parsing into Δψ and ΔpH by ionic strength. Biochemistry. 2001;40(5):1226–1237. doi: 10.1021/bi0018741. [DOI] [PubMed] [Google Scholar]
- 14.de Filippis LF, Ziegler H. Effect of sublethal concentrations of zinc, cadmium and mercury on the photosynthetic carbon reduction cycle of Euglena . J Plant Physiol. 1993;142(2):167–172. doi: 10.1016/S0176-1617(11)80958-2. [DOI] [Google Scholar]
- 15.de Filippis LF, Hampp R, Ziegler H. The effects of sublethal concentrations of zinc, cadmium and mercury on Euglena . Arch Microbiol. 1981;128(4):407–411. doi: 10.1007/BF00405922. [DOI] [Google Scholar]
- 16.de la Rosa-Manzano E, Andrade JL, Garcia-Mendoza E, et al. Photoprotection related to xanthophyll cycle pigments in epiphytic orchids acclimated at different light microenvironments in two tropical dry forests of the Yucatan Peninsula, Mexico. Planta. 2015;242(6):1425–1438. doi: 10.1007/s00425-015-2383-4. [DOI] [PubMed] [Google Scholar]
- 17.Deng C, Zhang D, Pan X, et al. Toxic effects of mercury on PSI and PSII activities, membrane potential and transthylakoid proton gradient in Microsorium pteropus . J Photochem Photobiol B. 2013;127:1–7. doi: 10.1016/j.jphotobiol.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Dzbek J, Korzeniewski B. Control over the contribution of the mitochondrial membrane potential (Δψ) and proton gradient (ΔpH) to the proton motive force (Δp). In silico studies. J Biol Chem. 2008;283(48):33232–33239. doi: 10.1074/jbc.M802404200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foyer CH, Neukermans J, Queval G, et al. Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot. 2012;63(4):1637–1641. doi: 10.1093/jxb/ers013. [DOI] [PubMed] [Google Scholar]
- 20.Gao S, Niu J, Chen W, et al. The physiological links of the increased photosystem II activity in moderately desiccated Porphyra haitanensis (Bangiales, Rhodophyta) to the cyclic electron flow during desiccation and re-hydration. Photosynth Res. 2013;116(1):45–54. doi: 10.1007/s11120-013-9892-4. [DOI] [PubMed] [Google Scholar]
- 21.Guissé B, Srivastava A, Strasser RJ. Effects of high temperature and water stress on the polyphasic chlorophyll a fluorescence transient of potato leaves. J Gansu Agric Univ. 1995;324(3):3877–3880. doi: 10.1007/978-94-009-0173-5_914. [DOI] [Google Scholar]
- 22.Heber U, Gerst U, Krieger A, et al. Coupled cyclic electron transport in intact chloroplasts and leaves of C3 plants: does it exist? If so, what is its function? Photosynth Res. 1995;46(1-2):269–275. doi: 10.1007/BF00020440. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickson L, Furbank RT, Chow WS. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res. 2004;82(1):73–81. doi: 10.1023/B:PRES.0000040446.87305.f4. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Zhang SB, Cao KF. The different effects of chilling stress under moderate light intensity on photosystem II compared with photosystem I and subsequent recovery in tropical tree species. Photosynth Res. 2010;103(3):175–182. doi: 10.1007/s11120-010-9539-7. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Zhang SB, Cao KF. Stimulation of cyclic electron flow during recovery after chilling-induced photoinhibition of PSII. Plant Cell Physiol. 2010;51(11):1922–1928. doi: 10.1093/pcp/pcq144. [DOI] [PubMed] [Google Scholar]
- 26.Ioannidis NE, Kotzabasis K. Polyamines in chemiosmosis in vivo: a cunning mechanism for the regulation of ATP synthesis during growth and stress. Front Plant Sci. 2014;5:71. doi: 10.3389/fpls.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannidis NE, Cruz JA, Kotzabasis K, et al. Evidence that putrescine modulates the higher plant photosynthetic proton circuit. PLoS ONE. 2012;7(1):e29864. doi: 10.1371/journal.pone.0029864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov AG, Morgan-Kiss RM, Krol M, et al. Photoinhibition of photosystem I in a pea mutant with altered LHCII organization. J Photochem Photobiol B. 2015;152(3):335–346. doi: 10.1016/j.jphotobiol.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, Qiu B. Inhibition of photosynthesis by UV-B exposure and its repair in the bloom-forming cyanobacterium Microcystis aeruginosa . J Appl Phycol. 2010;23(4):691–696. doi: 10.1007/s10811-010-9562-2. [DOI] [Google Scholar]
- 30.Jin SH, Li XQ, Hu JY, et al. Cyclic electron flow around photosystem I is required for adaptation to high temperature in a subtropical forest tree, Ficus concinna . J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2009;10(10):784–790. doi: 10.1631/jzus.B0820348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson GN. Cyclic electron transport in C3 plants: fact or artefact? J Exp Bot. 2005;56(411):407–416. doi: 10.1093/jxb/eri106. [DOI] [PubMed] [Google Scholar]
- 32.Johnson MP, Ruban AV. Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth Res. 2014;119(1-2):233–242. doi: 10.1007/s11120-013-9817-2. [DOI] [PubMed] [Google Scholar]
- 33.Kanazawa A, Kramer DM. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA. 2002;99(20):12789–12794. doi: 10.1073/pnas.182427499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klughammer C, Schreiber U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl Notes. 2008;1:27–35. [Google Scholar]
- 35.Kramer DM, Avenson TJ, Edwards GE. Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci. 2004;9(7):349–357. doi: 10.1016/j.tplants.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Liu D, Li S, Islam E, et al. Lead accumulation and tolerance of Moso bamboo (Phyllostachys pubescens) seedlings: applications of phytoremediation. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(2):123–130. doi: 10.1631/jzus.B1400107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu DF, Zhang D, Liu GQ, et al. Influence of heat stress on leaf ultrastructure, photosynthetic performance, and ascorbate peroxidase gene expression of two pear cultivars (Pyrus pyrifolia) J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2013;14(12):1070–1083. doi: 10.1631/jzus.B1300094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyake C, Shinzaki Y, Miyata M, et al. Enhancement of cyclic electron flow around PSI at high light and its contribution to the induction of non-photochemical quenching of chl fluorescence in intact leaves of tobacco plants. Plant Cell Physiol. 2004;45(10):1426–1433. doi: 10.1093/pcp/pch163. [DOI] [PubMed] [Google Scholar]
- 39.Munekage Y, Hojo M, Meurer J, et al. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis . Cell. 2002;110(3):361–371. doi: 10.1016/S0092-8674(02)00867-X. [DOI] [PubMed] [Google Scholar]
- 40.Pagliano C, Raviolo M, Dalla Vecchia F, et al. Evidence for PSII donor-side damage and photoinhibition induced by cadmium treatment on rice (Oryza sativa L.) J Photochem Photobiol B. 2006;84(1):70–78. doi: 10.1016/j.jphotobiol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Partelli FL, Batista-Santos P, Scotti-Campos P, et al. Characterization of the main lipid components of chloroplast membranes and cold induced changes in Coffea spp. Environ Exp Bot. 2011;74(2):194–204. doi: 10.1016/j.envexpbot.2011.06.001. [DOI] [Google Scholar]
- 42.Pospisil P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim Biophys Acta. 2012;1817(1):218–231. doi: 10.1016/j.bbabio.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Schreiber U, Klughammer C. New accessory for the Dual-PAM-100: the P515/535 module and examples of its application. PAM Appl Notes. 2008;1:1–10. [Google Scholar]
- 44.Setif P. Electron-transfer kinetics in cyanobacterial cells: methyl viologen is a poor inhibitor of linear electron flow. Biochim Biophys Acta. 2015;1847(2):212–222. doi: 10.1016/j.bbabio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Shikanai T. Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol. 2007;58:199–217. doi: 10.1146/annurev.arplant.58.091406.110525. [DOI] [PubMed] [Google Scholar]
- 46.Shikanai T. Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr Opin Biotechnol. 2014;26:25–30. doi: 10.1016/j.copbio.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi S, Badger MR. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 2011;16(1):53–60. doi: 10.1016/j.tplants.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Tu W, Li Y, Liu W, et al. Spring ephemerals adapt to extremely high light conditions via an unusual stabilization of photosystem II. Front Plant Sci. 2015;6:1189. doi: 10.3389/fpls.2015.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang P, Ye J, Shen Y, et al. The role of chloroplast NAD(P)H dehydrogenase in protection of tobacco plant against heat stress. Sci China C Life Sci. 2006;49(4):311–321. doi: 10.1007/s11427-006-2005-2. [DOI] [PubMed] [Google Scholar]
- 50.Yamori W, Sakata N, Suzuki Y, et al. Cyclic electron flow around photosystem I via chloroplast NAD(P)H dehydrogenase (NDH) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice. Plant J. 2011;68(6):966–976. doi: 10.1111/j.1365-313X.2011.04747.x. [DOI] [PubMed] [Google Scholar]
- 51.Yamori W, Makino A, Shikanai T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci Rep. 2016;6:20147. doi: 10.1038/srep20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi X, McChargue M, Laborde S, et al. The manganese-stabilizing protein is required for photosystem II assembly/stability and photoautotrophy in higher plants. J Biol Chem. 2005;280(16):16170–16174. doi: 10.1074/jbc.M501550200. [DOI] [PubMed] [Google Scholar]
- 53.Yin HL, Tian CY. Effects of nitrogen regulation on photosystem II chlorophyll fluorescence characteristics of functional leaves in sugar beet (Beta vulgaris) under salt environment. Chin J Plant Ecol. 2013;37(2):122–131. doi: 10.3724/SP.J.1258.2013.00013. [DOI] [Google Scholar]
- 54.Zhang G, Liu Y, Ni Y, et al. Exogenous calcium alleviates low night temperature stress on the photosynthetic apparatus of tomato leaves. PLoS ONE. 2014;9(5):e97322. doi: 10.1371/journal.pone.0097322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou YH, Huang LF, Yu JQ. Effects of sustained chilling and low light on gas exchange, chlorophyll fluorescence quenching and absorbed light allocation in cucumber leaves. J Plant Physiol Mol Biol. 2004;30(2):153–160. (in Chinese) [PubMed] [Google Scholar]
- 56.Zhou YH, Lam HM, Zhang JH. Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J Exp Bot. 2007;58(5):1207–1217. doi: 10.1093/jxb/erl291. [DOI] [PubMed] [Google Scholar]
- 57.Zhou YH, Zhang YL, Wang XM, et al. Effects of nitrogen form on growth, CO2 assimilation, chlorophyll fluorescence, and photosynthetic electron allocation in cucumber and rice plants. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(2):126–134. doi: 10.1631/jzus.B1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zivcak M, Brestic M, Kalaji HM, et al. Photosynthetic responses of sun- and shade-grown barley leaves to high light: is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth Res. 2014;119(3):339–354. doi: 10.1007/s11120-014-9969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]