Abstract
Background
The aim was to study changes in immunohistochemical expression markers of synovial and skin inflammation, clinical outcomes and magnetic resonance imaging (MRI) scores with abatacept treatment in patients with psoriatic arthritis (PsA).
Methods
Biological-treatment-naïve PsA patients with active disease including synovitis of a knee were enrolled in this single-centre, crossover study. Patients were randomised to receive intravenous abatacept 3 mg/kg of body weight or placebo infusion on day 1, 15 and 29; thereafter abatacept 10 mg/kg of body weight was administered every 28 days for 5 months. Clinical data were collected at each visit. Synovial biopsy of the involved knee was obtained at baseline and 2 and 6 months. MRI of the same knee and skin biopsy was performed prior to arthroscopy.
Results
Fifteen patients were recruited. Significant improvements in the joint-related measures were observed; 90% were European League Against Rheumatism criteria responders and 30% achieved psoriasis area severity index (PASI)50 at 6 months. Reduction in synovitis (P = 0.016) and vascularity (P = 0.039) macroscopic scores consistent with decrease in total MRI score (P = 0.016) were noticed. Abatacept decreased the immunohistological expression of FOXP3+ cells (P = 0.027), specifically the expression of CD4+FOXP3+ regulatory T cells (Tregs) (P = 0.008) in the synovium over 6 months. There was no significant clinical or immunohistological change in any of the skin measures.
Conclusion
This is the first study assessing synovial and psoriatic skin immunpathological changes following abatacept treatment in PsA. Reduction in Treg expression in the synovium but not in the psoriatic lesion suggests abnormal Treg function in PsA with differential suppressive capacity in the synovium compared to the lesional skin. The results of this study demonstrate that abatacept 10 mg/kg of body weight might be an effective treatment option for joint disease in patients with PsA.
Trial registration
Irish Health Products Regulatory Authority. Trial registration number: CT 900/489/1 – Abatacept (case number: 2077284, EudraCT Number: 2009-017525-19, Protocol number: 77777). Registered on 12 March 2010.
Keywords: Abatacept, Psoriatic arthritis, Synovium, Skin, Regulatory T-cell, FOXP3
Background
Psoriatic arthritis (PsA) is a chronic immune-mediated inflammatory disease that affects peripheral joints, entheses and axial sites along with skin and nails. In addition to the characteristic extra-articular features, such as uveitis and inflammatory bowel disease, PsA is also associated with comorbidities [1, 2].
Interplay between the adaptive and innate immune system, activation of T cells and subsequent production of inflammatory cytokines such as TNF, interferon (IFN)-γ, IL-17 and IL-22 have a dominant pathogenic role in psoriatic plaques [3, 4]. In PsA, CD8+ T cells may represent key players that recognise antigens presented by human leukocyte antigen (HLA) class 1 molecules [5]. CD8+ T cells are found in greater numbers in synovial fluid and in entheseal sites while CD4+ T cells are more abundant in synovial tissue [5–7].
Regulatory T cells (Tregs) are a subset of CD4+ CD25high T lymphocytes that require forkhead box transcription factor (FOXP3) for their suppressive function [4, 8, 9]. Tregs derived from the thymus mediate suppression through a contact-dependent mechanism including cytotoxic T-lymphocyte antigen 4 (CTLA-4) [10]. Increased Treg expression compared to normal skin has been reported in psoriatic lesions, irrespective of the disease severity. Previous studies indicated an abnormal phenotype and impaired function of synovial Tregs in rheumatoid arthritis (RA) and increased FOXP3 expression in synovial CD4+ T cells in rheumatoid factor (RF)-negative RA and PsA compared to seropositive RA [10–12]. Tregs isolated from peripheral blood and psoriatic skin have dysfunctional activity in suppressing effector T-cell responses in psoriasis [13–16].
Due to the heterogeneity of PsA, identifying appropriate treatment for each individual can be challenging. Patients with peripheral arthritis that does not respond to disease-modifying antirheumatic drugs (DMARDs) are recommended for treatment with apremilast or biologic agents (TNFi, IL-12/23i and IL17i), IL-12/23i and IL17i) are recommended [17]. Since not all patients respond to these agents and many patients’ initial responses wane over time due to immunogenicity, poor tolerability and/or adverse events, it has been important to develop new therapies that target other key cytokines and immunologic pathways [18]. Targeting T cells, the main mediators of the pathogenic processes in PsA, can potentially have a significant role in the future immunotherapy of the disease.
Abatacept is a soluble, fully human fusion protein consisting of the extracellular domain of CLTA-4 linked to human IgG1 [19]. Abatacept selectively binds to CD80/86 on antigen-presenting cells blocking the costimulatory interaction with CD28 on T-cells, and thereby inhibits optimal T-cell activation and decreases production of inflammatory cytokines such as TNFα, INFγ, IL-2 and IL-6 [20–22]. Abatacept is approved in RA and juvenile idiopathic arthritis [23, 24]. One phase 2 randomised controlled trial of abatacept in PsA showed significant American College of Rheumatologists (ACR)20 responses, but only modest improvement in skin measures compared to placebo (PBO) [25]. More recently, a randomised, placebo-controlled, phase 3 trial of abatacept in PsA reported benefits of abatacept in ACR20 responses, regardless of TNFi exposure, with modest benefit on psoriasis lesions [26].
A few studies have evaluated synovial changes after different therapies in PsA [27], but the effect of abatacept on synovial and skin tissue has not been previously studied. Therefore, our aim was to study changes in immunohistochemical markers of synovial and skin inflammation, including FOXP3 expression, after introducing abatacept treatment in PsA. Secondary objectives were to (1) assess clinical outcomes and magnetic resonance imaging (MRI) changes over time, (2) evaluate the impact of a short period of abatacept 3 mg/kg of body weight treatment compared to PBO and (3) investigate if cell markers of synovial inflammation correlate with disease activity measures and MRI synovitis scores.
Methods
Patients
PsA patients fulfilling the classification criteria for psoriatic arthritis (CASPAR) [28] with active disease of ≥3 months duration (tender joint count ≥3 and swollen joint count ≥3), aged 18 to 80 years were recruited. Patients had to have clinical synovitis of the knee and have agreed to undergo synovial biopsies. Presence of a psoriatic skin lesion was not requisite. Participants must not have been exposed to a previous biologic DMARD. Methotrexate (MTX) was the only concomitant synthetic DMARD (sDMARD) permitted in the study. Patients who were on a stable dose of MTX (7.5–25 mg/week) for >3 months prior to randomisation remained on this stable dose for the duration of the study. A decrease in the MTX dosage was allowed in cases of toxicity. The use of a stable dose of prednisolone <10 mg/day or its equivalent, and of a nonsteroidal anti-inflammatory drug (NSAID) was permitted during the study. Decrease in NSAIDs was permitted but only if due to toxicity.
Key exclusion criteria were concurrent autoimmune diseases or skin conditions other than plaque psoriasis, arthritis mutilans, pregnancy, breast feeding, evidence of latent or active tuberculosis, evidence of chronic or clinically significant infection or malignancy.
Informed, written consent was obtained from all study participants according to the Declaration of Helsinki. The study was approved by St. Vincent’s Healthcare Group Ethics and Medical Research Committee.
Study design and treatment
This study was a single-centre, placebo-controlled, crossover study of 15 patients with PsA. Patients were randomised to receive abatacept 3 mg/kg of body weight (approved dosing for RA) or PBO infusions on day 1, 15 and 29; thereafter abatacept 10 mg/kg of body weight was administered every 28 days for 5 months (on day 57, 85, 113, 141 and 169). Treatments were administered as a 30-minute intravenous infusion; dosing was calculated based on body weight at screening (500 mg, 750 mg and 1000 mg for patients weighing <60 kg, 60–100 kg and >100 kg, respectively).
Clinical and laboratory assessments
Demographic data collected included age, sex, psoriasis and psoriatic arthritis disease duration, menopausal status, previous use of sDMARDs, current use of MTX, oral corticosteroids and calcium and vitamin D supplements. Clinical assessments were performed at each visit. Clinical variables included disease activity score in 28 joints (DAS28) with four variables (DAS28-erythrocyte sedimentation rate (ESR) and DAS28-C-reactive protein (CRP)), tender joint count (TJC) 28 and 68, swollen joint count (SJC) 28 and 66, number of digits with dactylitis, enthesitis score based on the Leeds enthesitis index (LEI), number of affected nails on the hands (0–10) and psoriasis area severity index (PASI) [29, 30]. Patients’ self-reported parameters such as duration of early morning stiffness (EMS), visual analogue scale for global health (GVAS), pain and fatigue scores, health assessment questionnaire (HAQ), dermatology life quality index (DLQI) and PsA-specific quality of life score (PsAQol) were recorded [30, 31]. Laboratory assessments included a recording of the following parameters: RF positivity, antibodies against cyclic citrullinated peptides (aCCP), ESR and CRP.
Efficacy and safety assessments
Joint and skin-related response to abatacept or PBO was assessed using the European League Against Rheumatism (EULAR) response criteria and a 50% improvement in the PASI score (PASI50) [32]. Safety evaluations were obtained at each visit including routine laboratory tests and monitoring of adverse events (AEs) for severity of the event (mild, moderate, severe or very severe), the investigator’s opinion of its relationship to abatacept/PBO treatment (not related, unlikely or possibly related) and information on outcomes.
Synovial and skin biopsies
Arthroscopy of the same involved knee joint was performed using a Wolf (Illinois, USA) 2.7-mm needle arthroscope under local anaesthesia at three time points (day 1, 57 and 169). Macroscopic synovitis and vascularity were scored on a visual analogue scale (VAS) (0–100 mm). Synovial membrane biopsies were obtained from at least six sites within the joint (suprapatellar pouch, medial and lateral gutters, infrapatellar and tibiofemoral regions and cartilage pannus junction near the patellar rim) using 1.5-mm grasping forceps [33, 34].
Under local anaesthesia, patients underwent a 6-mm-punch skin biopsy at three time points (day -28, 54 and 166). Biopsies were taken from the centre of a target psoriasis lesion from the same plaque [15].
Immunohistochemical analysis
Synovial and skin biopsies were embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura, Zoeterwoude, The Netherlands) and stored at -80 °C. Cryostat sections (7 μm) were mounted on slides coated with 3-aminopropyltriethoxysilane (Sigma-Aldrich St. Louis, MO, USA), air-dried overnight, wrapped in foil and stored at -80 °C. A routine three-stage immunoperoxidase labelling technique was used [35]. Tissue sections were thawed and acetone-fixed for 10 minutes. Immunostaining was performed on the Dako Autostainer 48 Link. Sections were processed using the Dako Envision FLEX Mouse Linker kit according to the manufacturer’s instructions. DAB was used as the chromogen and slides were counterstained with hematoxylin. The synovial and skin sections were incubated with mouse monoclonal antibodies against T-cell-specific markers CD3 (1:25), CD4 (1:20), CD8 (1:50), CD31 (Dako Ready to use Antibody, Clone numbers: CD3 – F7.2.38, CD4 – 4B12, CD8 – C8/144B, CD31 – JC70A) (Dako, Glostrup, Denmark), and FOXP3 (1:500) (Abcam, Cambridge, UK, Clone: 236A/E7). Sections were also incubated with an appropriate isotype-matched mouse monoclonal antibody (IgG1). Negative control slides omitting the secondary antibodies were also stained.
CD3, CD4 and CD8 expression in the biopsies were scored using a well-established semi-quantitative scoring method on a 5-point scale ranging from 0 to 4 (0 = no staining, 1 ≤ 25%, 2 = 25–50%, 3 = 50–75%, 4 ≥ 75% staining). For evaluation of CD4+ cells, only cells with lymphocyte morphology were included as CD4 can be expressed by macrophages [36, 37]. CD31-expressing microvessels were assessed under × 400 magnification by counting the number of vessels staining per five high power fields (HPF) on two levels when available (=10 HPF/slides). The result for one biopsy was extrapolated from two HPFs, due to the size of the tissue sample. Intranuclear staining for FOXP3 expression was assessed in a semi-quantitative manner as a percentage over 300 cells. (More specifically, 300 lymphocytes were counted at objective × 40 in “hot spots”, both in lymphoid aggregates and lymphoid infiltrates, and the number of FOXP3+ cells was divided by 3 to obtain a percentage; the result was then the average rounded up or down to the nearest whole number.)
Immunofluorescence staining of synovial tissue
To examine synovial tissue co-localization of CD4 with FOXP3, dual immunofluorescence staining was performed on cryostat synovial sections. Cryostat sections were defrosted at room temperature for 20 minutes and fixed in acetone for 10 minutes. Slides were washed in PBS for 5 minutes. Non-specific binding was blocked using 1% casein in PBS for 20 minutes. Slides were co-incubated with rabbit monoclonal antibody against CD4 (1:500, Abcam, Clone: EPR6855) and mouse monoclonal anti-FOXP3 antibody (1:500, Abcam, Clone: 236A/E7). IgG control antibodies were used as negative controls. Following overnight incubation in a humidified chamber at 4 °C, immunofluorescence sections were co-incubated with Alexa 488 goat anti-mouse superclonal™ secondary antibody (1:200, Invitrogen) and CyTM3-conjugated goat anti-rabbit secondary antibody (1:500, Jackson ImmunoResearch) for 60 minutes. Sections were mounted with Antifade (Molecular Probes) and assessed by immunofluorescence microscopy (Olympus BX51). The number of CD4+FOXP3+ Tregs at baseline and 6 months was scored manually by two independent readers on cryostat sections by identifying cells that had clear signals for co-expression of CD4 and FOXP3 on merged images.
Magnetic resonance imaging
MRI scan of the same involved knee was performed in each patient 3 days prior to arthroscopy at baseline, 2 and 6 months using a 1.5 T Signa Excite HD MRI scanner (GE Healthcare, UK) and a dedicated eight-channel array HD knee surface coil, with patients lying supine. The examinations performed included intravenous contrast (Gadolinium diethylenetriamine penta-acetic acid) enhanced, T1-weighted, fat-suppressed pulse sequences in coronal, sagittal and axial planes. The following scanning parameters were used: coronal, repetition time (TR) 640 ms; echo time (TE) 16; slice thickness 4/1 mm; field of view (FOV) 18; NEX 2; matrix 512 × 256; sagittal, TR 500; TE 16; slice thickness 4/1 mm; FOV 22; NEX 2; matrix 256 × 192 axial, TR 440; TE 11; slice thickness 3/1.5 mm; FOV 16; NEX 3; matrix 224 × 192.
Images were scored using the psoriatic arthritis MRI synovitis score (PsAMRIS) semi-quantitative method by one consultant radiologist with a special interest in musculoskeletal radiology, who was blind to patient identity and scan chronology. Each knee was divided into four anatomical regions ((medial (MED) and lateral (LAT) parapatellar recesses, intercondylar notch (ICN) and suprapatellar pouch (SPP)) and a synovitis score ranging from 0 to 3 was assigned to each region (0 = normal synovium, 1 = diffuse, even thickening, 2 = nodular thickening, 3 = gross, nodular thickening) based on the overall impression of the severity of synovial abnormality in the three orthogonal scanning planes. A total MRI synovitis score (MRIS) from the four regional scores ranging from 0 to 12 was calculated and used for analysis [38, 39].
Statistical analysis
Statistical analysis was performed using SPSS for Windows version 20.0 (Armonk, NY, USA). Baseline characteristics were described using mean (SD), median (IQR) for continuous variables and percentage for counts. The Wilcoxon test and the paired sample t test were used to compare responses to abatacept treatment for clinical measures, synovitis macroscopic scores and immunohistological staining scores at 0 vs 2 months, 2 vs 6 months and 0 vs 6 months. To measure the effect of the first 2 months of abatacept or PBO treatment over time on cell markers of inflammation in the group we used a general linear model (GLM) repeated measure (within-subject factor: 0, 2 and 6-month time points, between-subject factors: abatacept and PBO treatment groups, and treatment duration). Correlation between synovial cell markers, macroscopic scores, clinical parameters and MRI scores were analysed using Pearson correlation. P < 0.05 was considered as statistically significant.
Results
Demographic and disease-specific characteristics of the patients
Fifteen patients (8 female and 7 male) with mean age of 45 (±14.6) years were recruited, and 14 subjects were randomised. Seven patients received abatacept 3 mg/kg of body weight and seven patients were given PBO infusions on day 1, 15 and 29. Eleven patients received he abatacept dose of 10 mg/kg of body weight from day 57. In total 10 patients completed the study (Fig. 1). Out of 14 randomised subjects, 2 patients dropped out due to commitment issues (1 patient in the 3 mg/kg of body weight treatment arm and 1 in the PBO arm) and 2 patients were withdrawn because of an acute disease flare (1 patient in the 10 mg/kg of body weight treatment arm on day 71 and 1 in the PBO arm on day 40). Demographic and disease-specific characteristics of the group are summarised in Table 1. All patients were of Caucasian race. Mean disease duration was 18 years for psoriasis and 10 years for PsA. Four patients were on a stable dose of MTX at the time of recruitment (one patient in the 3 mg/kg of body weight treatment arm and three in the PBO arm) and stayed on the same dose of MTX, the remainder had not received any prior DMARDs; one patient (PBO arm) was on a stable dose of prednisolone 5 mg/day.
Fig. 1.
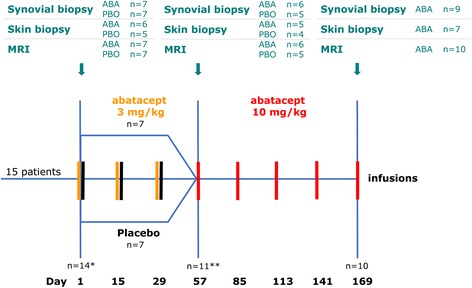
Study design. Numbers of patients who underwent synovial and skin biopsies, and magnetic resonance imaging (MRI) in the abatacept (ABA) and placebo (PBO) study arms at each time point. *One patient was not randomized (screening visit and first MRI only). **Four patients were withdrawn from the study (two patients due to commitment issues (one in the 3 mg/kg of body weight treatment arm and one in the PBO arm)
Table 1.
Patients’ demographic and clinical characteristics at baseline
| Variable | Value |
|---|---|
| Demographic parameters | |
| Age (years) | 45 (±14.6) |
| Female/male, n (%) | 8 (53)/7 (47) |
| Age onset of psoriasis (years) | 23 (±15) |
| Psoriasis disease duration (years) | 18 (±12) |
| Age onset of PsA (years) | 35 (±15) |
| PsA disease duration (years) | 10 (±9.6) |
| Previous sDMARDs, n (%) | 3 (20) |
| Concomitant sDMARDs (methotrexate), n (%) | 4 (27) |
| Currently taken corticosteroids, n (%) | 1 (7) |
| Clinical parameters, physician’s assessment | |
| aCCP+, n (%) | 0 |
| RF+, n (%) | 1 (7) |
| ESR (mm/h) | 27 (±11) |
| CRP (mg/L) | 19 (±10.4) |
| DAS28-ESR | 4.9 (±1) |
| DAS28-CRP | 4.7 (±0.9) |
| TJC 68 | 11.5 (±11) |
| SJC 66 | 6.9 (±7.4) |
| Dactylitis (0–20) | 1.1 (±1.8) |
| Enthesitis (0–6) | 1.6 (±1.6) |
| Nail (0–10) | 5 (±3.8) |
| PASI (0–72) | 4.5 (±4.6) |
| Clinical parameters, patient-reported | |
| Stiffness (minutes) | 46 (±35.9) |
| Pain (0–10) | 5.9 (±3.1) |
| GVAS (0 − 100) | 56 (±28.6) |
| Fatigue (0–10) | 5.9 (±2.8) |
| HAQ (0–3) | 0.9 (±0.7) |
| DLQI (0–30) | 4.8 (±7.2) |
| PsAQol (0–20) | 8.6 (±5.7) |
Results are presented as mean ± SD, median (IQR) or percentage. Dactylitis, the number of affected digits. Enthesitis score is based on the Leeds enthesitis index. Nail, the number of affected nails on the hands. Stiffness is early morning stiffness. Pain scale ranges from 0 = no pain to 10 = pain as bad as it could be. The visual analogue scale for global health (GVAS) ranges from 0 = worst imaginable health state to 100 = best imaginable health state. The fatigue scale ranges from 0 = no fatigue to 10 = fatigue as bad as it could be. The health assessment questionnaire HAQ scale ranges from 0 = no difficulty to 3 = unable to perform activity. PsA psoriatic arthritis, aCCP antibodies against cyclic citrullinated peptides, RF rheumatoid factor, sDMARD synthetic disease-modifying antirheumatic drug, aCCP antibodies against cyclic citrullinated peptides, RF rheumatiod factor, ESR erythrocyte sedimentation rate, CRP C-reactive protein, DAS28-ESR/CRP disease activity score based on a 28-joint assessment of pain or swelling using the ESR/CRP-based formula (www.das-score.nl), TJC tender joint count, SJC swollen joint count, PASI psoriasis area and severity index, DLQI dermatology life quality index, PsAQol psoriatic arthritis-specific quality of life score
Clinical responses of arthritis and psoriasis
All patients had active joint disease at baseline as reflected by both physicians’ assessments and patients’ reported outcome measures (PROMs), such as morning stiffness, pain, global health, fatigue, HAQ or PsAQoL scores. Mean TJC68, SJC66, DAS28-ESR and DAS28-CRP were 11.5 (±11), 6.9 (±7.4), 4.9 (±1) and 4.7 (±0.9), respectively. Skin-related measures showed mild to moderate psoriasis with mean PASI, DLQI and nail scores 4.5 (±4.6), 4.8 (±7.2) and 5 (±3.8), respectively (Table 1). DAS28-ESR (P = 0.01) and DAS28-CRP (P = 0.042) improved as early as 2 months. Significant improvements in most of the joint-related measures and PROMs were observed at 6 months compared to 2 months and to baseline (data not shown). There was very little change in skin-related outcomes. Change in PASI and DLQI from 2 to 6 months was -5.4 (±11.7) and -4.2 (±11.1) in the treatment, and -1.15 (±2.6) and 0.17 (±2.2) in the PBO group, respectively.
According to the EULAR response criteria 73% and 90% of patients were EULAR responders after 2 and 6 months of treatment (46% and 80% good responders, 27% and 10% moderate responders, respectively). PASI50 response at 2 and 6 months was seen in only 9% and 30% of the patients.
Improvement of knee synovitis on MRI
MRI scans were available at 0, 2 and 6 months for 15, 11 and 10 patients, respectively. MRI synovitis scores improved in each anatomical region of the knee throughout the study. Mean total MRIS at baseline, 2 and 6 months were 8.1 (±3), 7.3 (±3.8) and 6.2 (±4.3), respectively. There was a significant reduction in total MRIS from 2 to 6 months (P = 0.047) and from 0 to 6 months (P = 0.016). MRIS at 6 months was associated with change in DAS28-CRP from 2 to 6 months (rho = -0.63, P = 0.049).
Macroscopic evaluation of the synovium
Synovial inflammation as assessed by arthroscopy showed significant improvement throughout the study. Mean macroscopic scores at baseline and 2 and 6 months were 73 (±22.1), 41 (±29.7) and 44.4 (±34.3) for synovitis, and 59 (±29.7), 46 (±27.6) and 34 (±29.6) for vascularity, respectively. Significant reduction in synovitis scores was noted from 0 to 2 months and from 0 to 6 months (P = 0.016), and in vascularity scores from 0 to 6 months (P = 0.039).
Immunohistological analyses of synovial and skin tissues
Synovial and skin biopsies were available at 0, 2 and 6 months for 14, 11 and 9 patients, and for 11, 9 and 7 patients, respectively. Mean scores of immunohistological expression of CD3, CD4, CD8, FOXP3 and CD31 in the synovium and in the skin prior to and following treatment are summarised in Table 2. There was a trend toward reduction in CD8+ T cells, a reduction in CD4 expression (P = 0.073) and a significant reduction in FOXP3 expression (P = 0.027) in the synovium following treatment at 6 months. Figure 2 shows representative, single-stained images of decrease in CD4+ cells and in FOXP3+ cells prior to and at 6 months after abatacept treatment. FOXP3 expression was observed mostly in lymphoid follicles; some peri-vascular staining was seen and some lining layer staining was occasionally observed.
Table 2.
Synovial and skin immunohistological expression of CD3, CD4, CD8, FOXP3 and CD31 prior to and following abatacept treatment
| Baseline | 2 Months | 6 Months | P value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 vs 2 months | 0 vs 6 months | |||||||
| ABA | PBO | ABA | PBO | ABA | ABA | PBO | ABA | |
| Synovium | ||||||||
| CD3 | 2.66 (±1.2) | 1.8 (±0.8) | 2.3 (±1) | 2.25 (±1.5) | 2.22 (±1.3) | 0.679 | 0.824 | 0.739 |
| CD4 | 2.83 (±1.2) | 2.4 (±1.5) | 2.3 (±0.8) | 2.75 (±1.3) | 1.78 (±1.1) | 0.296 | 1 | 0.073 |
| CD8 | 1.66 (±0.8) | 2 (±1) | 1.3 (±1) | 1.75 (±1) | 1.33 (±0.7) | 0.576 | 0.604 | 0.129 |
| FOXP3 | 5 (±6) | 4.4 (±4.4) | 2.8 (±2.2) | 4.6 (±4) | 1.13 (±2.4) | 0.407 | 0.807 | 0.027* |
| CD31 | 78.3 (±53.9) | 85.6 (±50.2) | 67.6 (±75.9) | 155.5 (±136) | 73.22 (±69.9) | 0.621 | 0.482 | 0.813 |
| Skin | ||||||||
| CD3 | 2.6 (±0.8) | 2.4 (±0.9) | 2.25 (±1.5) | 2.5 (±2.1) | 2.43 (±1.1) | 0.718 | 0.717 | 0.891 |
| CD4 | 3.5 (±0.5) | 2.8 (±1.1) | 2.75 (±0.9) | 4 (±0) | 3.14 (±0.9) | 0.182 | 1 | 0.891 |
| CD8 | 2 (±0.6) | 1.75 (±1) | 1.5 (±0.6) | 2 (±1.4) | 1.71 (±0.8) | 0.718 | 0.423 | 0.577 |
| FOXP3 | 16 (±9.5) | 9.2 (±10.7) | 9.5 ±4.5 | 17.5 ±2.1 | 13.14 ±8.3 | 0.743 | 0.709 | 0.497 |
| CD31 | 61.2 (±38) | 65.4 (±20.5) | 75.5 ±17.1 | 64.5 ±10.6 | 54.71 ±17.2 | 0.788 | 0.749 | 0.612 |
The Wilcoxon test and the paired sample t test were used for analysis. Results are presented as mean ± SD
Number of ABA samples at 0, 2 and 6 months: synovial n = 7, n = 6, n = 9; skin n = 6, n = 5, n = 7, respectively. Number of PBO samples at 0 and 2 months: synovial n = 7, n = 5; skin n = 5, n = 4, respectively. Expression of CD3, CD4 and CD8 was scored on a 5-point scale (0–4). CD31 expression was scored by the number of positive vessels/10 high power fields. Forkhead box protein transcription factor 3 (FOXP3) expression was scored as %/300 cells. ABA abatacept group, PBO placebo group. *Statistically significant
Fig. 2.
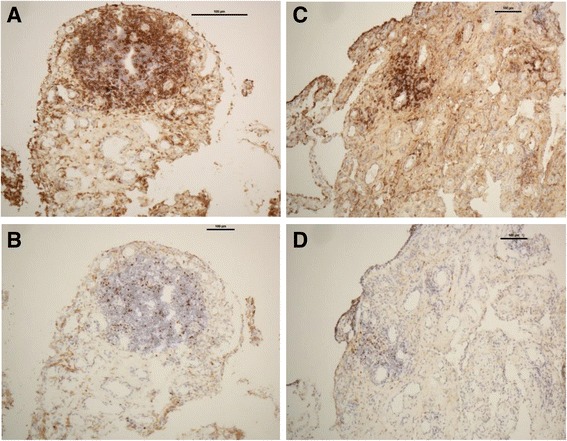
Immunohistological comparison of the expression of CD4+ and forkhead box protein transcription factor 3-positive (FOXP3+) T cells (magnification × 10) in the synovial tissue prior to and at 6 months after abatacept treatment (patient #6). Baseline images show focal infiltration of CD4+ T cells (a) and FOXP3+ T cells (b) in a villus. Decrease in CD4 expression and significant reduction in the number of FOXP3+ T cells are shown (c and d, respectively)
We did not find significant change in synovial CD3, CD8 or CD31 expression during the study period. A trend toward reduction in CD3+, CD4+ and CD8+ T cells was also seen in the lesional skin in particular in the first 2 months; however, significant change in expression was not observed for any of the cell markers over the 6-month treatment period.
Dual immunofluorescence staining of synovial tissue
Recent studies have proposed that FOXP3 is not only expressed by Tregs, but can be expressed by other types of lymphoid or myeloid cells, and by some non-haematopoietic cells such as epithelial cells [40]. To further specify the phenotype of FOXP3+ cells we performed dual immunofluorescence staining on synovial tissue sections with anti-CD4 and anti-FOXP3 antibodies.
Figure 3a-c shows representative images of immunofluorescent staining in synovial tissue (nuclear staining of FOXP3 (green), CD4+ T lymphocytes (red), and double-stained CD4+FOXP3+ T lymphocytes (green nuclear staining surrounded by red cytoplasmic staining). CD4 co-localized with FOXP3 mostly in lymphoid follicles. Figure 3d demonstrates quantification of double-stained CD4+FOXP3+ cells in synovial tissue at baseline and 6- months post treatment. Significant reduction (P = 0.008) in the number of double stained CD4+FOXP3+ T-cells was found in patients (n = 9) following 6 months abatacept treatment compared to baseline.
Fig. 3.
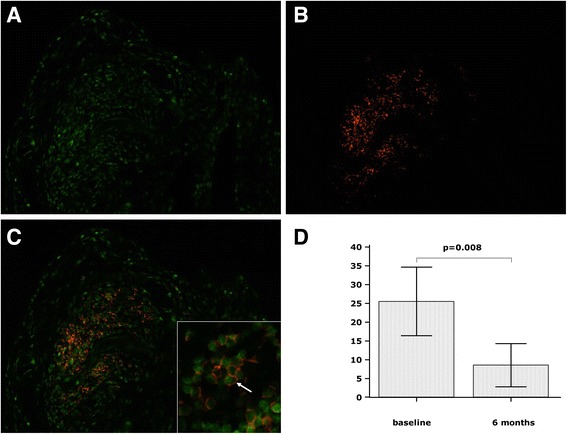
Representative images of immunofluorescent staining in cryostat synovial sections in a patient. a Nuclear staining of forkhead box protein transcription factor 3 (FOXP3) (green), b CD4+ T lymphocytes (red), c double-stained CD4+FOXP3+ T lymphocytes (green nuclear staining surrounded by red cytoplasmic staining), insert shows magnification × 40, white arrow points to a double-stained CD4+FOXP3+ T cell. d Reduction in the number of double-stained CD4+FOXP3+ T cells from baseline to 6 months. Magnification of photomicrographs × 20
The impact of a short period of treatment with abatacept 3 mg/kg of body weight or placebo on clinical outcomes and cell markers
Abatacept 3 mg/kg of body weight or PBO was given to 7 patients in each arm on day 1, 15 and 29. Clinical outcomes, PROMs, and cell markers of synovial and skin inflammation were compared between the two treatment arms at 0 and 2 months. Disease activity measures and cell markers or their change over 2 months did not show significant difference between the abatacept and PBO groups (data not shown), with two exceptions: the MRIS was higher at baseline (P = 0.02) and FOXP3 expression in the skin was lower at 2 months (P = 0.028) in the treatment group compared to the PBO arm. The GLM repeated measure showed that the short PBO treatment did not have a significant effect on the changes in expression of cell markers of synovial and skin inflammation between baseline and 2 and 6 months in the whole group.
Macroscopic evaluation of synovial inflammation correlates with reduction in synovial cell markers
Both synovitis and vascularity scores correlated with FOXP3 expression at 2 months (rho = 0.7, P = 0.024 and rho = 0.65, P = 0.041, respectively), and improvement in vascularity score correlated with reduction in FOXP3 expression from 2 to 6 months (rho = 0.64, P = 0.034).
Safety
AEs were reported in a total of 12 patients (86%). Four patients (57%) in the treatment arm receiving abatacept 3 mg/kg of body weight, 6 patients (86%) in the PBO arm, and 8 patients (73%) who received the dose of 10 mg/kg of body weight from day 57 experienced mild or moderate AEs. There were no cases of acute infusion reaction. AEs possibly related to abatacept or PBO as per the investigator’s opinion were reported in one (headache, influenza-like symptoms) and two (diarrhea, flare of psoriasis and arthritis) cases, respectively. There were no serious adverse events.
Discussion
This single-centre, placebo-controlled, crossover study demonstrates that abatacept reduced the immunohistological expression of CD4+ T cells and specifically reduced FOXP3+ Treg expression in the synovium over 6 months. Abatacept 10 mg/kg treatment significantly improved clinical measures including MRI synovitis scores and arthritis-related PROMs in PsA with little effect on skin-related outcomes.
Clinical efficacy of abatacept has been reported in patients with active RA, including those with an inadequate response to MTX or TNF inhibitors [41], in early type 1 diabetes mellitus, and more recently, in primary Sjögren’s syndrome [21, 42]. Whilst there was no major clinical response to treatment with abatacept 10 mg/kg of body weight in a phase-2 open-label 24-week trial in ankylosing spondylitis [43], Mease et al. reported efficacy of abatacept on joint symptoms in PsA and identified a differential dosage effect of abatacept, with the dose of 10 mg/kg of body weight having more therapeutic effect on arthritis than the 3 mg/kg regimen [25]. In line with this study, we did not notice significant improvement in joint-related outcomes with the dose of 3 mg/kg of body weight, whereas the 10 mg/kg regimen led to significant improvements in most joint-related measures at 6 months. Similarly, MRI synovitis scores improved significantly over time only after administering the dose of 10 mg/kg of body weight. Abatacept has been shown to have some clinical benefit in the treatment of psoriasis [4]. In an open-label phase-1 study of abatacept in psoriasis, 50% or greater improvement of skin involvement was achieved in patients using doses of 25 and 50 mg/kg of body weight, and the same doses correlated best with the reduction of epidermal hyperplasia and the decrease in activated T cells in the skin [44, 45]. We observed very little improvement in the PASI or DLQI over 6 months, but the treatment doses of 3 and 10 mg/kg of body weight may have been too low to be of clinical benefit.
Studies assessing synovial changes after initiating antirheumatic therapy are limited in PsA. From these studies it can be concluded that successful treatment leads to deactivation of endothelium, reduced vascularity and reduction in infiltrating immune cells [27]. Immunpathological changes in the synovium following abatacept treatment in PsA was evaluated in a single case report, and suggested regression of synovial inflammation in a patient refractory to TNF inhibitors [46]. We found significant reduction in macroscopic scores for both synovitis and vascularity after 6 months of abatacept therapy. A trend toward reduction in CD8+ and CD4+ T cells was observed throughout the study, but a statistically significant effect of the treatment was only detected for the reduction in the number of FOXP3+ Tregs in the synovium.
Tregs play a central role in immune tolerance, suppressing not only autoimmune responses but also aberrant or excessive immune responses to non-self-antigens [4] Data on the presence of FOXP3+ Tregs in inflamed synovial tissue are controversial. Enrichment of Tregs in the inflamed joints was reported in a large cohort of patients with chronic inflammatory arthritis, including patients with PsA [47], whilst another study showed only scarce synovial membrane expression of FOXP3+ T cells during relapse in inflammatory arthritis [12]. Deficiencies in Treg function have been also identified in different autoimmune diseases, including RA and psoriasis. Anti-TNF therapy has been shown to induce a potent population of Tregs in patients with RA; however, after cessation of treatment the natural Treg defect persisted [11, 13–15]. It has been suggested that reduced expression and functional abnormalities in Treg-associated CTLA-4 could contribute to abnormal Treg function in RA and may represent a target for therapy [11]. Data for the effects of abatacept on Treg cell function are limited and conflicting. Bonelli et al. showed that abatacept increased the frequency of Treg cells in RA and inhibited their suppressive capacity [48], whilst another study in RA reported enhanced suppressive capacity of Tregs isolated from peripheral blood [49]. Whether the reduction in FOXP3 expression in the synovium we observed after abatacept treatment is protective or pathogenic remains unclear, since our study did not include detailed phenotypic or functional analyses of the Tregs. However, in view of the significant improvement seen in arthritis measures we speculate that the reduction in potentially dysfunctional Tregs may be protective. In line with our observations, a lack of retention of Tregs has been reported after successful anti-inflammatory treatment with intra-articular glucocorticoid injection, suggesting that a specific treatment that modulates local FOXP3+ Treg numbers and function would be of benefit for patients with joint inflammation [12].
A study investigating the role of Tregs in the pathogenesis of psoriasis found increased Treg expression in lesional skin compared to normal skin, in particular in the chronic plaque type of psoriasis, irrespective of disease severity [15]. It has been proposed that the differential dosage effect of abatacept in PsA that was reported in a previous study may be due to the suppression of Tregs at higher doses [19, 25]. In this study, we did not observe a better skin response with low-dose abatacept, possibly due to the small sample size, although the study by Abrams et al. [45] suggests that the abatacept doses used in our study may well have been too low to achieve meaningful skin response.
In contrast to changes seen in the synovial tissue, we did not demonstrate significant immunohistological change in skin involvement in response to abatacept. Different cytokine milieu in the joints compared to the skin may account for this. A recent comparative genomic profiling study of the synovium versus skin lesions in PsA revealed clearly distinct gene expression patterns in the skin compared to the synovium with a stronger IL-17 gene signature in the skin, and more equivalent TNF and IFN-γ gene signatures in both tissues. The authors found fewer Tregs in the synovium compared to the skin; however, the difference was not significant [50].
Conclusions
In conclusion, this is the first study to assess both synovial and psoriatic skin immunopathological changes following abatacept treatment in PsA. The results of the study suggest that treatment with abatacept 10 mg/kg of body weight is associated with significant improvement in the joint-related clinical outcomes but not skin-related outcomes in biologic DMARD-naïve PsA patients and might be an effective treatment option in patients with PsA. We observed different patterns of efficacy of abatacept in the joints and in lesional skin. Reduction in FOXP3+ T-cell expression in the synovium but not in the psoriatic lesion following abatacept treatment may indicate abnormal Treg function in PsA with differential suppressive capacity in the synovium compared to the skin. Comprehensive studies are required on both the distribution and function of Tregs, together with their complex interactions with the local inflammatory milieu present in the psoriatic skin lesion and inflamed synovium in PsA.
Acknowledgements
The authors thank all the patients who participated in the study. We are grateful to Prof. Douglas Veale, Dr Carl Orr, Dr Len Harty and Dr Emese Balogh for obtaining the synovial biopsies and to Dr Anne Marie Tobin and Dr Karoline Adamzik for performing skin biopsies. We would also like to thank Ms Katalin Hodosi for reviewing the statistical analyses. The authors gratefully acknowledge Ms Anne Marie Baker, Ms Martina Cooney and Dr Monika Biniecka their valuable contribution to the study.
Funding
Bristol Myers Squibb provided the study drug and an unrestricted research grant.
Availability of data and materials
The datasets acquired and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
OF was the principal investigator who obtained the research grant, and was responsible for the study design and overseeing the study. AS was responsible for study recruitment and data collection, performed the statistical analysis and drafted the manuscript. EH carried out MRI scoring and contributed to data interpretation. MG carried out immunohistochemical analysis. LM assisted with immunohistochemical analysis and data acquisition. JMc carried out immunohistochemical analysis and contributed to data interpretation. MH was responsible for study recruitment and data collection. ME contributed to data collection and interpretation. PG participated in designing the study and its coordination. GK was responsible for obtaining skin biopsies and contributed to data interpretation. AF was responsible for overseeing immunohistochemical analysis and carried out immunohistochemical scoring. BK was the co-principal investigator of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Competing interests
OF was supported by research grants from Pfizer, AbbVie, BMS and UCB. AS, EH, MG, LM, JMcC, MH, ME, PG, GK, AF and BK declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by St. Vincent’s Healthcare Group Ethics and Medical Research Committee. Informed, written consent was obtained from all study participants according to the Declaration of Helsinki.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- aCCP
Antibodies against cyclic citrullinated peptides
- AEs
Adverse events
- CASPAR
Classification criteria for psoriatic arthritis
- CRP
C-reactive protein
- CTLA-4
Cytotoxic T-lymphocyte Antigen 4
- DAS28
Disease activity score 28
- DLQI
Dermatology life quality index
- DMARDs
Disease-modifying antirheumatic drugs
- EMS
Early morning stiffness
- ESR
Erythrocyte sedimentation rate
- EULAR
European League Against Rheumatism
- FOXP3
Forkhead box transcription factor
- GVAS
Visual analogue scale for global health
- HAQ
Health assessment questionnaire
- HPF
High-power fields
- IgG1
Immunoglobulin G1
- IL
Interleukin
- INFγ
Interferon gamma
- JIA
Juvenile idiopathic arthritis
- LEI
Leeds enthesitis index
- MRI
Magnetic resonance imaging
- MRIS
Total MRI synovitis score
- MTX
Methotrexate
- NSAID
Nonsteroidal anti-inflammatory drug
- PASI
Psoriasis Area Severity Index
- PBO
Placebo
- PBS
Phosphate-buffered saline
- PDE4i
Phosphodiesterase 4 inhibitor
- PROMs
Patients’ reported outcome measures
- PsA
Psoriatic arthritis
- PsAQol
PsA-specific quality of life score
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- SJC
Swollen joint count
- TJC
Tender joint count
- TNFi
Tumour necrosis factor alpha inhibitor Tregs, Regulatory T cells
Contributor Information
Agnes Szentpetery, Email: agnes.szentpetery@hotmail.com.
Eric Heffernan, Email: e.heffernan@st-vincents.ie.
Martina Gogarty, Email: gogartm@tcd.ie.
Lisa Mellerick, Email: lisamellerick@gmail.com.
Janet McCormack, Email: janet.mccormack@ucd.ie.
Muhammad Haroon, Email: mharoon301@hotmail.com.
Musaab Elmamoun, Email: mosab100@yahoo.com.
Phil Gallagher, Email: p.gallagher@st-vincents.ie.
Genevieve Kelly, Email: gkellywinter@gmail.com.
Aurelie Fabre, Email: a.fabre@st-vincents.ie.
Brian Kirby, Email: b.kirby@st-vincents.ie.
Oliver FitzGerald, Email: oliver.fitzgerald@ucd.ie.
References
- 1.Fitzgerald O, Winchester R. Psoriatic arthritis: from pathogenesis to therapy. Arthritis Res Ther. 2009;11:214. doi: 10.1186/ar2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haroon M, FitzGerald O. Psoriatic arthritis: complexities, comorbidities and implications for the clinic. Expert Rev Clin Immunol. 2016;12:406–16. [DOI] [PubMed]
- 3.Diani M, Altomare G, Reali E. T cell responses in psoriasis and psoriatic arthritis. Autoimmun Rev. 2015;14:286–92. doi: 10.1016/j.autrev.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol. 2012;9:302–9. doi: 10.1038/cmi.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FitzGerald O, Haroon M, Giles JT, Winchester R. Concepts of pathogenesis in psoriatic arthritis: genotype determines clinical phenotype. Arthritis Res Ther. 2015;17:115. doi: 10.1186/s13075-015-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veale DJ. New therapies and new goals for psoriatic arthritis. Arthritis Rheum. 2011;63:874–6. doi: 10.1002/art.30173. [DOI] [PubMed] [Google Scholar]
- 7.Mortezavi M, Thiele R, Ritchlin C. The joint in psoriatic arthritis. Clin Exp Rheumatol. 2015;33:S20–5. [PubMed] [Google Scholar]
- 8.Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front Immunol. 2015;6:61. doi: 10.3389/fimmu.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appel H, Wu P, Scheer R, Kedor C, Sawitzki B, Thiel A, et al. Synovial and peripheral blood CD4 + FoxP3+ T cells in spondyloarthritis. J Rheumatol. 2011;38:2445–51. doi: 10.3899/jrheum.110377. [DOI] [PubMed] [Google Scholar]
- 10.Ryder LR, Bartels EM, Woetmann A, Madsen HO, Odum N, Bliddal H, et al. FoxP3 mRNA splice forms in synovial CD4+ T cells in rheumatoid arthritis and psoriatic arthritis. APMIS. 2012;120:387–96. doi: 10.1111/j.1600-0463.2011.02848.x. [DOI] [PubMed] [Google Scholar]
- 11.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105:19396–401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghavan S, Cao D, Widhe M, Roth K, Herrath J, Engstrom M, et al. FOXP3 expression in blood, synovial fluid and synovial tissue during inflammatory arthritis and intra-articular corticosteroid treatment. Ann Rheum Dis. 2009;68:1908–15. doi: 10.1136/ard.2008.100768. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+ CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soler DC, McCormick TS. The dark side of regulatory T cells in psoriasis. J Invest Dermatol. 2011;131:1785–6. doi: 10.1038/jid.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun WJ, Lee DW, Chang SE, Yoon GS, Huh JR, Won CH, et al. Role of CD4CD25FOXP3 regulatory T cells in psoriasis. Ann Dermatol. 2010;22:397–403. doi: 10.5021/ad.2010.22.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–59. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta Felquer M, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis: treatment recommendations for psoriatic arthritis 2015. Arthritis Rheumatol. 2016;68:1060–71. [DOI] [PubMed]
- 18.Mease P. A short history of biological therapy for psoriatic arthritis. Clin Exp Rheumatol. 2015;33:S104–8. [PubMed] [Google Scholar]
- 19.Iannone F, Lapadula G. The inhibitor of costimulation of T cells: Abatacept. J Rheumatol. 2012;39:100–2. doi: 10.3899/jrheum.111125. [DOI] [PubMed] [Google Scholar]
- 20.Moreland L, Bate G, Kirkpatrick P. Abatacept. Nat Rev Drug Discov. 2006;5:185–6. doi: 10.1038/nrd1989. [DOI] [PubMed] [Google Scholar]
- 21.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–9. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisman MH, Durez P, Hallegua D, Aranda R, Becker JC, Nuamah I, et al. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J Rheumatol. 2006;33:2162–6. [PubMed] [Google Scholar]
- 23.Ruderman EM, Pope RM. The evolving clinical profile of abatacept (CTLA4-Ig): a novel co-stimulatory modulator for the treatment of rheumatoid arthritis. Arthritis Res Ther. 2005;7(Suppl 2):S21–5. doi: 10.1186/ar1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 25.Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939–48. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 26.Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017:1–9. doi:10.1136/annrheumdis-2016-210724. [DOI] [PMC free article] [PubMed]
- 27.van Kuijk AW, Tak PP. Synovitis in psoriatic arthritis: immunohistochemistry, comparisons with rheumatoid arthritis, and effects of therapy. Curr Rheumatol Rep. 2011;13:353–9. doi: 10.1007/s11926-011-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 29.Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59:686–91. doi: 10.1002/art.23568. [DOI] [PubMed] [Google Scholar]
- 30.Coates LC, Mumtaz A, Helliwell PS, Mease PJ, Callis-Duffin K, Krueger GG, et al. Development of a disease severity and responder index for psoriatic arthritis (PsA)–report of the OMERACT 10 PsA special interest group. J Rheumatol. 2011;38:1496–501. doi: 10.3899/jrheum.110278. [DOI] [PubMed] [Google Scholar]
- 31.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 32.Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35:745–57. doi: 10.1016/j.rdc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 33.van de Sande MG, Gerlag DM, Lodde BM, van Baarsen LG, Alivernini S, Codullo V, et al. Evaluating antirheumatic treatments using synovial biopsy: a recommendation for standardisation to be used in clinical trials. Ann Rheum Dis. 2011;70:423–7. doi: 10.1136/ard.2010.139550. [DOI] [PubMed] [Google Scholar]
- 34.Veale DJ. The role of arthroscopy in early arthritis. Clin Exp Rheumatol. 1999;17(1):37–8. [PubMed] [Google Scholar]
- 35.Gogarty M, Fitzgerald O. Immunohistochemistry of the inflamed synovium. Methods Mol Med. 2007;135:47–63. doi: 10.1007/978-1-59745-401-8_3. [DOI] [PubMed] [Google Scholar]
- 36.van Kuijk AW, Reinders-Blankert P, Smeets TJ, Dijkmans BA, Tak PP. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis. 2006;65:1551–7. doi: 10.1136/ard.2005.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tak PP, Smeets TJ, Daha MR, Kluin PM, Meijers KA, Brand R, et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–25. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes LA, Grainger AJ, Keenan AM, Thomas C, Emery P, Conaghan PG. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MRI in knee osteoarthritis. Rheumatology (Oxford) 2005;44:1569–73. doi: 10.1093/rheumatology/kei094. [DOI] [PubMed] [Google Scholar]
- 39.Glinatsi D, Bird P, Gandjbakhch F, Mease PJ, Boyesen P, Peterfy CG, et al. Validation of the OMERACT Psoriatic Arthritis Magnetic Resonance Imaging Score (PsAMRIS) for the hand and foot in a randomized placebo-controlled trial. J Rheumatol. 2015;42:2473–9. doi: 10.3899/jrheum.141010. [DOI] [PubMed] [Google Scholar]
- 40.Devaud C, Darcy PK, Kershaw MH. Foxp3 expression in T regulatory cells and other cell lineages. Cancer Immunol Immunother. 2014;63:869–76. doi: 10.1007/s00262-014-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massarotti EM. Clinical and patient-reported outcomes in clinical trials of abatacept in the treatment of rheumatoid arthritis. Clin Ther. 2008;30:429–42. doi: 10.1016/j.clinthera.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Meiners PM, Vissink A, Kroese FG, Spijkervet FK, Smitt-Kamminga NS, Abdulahad WH, et al. Abatacept treatment reduces disease activity in early primary Sjogren’s syndrome (open-label proof of concept ASAP study) Ann Rheum Dis. 2014;73:1393–6. doi: 10.1136/annrheumdis-2013-204653. [DOI] [PubMed] [Google Scholar]
- 43.Song IH, Heldmann F, Rudwaleit M, Haibel H, Weiss A, Braun J, et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis. 2011;70:1108–10. doi: 10.1136/ard.2010.145946. [DOI] [PubMed] [Google Scholar]
- 44.Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–52. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrams JR, Kelley SL, Hayes E, Kikuchi T, Brown MJ, Kang S, et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J Exp Med. 2000;192:681–94. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canete JD, Celis R, Hernandez V, Pablos JL, Sanmarti R. Synovial immunopathological changes associated with successful abatacept therapy in a case of severe refractory psoriatic arthritis. Ann Rheum Dis. 2010;69:935–6. doi: 10.1136/ard.2009.113233. [DOI] [PubMed] [Google Scholar]
- 47.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–46. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonelli M, Goschl L, Bluml S, Karonitsch T, Hirahara K, Ferner E, et al. Abatacept (CTLA-4Ig) treatment reduces T cell apoptosis and regulatory T cell suppression in patients with rheumatoid arthritis. Rheumatology (Oxford). 2016;55:710–20. [DOI] [PubMed]
- 49.Alvarez-Quiroga C, Abud-Mendoza C, Doniz-Padilla L, Juarez-Reyes A, Monsivais-Urenda A, Baranda L, et al. CTLA-4-Ig therapy diminishes the frequency but enhances the function of Treg cells in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:588–95. doi: 10.1007/s10875-011-9527-5. [DOI] [PubMed] [Google Scholar]
- 50.Belasco J, Louie JS, Gulati N, Wei N, Nograles K, Fuentes-Duculan J, et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol. 2015;67:934–44. doi: 10.1002/art.38995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets acquired and/or analysed during the current study are available from the corresponding author on reasonable request.


