Abstract
Objective: To investigate the effect of peritoneal macrophage autophagy on immune function in sepsis mice. Methods: Seventy-two male BALB/C mice were intraperitoneally injected with LPS to induce sepsis. The mice were randomly divided into six groups: LPS+2 h, LPS+6 h, LPS+12 h, LPS+24 h and LPS+36 h. LPS with a dose of 10 mg/kg was intraperitoneally injected into the abdominal cavity of the sepsis mice, and the control group was injected with the same dose of saline. ELISA was used to detect the concentrations of inflammatory factors IL-2, IL-10 and TNF-α in the peripheral blood, and the CD4+T/CD8+T ratio in the peripheral blood was detected by flow cytometry. The expression levels of LC3II and Beclin-1/beta-action in the mouse macrophages were measured using Western blot to determine the level of autophagy. Results: The expression levels of LC3II and Beclin-1 were significantly higher in the peritoneal macrophages of the mice from the LPS+2 h group than in those of the mice from the normal group (P<0.05). Meanwhile, these levels continuously declined in the LPS+6 h, LPS+12 h, LPS+24 h and LPS+36 h groups (P<0.05). The peripheral blood CD4+T/CD8+T cell ratio was significantly higher in the LPS+2 h and LPS+6 h groups than in the normal group (P<0.05). The ratio peaked at 6 h and then continuously declined (P<0.05). Furthermore, the concentrations of IL-2 and Tnf-α were significantly higher in the peripheral blood serum of the LPS+2 h, LPS+6 h and LPS+12 h groups than in those of the normal group (P<0.05). The peak was observed at 12 h followed by a continuous decline in the LPS+24 h and 3 LPS+6 h groups (P<0.05). The peripheral serum IL-10 concentration was significantly higher in the LPS+2 h, LPS+6 h, LPS+12 h, LPS+24 h and LPS+36 h groups than in the normal group (P<0.05). In the LPS+6 h, LPS+12 h, LPS+24 h and LPS+36 h groups, the peritoneal macrophages LC3II, Beclin-1 and peripheral serum CD+4T/CD+8T ratio correlation index R2=0.716 (P=0.043), R2=0.954 (P=0.023). Conclusion: Autophagy in peritoneal macrophages plays an important role in the immune function of sepsis mice. In addition, the autophagy of peritoneal macrophages and the immune function of sepsis mice are strongly correlated. Furthermore, macrophage autophagy plays an important role in the immune function changes in sepsis mice, and the underlying mechanism may be involved in inflammation and macrophage antigen presentation by regulating the secretion of inflammatory cytokines and lymphocyte apoptosis antagonism.
Keywords: Macrophage, autophagy immune function change, sepsis disease
Introduction
Sepsis is a complex clinical syndrome characterised by sepsis shock and organ dysfunction. The occurrence of sepsis is closely related to immune function disorder, which induces excessive inflammatory response accompanied by low immune function [1]. Immune cell apoptosis is the main cause of immunosuppression in sepsis development and immunosuppression prevents the immune system from controlling primary and secondary infections, leading to death [2]. As an important part of the immune system macrophages play various roles in infection control and pathogen identification these cells also assist the antigen-presenting function, release of inflammatory cytokines and activation of the specific immune system [3]. Autophagy is activated in sepsis, and macrophage heterophagy way not only through the clearance of apoptotic lymphocytes, but also through the autophagy pathway, macrophage autophagy has direct and indirect participations in the removal of apoptotic lymphocytes [4,5]. The present study uses a mouse model of LPS-induced sepsis to investigate the effect of peritoneal macrophage autophagy on the immune function of sepsis mice, elucidate the mechanisms underlying the occurrence and development of sepsis and provide a theoretical basis for sepsis treatment.
Materials and methods
Materials
Laboratory animals and materials
Animal origin and management
Clean-grade BALB/c mice, aged 6-8 weeks, with a body mass (of 200 ± 10) g, were purchased from Xinjiang Medical University (experimental animal production license No. XJYK (New) 0011, 2011). Randomisation of the groups was conducted before the operation. The mice were housed in a special cage with 12 randomly selected rats per cage.
Main reagent
IL-2 was purchased from Shanghai West Tang Company. IL-10 and TNF-α were obtained from Cloud-Clone Corp. LC3B and Beclin-1 were procured from CST. LPS, RIPA and PMSF were purchased from Sigma Company.
Animal grouping
Seventy-two rats were randomly divided into the LPS+2 h, LPS+6 h, LPS+12 h, LPS+24 h, LPS+36 h and normal control groups.
Processing methods
The mice were fasted for 12 h and allowed access to drinking water before the experiment.
Normal group
The normal control group was injected with the same dose of normal saline.
Sepsis group
The abdomen of the experimental mouse group was intraperitoneally injected with LPS at a dose of 10 mg/kg. The mice were divided into the LPS+2 h, LPS+6 h, LPS+12 h, LPS+24 h and LPS+36 h groups, and each mouse was injected with LPS solution with a volume of about 180-250 µL.
Sepsis group
Experimental group of mouse abdominal line LPS.
Model preparation
LPS (10 mg/kg) was intraperitoneally administered, and the control group received the same amount of normal saline. After LPS injection, the animals were observed for malaise, diarrhoea, piloerection, incontinence and eating drinking activities.
Specimen collection
The mice were sacrificed by draining the orbital vein blood.
Methods
ELISA of peripheral blood to detect the inflammatory factors secreted in sepsis mice
Blood was removed from the eyes of the sepsis mice using ophthalmic forceps. The peripheral blood obtained was placed in an EP tube, centrifuged (12,000 × g, 10 min) and then stored in a refrigerator at 70°C for further use. ELISA was used to detect the inflammatory factors in each group.
Flow cytometry to detect the CD4+T/CD8+T ratio in the peripheral blood of sepsis mice
Peripheral blood T cell subsets of the blood samples were collected in sterile EDTA containing blood vessels and then added with FITC-CD4+T and PE-CD8+T. The expression levels of CD4+T and CD8+T were detected using flow cytometry.
Extraction of peritoneal macrophages from different groups of sepsis mice
The mice were killed by cervical dislocation, submerged in 75% ethanol for 3 min, placed in a sterile paper and then fixed in supine position after collecting peritoneal macrophages.
(1) The abdominal skin was removed, and the peritoneum was exposed. A 5 mL aliquot of pre-cold serum-free medium was injected into the abdominal cavity. The abdomen was gently massaged for 5 min after the slow return to the peritoneal lavage fluid. The above operation was repeated two times with about 10-15 mL in a liquid suction centrifugal tube.
(2) The 300 g/min samples were centrifuged for 5 min, and the upper suspension was discarded. Culture medium with RPMI-1640 (10% foetal bovine serum).
(3) The liquid was cleared of the suspension cells to adjust the cell density to 5 × 10^5/mL after inoculation in the six-well plate that was cultured overnight.
(4) High-purity peritoneal macrophages were obtained.
Western blot detection of the expression of LC3-II and Beclin-1 in each group of mice to detect the autophagy level
After reaching the boiling point in 12% sodium dodecyl sulphate, the total protein of the sepsis mouse peritoneal macrophages was extracted. The protein was subjected to polyacrylamide gel electrophoresis, electrotransferred to a PVDF membrane and then incubated with anti-LC3 antibody (1:1000) and two goat anti-rabbit antibodies (1:20000). After washing with TBST, the specimens were subjected to anti-chemiluminescence, exposed to a dark room and then developed. The film was fixed and then subjected to a gel imaging system. The amount of autophagosomes and the expression of LC3II are proportional to the calculated LC3 II/beta-actin LC3II expression that represent the absorbance ratio.
Statistical analysis
SPSS 19 software was used for data analysis. Data are presented as mean ± S.D. Different time points were compared through single-factor ANOVA, and the two groups were compared by using t test and curve fitting regression analysis. Differences at P<0.05 were statistically significant.
Results
Basic situation of mice
Normal control mice were in good general condition. The LPS-treated group exhibited poor mental response, shortness of breath, vertical hair, body temperature, decreased muscle tone, incontinence and other performances.
Peripheral serum inflammatory mediator expression
The peripheral serum IL-2 and Tnf-α concentrations were significantly higher in the LPS+2 h, LPS+12 h and LPS+6 h groups than in the normal group (P<0.05). The concentrations peaked in the LPS+12 h group and then continued to decline in the LPS+24 h and 36 h groups (P<0.05) (Figures 1, 3). The serum IL-10 concentration significantly elevated in the LPS+2 h, LPS+6 h, LPS+12 h, LPS+24 h and LPS+36 h groups compared with the normal group (P<0.05) (Figure 2).
Figure 1.

Expression of IL-2 in the peripheral blood of each group. Note: *compared with the normal group P<0.05.
Figure 3.
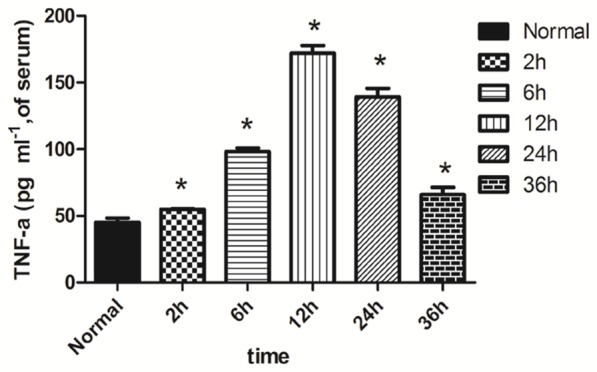
Expression of Tnf-α in the peripheral serum of each group. Note: *compared with the normal group P<0.05.
Figure 2.
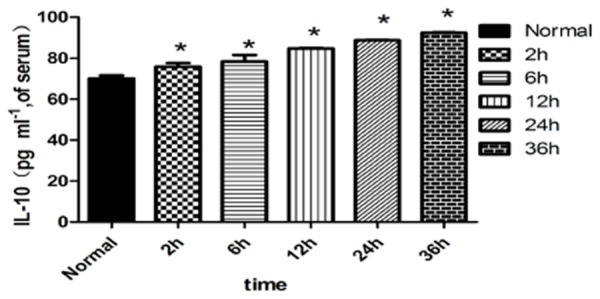
Expression of IL-10 in the peripheral blood of each group. Note: *compared with the normal group P<0.05.
Peripheral serum CD4+T/CD8+T cell ratio
The peripheral blood CD4+T/CD8+T cell ratio was significantly higher in the LPS+2 h and LPS+6 h groups than in the normal group (P<0.05). This ratio peaked in the LPS+6 h group and then continued to decline (P<0.05) (Figures 4, 5).
Figure 4.
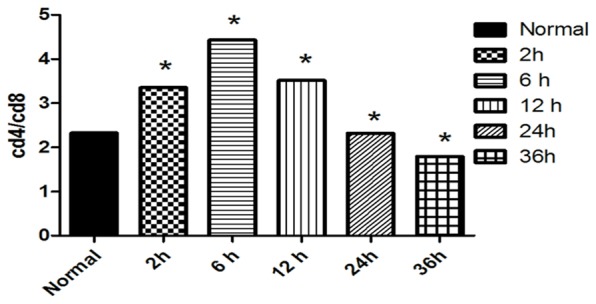
CD4+T/CD8+T cell ratio in the peripheral serum of each group. Note: *compared with the normal group P<0.05.
Figure 5.

Flow cytometry of CD4+T and CD8+T in the peripheral blood of each group.
Autophagy level in the peritoneal macrophages of sepsis mice
The expression of Beclin-1 in the peritoneal macrophages of mice LC3II significantly increased in the LPS+2 h group compared with that in the normal group (P<0.05). The expression continued to decline in the LPS+6 h, LPS+12 h, LPS+24 h and LPS+36 h groups (P<0.05) (Figure 6). The LC3II, Beclin-1 and peripheral serum CD+4T/CD+8T ratio (Figure 4) in the peritoneal macrophages have a correlation index of R2=0.716 (P=0.043) and R2=0.954 in the LPS+6 h, LPS+12 h, LPS+24 h and LPS+36 h groups (P=0.023).
Figure 6.

A. Expression levels of the autophagy-related protein LC3II and Beclin-1 in the peritoneal macrophages of each group. B. Expression levels of the autophagy-related protein LC3II in the peritoneal macrophages of each group. Note: *compared with the normal group P<0.05. C. Expression levels of the autophagy-related protein Beclin-1 in the peritoneal macrophages of each group. Note: *compared with the normal group P<0.05.
Discussion
Many mechanisms are involved in the development of immune paralysis in sepsis, in which lymphocyte dysfunction plays an important role. The development of sepsis is accompanied by inflammatory reaction. Early in the main inflammatory reaction, TNF-α, IL-2, IL-4, IL-10 and other cytokines induce the apoptosis of lymphocytes in the blood. High rates of lymphocyte proliferation and apoptosis show immunity to helper type T2 cell reaction and Th1/Th2 drift, rendering the body immune to paralysis [6,7]. As an important cytokine of Thl cells, IL-2 is involved in cellular immunity and stimulates the proliferation and differentiation of CD8+T cells. IL-10 is an indicator of cytokines in type-Th2 cells; its activation stimulates B cells and promotes CD4+T cells to differentiate into Th2 cells. The regulation of Th1-type/Th2 cytokine balance is conducive to the stability of the body immune system [8]. T cells are the most important regulatory components in cellular immunity; these cells contribute to the up- and down-regulation of immune response. Peripheral mature T cells are divided into CD4+ helper T cells and CD8+ cytotoxic T cells. CD4+T cells often assist B cells to differentiate and produce antibodies that are involved in the promotion of cell-mediated immune responses. CD8+T cells kill and inhibit, and the ratio of CD4+T/CD8+T is used to evaluate the immune status of the body. The value of the positive regulation of the immune response is dominant [9]. The immune response is enhanced by regulating the Th1/Th2 balance in the peripheral blood and increasing the CD4+T/CD8+T ratio. The balance changes of Thl/Th2 reflect the imbalance of SIRS/CARS (compensatory anti-inflammatory response syndrome) and the immune function of the body [10].
The immune response function in sepsis state of T lymphocytes on pathogenic microorganisms decreased, the cell factor including IL-2 secretion decreased, due to a large number of apoptotic CD4+T lymphocytes, T cells decreased in number, to further reduce the secretion of IL-2, IL-2 levels in peripheral blood decreased, decreased the level of IL-2 and further makes the activation and proliferation of T cells disorder that exacerbated the occurrence of immunosuppression [11,12]. The growth and decline of CD4+T and CD8+T are closely related to the direction and degree of inflammation/inflammation inhibition [13]. The early stage of sepsis is characterised by the expression of TNF-α and IL-2 pro-inflammatory cytokines, particularly the explosive release of pro-inflammatory response. As the disease progresses, the inflammatory reaction is gradually replaced by the early inflammatory response, which includes reduced TNF-α and IL-2 levels and elevated IL-10 levels. These phenomena inhibit the secretion of macrophage TNF-α and the expression of macrophage and T cell costimulatory molecules. Therefore, in the late stage of sepsis, the elevated IL-10 levels are associated with immunosuppression [14,15].
The autophagy of macrophages is involved in the normal growth and development of cells and in the physiological and pathological processes. Autophagy plays an important role in the development of neurodegenerative diseases, tumours, cardiomyopathy and infectious diseases. Autophagy is involved in catabolic processes and mainly functions in the recycling of intracellular macromolecules and the removal of damaged organelles and intracellular pathogens; thus, autophagy maintains cell homeostasis, ensures cell energy supply and exerts a cell protective effect [16,17]. In addition, autophagy assists antigen-presenting cells for antigen presentation, confers resistance to intracellular bacterial and viral infections, stimulates innate and acquired immune responses and maintains immunity to steady-state yeast [18,19].
The mice were injected with LPS to establish a sepsis model. The CD4+T/CD8+T cell ratio increased in the LPS+2 h group and peaked in the LPS+6 h group. The immune response was up-regulated and then continued to decline. The peripheral serum levels of TNF-α and IL-2 increased, peaked at 12 h, and then declined thereafter. The explosive release of pro-inflammatory response is the main characteristic of immunity state in hyperthyroidism. The immune response was reduced, the CD4+T/CD8+T ratio decreased, the Th1/Th2 drift persistently increased and the expression of IL-10, TNF-α and IL-2 continued to decline. The performance and anti-inflammatory reaction of lymphocyte apoptosis were significantly induced, which rendered the body immune to paralysis. Macrophage autophagy was activated in the sepsis model mice at 2 h. At 6 h, the autophagy level continued to decline; the immune response of the sepsis mice was reduced. The effects of macrophage phagocytosis, antigen presentation, secretion of inflammatory cytokines and removal of apoptotic lymphocyte function are due to the up- and down-regulation of macrophage autophagy in the abdominal cavity, thereby affecting the immune function. Autophagy also maintains T lymphocyte homeostasis and promotes T cell differentiation and maturation. Autophagy defects lead to apoptosis. The lack of autophagy-related proteins Atg5 and Atg7 leads to the development of CD4+ and CD8+T lymphocyte maturation disorder and significantly increases the apoptosis rate in peripheral lymphoid organs [20,21].
The protective role of autophagy against various pathophysiological conditions may be related to its regulatory effect on lymphocyte apoptosis [22]. Studies have shown that the protective mechanism of autophagy is associated with the inhibition of apoptosis [23]. Some common upstream regulatory factors are activated during autophagy and apoptosis to maintain dynamic balance [24,25]. Macrophages are the major effector cells of innate immune responses and are also the hub of innate and adaptive immunities. Macrophage phagocytosis involves antigen presenting and secretion of various cytokines in senescent cells. This process removes pathogens, causes inflammation, induces adaptive immune response and tissue damage repair and reconstruction [25].
Conclusions
(1) The autophagy of peritoneal macrophages protects immune function in sepsis mice. (2) The autophagy of peritoneal macrophages is strongly dependent on immune function. (3) The autophagy of peritoneal macrophages plays an important role in the changes of immune function in sepsis mice. Its underlying mechanism may involve inflammation and macrophage antigen presentation, regulation of the secretion of inflammatory cytokines and lymphocyte apoptosis antagonism.
Acknowledgements
The authors would like to acknowledgment the help from the Medical College of Shihezi University.
Disclosure of conflict of interest
None.
References
- 1.Chen Y, Li CS. The effectiveness of XueBiJing injection in therapy of sepsis: a multicenter clinical study. Chinese Journal of Emergency Medicine. 2013;22:130–135. [Google Scholar]
- 2.Gao YL, Chai YF, Yao YM. [Advancement in the research of mechanism of immune dysfunction in sepsis and the regulatory effects of Xuebijing injection] . Zhonghua Shao Shang Za Zhi. 2013;29:162–165. [PubMed] [Google Scholar]
- 3.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 4.Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–53. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irla M, Küpfer N, Suter T, Lissilaa R, Benkhoucha M, Skupsky J, Lalive PH, Fontana A, Reith W, Hugues S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulcsar KA, Griffin DE. T cell-derived interleukin-10 is an important regulator of the Th17 response during lethal alphavirus encephalomyelitis. J Neuroimmunol. 2016;295:60–67. doi: 10.1016/j.jneuroim.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang SW, Sun CD, Wen Y, Yin CH. [Effect of treatment with Xuebijing injection on serum inflammatory mediators and Th1/2 of spleen in rats with sepsis] . Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18:673–6. [PubMed] [Google Scholar]
- 9.Banuelos J, Lu NZ. A gradient of glucocorticoid sensitivity among helper T cell cytokines. Cytokine Growth Factor Rev. 2016;31:27–35. doi: 10.1016/j.cytogfr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suni MA, Picker LJ, Maino VC. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J Immunol Methods. 1998;212:89–98. doi: 10.1016/s0022-1759(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 11.Chao YH, Wu HP, Wu KH, Tsai YG, Peng CT, Lin KC, Chao WR, Lee MS, Fu YC. An increase in CD3+CD4+CD25+ regulatory T cells after administration of umbilical cord-derived mesenchymal stem cells during sepsis. PLoS One. 2014;9:e110338. doi: 10.1371/journal.pone.0110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng MWL, Bowman EP, Mcelwee JJ, Smyth MJ, Casanova JL, Cooper AM, Cua DJ. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–29. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 13.Finke JH, Rayman P, Alexander J, Edinger M, Tubbs RR, Connelly R, Pontes E, Bukowski R. Characterization of the cytolytic activity of CD4+ and CD8+Tumor-infiltrating lymphocytes in human renal cell carcinoma. Cancer Res. 1990;50:2363–2370. [PubMed] [Google Scholar]
- 14.Giamarellos-Bourboulis EJ, Tsaganos T, Spyridaki E, Mouktaroudi M, Plachouras D, Vaki I, Karagianni V, Antonopoulou A, Veloni V, Giamarellou H. Early changes of CD4-positive lymphocytes and NK cells in patients with severe Gram-negative sepsis. Crit Care. 2006;10:R166. doi: 10.1186/cc5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De MI, Martinet W, Schrijvers DM, Timmermans JP, Bult H, De Meyer GR. Toll-like receptor 7 stimulation by imiquimod induces macrophage autophagy and inflammation in atherosclerotic plaques. Basic Res Cardiol. 2012;107:269. doi: 10.1007/s00395-012-0269-1. [DOI] [PubMed] [Google Scholar]
- 16.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 17.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid D, Münz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Liu XD, Gong X, Eissa NT. Signaling pathway of autophagy associated with innate immunity. Autophagy. 2008;4:110–112. doi: 10.4161/auto.5225. [DOI] [PubMed] [Google Scholar]
- 20.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe K, Ichinose S, Hayashizaki K, Tsubata T. Induction of autophagy by B cell antigen receptor stimulation and its inhibition by costimulation. Biochem Biophys Res Commun. 2008;374:274–281. doi: 10.1016/j.bbrc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Gonzálezpolo RA, Boya P, Pauleau AL, Jalil A, Larochette N, Souquère S, Eskelinen EL, Pierron G, Saftig P, Kroemer G. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- 23.Deng B, Wehlinghenricks M, Villalta SA, Wang Y, Tidball JG. Interleukin-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh YC, Athar M, Chaudry IH. When apoptosis meets autophagy: deciding cell fate after trauma and sepsis. Trends Mol Med. 2009;15:129–138. doi: 10.1016/j.molmed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]


