Abstract
Awake surgery with electrocorticosubcortical stimulation is the golden standard treatment for gliomas in eloquent areas. Preoperatively, mostly mild cognitive disturbances are observed with postoperative deterioration. We describe pre- and postoperative profiles of 4 patients (P1–P4) with gliomas in “critical” language areas (“Broca,” “Wernicke,” and the arcuate fasciculus) undergoing awake surgery to get insight into the underlying mechanism of neuroplasticity. Neuropsychological examination was carried out preoperatively (at T1) and postoperatively (at T2, T3). At T1, cognition of P1 was intact and remained stable. P2 had impairments in all cognitive domains at T1 with further deterioration at T2 and T3. At T1, P3 had impairments in memory and executive functions followed by stable recovery. P4 was intact at T1, followed by a decline in a language test at T2 and recovery at T3. Intraoperatively, in all patients language positive sites were identified. Patients with gliomas in “critical” language areas do not necessarily present cognitive disturbances. Surgery can either improve or deteriorate (existing) cognitive impairments. Several factors may underlie the plastic potential of the brain, for example, corticosubcortical networks and tumor histopathology. Our findings illustrate the complexity of the underlying mechanism of neural plasticity and provide further support for a “hodotopical” viewpoint.
1. Introduction
Awake surgery is considered the golden standard treatment for low-grade gliomas (LGG) in eloquent regions to optimize tumor resection while preserving neurological and cognitive functions and hence quality of life [1, 2]. However, deficits in cognitive functions, that is, language, memory, attentional, and executive functions, occur in the (pre- and) postoperative phase of awake glioma surgery [3–5].
Eloquent regions typically include the left dominant perisylvian brain regions. DES has provided evidence for a “hodotopical” (i.e., dynamic) view of the organization of brain functions as opposed to a “topological” viewpoint (i.e., static organization of brain functions) [6–8]. Language functions are “classically” represented in cortical areas such as Broca's and Wernicke's area and in the subcortical tracts that connect different eloquent cortical regions. LGGs typically invade functional subcortical white matter tracts. However, due to the relative slow growth rate (i.e., 4 mm a year) of LGG, neural plasticity can be facilitated [9, 10]. This may be the reason that, instead of moderate to severe language problems, typically mild language disorders are observed in this patient group [11]. Despite intense intraoperative monitoring, brain tumor surgery resection may induce or aggravate the existing cognitive deficits. For a long time, complete recovery within 3 months was claimed to take place, but Satoer et al. [5]. found that cognitive recovery can continue until up to at least 1 year postoperatively. A recent review of cognition in glioma patients showed various pre- and postoperative cognitive profiles with deficits in various domains at different time-moments [12]. These findings point towards differential postoperative recovery courses of cognitive functions. Apart from individual variability in functional organization and language lateralization, other factors accounting for the potential of neuroplasticity are under debate. Tumor related characteristics (e.g., tumor volume, grade) may interfere with the course of cognitive recovery [13, 14]. Anticonvulsants and adjuvant therapy (radio- and chemotherapy) as well as the degree of seizures (frequency) may have impact on the functional cerebral network in brain tumor patients [15]. In this article, we describe 4 patients with a brain tumor in dominant perisylvian language areas in proximity of the arcuate fasciculus with differential pre- and postoperative cognitive profiles illustrating the diversity of neural plasticity processes.
2. Materials and Methods
2.1. Case Reports
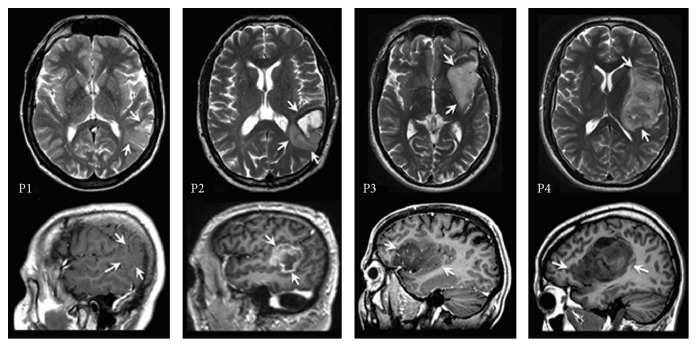
This is a follow-up study of 4 patients that we selected based on tumor localization in perisylvian language areas. The patients (P1, P2, P3, and P4) were diagnosed with a glioma in the language dominant left hemisphere as identified with fMRI (see structural MRI scans for tumor localization in Figure 1 and resection cavity in Figure 2). The demographic and clinical characteristics of the patients are shown in Table 1. The tumor was in proximity to the posterior temporoparietal language regions “Wernicke” in patients P1, P2, and P4. In P4, the tumor extended frontally towards the frontal language region of “Broca” as well. In P3, the tumor was located in the frontal and insular gyrus, in proximity or possibly with minimal involvement of the inferior frontal gyrus, that is, “Broca,” but not the posterior temporoparietal regions. Tumor locations in all patients were in the vicinity of the arcuate fasciculus (AF).
Figure 1.
Preoperative MRI scans axial T2 weighted and sagittal T1 weighted (contrast-enhanced in P1 and in P3) sections depicting tumor localization (arrows).
Figure 2.
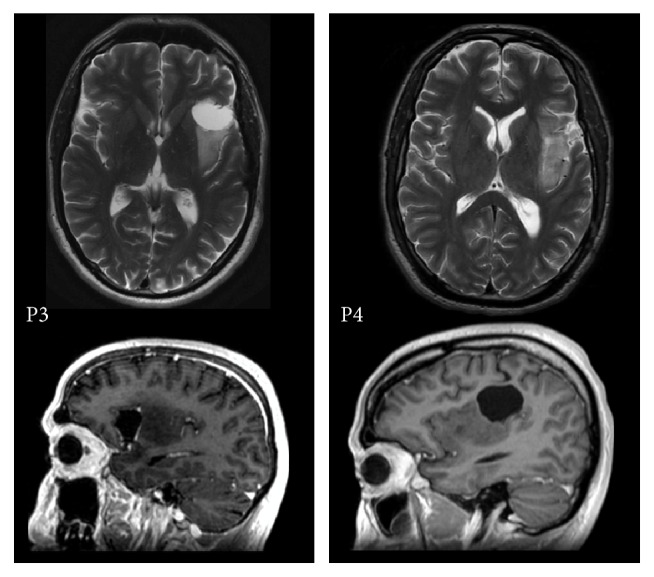
Postoperative MRI scans axial T2 weighted and sagittal T1 weighted P3 and P4.
Table 1.
Demographic and clinical characteristics. Y = years, Hem. Dom. = hemispheric dominance, M = male, L = left, R = right, AF = arcuate fasciculus, O = oligodendroglioma, GBM = glioblastoma, AO = anaplastic oligodendroglioma, EoR = extent of resection, and AED = antiepileptic drugs.
| Case | Gender | Age (y) |
Education (y) | Handedness | Onset symptoms | Hem. Dom. | Cortical area | Subcortical area |
WHO grade (2007) |
Histology | EoR (%) |
AED | Adjuvant therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | M | 45 | 15 | L | Seizure | L | Temporoparietal (“Wernicke”) |
AF | II (low) | O | 58.34 | Yes | Radio |
| P2 | M | 63 | 12 | R | Language problems | L | Temporoparietal (“Wernicke”) |
AF | IV (high) | GBM | 88.68 | Yes | Radio |
| P3 | M | 41 | 16 | R | Seizure | L | Frontal and insular (“Broca”) |
AF | II (low) | O | 52.73 | Yes | No |
| P4 | M | 33 | 15 | R | Seizure | L | Temporoparietal and frontal (“Wernicke” and “Broca”) |
AF | III (high) | AO | 81.48 | Yes | Radio |
2.2. Procedure: Operation, Neuroimaging, and Pathological Findings
Between July 2011 and June 2013, patients were treated with awake brain surgery given the tumor location in or near presumed critical language regions. Electrical stimulation was carried out at cortical and subcortical level with a bipolar electrode. Object naming and repetition tasks were administered during stimulation, whereas more extensive language testing was conducted using the Dutch Linguistic Intraoperative Protocol (DuLIP) with spontaneous speech monitoring during resection [16].
Localization of the tumor was determined by a neuroradiologist using 3D T1-weighted images and 2D T2-weighted images. The pre- and postoperative tumor volume was calculated by manual delineation of 3-dimensional deviant signal intensity on T2-weighted MR images using Osirix version 4.1.2. (http://www.osirix-viewer.com/). Postoperative MRI scans were assessed at 6 months after surgery. The extent of the resection was calculated as the fraction (%) of the difference between the preoperative and postoperative volume divided by the preoperative volume. The histological type of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma) and the World Health Organization (WHO) grade (2007) were determined by a neuropathologist, from tissue obtained during the tumor resection.
2.3. Neuropsychological Assessment
Pre- and postoperatively, we administered an extensive neuropsychological test-protocol (Table 2). Language tests are as follows: Boston Naming Test (BNT) or object naming (DuLIP), action naming, category and letter fluency, and Aachen Aphasia Test (AAT) subtests: repetition, writing to dictation, reading aloud, and Token Test. Memory tests are as follows: 15-word test (imprinting, recall); digit span. Attentional and executive functions tests are as follows: design fluency, Trail Making Tests A and B, and Stroop Color Word Tests I–III. Based on the normative data, z-scores were computed to compare performance of the patients to healthy controls. A clinical impairment is reflected by a z-score between −1.5 and −2; a pathological impairment is reflected by a z-score of ≥−2. Postoperatively, P1 and P2 were tested at 6 weeks and 6 months and P3 and P4 at 3 months and 1 year. The study was approved by the Ethical Committee of Erasmus MC Rotterdam and University of Brussels. All patients gave written informed consent.
Table 2.
Neuropsychological assessment.
| Cognitive abilities and description of task | |
|---|---|
| Language tests | |
| AAT [17] | |
| Repetition | Repeating phonemes, words, and sentences |
| Writing to dictation | Writing words and sentences on dictation |
| Reading out loud | Reading aloud words and sentences |
| Token Test | Comprehension of, pointing to, and manipulating geometric forms |
| Boston Naming Test [18] | Naming 60 pictures, presented in order of word frequency and word difficulty |
| Category fluency | Flexibility of verbal semantic thought: categories (e.g., animals) (within 1 min) |
| Letter fluency | Flexibility of verbal phonological thought: letters D, A, and T (within 1 min) |
| DuLIP [16] | |
| Syntactic fluency | Flexibility of verbal grammatical thought, producing verbs (within 1 min) |
| Object naming1 | Word finding: naming objects |
| Action naming | Word finding, grammar: naming actions |
| Memory tests | |
| Digit span for/backward [19] | Verbal learning of digits: repeating the list of digits forward/backward |
| 15-word test [19] | Verbal learning of words |
| Learning | Immediate recall: learning a list of 15 words, immediate recall for 5 times |
| Recall | Delayed recall: learning a list of 15 words, 1 delayed recall |
| Recognition | Delayed recognition: 1 delayed recognition out of 30 words |
| Attentional & executive tests | |
| Trail Making Test (TMT) [19] | |
| Trail Making Test A | Visuomotor speed, attention: connecting numbers in ascending order |
| Trail Making Test B | Divided attention/mental flexibility: connecting alternating numbers and letters |
| Stroop Color Word Test [19] | |
| Stroop I | Mental speed, selective attention: reading color words |
| Stroop II | Mental speed, selective attention: naming colors |
| Stroop III | Mental speed, selective attention: naming colors of printed words denoting another color |
| Design fluency [20] | Nonverbal fluency, attention, motor speed, visuoperceptual and constructional abilities |
1Flanders: object naming from DuLIP, the Netherlands: Boston Naming Test.
3. Results
Tumor volume ranged from 1.46 cm3 to 108 cm3. Pathological examination of tumor tissue obtained during resection revealed a LGG (WHO grade II) in P1 and P3 and a HGG in P2 (WHO grade IV) and in P4 (WHO grade III). The extent of resection (EoR) ranged from 58 to 89%. P1, P2, and P4 underwent postoperative radiotherapy (33 fraction doses of 1.8 Gy). All patients used anticonvulsants pre- and postoperatively (see Table 2).
3.1. Neuropsychological Assessment: Pre-, Intra-, and Postoperative Course (See Table 3)
Table 3.
Pre- and postoperative neuropsychological test-scores P1–P4 (a–d). w = weeks, m = months, and y = years. ∗∗Pathological (severe) impairment: z-score ≤−2 (marked with bold). ∗Clinical (mild) impairment: z-score ≤−1.5 (marked with italic).
(a).
| P1 | |||
|---|---|---|---|
| Preoperative results | Postoperative results (6 w) |
Postoperative results (6 m) |
|
| Language | |||
| Object naming (DuLIP) | 0.13 | 0.63 | 0.63 |
| Action naming (DuLIP) | 0.76 | 0.76 | 0.76 |
| Category fluency | 0.10 | −1.00 | 0.45 |
| Letter fluency | 1.10 | −0.10 | 0.50 |
| AAT Token Test | 0.83 | 0.83 | 0.83 |
| Memory | |||
| 15 WT imprinting | −0.10 | −1.60 ∗ | 0.10 |
| 15 WT recall | −0.60 | −0.60 | −0.20 |
| Digit span | 0.67 | 0.33 | 1.00 |
| Attention/executive functions | |||
| Design fluency productivity | 2.05 | 1.41 | 2.33 |
| Design fluency flexibility | 1.28 | 0.58 | 0.67 |
| Design fluency strategy | 1.08 | 2.05 | 1.28 |
| TMTA | 1.10 | 1.20 | 1.60 |
| TMTB | 1.40 | 1.70 | 2.10 |
| TMTBA | 0.90 | 1.20 | 1.40 |
| Stroop I | −0.30 | −0.40 | −0.20 |
| Stroop II | 1.50 | 1.20 | 1.80 |
| Stroop III | 1.60 | 2.40 | 1.90 |
| Stroop interference | 1.00 | 2.30 | 1.20 |
(b).
| P2 | |||
|---|---|---|---|
| Preoperative results | Postoperative results (6 w) | Postoperative results (6 m) | |
| Language | |||
| Object naming (DuLIP) | −0.64 | −6.28 ∗∗ | −4.40 ∗∗ |
| Action naming (DuLIP) | −0.80 | −2.94 ∗∗ | −1.37 |
| Category fluency | −1.20 | −2.50 ∗∗ | −2.30 ∗∗ |
| Letter fluency | −1.90 ∗ | −2.70 ∗∗ | −2.40 ∗∗ |
| AAT Token Test | −5.35 ∗∗ | −10.81 ∗∗ | −5.35 ∗∗ |
| Memory | |||
| 15 WT imprinting | −0.64 | −3.90 ∗∗ | −3.50 ∗∗ |
| 15 WT recall | −0.80 | −3.10 ∗∗ | −2.70 ∗∗ |
| Digit span | −1.20 | −3.00 ∗∗ | −3.00 ∗∗ |
| Attention/executive functions | |||
| Design fluency productivity | 0.13 | −1.88 ∗ | −2.05 ∗∗ |
| Design fluency flexibility | −0.25 | −1.13 | 0.00 |
| Design fluency strategy | 0.84 | −0.47 | 0.25 |
| TMTA | 0.20 | −4.10 ∗∗ | −1.50 ∗ |
| TMTB | −1.60 ∗ | −4.30 ∗∗ | −3.40 ∗∗ |
| TMTBA | −1.90 ∗ | −2.70 ∗∗ | −3.10 ∗∗ |
| Stroop I | −2.00 ∗∗ | −5.40 ∗∗ | −5.00 ∗∗ |
| Stroop II | −2.20 ∗∗ | −4.40 ∗∗ | −5.10 ∗∗ |
| Stroop III | −1.90 ∗ | −3.40 ∗∗ | −3.40 ∗∗ |
| Stroop interference | −0.60 | −1.20 | −0.30 |
(c).
| P3 | |||
|---|---|---|---|
| Preoperative results | Postoperative results (3 m) | Postoperative results (1 y) | |
| Language | |||
| Boston Naming Test | −0.60 | −0.27 | −1.27 |
| Category fluency | 1.10 | 1.57 | −0.46 |
| Letter fluency | 0.00 | 0.54 | 1.08 |
| AAT Token Test | −0.10 | −0.83 | −0.83 |
| AAT repetition | 1.39 | 0.83 | 0.83 |
| AAT reading aloud | 0.54 | 0.54 | 0.54 |
| AAT writing to dictation | 0.54 | 0.54 | 0.54 |
| Memory | |||
| 15 WT imprinting | −1.40 | −0.40 | 0.40 |
| 15 WT recall | −1.60 ∗ | −0.50 | 1.00 |
| Attention/executive functions | |||
| TMTA | 0.50 | 1.10 | 1.00 |
| TMTB | −2.40 ∗∗ | 0.30 | −0.20 |
| TMTBA | −3.00 ∗∗ | −0.40 | −0.90 |
| Stroop I | 0.60 | 0.60 | 1.10 |
| Stroop II | −0.30 | −0.30 | 0.70 |
| Stroop III | 0.70 | 0.10 | 1.10 |
| Stroop interference | 1.10 | 0.30 | 0.80 |
(d).
| P4 | |||
|---|---|---|---|
| Preoperative results | Postoperative results (3 m) | Postoperative results (1 y) | |
| Language | |||
| Boston Naming Test | −0.21 | −0.74 | 0.05 |
| Category fluency | 0.75 | −1.16 | 0.73 |
| Letter fluency | −1.08 | −1.26 | −1.44 |
| AAT Token Test | −0.47 | 0.99 | −0.10 |
| AAT repetition | 0.28 | −4.17 ∗∗ | −1.39 |
| AAT reading aloud | −0.49 | −0.49 | 0.54 |
| AAT writing to dictation | 0.27 | −0.27 | 0.00 |
| Memory | |||
| 15 WT imprinting | −1.30 | 1.50 | −0.10 |
| 15 WT recall | −0.70 | 0.00 | 0.40 |
| Attention/executive functions | |||
| TMTA | 0.70 | 0.00 | 0.90 |
| TMTB | −0.60 | −1.40 | 0.00 |
| TMTBA | −1.10 | −1.60 ∗ | −0.60 |
| Stroop I | 0.80 | −0.90 | −0.30 |
| Stroop II | 1.10 | 0.00 | −0.50 |
| Stroop III | 1.20 | −0.50 | −0.30 |
| Stroop interference | 0.60 | −0.70 | 0.00 |
3.1.1. P1: Low-Grade Glioma in “Wernicke's” Area and Near AF
Preoperatively, the cognitive functions of P1 were intact (z ≥ −1.5). During operation, speech arrest occurred after stimulation at the precentral gyrus (primary motor cortex) at the level of the mouth. Postoperatively, at 6 weeks a clinical deficit in a memory test was observed (15 WT imprinting; z = −1.60) which recovered at 6 months. No other cognitive deficits were observed (z ≥ −1.5).
3.1.2. P2: High-Grade Glioma in “Wernicke's” Area Near AF
Preoperatively, P2 had clinically or pathologically significant impairments in language (letter fluency; z = −1.90, AAT Token Test; z = −5.35), and attention and executive deficits (TMT B: z = −1.60, TMT BA: z = −1.90, Stroop I: z = −2.00, Stroop II: z = −2.20, and Stroop III: z = −1.90). During surgery cortical stimulation in the posterior superior temporal gyrus/angular gyrus triggered speech arrest. During stimulation of the AF, phonemic paraphasia occurred; these increased during resection near the AF, at which point resection was terminated. At 6 weeks postoperatively new deficits were found in language (object naming: z = −6.28, action naming: z = −2.94, and category fluency: z = −2.50), in memory (15 WT imprinting: z = −3.90, 15 WT recall: z = −3.10, and digit span: z = −3.00), and in attention and executive functions (design fluency: productivity: z = −1.88, TMT A: z = −4.10). There was an increase of the preoperative deficits in language (letter fluency: z = −2.70, AAT Token Test: z = −10.81) and in attention and executive functions (TMT B: z = −4.30, TMT BA: z = −2.70, Stroop I: z = −5.40, Stroop II: z = −4.40, and Stroop III: z = −3.40). At 6 months postoperatively, improvement was observed in 1 subtest within the language domain (action naming: z = −1.37) and in the attention and executive functions (TMT A: z = −1.50), but further deterioration was found in another subtest (design fluency: productivity: z = −2.05).
3.1.3. P3: Low-Grade Glioma in “Broca's” Area and Near AF
Preoperatively P3 was clinically impaired in memory (15 WT recall: z = −1.60) and had selective pathological impairments in executive functioning (TMT B: z = −2.40, TMT BA: z = −3.00). During surgery speech arrest occurred with stimulation of the inferior frontal gyrus, below the motor cortex, and the parietal lobe (see Figure 3). Phonemic paraphasia and neologisms were elicited at the temporoparietal junction. At the subcortical level near the AF also phonemic paraphasia was elicited. At the motor cortex, stimulation triggered dysarthria and contraction of the tongue. At the end of resection, perseverations occurred at which point resection was terminated (see Figure 4). Postoperatively at 3 months, the patient had recovered from the observed preoperative impairments in memory (15 WT recall: z = −0.50) and executive functioning (TMT B: z = 0.30, TMT BA: z = −0.40), which remained stable during the follow-up of 1 year (15 WT: z = 1.00, TMT B: z = −0.20, and TMT BA: z = −0.90). No other impairments were present.
Figure 3.
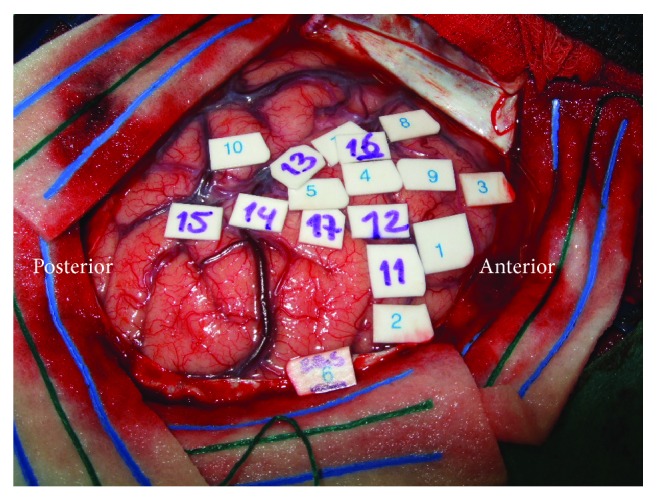
Intraoperative mapping P3. Cortical positive sites: speech arrest (1, 5, 7–9, and 13-14), dysarthria (2–4), neologism (10, 15), phonemic paraphasia (10, 15), and contraction of tongue (11-12, 16-17).
Figure 4.
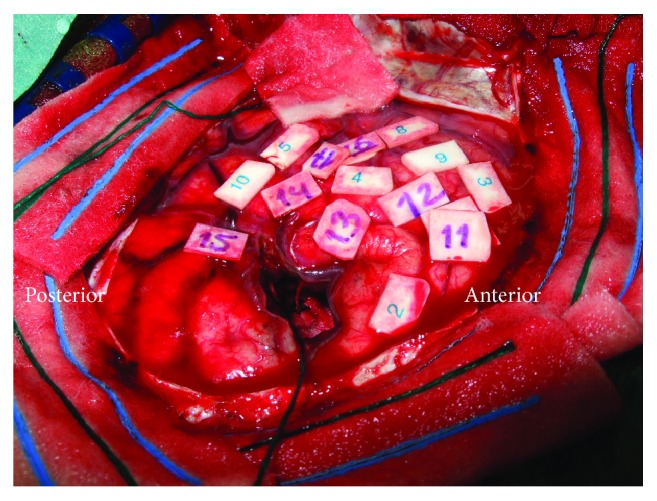
Resection cavity P3.
3.1.4. P4: High-Grade Glioma in “Wernicke's” Area with Extension to “Broca's” Area and Near AF
Preoperatively, P4 had no cognitive disorders. During surgery speech arrest was found when the inferior frontal gyrus was stimulated, below the motor cortex and in the temporal lobe. Phonemic paraphasia was elicited in the parietal lobe. Resection was terminated when perseverations occurred. At 3 months postoperatively, P4 developed a deficit in language (AAT repetition: z = −4.17) which had recovered at 1 year (z = −1.39) and in attention and executive functions (TMT BA: z = −1.60) which also recovered at 1 year (z = −0.60).
4. Discussion
A detailed examination of cognitive functions was conducted in 4 patients with brain tumors in or near “classical” language areas Broca, Wernicke, and the AF before and after awake surgery. Given the tumor localization it is remarkable that only in one patient (P2) a language disorder was present preoperatively. Our study revealed mixed cognitive profiles at pre- and postoperative time-points. Two patients (P1 and P4) showed relatively intact cognitive performance, which may be explained by neural plasticity (i.e., reorganization of functions). By contrast, the other two cases (P2 and P3) demonstrated impairments in several cognitive domains pre- and/or postoperatively. These different findings are in line with a “dynamic” or “hodotopical” brain as opposed to a “static” or “topological” viewpoint [6]. Several factors may be related to the plastic potential of the brain such as different corticosubcortical networks (localization), tumor grade (low versus high), tumor volume, EoR, and the use of anticonvulsants and/or adjuvant therapy with irradiation.
P1 with a tumor in Wernicke's area appeared to have intact cognition, apart from a temporary clinical memory deficit at 6 weeks postoperatively. Intact cognition after glioma surgery in Wernicke's area has been reported previously [21]. By contrast, a multicognitive disturbed profile at pre- and postoperative level was found in P2 who had a very similar tumor localization. This implies that the “classical” language area Wernicke can also be related to other cognitive functions or that cognitive functions are disturbed when language is impaired (partly) in line with the model proposed by Coello et al. [22]. However, it is not possible to make strong assumptions about the interdependency of deficits in different cognitive domains. In P2, a simultaneous decline of language, memory, and executive functions at T2 illustrates this phenomenon where both verbal and nonverbal tasks deteriorated (verbal fluency and design fluency). Surprisingly, P3 with a tumor near Broca's area did not suffer from preoperative language deficits probably due to functional reorganization. Instead, impairments in the domains of memory and executive functions were observed which recovered within 3 months. P4 with a large tumor extending to Broca's and Wernicke's area had generally intact language performance, again in contrast with the “classical” language model, apart from a temporary decline on a repetition task at 3 months after surgery. Recently, the sensitivity of a repetition task was demonstrated in the intraoperative stimulation setting especially in or near the AF [23]. In all our patients the tumor was also located in or near the AF. Surgery in this area can cause a decline in phonological language performance [24]. A variety of pre- and postoperative cognitive disturbances in our patients demonstrate that this subcortical tract (AF) is not only associated with phonology. This has previously been observed in patients with lesions with a different etiology [25]. Hence, preservation of AF during surgery appears to be mandatory for the surveillance of (further) cognitive decline. Despite the detection of intraoperative language positive sites in all patients, different postoperative cognitive outcomes were observed. Tumor resection in proximity of a language positive site, but also preoperative language deficits, can be a risk factor for postoperative aphasia [26].
Apart from localization and the intraoperative procedure, the differential pre- and postoperative cognitive profiles in our patients could be attributed to tumor related factors, such as tumor grade. Noll et al. [27] found that patients with grade IV gliomas present with poorer preoperative cognitive performance (verbal learning, processing speed, executive functioning, and language) than patients with lower-grade gliomas (II, III). These differences were not related to tumor size, seizure status, and anticonvulsants or steroid use which points to evidence of a so-called “lesion momentum”: faster growing tumors may be associated with more severe cognitive impairments. Our results are partly consistent with this line of reasoning. P2 with a high-grade glioma showed a preoperative disturbed cognitive profile (deficits in language, memory, and attention/executive functioning). Preserved cognitive functions in P1 were possibly facilitated by the slow growth rate of a low-grade tumor allowing “typical” functional reorganization (i.e., 4 mm p/y), that is, preoperative cognitive plasticity. A faster growth rate of a high-grade tumor, as in P2, could have more aggressively affected these preoperative cognitive functions. However, results in P3 and P4 do not concur with this hypothesis: P3 with a LGG presented disturbances of memory, attention, and executive functions whereas P4 with a HGG demonstrated overall intact cognitive performance. It is possible in this case (P4) that, due to fast tumor growth, mainly suppression of functional areas occurs, whereas the integrity of white matter bundles associated with function remains intact. Herbet et al. [28] showed via a probabilistic atlas that reorganization at subcortical level, in proximity to white matter tracts, could be less optimal than at cortical level. In addition, Trinh et al. [29] demonstrated that a subcortical injury was an independent predictor for longer-term neurological impairments underlining the importance of preservation of subcortical tracts. It may also be possible that genetic tumor mutation is associated with cognition: IDH1-mutant wild-type (isocitrate dehydrogenase), more aggressive than IDH1-mutant tumors, appeared to be associated with more severe cognitive impairments possibly hindering neuroplasticity [30].
Another intervening factor influencing preoperative cognitive performance could be tumor volume. Habets et al. [13] found that larger brain tumors in the left hemisphere were associated with poorer executive functioning. This explanation may hold for P2 who has a relatively large preoperative tumor volume and is suffering from more serious cognitive deficits compared to P1 with a smaller tumor and intact cognition. However, P3 and P4 showed the reversed pattern, with P4 having a larger tumor with intact cognitive performance and P3 with a smaller tumor and deficits.
EoR may have played a role as P2 and P4 underwent a more extensive tumor resection than P1 and P3. However, a recent follow-up study did not reveal a relation between EoR and cognitive decline [5]. Currently, there is only evidence that a more extensive resection is associated with longer survival in both LGG and HGG patients [31]. In addition, in all patients resection was conducted according to individual subcortical functional boundaries.
In general, (stimulation-induced) seizures and the use of anticonvulsants can be a risk factor for deficits in cognitive performance [32]. Deficits in information processing, attention, and executive functions were found to be related to the use of anticonvulsants in long-term glioma survivors (at least 1 year after diagnosis) in the absence of seizures [33]. In our patients, the use of medication may have added to cognitive defects or postoperative decline. However, all patients took anticonvulsants both before and after surgery, which makes it hard to draw any firm conclusions. Apart from antiepileptic drugs, radiotherapy may also have had negative effects on cognitive performance [34]. P1, P2, and P4 were treated with radiotherapy, of whom P2 and P4, but not P1, showed postoperative cognitive deterioration during the administration of irradiation. In addition, in all patients a “safe” fraction dose of maximally 2 Gy per session was administered, which is known to be associated with relative stable cognition for several years after irradiation [35].
Finally, some other factors should be taken into account when interpreting our results. Handedness may have interfered with the results, as P1 was left-handed as opposed to P2– P4. Language organization in left-handed people is not always consistent and can be represented in a more widespread network than in right-handed people [36]. However, all patients had tumors in the language dominant hemisphere as attested with fMRI. The detection of crossed cerebellar activation may add to the identification of language lateralization in (left-handed) brain tumor patients [37]. From these 4 cases, it is clear that cognitive functions cannot be related to a certain location in the brain. Unfortunately, we do not know when and until which period improvement of specific cognitive functions exactly takes place. Follow-up measurements were not administered at similar time-points in all patients, namely, 6 weeks or 3 months for early and 6 months or 1 year for late follow-up. However, a recent outcome study found that the postoperative interval of 3 and 6 months is crucial for language improvement, whereas recovery of the executive functions appeared to take longer than 6 months [38]. Evidently, larger subgroups with patients with a comparable brain tumor localization and, for instance, tumor grade need to be analyzed to investigate the different courses that underlie functional neural plasticity. No postoperative fMRI and diffusion tensor imaging studies were available; therefore, it is difficult to account for reorganization at both the structural and functional level.
Patients with brain tumors in “classical” language areas do not necessarily present language (or other cognitive) disturbances. Surgery can either improve or deteriorate (existing) cognitive (impairments) functions. The findings of these case studies provide therefore further support for neural plasticity within a “hodotopical” framework. It remains uncertain to which extent and which factors, such as localization, tumor grade, volume, EoR, and/or adjuvant therapy, contribute to neural plasticity. Hence, an extensive examination of cognitive functions with larger (sub)groups taking into account localization, tumor, and treatment related factors will elucidate prognostic factors of the plastic potential of the brain.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.De Witt Hamer P. C., Robles S. G., Zwinderman A. H., Duffau H., Berger M. S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. Journal of Clinical Oncology. 2012;30(20):2559–2565. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 2.Duffau H. Surgery of low-grade gliomas: towards a ‘functional neurooncolngy’. Current Opinion in Oncology. 2009;21(6):543–549. doi: 10.1097/cco.0b013e3283305996. [DOI] [PubMed] [Google Scholar]
- 3.Talacchi A., Santini B., Savazzi S., Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. Journal of Neuro-Oncology. 2011;103(3):541–549. doi: 10.1007/s11060-010-0417-0. [DOI] [PubMed] [Google Scholar]
- 4.Papagno C., Casarotti A., Comi A., Gallucci M., Riva M., Bello L. Measuring clinical outcomes in neuro-oncology. A battery to evaluate low-grade gliomas (LGG) Journal of Neuro-Oncology. 2012;108(2):269–275. doi: 10.1007/s11060-012-0824-5. [DOI] [PubMed] [Google Scholar]
- 5.Satoer D., Visch-Brink E., Smits M., et al. Long-term evaluation of cognition after glioma surgery in eloquent areas. Journal of Neuro-Oncology. 2014;116(1):153–160. doi: 10.1007/s11060-013-1275-3. [DOI] [PubMed] [Google Scholar]
- 6.Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325–337. doi: 10.1016/j.cortex.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Tate M. C., Herbet G., Moritz-Gasser S., Tate J. E., Duffau H. Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain. 2014;137, part 10:2773–2782. doi: 10.1093/brain/awu168. [DOI] [PubMed] [Google Scholar]
- 8.Bizzi A., Nava S., Ferrè F., et al. Aphasia induced by gliomas growing in the ventrolateral frontal region: Assessment with diffusion MR tractography, functional MR imaging and neuropsychology. Cortex. 2012;48(2):255–272. doi: 10.1016/j.cortex.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Mandonnet E., Delattre J.-Y., Tanguy M.-L., et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Annals of Neurology. 2003;53(4):524–528. doi: 10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- 10.Desmurget M., Bonnetblanc F., Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130(4):898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- 11.Satoer D., Vincent A., Smits M., Dirven C., Visch-Brink E. Spontaneous speech of patients with gliomas in eloquent areas before and early after surgery. Acta Neurochirurgica. 2013;155(4):685–692. doi: 10.1007/s00701-013-1638-8. [DOI] [PubMed] [Google Scholar]
- 12.Satoer D., Visch-Brink E., Dirven C., Vincent A. Glioma surgery in eloquent areas: can we preserve cognition? Acta Neurochirurgica. 2016;158(1):35–50. doi: 10.1007/s00701-015-2601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habets E. J. J., Kloet A., Walchenbach R., Vecht C. J., Klein M., Taphoorn M. J. B. Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochirurgica. 2014;156(8):1451–1459. doi: 10.1007/s00701-014-2115-8. [DOI] [PubMed] [Google Scholar]
- 14.Miotto E. C., Junior A. S., Silva C. C., et al. Cognitive impairments in patients with low grade gliomas and high grade gliomas. Arquivos de Neuro-Psiquiatria. 2011;69(4):596–601. doi: 10.1590/S0004-282X2011000500005. [DOI] [PubMed] [Google Scholar]
- 15.Heimans J. J., Taphoorn M. J. B. Impact of brain tumour treatment on quality of life. Journal of Neurology. 2002;249(8):955–960. doi: 10.1007/s00415-002-0839-5. [DOI] [PubMed] [Google Scholar]
- 16.De Witte E., Satoer D., Robert E., et al. The Dutch linguistic intraoperative protocol: a valid linguistic approach to awake brain surgery. Brain and Language. 2015;140:35–48. doi: 10.1016/j.bandl.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Graetz S., De Bleser P., Willmes K. Akense Afasie Test. Dutch edition. Lisse: Swets & Zeitlinger; 1991. [Google Scholar]
- 18.Kaplan E., Goodglass H., Weintraub S. Boston Naming Test. Philadelphia-Tokyo: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- 19.Lezak M. D. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 20.Goebel S., Fischer R., Ferstl R., Mehdorn H. M. Normative data and psychometric properties for qualitative and quantitative scoring criteria of the Five-point Test. Clinical Neuropsychologist. 2009;23(4):675–690. doi: 10.1080/13854040802389185. [DOI] [PubMed] [Google Scholar]
- 21.Sarubbo S., Latini F., Sette E., et al. Is the resection of gliomas in Wernicke's area reliable? Wernicke's area resection. Acta Neurochirurgica. 2012;154(9):1653–1662. doi: 10.1007/s00701-012-1416-z. [DOI] [PubMed] [Google Scholar]
- 22.Coello A. F., Moritz-Gasser S., Martino J., Martinoni M., Matsuda R., Duffau H. Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks: A review. Journal of Neurosurgery. 2013;119(6):1380–1394. doi: 10.3171/2013.6.JNS122470. [DOI] [PubMed] [Google Scholar]
- 23.Sierpowska J., Gabarros A., Fernandez-Coello A., et al. Words are not enough: nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection. Journal of Neurosurgery. 2016:1–11. doi: 10.3171/2016.2.JNS151592. [DOI] [PubMed] [Google Scholar]
- 24.Sarubbo S., De Benedictis A., Merler S., et al. Towards a functional atlas of human white matter. Human Brain Mapping. 2015;36(8):3117–3136. doi: 10.1002/hbm.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauschecker A. M., Deutsch G. K., Ben-Shachar M., Schwartzman A., Perry L. M., Dougherty R. F. Reading impairment in a patient with missing arcuate fasciculus. Neuropsychologia. 2009;47(1):180–194. doi: 10.1016/j.neuropsychologia.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilmberger J., Ruge M., Kreth F.-W., Briegel J., Reulen H.-J., Tonn J.-C. Intraoperative mapping of language functions: A longitudinal neurolinguistic analysis - Clinical article. Journal of Neurosurgery. 2008;109(4):583–592. doi: 10.3171/JNS/2008/109/10/0583. [DOI] [PubMed] [Google Scholar]
- 27.Noll K. R., Sullaway C., Ziu M., Weinberg J. S., Wefel J. S. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro-Oncology. 2014;17(4):580–587. doi: 10.1093/neuonc/nou233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbet G., Maheu M., Costi E., Lafargue G., Duffau H. Mapping neuroplastic potential in brain-damaged patients. Brain. 2016;139(3):829–844. doi: 10.1093/brain/awv394. [DOI] [PubMed] [Google Scholar]
- 29.Trinh V. T., Fahim D. K., Shah K., et al. Subcortical injury is an independent predictor of worsening neurological deficits following awake craniotomy procedures. Neurosurgery. 2013;72(2):160–169. doi: 10.1227/NEU.0b013e31827b9a11. [DOI] [PubMed] [Google Scholar]
- 30.Wefel J. S., Noll K. R., Rao G., Cahill D. P. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncology. 2016 doi: 10.1093/neuonc/now165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanai N., Berger M. S. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 32.Meador K. J. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002;58(8):S21–S26. doi: 10.1212/WNL.58.8_suppl_5.S21. [DOI] [PubMed] [Google Scholar]
- 33.Klein M., Engelberts N. H. J., Van der Ploeg H. M., et al. Epilepsy in low-grade gliomas: The impact on cognitive function and quality of life. Annals of Neurology. 2003;54(4):514–520. doi: 10.1002/ana.10712. [DOI] [PubMed] [Google Scholar]
- 34.Taphoorn M. J. B., Klein M. Cognitive deficits in adult patients with brain tumours. The Lancet Neurology. 2004;3(3):159–168. doi: 10.1016/s1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 35.Douw L. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 36.Szaflarski J. P., Binder J. R., Possing E. T., McKiernan K. A., Ward B. D., Hammeke T. A. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–244. doi: 10.1212/WNL.59.2.238. [DOI] [PubMed] [Google Scholar]
- 37.Méndez Orellana C., Visch-Brink E., Vernooij M., et al. Crossed Cerebrocerebellar Language Lateralization: An Additional Diagnostic Feature for Assessing Atypical Language Representation in Presurgical Functional MR Imaging. American Journal of Neuroradiology. 2015;36(3):518–524. doi: 10.3174/ajnr.A4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Witte E. Speaking the language of the brain. Aneurolinguistic approach to the assessment of braintumour patients undergoing awake surgery. Doctoral Dissertation [Doctoral, thesis] Brussels University Press: VUBPRESS; 2015. [Google Scholar]