Abstract
Permeant cGMP analogs prevent the mitochondria permeability transition (MPT) in vitro. In this study, we explored whether 8-pCPT-cGMP prevents the MPT and decreases post-transplant damage to fatty partial liver grafts (FPG) in vivo. Rats were fed a control or high-fat, high-fructose diet for 2-week. Lean and fatty liver explants were reduced in size ex vivo to ~35% and stored in the University of Wisconsin solution with and without 8-pCPT-cGMP (300 µM) for 2 h. After transplantation, alanine aminotransferase release (indicator of hepatocellular injury), hyperbilirubinemia (indicator of poor liver function), and cell death were all higher in FPG than in lean partial grafts (LPG). Liver regeneration increased in LPG but was suppressed in FPG. 8-pCPT-cGMP blunted graft injury, improved liver regeneration and function, and increased survival of FPG. Hepatic mitochondrial depolarization detected by intravital multiphoton microscopy of rhodamine 123 in living rats was ~3.5-fold higher in FPG than in LPG. 8-pCPT-cGMP decreased mitochondrial depolarization in FPG almost to the level of LPG. Activation of mammalian target of rapamycin (mTOR), an energy sensitive kinase that stimulates cell proliferation and growth, and p70S6 kinase, a downstream signaling molecule of mTOR, was increased in LPG but suppressed in FPG. 8-pCPT-cGMP restored the activity of mTOR and p70S6 kinase in FPG. 8-pCPT-cGMP also increased activation of cAMP response element-binding protein (CREB) and expression of cyclins D1 and E in FPG. Non-alcoholic steatosis increases injury and suppresses regeneration after partial liver transplantation, at least in part, due to more severe mitochondrial dysfunction. Protection of mitochondria with a cGMP analog effectively improves outcomes of FPG transplantation.
Keywords: Cyclic GMP, fatty liver, liver regeneration, partial liver graft, mitochondrial permeability transition, steatosis
Introduction
Hepatic steatosis is characterized by excessive accumulation of fat in hepatocytes of the liver and is a common feature of many liver diseases, such as non-alcoholic fatty liver disease (NAFLD, including non-alcoholic steatohepatitis [NASH]), alcoholic fatty liver disease, and drug-induced fatty liver disease. Obesity, nutritional factors, altered gut microbiome, insulin resistance, physical inactivity, metabolic dyslipidemia, and genetic factors are risk factors contributing to development of NAFLD [1-4]. Western diet, which is characterized by high fat, high fructose and high cholesterol intake, plays an important role in development of obesity and NAFLD [5-7]. Due to an epidemic of obesity, NAFLD has become the leading cause of liver disease in Western countries with an estimated prevalence of 19-31% and affecting >30,000,000 people in the U.S. alone [6,8,9]. Since fatty liver grafts experience more dysfunction and primary non-function after transplantation, the high prevalence of NAFLD has become a significant problem and burden for liver transplantation [10,11].
Due to the high incidence of NAFLD and other fatty liver diseases, up to one-half potential donor livers have some extent of steatosis [12,13]. Severe steatosis is well known to increase markedly the vulnerability of livers to ischemia/reperfusion (I/R) injury and the risk of mortality after liver transplantation [14-18]. Therefore, severely steatotic livers (steatosis in >60% hepatocytes) are not used for transplantation. However, a recent analysis of the UNOS data base shows that even >30% macrovesicular steatosis decreases 1-year graft survival [19]. The use of donor livers with mild to moderate steatosis remains problematic, and most transplant centers use fatty liver donors only in the absence of other known risk factors [17,20,21]. Accordingly, steatotic grafts are generally excluded for use in partial liver transplantation. Thus, steatosis limits the availability of partial liver grafts, and development of effective therapies to improve the survival of fatty partial grafts (FPG) would increase the number of usable marginal liver grafts, thus alleviating the current severe shortage of donor livers.
Onset of the mitochondrial permeability transition (MPT) is an important mediator of hepatic I/R injury both in vitro and in vivo by causing necrotic and apoptotic cell death [22-25]. The MPT also mediates primary liver graft non-function after liver transplantation and occurs after major liver resection [26-28]. Previous studies show that cell-permeable cGMP analogs (e.g. 8-BrcGMP and 8-pCPT-cGMP) prevent onset of the MPT after I/R in cultured hepatocytes and in isolated liver mitochondria incubated with cytosol [29,30]. Moreover, cGMP and cAMP analogs in the presence of liver cytosol delay onset of the Ca+2-induced MPT in isolated rat mitochondria [31]. Both cGMP and cAMP analogs appear to exert protection through activation of protein kinase A (PKA) [31]. Whether cell-permeable cGMP analogs prevent liver graft failure in vivo is not known. Here, we show that 8-pCPT-cGMP decreases injury and improves survival after partial transplantation of fatty livers harvested from rats fed a high-fat, high fructose diet, a model of NAFLD [32].
Methods
Induction of non-alcoholic liver steatosis
Non-alcoholic liver steatosis was induced in male Lewis rats (100-150 g, Charles River, Raleigh, NC) by feeding a high-fat, high-fructose diet (HFFr diet, Dyets, Bethleham, PA) for 2 weeks, as described previously [32]. Control rats were fed a low-fat, low-fructose control diet (Dyets) for the same time. Food was available at libitum.
Reduced-size liver transplantation
Under isoflurane anesthesia, the abdomen was opened, and the median lobe and the left lateral lobe were ligated with 4-0 suture and removed. The right superior and inferior lateral lobes and the caudate lobes remained. This technique decreased liver mass by ~65%. Reduced-size livers were then infused with 5 ml ice-cold University of Wisconsin (UW) solution (Barr Laboratories Inc. Pomona, NY) and back-table preparation was performed as described previously [28,33]. Reduced-size explants were weighed and stored in UW storage solution with and without 8-(4-chlorophenylthio)-guanosine 3’,5’-cyclic monophosphate (8-pCPT-cGMP, 300 mM, BioLog Life Sciencs Institute, Flughafendamm, Germany) at 0-1°C for 2 h. After cold storage, donor explants were rinsed with 5 mL of lactated Ringer’s solution (Baxter Corp., Deerfield, IL) with and without 8-pCPT-cGMP (300 mM). Sham operation and implantation were performed as described previously [28]. The hepatic artery and the bile duct were reconnected. Lean partial grafts (LPG) and FPG were implanted into male Lewis rats fed control diet and weight-matched to the corresponding donor rats. 8-pCPT-cGMP treatment was randomly assigned. Rats were observed for 7 days after surgery for survival. All animals were given humane care in compliance with institutional guidelines using protocols approved by the Institutional Animal Care and Use Committee.
Clinical chemistry and hepatic triglycerides
At 18 and 38 h after implantation, blood samples were collected from the vena cava under pentobarbital anesthesia (50 mg/kg, ip). Serum ALT and total bilirubin were determined as described previously [33].
Triglycerides were measured in lean and fatty livers harvested from rats fed control and HFFr diets, respectively, for 2 weeks without transplantation and in transplanted LPG and FPG collected at 38 h after implantation using an analytical kit from Enzymatic Standbio (Boerne, TX) [32].
Histology
Livers were frozen-sectioned and stained with Oil-Red-O staining to evaluate steatosis. At 38 h after transplantation, livers were harvested, fixed and processed in paraffin for hematoxylin and eosin (H&E) staining [32]. Images captured with a 10× objective lens were evaluated in 10 random fields for necrosis.
Immunohistochemistry
To assess liver regeneration, 5-bromo-2’-deoxyuridine (BrdU) was injected (100 mg/kg i.p.) at 37 h after transplantation, liver grafts were harvested at 38 h. BrdU-labeling, which detects cells synthesizing DNA, was revealed by immunohistochemistry in paraffin sections as described elsewhere [28,34]. BrdU-positive and negative cells were counted in 10 random fields under the light microscope using a 40× objective lens.
Intravital multiphoton microscopy
Onset of the MPT leads to mitochondrial depolarization. Therefore, the polarization status of mitochondria after partial liver transplantation was detected by intravital multiphoton microscopy of rhodamine 123 (Rh123), as described previously [35]. At 18 h after implantation or sham operation, rats were connected to a small animal ventilator via a respiratory tube (14-gauge catheter) after tracheotomy under pentobarbital anesthesia (50 mg/kg, ip), as described [35]. The cationic fluorophore Rh123 (Sigma, St. Louis, MO, 6 µmol/rat), which labels polarized mitochondria (green), and propidium iodide (PI, Sigma, 0.12 µmol/rat), which labels nuclei of non-viable cells (red), were infused into the carotid artery over ~10 min. After laparotomy, intravital multiphoton microscopy was performed, and nonviable hepatocytes (with nuclear PI staining) and viable hepatocytes with mitochondrial depolarization (diffused and/or dim Rh123 fluorescence without nuclear PI staining) were quantified as described previously [35].
Immunoblotting
Liver tissue was snap-frozen in liquid nitrogen after harvest and stored at -80°C until analysis. Immunoblotting was performed as described previously [36]. Proteins of interest were detected using primary antibodies specific for mammalian target of rapamycin (mTOR), phospho-mTOR (p-mTOR), phospho-p70S6 kinase (p-p70S6K), proliferating cell nuclear antigen (PCNA) (Dako, Glostrub, Denmark), cyclin D1, cyclin E (Santa Cruz Biotech., Santa Cruz, CA), cleaved caspase-3 (CC3), cAMP response element-binding protein (CREB), and phospho-CREB (Cell Signaling, Danvers, MA) at dilutions of 1:100 to 1000, and actin (ICN, Costa Mesa, CA) at a dilution of 1:3000 at 4°C overnight, respectively.
Statistical analysis
Groups were compared using the Kaplan-Meier test or ANOVA plus a Student-Newman-Keuls posthoc test, as appropriate. Data shown are means ± SEM. Differences were considered significant at P<0.05.
Results
8-pCPT-cGMP attenuates injury of non-alcoholic fatty partial liver grafts after transplantation
To determine the effects of a cell permeable cGMP analog on FPG transplantation, we induced steatosis with a high-fat, high-fructose (HFFr) diet, since high fat and fructose intake are common in Western diet and an apparent cause of NAFLD [4,37]. After feeding the HFFr diet for 2 weeks, hepatic triglycerides increased from a basal level of 15 mg/g liver to 116 mg/g liver (P<0.01) (Table 1). Oil-Red-O staining revealed fat droplets in ~50% of hepatocytes (data not shown), as previously reported [32]. Fat droplets were primarily microvesicular with a small number of macrovesicular droplets. Livers from rats fed the control diet showed markedly less Oil-Red-O staining that was confined to small fat droplets [32]. After FPG transplantation with and without 8-pCPT-cGMP treatment hepatic triglycerides decreased slightly (18-22%), but differences with and without 8-pCPT-treatment were not statistically significant (Table 1).
Table 1.
8-pCPT-cGMP did not decrease triglycerides in liver grafts after partial liver transplantation
| Groups | Hepatic triglycerides (mg/g liver; mean ± SEM) | Statistical significance |
|---|---|---|
| Lean | 15.0 ± 2.3 | |
| Fatty | 116.3 ± 8.0 | P<0.01 vs Lean |
| LPG | 17.1 ± 2.2 | P>0.05 vs Lean |
| FPG | 96.5 ± 8.1 | P<0.01 vs LPG; P<0.05 vs Fatty |
| FPG + 8-pCPT-cGMP | 91.0 ± 8.8 | P>0.05 vs FPG; P<0.05 vs Fatty |
Lean and Fatty: lean and fatty livers were harvested from rats fed control and high-fat, high-fructose diets, respectively, for 2 weeks without transplantation. LPG and FPG: transplanted lean and fatty partial liver grafts from rats fed control or high-fat, high-fructose diet, respectively, were collected at 38 h after implantation. Values are mean ± S.E.M.
Histology was normal in sham-operated lean livers (Figure 1A). After LPG transplantation, scattered focal hepatic necrosis occurred (Figure 1A). By contrast, substantially greater necrosis occurred after FPG transplantation, primarily in periportal and midzonal regions of the liver lobules (Figure 1A). Some eosinophilic inclusion bodies were also observed. How these eosinophilic inclusion bodies formed remains unclear. After FPG transplantation with 8-pCPT-cGMP treatment, necrosis and eosinophilic inclusion bodies were markedly decreased (Figure 1A).
Figure 1.
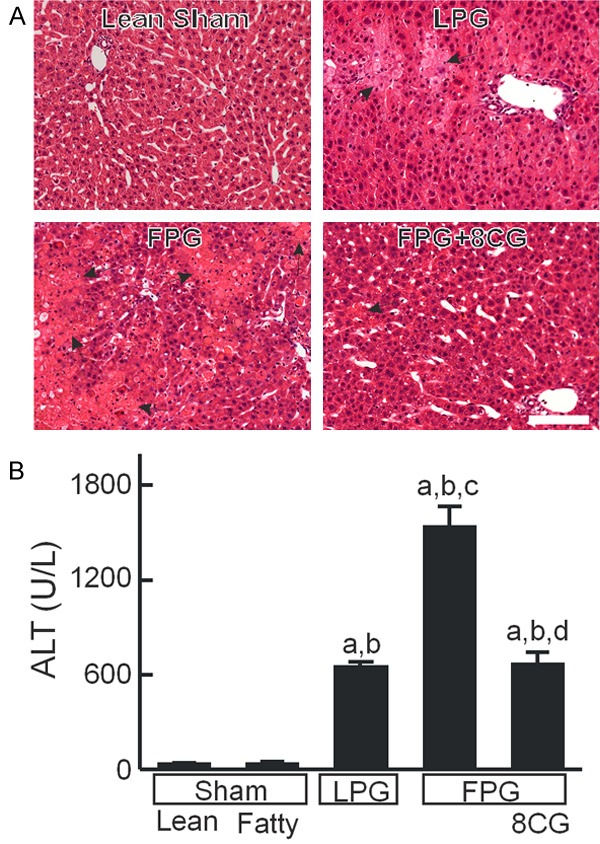
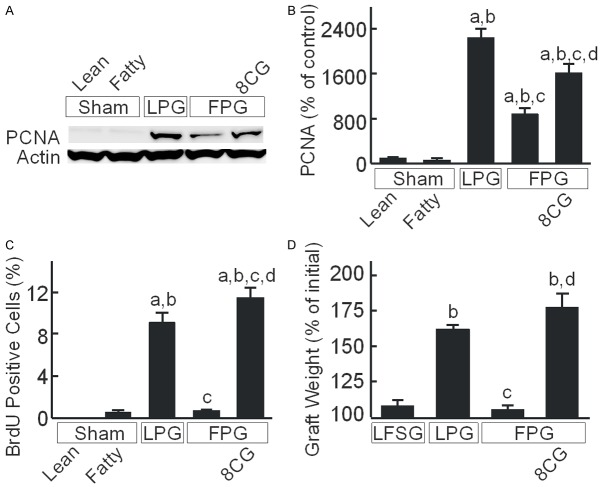
8-pCPT-cGMP attenuates injury of fatty partial liver grafts after transplantation. Rats were fed a low-fat, low-fructose control diet or a high-fat, high-fructose diet for 2 weeks. A: Livers were collected 38 h after sham operation or transplantation. Shown are representative images of H+E-stained liver sections. Arrows identify necrotic areas. Bar is 100 µm. B: Blood was collected at 18 h after sham operation or transplantation for measurement of alanine aminotransferase (ALT) in sera. Sham, sham operation; Lean, lean liver; Fatty, fatty liver; LPG, lean partial graft; FPG, fatty partial graft; 8CG, 8-pCPT-cGMP. a, P<0.05 vs sham-operated lean livers; b, P<0.05 vs sham-operated fatty livers; c, P<0.05 vs LPG, and d, P<0.05 vs FPG (n = 4/group).
Serum ALT, a marker of hepatocellular injury, was 36-40 U/L after sham operation in rats fed control and HFFr diets (Figure 1B). Previously, we showed that serum ALT peaks at ~18 h after partial liver transplantation and then decreases gradually afterwards [33]. Therefore, we compared ALT release at 18 h after LPG and FPG transplantation. After LPG transplantation, ALT increased to 655 U/L, which increased to 1540 U/L after FPG transplantation. After transplantation of FPG preserved in UW solution containing 8-pCPT-cGMP, ALT was 675 U/L, nearly identical to that of the LPG group (Figure 1B).
Apoptosis was assessed by TUNEL staining. TUNEL-positive cells were 0.3% and 0.4% in lean and fatty livers after sham-operation, respectively (Figure 2A, 2B). At 38 h after transplantation, TUNEL-positive cells increased to 0.7% in LPG. After FPG transplantation, apoptosis further increased to 2.8%, which storage with 8-pCPT-cGMP decreased to 1.2% (Figure 2A, 2B). Activation of caspase-3 mediates apoptosis. Cleaved caspase-3 was not significantly different between lean and fatty livers after sham-operation (Figure 2C, 2D). Cleaved caspase-3 increased to 172% after LPG transplantation, which further increased to 362% after FPG transplantation. After 8-pCPT-cGMP treatment of FPG, cleaved caspase-3 increased only 154% (Figure 2C, 2D).
Figure 2.
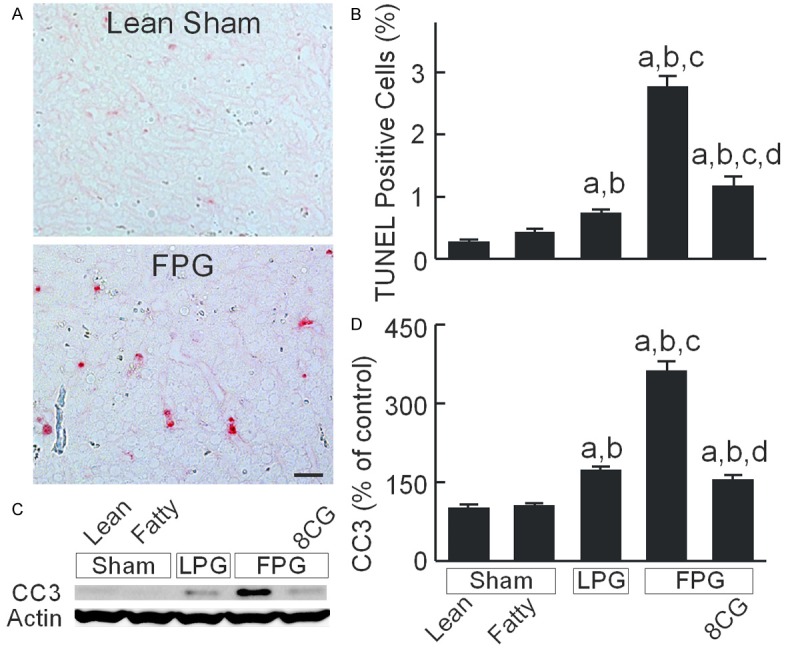
8-pCPT-cGMP decreases apoptosis after transplantation of fatty partial liver grafts. Livers were collected 38 h after sham operation (Sham) or transplantation. A: Apoptosis revealed by TUNEL staining. Shown are representative images of TUNEL stained liver sections from a lean sham liver and a transplanted FPG. Bar is 20 µm. B: Percentage of TUNEL-positive cells; C: Representative immunoblot images of cleaved caspase-3 (CC3) and actin; D: Quantification of CC3 image by densitometry. Sham, sham operation; Lean, lean liver; Fatty, fatty liver; LPG, lean partial graft; FPG, fatty partial graft; 8CG, 8-pCPT-cGMP. a, P<0.05 vs sham-operated lean livers; b, P<0.05 vs sham-operated fatty livers; c, P<0.05 vs LPG, and d, P<0.05 vs FPG (n = 4/group).
8-pCPT-cGMP improves liver regeneration after fatty partial liver transplantation
Liver regeneration was evaluated at 38 h after transplantation (Figure 3). PCNA is a marker of cell proliferation [38]. PCNA was barely detectable after sham operation in either lean or fatty livers but increased ~22 fold after LPG transplantation (Figure 3A, 3B). However after FPG transplantation, PCNA increased only 8-fold in the absence of 8-pCPT-cGMP treatment, but with treatment PCNA increased ~16-fold (Figure 3A, 3B). BrdU nuclear labeling, an indicator of synthesis of DNA in the S-phase of the cell cycle, was also barely detectable in sham-operated lean and fatty livers. BrdU labeling increased to 9% of nuclei after LPG transplantation but was less than 1% in FPG (Figure 3C). However with 8-pCPT-cGMP treatment, BrdU labeling in FPG was restored to ~11% (Figure 3C).
Figure 3.
8-pCPT-cGMP improves regeneration of FPG after transplantation. Livers were collected 38 h after sham operation or transplantation. Proliferating cell nuclear antigen (PCNA) was detected by immunoblotting (A) and quantified by densitometry (B). 5-Bromo-2’-deoxyuridine (BrdU) incorporation was detected immunohistochemically. BrdU-positive cells (C) were counted in 10 random fields per slide in a blinded manner. Partial grafts were weighed before implantation and at 38 h after transplantation to calculate graft weight increases (D). Sham, sham operation; Lean, lean liver; Fatty, fatty liver; LFSG, lean full-size graft; LPG, lean partial graft; FPG, fatty partial graft; 8CG, 8-pCPT-cGMP. a, P<0.05 vs sham-operated lean livers; b, P<0.05 vs sham-operated fatty livers or LFSG; c, P<0.05 vs LPG, and d, P<0.05 vs FPG (n = 4/group).
We also compared graft weight changes after partial liver transplantation to lean full-size liver grafts (LFSG). After 38 h, graft weight increased 8% after transplantation of LFSG and 62% after LPG transplantation. By contract, after FPG transplantation, graft weight increased only 6%, which 8-pCPT-cGMP treatment increased to 66% (Figure 3D).
8-pCPT-cGMP improves graft function and survival after fatty partial liver transplantation
Hyperbilirubinemia is an indicator of poor liver function. In rats after LPG transplantation, total bilirubin increased from basal levels of <0.1 mg/dL to 1.4 mg/dL at 18 h, which decreased to 0.5 mg/dL at 38 h after transplantation (Figure 4A). In contrast, total bilirubin increased to 3 mg/dL at 18 h after FPG transplantation, which further increased to 5.2 mg/dL at 38 h. However, in rats transplanted with 8-pCPT-cGMP-treated FPG, total bilirubin only increased to 1.6 mg/dL at 18 h, which declined to 1.2 mg/dL at 38 h (Figure 4A).
Figure 4.
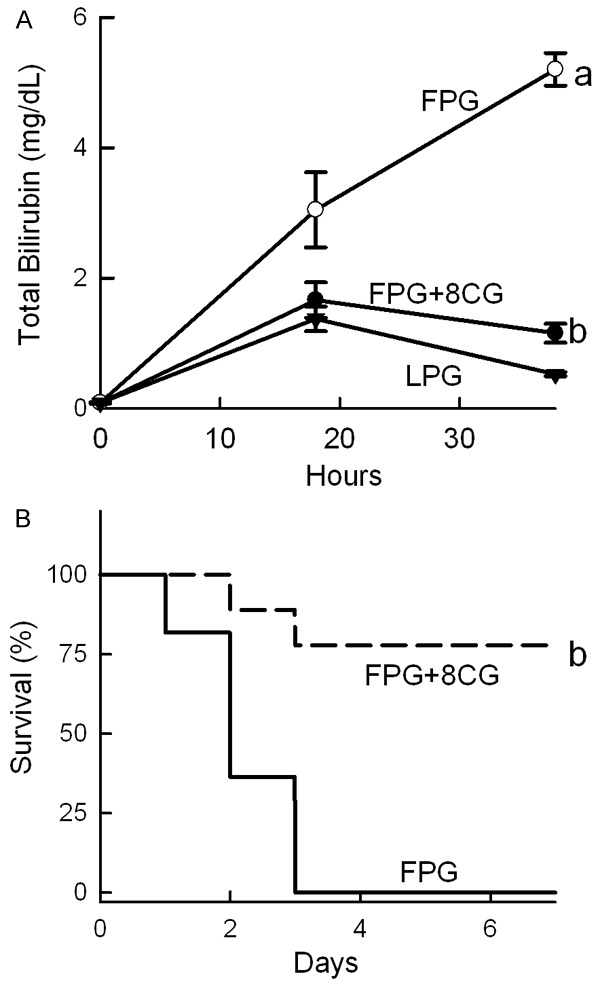
8-pCPT-cGMP improves function and increases survival of transplanted fatty partial liver grafts. Blood was collected at 18 and 38 h after implantation for measurement of total bilirubin (A). LPG, lean partial graft; FPG, fatty partial graft; 8CG, 8-pCPT-cGMP. a, P<0.05 vs LPG, and b, P<0.05 vs FPG (n = 4/group). Rats were observed 7 days for survival (B). b, P<0.05 vs FPG by the Kaplan-Meier test (n = 9-11/group).
All rats survived after sham operation (data not shown). Our previous study showed that all rats survived after transplantation of LPG cold stored for 2 h [32]. By contrast, survival decreased to 0% by 3 days after transplantation of FPG (Figure 4B). 8-pCPT-cGMP increased the survival of FPG to 77% (Figure 4B). Therefore, 8-pCPT-cGMP effectively prevented failure of FPG after transplantation.
8-pCPT-cGMP blunts mitochondrial depolarization in hepatocytes of transplanted fatty partial liver grafts
Energy supply is essential for maintaining cell function and supporting cell proliferation. Onset of the MPT plays an essential role in liver graft failure from preservation injury [26]. We therefore examined mitochondrial polarization status in transplanted liver grafts by intravital multiphoton microscopy using the fluorescence of Rh123, a membrane-permeant cationic fluorophore that labels polarized mitochondria. After sham operation, mitochondria were polarized in virtually every hepatocyte, as indicated by punctate Rh123 green fluorescence and in confirmation of previous studies (Figure 5A) [26,28,35]. In implanted LPG at 18 h after transplantation, mitochondria in some hepatocytes (~20%) became depolarized, as indicated by dim and diffuse instead of punctate Rh123 fluorescence (Figure 5A). After FPG transplantation, mitochondrial depolarization increased to 69% of hepatocytes, an about 3.5-fold increase compared to LPG. 8-pCPT-cGMP treatment of FPG during storage decreased mitochondrial depolarization after transplantation to 21% (Figure 5B). At this early stage, nonviable cells (with red PI fluorescence in nuclei) were rare, indicating mitochondrial depolarization occurred before cell death.
Figure 5.
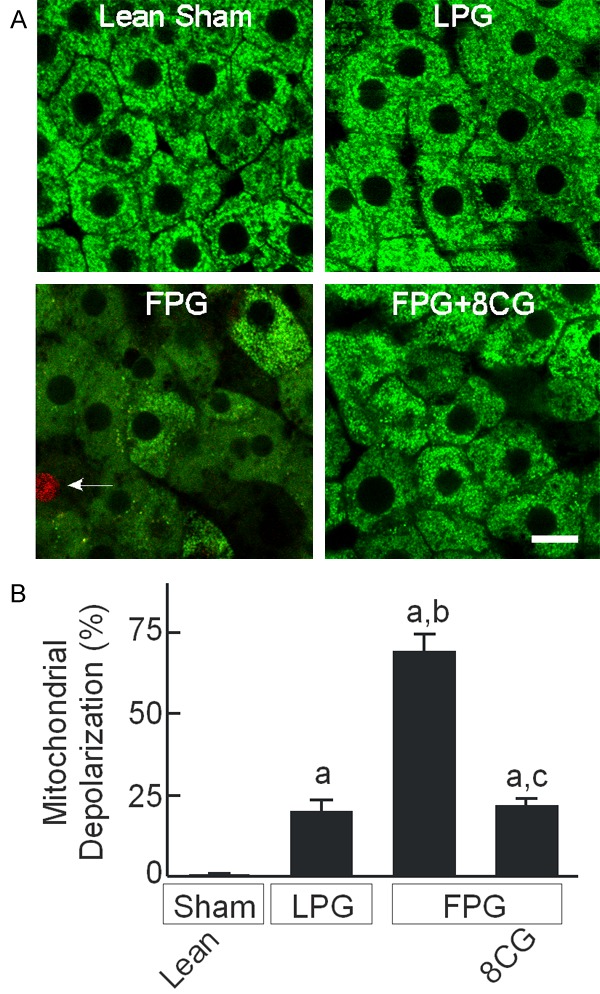
8-pCPT-cGMP blocks mitochondrial depolarization in transplanted fatty partial liver grafts. Intravital multiphoton microscopy of Rh123 and PI was performed at 18 h after sham operation or transplantation, as described in “METHODS”. A: Representative multiphoton images. Bar is 10 µm. Arrow identifies a PI-positive non-viable hepatocyte. B: Hepatocytes with depolarized mitochondria were counted in 10 random fields per rat as the percentage of the total. a, P<0.05 vs sham-operated lean livers; b, P<0.05 vs LPG and c, P<0.05 vs FPG (n = 4/group).
8-pCPT-cGMP increases mTOR signaling in transplanted fatty partial liver grafts
The mTOR pathway modulates cell proliferation and is highly sensitive to nutrient/energy alterations [39,40]. At 38 h after transplantation, total mTOR protein expression was not significantly different between LPG, FPG and sham (data not shown). Weak phospho-mTOR bands were detected in sham-operated lean and fatty livers, which increased 4.4-fold after LPG transplantation (Figure 6A, 6B). In contrast, phospho-mTOR increased only 1.8-fold after FPG transplantation, indicating lower mTOR activation compared to LPG. 8-pCPT-cGMP treatment of FPG, however, increased phospho-mTOR 3.2-fold after transplantation compared to sham (Figure 6A, 6B). Phosphorylation of p70S6K, a kinase downstream of mTOR, also increased 5.6-fold after LPG transplantation (Figure 6A, 6C). After FPG transplantation, p70S6K phosphorylation increased only 1.6-fold, which 8-pCPT-cGMP treatment increased to 4.9-fold (Figure 6A, 6C).
Figure 6.
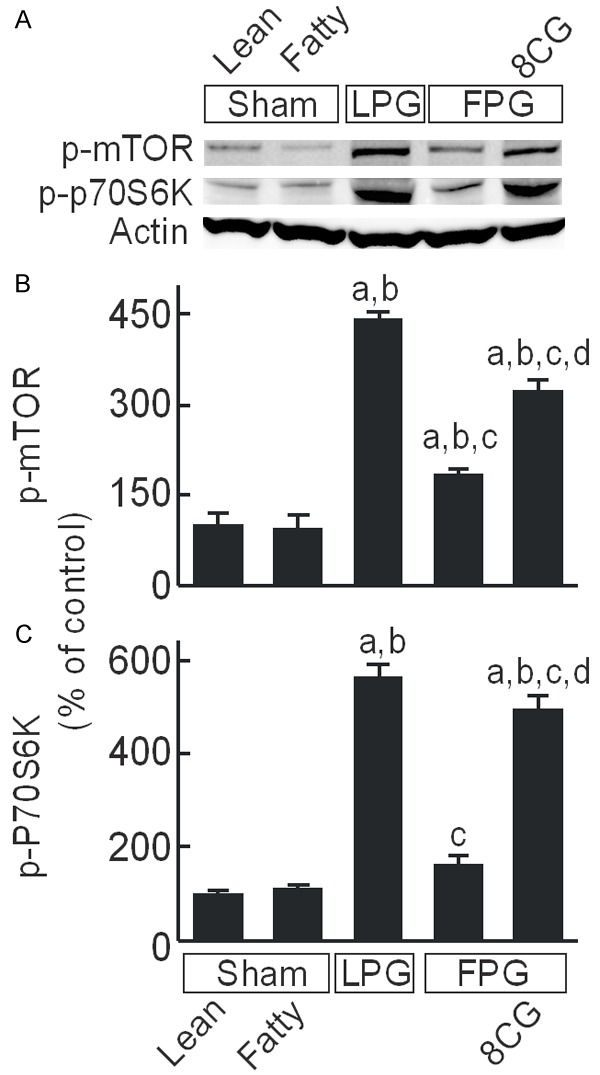
8-pCPT-cGMP increases mTOR signaling in transplanted fatty partial liver grafts. Livers were collected 38 h after sham operation or transplantation. Phospho-mammalian target of rapamycin (p-mTOR), phospho-p70S6 kinase (p-p70S6K), and actin were detected by immunoblotting (A, representative blots). (B) Quantification of p-mTOR by densitometry; (C) Quantification of p-p70S6K. Sham, sham operation; Lean, lean liver; Fatty, fatty liver; LPG, lean partial graft; FPG, fatty partial graft; 8CG, 8-pCPT-cGMP. a, P<0.05 vs sham-operated lean livers; b, P<0.05 vs sham-operated fatty livers; c, P<0.05 vs LPG, and d, P<0.05 vs FPG (n = 4/group).
8-pCPT-cGMP increases CREB activation in transplanted fatty partial liver grafts
In hepatocytes, 8-pCPT-cGMP activates PKA, an event that suppresses onset of the MPT [31]. PKA activation also stimulates cell proliferation/growth through the activation of CREB [41,42]. We therefore explored whether 8-pCPT-cGMP alters CREB activation. Total CREB protein expression was not different between lean and fatty livers and did not change after partial liver transplantation (Figure 7A). After LPG transplantation, phospho-CREB increased 1.5-fold compared to sham operation but decreased 53% after FPG transplantation. After 8-pCPT-cGMP treatment, phospho-CREB instead increased 1.4-fold after FPG transplantation (Figure 7A, 7B).
Figure 7.
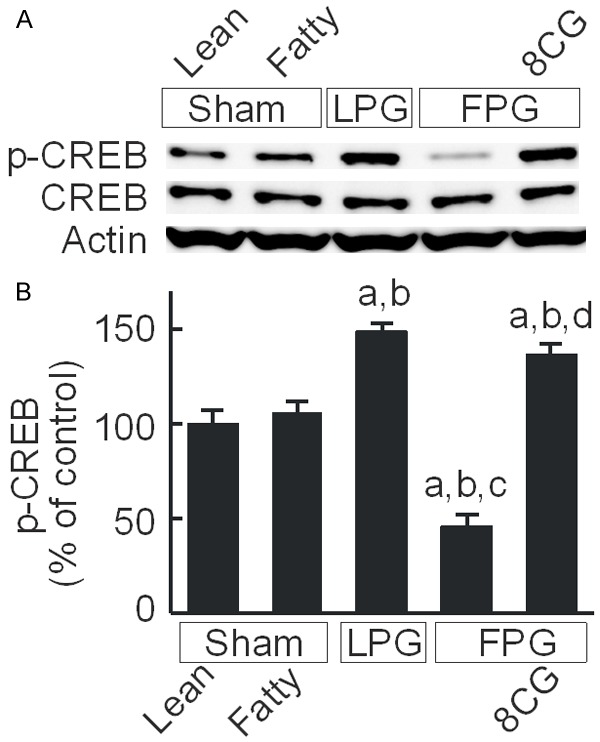
8-pCPT-cGMP increases CREB activation in transplanted fatty partial liver grafts. Livers were collected 38 h after sham operation or transplantation. cAMP response element-binding protein (CREB), phospho-CREB (p-CREB), and actin were detected by immunoblotting (A, representative images). (B) Quantification of p-CREB by densitometry; Sham, sham operation; Lean, lean liver; Fatty, fatty liver; LPG, lean partial graft; FPG, fatty partial graft; 8CG, 8-pCPT-cGMP. a, P<0.05 vs sham-operated lean livers; b, P<0.05 vs sham-operated fatty livers; c, P<0.05 vs LPG, and d, P<0.05 vs FPG (n = 4/group).
8-pCPT-cGMP increases cyclin expression in transplanted fatty partial liver grafts
Cyclins are important governors of cell cycle progression [43]. We explored the effects of 8-pCPT-cGMP on cyclin D1 and E expression in FPG (Figure 8). Weak bands of cyclin D1 and E were observed in sham-operated lean livers, which were not significantly different from sham-operated fatty livers (Figure 8A). Cyclin D1 increased 7.5-fold in transplanted LPG but increased only 1.9-fold in transplanted FPG (Figure 8A, 8B). In transplanted FPG treated with 8-pCPT-cGMP, cyclin D1 increased 6.1-fold (Figure 8A, 8B). Cyclin E changed in a similar fashion after the various treatments (Figure 8A, 8C).
Figure 8.
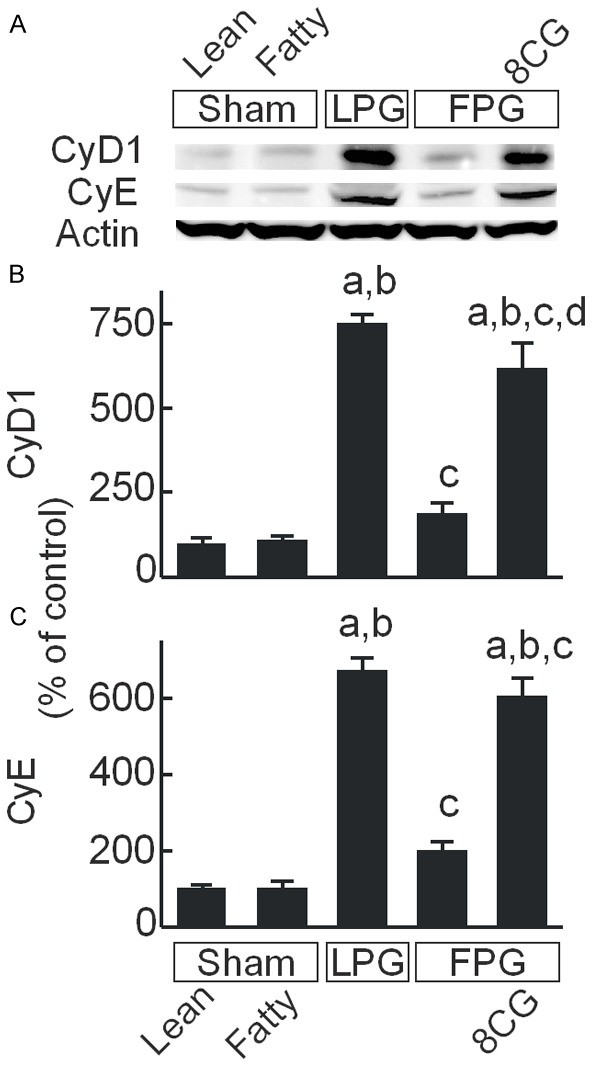
8-pCPT-cGMP increases cyclin D1 and E expression in transplanted fatty partial liver grafts. Livers were collected 38 h after sham operation or transplantation. Cyclin D1 (CyD1), cyclin E (CyE), and actin were detected by immunoblotting (A, representative images). (B) Quantification of cyclin D1 by densitometry; (C) Quantification of cyclin E. Sham, sham operation; Lean, lean liver; Fatty, fatty liver; LPG, lean partial graft; FPG, fatty partial graft; 8CG, 8-pCPT-cGMP. a, P<0.05 vs sham-operated lean livers; b, P<0.05 vs sham-operated fatty livers; c, P<0.05 vs LPG, and d, P<0.05 vs FPG (n = 4/group).
Discussion
8-pCPT-cGMP attenuates injury and improves regeneration of non-alcoholic fatty partial liver grafts
The continuing shortage of donor livers has increased the number of patients waiting for liver transplantation and markedly increased waiting-list mortality in the last two decades, which approaches like living and split liver donation might help to alleviate [11,44,45]. However, the current epidemic of obesity and metabolic syndrome has increased the incidence of NAFLD and NASH, which when severe is a contraindication to organ donation in liver transplantation, especially in the context of living or split liver donation requiring partial liver transplantation. Therefore, development of effective therapies to improve outcomes of steatotic partial liver transplantation might significantly impact the shortage of usable donor livers and save lives. Steatotic partial grafts face two risk factors: fatty infiltration and small graft mass. Steatosis itself is among the most important factors that negatively impact the outcome of liver transplantation [20,21,46]. Steatotic grafts show substantially greater vulnerability to I/R injury during cold storage/transplantation, resulting in increased cell death, delayed recovery of graft function and higher primary non-function in clinical and experimental liver transplantation [47,48]. Compared to full size liver transplantation, grafts for partial liver transplantation usually experience shorter cold storage times. Clinically, cold preservation time is about 2 to 6 h for living donor transplantation whereas mean cold ischemic time for full-size transplantation is 6-12 h [49-51]. Although liver tissue is well known to have great regeneration capacity, liver regeneration is suppressed when liver graft mass is reduced to below a critical level even after short cold preservation times [33,52]. Therefore, strategies for successful FPG transplantation should both prevent liver injury and improve liver regeneration. Here, we demonstrated that the cell-permeable cGMP analog 8-pCPT-cGMP markedly decreased liver injury (less necrotic and apoptotic cell death and transaminase release), improved graft regeneration (increased BrdU incorporation, PCNA expression, and graft weight gain), and enhanced graft function (lower serum total bilirubin) (Figures 1, 2, 3, 4). Most importantly, 8-pCPT-cGMP markedly improved survival of FPG (Figure 4). Therefore, 8-pCPT-cGMP is a promising therapy to improve function and decrease failure of steatotic partial grafts.
8-pCPT-cGMP attenuates graft injury by decreasing mitochondrial depolarization after transplantation of fatty partial liver grafts
Onset of the MPT is a penultimate step leading to cell death after I/R injury, causing necrosis from ATP depletion and apoptosis from mitochondrial release of proapoptotic factors such as cytochrome c [25,53] (Figure 9). Cell death in turn leads to release of mitochondrial and other cellular damage-associated molecular pattern molecules (e.g., mitochondrial DNA and high mobility group box 1 protein), which are potent inflammatory mediators that attract leukocytes with subsequent production of reactive oxygen species, release of proteases and other activities to amplify liver injury [54-56] (Figure 9). Our previous study demonstrated that the MPT was more extensive in steatotic full-size grafts than in lean full-size grafts after transplantation [35]. However, this effect occurred after long cold storage. We also observed that the MPT occurred after lean partial grafts when liver graft size was reduced to 25% in rat (quarter-size grafts) with a longer cold storage (6 h) than current study (2 h) [36]. In this study we showed that steatosis increases the MPT in partial grafts even after short cold storage (Figure 5), demonstrating that co-existence of two risk factors (steatosis and reduced graft size) further increases the susceptibility to MPT onset, leading to severe graft injury and failure after transplantation. 8-pCPT-cGMP, which blocks the MPT in cultured hepatocytes after I/R and in isolated mitochondria after calcium uptake [31], also decreased mitochondrial depolarization in vivo after FPG transplantation, attenuated subsequent graft injury and improved graft survival (Figures 1, 2). These results are consistent with the conclusion that MPT onset plays an important role in FPG injury and failure and that blockade of MPT onset is an effective strategy to decrease FPG injury and improve survival.
Figure 9.
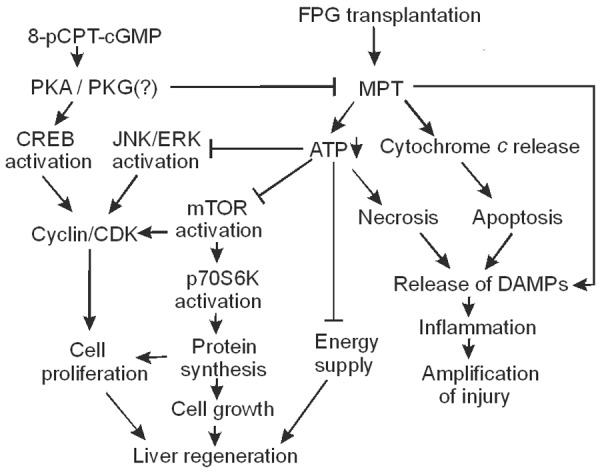
Potential mechanisms by which 8-pCPT-cGMP improves the outcome of fatty partial liver transplantation. Fatty liver transplantation causes onset of mitochondrial permeability transition (MPT), which decreases ATP formation and causes release of cytochrome c, leading to necrosis and apoptosis. Mitochondrial injury and cell death in turn cause release of mitochondrial and other cellular damage-associated molecular pattern molecules (DAMPs), leading to inflammation which further amplifies liver injury. ATP acts both as an energy source supporting liver regeneration and also a modulator of liver regeneration signaling. For example, mammalian target of rapamycin (mTOR) and its downstream p70S6 kinase (p70S6K), the pathway that regulates protein synthesis necessary for cell proliferation and growth, are highly sensitive to perturbations of nutrient/energy supply. Moreover, activation of a number of growth factor receptors and kinases that are involved in cell proliferation (e.g., extracellular signal-regulated kinase [ERK], c-Jun-N-terminal kinase [JNK], etc.) also requires high energy molecules. Formation of cyclin/cyclin-dependent kinase (CDK) complexes is a convergent point of multiple upstream signaling to control cell proliferation. Therefore, ATP depletion from the MPT occurring in FPG suppresses liver regeneration. Previous studies show that both cGMP and cAMP analogs protect against the MPT in cultured hepatocytes after ischemia/reperfusion through activation of protein kinase A (PKA). 8-pCPT-cGMP, a cell permeable cGMP analog, activates PKA in hepatocytes and possibly PKG in other cell types to block MPT onset, thus attenuating cell death and improving liver regeneration. 8-pCPT-cGMP also enhances cell proliferation through activation of cAMP response element-binding protein (CREB).
8-pCPT-cGMP is a cell-permeable cGMP analog, which typically acts via cGMP-dependent protein kinases (PKG) to regulate many physiological processes (e.g., smooth muscular cell relaxation and intracellular calcium concentration). Previous studies show in isolated rat liver mitochondria that cGMP analogs block the Ca2+-induced MPT in the presence of hepatic cytosol [29]. NO exerts its biological effects through activation of guanylate cyclase, producing cGMP [57], and NO donors and cGMP analogs prevent MPT-dependent necrotic killing of ischemic hepatocytes in culture after ischemia/reoxygenation [29]. Although PKG is typically the target of cGMP, hepatocytes do not express mRNA for either of the two isoforms of PKG [58]. Moreover, analogs of both cGMP and cAMP stimulate cytosolic PKA from hepatocytes, and a PKA peptide inhibitor but not a PKG peptide inhibitor abolishes cGMP and cAMP-stimulated kinase activity in hepatocyte cytosol [31]. Thus, by activation of cytosolic PKA, cGMP and cAMP inhibit MPT onset and protect hepatocytes against necrotic cell death after ischemia/reoxygenation [31].
These findings in vitro remain to be confirmed in vivo. In the present study, we showed that the cGMP analog, 8-pCPT-cGMP, also suppressed MPT onset in vivo after fatty partial liver transplantation, as visualized by intravital multiphoton microscopy of Rh123 (Figure 5). This protection of 8-pCPT-cGMP against the MPT in hepatocytes of FPG is thus likely mediated by PKA. However, since multiple cell types exist in the liver, we cannot rule out completely the possibility that 8-pCPT-cGMP also works through PKG in cells other than hepatocytes. Moreover, although NO and cGMP production are closely related, protection of 8-pCPT-cGMP is unlikely by increasing NO, since iNOS expression and production of reactive nitrogen species already increases dramatically in FPG after transplantation, effects which are associated with increased graft injury [32]. Moreover, 1400 W, a selective iNOS inhibitor, improves the outcome of FPG transplantation [32].
Potential mechanisms by which 8-pCPT-cGMP improves liver regeneration in FPG
In this study, we confirmed that FPG transplantation leads to poor liver regeneration (decreased BrdU incorporation, PCNA expression and liver graft weight gain), poor graft function (hyperbilirubinemia), and graft failure. 8-pCPT-cGMP improved all these adverse outcomes, including the enhancement of liver regeneration (Figure 3). Liver regeneration is essential for survival and recovery of graft mass and function after partial liver transplantation. Partial grafts face extra metabolic work load due to their reduced mass. Moreover, extra energy is needed to support liver regeneration. Therefore, mitochondrial dysfunction not only leads to cell death but can also suppress liver regeneration (Figure 9). In addition, ATP not only acts as an energy supplier for liver regeneration but also as a regeneration signaling modifier. Activation of a number of growth factor receptors and kinases by phosphorylation (e.g., epidermal growth factor receptor, MAP kinase kinase, extracellular signal-regulated kinase [ERK], c-Jun-N-terminal kinase [JNK], etc.) requires high energy molecules [59] (Figure 9). mTOR is a serine-threonine kinase that regulates the protein synthesis necessary for cell growth and proliferation [60-62]. Activation of its downstream p70S6 kinase in turn regulates the 40S ribosomal protein S6 to control protein synthesis and cell proliferation [60] (Figure 9). A previous study shows that inhibition of mTOR decreases DNA synthesis after partial hepatectomy [63]. The mTOR pathway is highly sensitive to perturbations of nutrient/energy supply, and decreased ATP can suppress the mTOR pathway [39,40]. Indeed, in FPG with severe mitochondrial dysfunction, we observed decreased mTOR and p70S6 kinase activation (Figure 6). 8-pCPT-cGMP, which prevented mitochondrial dysfunction, also improved mTOR and p70S6 kinase activation in FPG (Figure 6).
Additionally, we observed a suppression of CREB activation after FPG activation (Figure 7). Previous studies show that CREB activation regulates hepatocyte proliferation after partial hepatectomy [64,65]. PKA is an important regulator of CREB. Activation of G-protein coupled receptors stimulates the activity of membrane-associated adenylyl cyclase, which converts ATP to cAMP. Binding of cAMP to the two PKA catalytic subunits causes release of active catalytic subunits, which migrate into the nucleus to phosphorylate and thereby activate CREB, a transcriptional factor. Activated CREB then interacts with the cAMP response enhancer element of the promoters of cAMP-responsive genes to cause transcription of genes that regulates various physiological processes including proliferation [41,42] (Figure 9). Previous studies show that transient increases of cAMP also cause upregulation of genes expressed in the G1 phase of the cell cycle (e.g., c-fos), whereas decreased cAMP suppresses hepatic DNA synthesis after partial hepatectomy [66,67]. Interestingly, activation of PKG can also increase CREB phosphorylation. A previous study shows that cGMP-PKG signaling plays an important role preventing apoptosis and promoting cell proliferation in both normal and certain cancer cells and that PKG knockdown by siRNA decreases CREB phosphorylation [68]. Another study also shows that cGMP-PKG signaling pathway acts in parallel with the cAMP-PKA pathway to produce CREB phosphorylation [69]. Therefore, activation of either PKA or PKG can increase cell proliferation and 8-pCPT-cGMP may increase CREB activation via PKA and/or PKG (Figure 9).
Liver regeneration requires cell proliferation. Cyclins are a family of proteins that control cell cycle progression [43]. Cyclins bind corresponding cyclin-dependent kinases, forming complexes that phosphorylate other proteins (e.g., retinoblastoma protein). These phosphorylated proteins, in turn, regulate specific events during cell division [43]. Cyclin D1, which drives hepatocytes to enter cell cycle [70], is highly CREB-dependent, whereas cyclin B1 is co-regulated by both CREB-dependent and -independent mechanisms [71]. JNK1/2 also controls cyclin D1 expression, and inhibition of JNK activation suppresses hepatocyte mitosis after partial hepatectomy [72]. Inhibition of ERK activation also suppresses synthesis of cyclins E and A [73]. Inhibitors of mammalian target of rapamycin complex 1 (mTORC1, a protein complex composed of mTOR itself, regulatory-associated protein of mTOR [Raptor] and other proteins) [74], including rapamycin and siRNA for raptor, inhibit upregulation of cyclin D1 by inorganic polyphosphate [75]. Therefore, cyclins are a convergent point of multiple upstream signaling to control cell proliferation (Figure 9). In this study, we observed suppressed cyclin D1 and E expression in FPG compared to LPG transplantation. 8-pCPT-cGMP restored cyclin expression after FPG transplantation (Figure 8), which is consistent with improved FPG regeneration by 8-pCPT-cGMP (Figure 3).
Taken together, we observed more severe graft injury and suppression of regeneration after transplantation of FPG. Such increased injury and suppression of regeneration were associated with more severe mitochondrial dysfunction. 8-pCPT-cGMP improved the outcomes of FPG transplantation, most likely by blocking mitochondrial dysfunction and increasing proliferative signaling (Figure 9). Other cGMP analogs (e.g., 8-BrcGMP) also protect against the MPT onset in hepatocytes after ischemia/reperfusion in vitro [29,30]. Whether these analogs also improve the outcomes of FPG transplantation will be examined in future studies.
Acknowledgements
This study was supported, in part, by Grants from the National Institute of Health [DK70844, DK037034, DK073336], the Russian Federation [14.Z50.31.0028] and the Chinese National Natural Foundation [Grant 81470878]. The Cell & Molecular Imaging Core of the Hollings Cancer Center at the Medical University of South Carolina supported by NIH Grant 1P30 CA138313 and Shared Instrumentation Grant S10OD018113 provided instrumentation for multiphoton microscopy. Animals were housed in the Animal Resources at Medical University of South Carolina supported by NIH Grant C06 RR015455.
Disclosure of conflict of interest
None.
References
- 1.Yilmaz Y, Younossi ZM. Obesity-associated nonalcoholic fatty liver disease. Clin Liver Dis. 2014;18:19–31. doi: 10.1016/j.cld.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Goceri E, Shah ZK, Layman R, Jiang X, Gurcan MN. Quantification of liver fat: a comprehensive review. Comput Biol Med. 2016;71:174–189. doi: 10.1016/j.compbiomed.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Dongiovanni P, Lanti C, Riso P, Valenti L. Nutritional therapy for nonalcoholic fatty liver disease. J Nutr Biochem. 2016;29:1–11. doi: 10.1016/j.jnutbio.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Basciano H, Federico L, Adeli K. Fructose, insulin resistanceand metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825–G834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 7.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 8.Finkenstedt A, Graziadei IW. Steatosis after liver transplantation: is it really benign? Liver Transpl. 2016;22:585–7. doi: 10.1002/lt.24439. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 10.Canbay A, Sowa JP, Syn WK, Treckmann J. NASH cirrhosis-the new burden in liver transplantation: how should it be managed? Visc Med. 2016;32:234–238. doi: 10.1159/000446379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fayek SA, Quintini C, Chavin KD, Marsh CL. The current state of liver transplantation in the United States: perspective from American society of transplant surgeons (ASTS) scientific studies committee and endorsed by ASTS council. Am J Transplant. 2016;16:3093–3104. doi: 10.1111/ajt.14017. [DOI] [PubMed] [Google Scholar]
- 12.Rinella ME, Alonso E, Rao S, Whitington P, Fryer J, Abecassis M, Superina R, Flamm SL, Blei AT. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transpl. 2001;7:409–414. doi: 10.1053/jlts.2001.23787. [DOI] [PubMed] [Google Scholar]
- 13.Garcia Urena MA, Colina Ruiz-Delgado F, Moreno GE, Jimenez RC, Garcia GI, Loinzaz SC, Gonzalez P, Gomez SR. Hepatic steatosis in liver transplant donors: common feature of donor population? World J Surg. 1998;22:837–844. doi: 10.1007/s002689900479. [DOI] [PubMed] [Google Scholar]
- 14.Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 15.Mittler J, Pascher A, Neuhaus P, Pratschke J. The utility of extended criteria donor organs in severely ill liver transplant recipients. Transplantation. 2008;86:895–896. doi: 10.1097/TP.0b013e318186ad7a. [DOI] [PubMed] [Google Scholar]
- 16.Nocito A, El-Badry AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol. 2006;45:494–499. doi: 10.1016/j.jhep.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Urena MA, Moreno GE, Romero CJ, Ruiz-Delgado FC, Moreno SC. An approach to the rational use of steatotic donor livers in liver transplantation. Hepatogastroenterology. 1999;46:1164–1173. [PubMed] [Google Scholar]
- 18.Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan H, Jaruga B, Batkai S, Hoshino S, Tian Z, Kunos G, Diehl AM, Gao B. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125:125–215. doi: 10.1016/s0016-5085(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer AL, Lao OB, Dick AA, Bakthavatsalam R, Halldorson JB, Yeh MM, Upton MP, Reyes JD, Perkins JD. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16:874–884. doi: 10.1002/lt.22085. [DOI] [PubMed] [Google Scholar]
- 20.Marsman WA, Wiesner RH, Rodrigues L, Batts KP, Porayko MK, Hay JE, Gores GJ, Krom RA. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1997;62:1246–1251. doi: 10.1097/00007890-199611150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. doi: 10.1002/hep.1840200410. [DOI] [PubMed] [Google Scholar]
- 22.Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J Gastroenterol Hepatol. 2007;22(Suppl 1):S31–S37. doi: 10.1111/j.1440-1746.2006.04643.x. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb RA. Cell death pathways in acute ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther. 2011;16:233–238. doi: 10.1177/1074248411409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jassem W, Fuggle SV, Rela M, Koo DD, Heaton ND. The role of mitochondria in ischemia/reperfusion injury. Transplantation. 2002;73:493–499. doi: 10.1097/00007890-200202270-00001. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Z, Ramshesh VK, Rehman H, Currin RT, Sridharan V, Theruvath TP, Kim I, Wright GL, Lemasters JJ. Activation of the oxygensensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2008;295:G823–G832. doi: 10.1152/ajpgi.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theruvath TP, Zhong Z, Currin RT, Pediaditakis P, Lemasters JJ. Minocycline mitigates storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:235–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehman H, Sun J, Shi Y, Ramshesh VK, Liu Q, Currin RT, Lemasters JJ, Zhong Z. NIM811 prevents mitochondrial dysfunction, attenuates liver injury and stimulates liver regeneration after massive hepatectomy. Transplantation. 2011;91:406–412. doi: 10.1097/TP.0b013e318204bdb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong Z, Theruvath TP, Currin RT, Waldmeier PC, Lemasters JJ. NIM811, a mitochondrial permeability transition inhibitor, prevents mitochondrial depolarization in small-for-size rat liver grafts. Am J Transplant. 2007;7:1103–1111. doi: 10.1111/j.1600-6143.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide protects hepatocytes against mitochondrial permeability transitioninduced reperfusion injury. Hepatology. 2004;39:1533–1543. doi: 10.1002/hep.20197. [DOI] [PubMed] [Google Scholar]
- 30.Kim JS, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide: a signaling molecule against mitochondrial permeability transitionand pH-dependent cell death after reperfusion. Free Radic Biol Med. 2004;37:1943–1950. doi: 10.1016/j.freeradbiomed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Pediaditakis P, Kim JS, He L, Zhang X, Graves LM, Lemasters JJ. Inhibition of the mitochondrial permeability transition by protein kinase A in rat liver mitochondria and hepatocytes. Biochem J. 2010;431:411–421. doi: 10.1042/BJ20091741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He S, Rehman H, Wright GL, Zhong Z. Inhibition of inducible nitric oxide synthase prevents mitochondrial damage and improves survival of steatotic partial liver grafts. Transplantation. 2010;89:291–298. doi: 10.1097/TP.0b013e3181c99185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong Z, Connor HD, Froh M, Bunzendahl H, Lind H, Lehnert M, Mason RP, Thurman RG, Lemasters JJ. Free radical-dependent dysfunction of small-for-size rat liver grafts: prevention by plant polyphenols. Gastroenterology. 2005;129:652–664. doi: 10.1016/j.gastro.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 34.Zhong Z, Schwabe RF, Kai Y, He L, Yang L, Bunzendahl H, Brenner DA, Lemasters JJ. Liver regeneration is suppressed in small-for-size liver grafts after transplantation: involvement of JNK, cyclin D1 and defective energy supply. Transplantation. 2006;82:241–250. doi: 10.1097/01.tp.0000228867.98158.d2. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Rehman H, Krishnasamy Y, Ramshesh VK, Theruvath TP, Chavin KD, Schnellmann RG, Lemasters JJ, Zhong Z. Role of inducible nitric oxide synthase in mitochondrial depolarization and graft injury after transplantation of fatty livers. Free Radic Biol Med. 2012;53:250–259. doi: 10.1016/j.freeradbiomed.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rehman H, Connor HD, Ramshesh VK, Theruvath TP, Mason RP, Wright GL, Lemasters JJ, Zhong Z. Ischemic preconditioning prevents free radical production and mitochondrial depolarization in small-for-size rat liver grafts. Transplantation. 2008;85:1322–1331. doi: 10.1097/TP.0b013e31816de302. [DOI] [PubMed] [Google Scholar]
- 37.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 38.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Riehle KJ, Dan YY, Campbell JS, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;26(Suppl 1):203–212. doi: 10.1111/j.1440-1746.2010.06539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 41.Servillo G, Penna L, Foulkes NS, Magni MV, Della Fazia MA, Sassone-Corsi P. Cyclic AMP signalling pathway and cellular proliferation: induction of CREM during liver regeneration. Oncogene. 1997;14:1601–1606. doi: 10.1038/sj.onc.1200996. [DOI] [PubMed] [Google Scholar]
- 42.Servillo G, Della Fazia MA, Sassone-Corsi P. Coupling cAMP signaling to transcription in the liver: pivotal role of CREB and CREM. Exp Cell Res. 2002;275:143–154. doi: 10.1006/excr.2002.5491. [DOI] [PubMed] [Google Scholar]
- 43.Galderisi U, Jori FP, Giordano A. Cell cycle regulation and neural differentiation. Oncogene. 2003;22:5208–5219. doi: 10.1038/sj.onc.1206558. [DOI] [PubMed] [Google Scholar]
- 44.Alqahtani SA. Update in liver transplantation. Curr Opin Gastroenterol. 2012;28:230–8. doi: 10.1097/MOG.0b013e3283527f16. [DOI] [PubMed] [Google Scholar]
- 45.Jones PD, Hayashi PH, Sidney BI. Liver transplantation in 2013: challenges and controversies. Minerva Gastroenterol Dietol. 2013;59:117–131. [PubMed] [Google Scholar]
- 46.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 47.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, Stewart G, McCaughan G. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9:500–505. doi: 10.1053/jlts.2003.50099. [DOI] [PubMed] [Google Scholar]
- 48.Zhong Z, Connor H, Mason RP, Qu W, Stachlewitz RF, Gao W, Lemasters JJ, Thurman RG. Destruction of Kupffer cells increases survival and reduces graft injury after transplantation of fatty livers from ethanol-treated rats. Liver Transplant Surg. 1996;2:383–387. doi: 10.1002/lt.500020509. [DOI] [PubMed] [Google Scholar]
- 49.Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, Smeets P, Colle I, Van Vlierberghe H, Petrovic M, Voet D, Mortier E, Hesse UJ, de Hemptinne B. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237:429–436. doi: 10.1097/01.SLA.0000055277.78876.B7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Callaghan JM, Morgan RD, Knight SR, Morris PJ. The effect of preservation solutions for storage of liver allografts on transplant outcomes: a systematic review and meta-analysis. Ann Surg. 2014;260:46–55. doi: 10.1097/SLA.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 51.Bejaoui M, Pantazi E, Folch-Puy E, Baptista PM, Garcia-Gil A, Adam R, Rosello-Catafau J. Emerging concepts in liver graft preservation. World J Gastroenterol. 2015;21:396–407. doi: 10.3748/wjg.v21.i2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q, Rehman H, Krishnasamy Y, Haque K, Schnellmann RG, Lemasters JJ, Zhong Z. Amphiregulin stimulates liver regeneration after small-for-size mouse liver transplantation. Am J Transplant. 2012;12:2052–61. doi: 10.1111/j.1600-6143.2012.04069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immunemediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 57.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- 58.Kulhanek-Heinze S, Gerbes AL, Gerwig T, Vollmar AM, Kiemer AK. Protein kinase a dependent signalling mediates anti-apoptotic effects of the atrial natriuretic peptide in ischemic livers. J Hepatol. 2004;41:414–420. doi: 10.1016/j.jhep.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 59.Gabai VL, Meriin AB, Yaglom JA, Wei JY, Mosser DD, Sherman MY. Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stewss-induced inhibition of JNK dephosphorylation. J Biol Chem. 2000;275:38088–38094. doi: 10.1074/jbc.M006632200. [DOI] [PubMed] [Google Scholar]
- 60.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerasimovskaya EV, Tucker DA, Weiser-Evans M, Wenzlau JM, Klemm DJ, Banks M, Stenmark KR. Extracellular ATP-induced proliferation of adventitial fibroblasts requires phosphoinositide 3-kinase, Akt, mammalian target of rapamycin and p70 S6 kinase signaling pathways. J Biol Chem. 2005;280:1838–1848. doi: 10.1074/jbc.M409466200. [DOI] [PubMed] [Google Scholar]
- 62.Jaeschke A, Dennis PB, Thomas G. mTOR: a mediator of intracellular homeostasis. Curr Top Microbiol Immunol. 2004;279:283–298. doi: 10.1007/978-3-642-18930-2_17. [DOI] [PubMed] [Google Scholar]
- 63.Francavilla A, Starzl TE, Scotti C, Carrieri G, Azzarone A, Zeng QH, Porter KA, Schreiber SL. Inhibition of liver, kidney, and intestine regeneration by rapamycin. Transplantation. 1992;53:496–498. doi: 10.1097/00007890-199202010-00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boshart M, Weih F, Schmidt A, Fournier RE, Schutz G. A cyclic AMP response element mediates repression of tyrosine aminotransferase gene transcription by the tissue-specific extinguisher locus Tse-1. Cell. 1990;61:905–916. doi: 10.1016/0092-8674(90)90201-o. [DOI] [PubMed] [Google Scholar]
- 65.Zhao G, Wakabayashi R, Shimoda S, Fukunaga Y, Kumagai M, Tanaka M, Nakano K. Impaired activities of cyclic adenosine monophosphate-responsive element binding protein, protein kinase a and calcium-independent phospholipase A2 are involved in deteriorated regeneration of cirrhotic liver after partial hepatectomy in rats. Hepatol Res. 2011;41:1110–1119. doi: 10.1111/j.1872-034X.2011.00868.x. [DOI] [PubMed] [Google Scholar]
- 66.MacManus JP, Franks DJ, Youdale T, Braceland BM. Increases in rat liver cyclic AMP concentrations prior to the initiation of DNA synthesis following partial hepatectomy or hormone infusion. Biochem Biophys Res Commun. 1972;49:1201–1207. doi: 10.1016/0006-291x(72)90596-7. [DOI] [PubMed] [Google Scholar]
- 67.Kruijer W, Skelly H, Botteri F, van der Putten H, Barber JR, Verma IM, Leffert HL. Proto-oncogene expression in regenerating liver is simulated in cultures of primary adult rat hepatocytes. J Biol Chem. 1986;261:7929–7933. [PubMed] [Google Scholar]
- 68.Wong JC, Bathina M, Fiscus RR. Cyclic GMP/protein kinase G type-Ialpha (PKG-Ialpha) signaling pathway promotes CREB phosphorylation and maintains higher c-IAP1, livin, survivin, and Mcl-1 expression and the inhibition of PKG-Ialpha kinase activity synergizes with cisplatin in non-small cell lung cancer cells. J Cell Biochem. 2012;113:3587–3598. doi: 10.1002/jcb.24237. [DOI] [PubMed] [Google Scholar]
- 69.Lu YF, Hawkins RD. Ryanodine receptors contribute to cGMP-induced late-phase LTP and CREB phosphorylation in the hippocampus. J Neurophysiol. 2002;88:1270–1278. doi: 10.1152/jn.2002.88.3.1270. [DOI] [PubMed] [Google Scholar]
- 70.Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 2001;61:8564–8568. [PubMed] [Google Scholar]
- 71.Daniel P, Filiz G, Brown DV, Hollande F, Gonzales M, D’Abaco G, Papalexis N, Phillips WA, Malaterre J, Ramsay RG, Mantamadiotis T. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis. 2014;3:e108. doi: 10.1038/oncsis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 73.Bokemeyer D, Panek D, Kitahara M, Trzaskos JM, Muller CE, Hockemeyer J, Kunter U, Boor P, Floege J, Kramer HJ, Ostendorf T. The map kinase ERK regulates renal activity of cyclindependent kinase 2 in experimental glomerulonephritis. Nephrol Dial Transplant. 2007;22:3431–3441. doi: 10.1093/ndt/gfm428. [DOI] [PubMed] [Google Scholar]
- 74.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 75.Hassanian SM, Ardeshirylajimi A, Dinarvand P, Rezaie AR. Inorganic polyphosphate promotes cyclin D1 synthesis through activation of mTOR/Wnt/beta-catenin signaling in endothelial cells. J Thromb Haemost. 2016;14:2261–2273. doi: 10.1111/jth.13477. [DOI] [PMC free article] [PubMed] [Google Scholar]