Abstract
We present the case of a 79-year-old man who showed multiple pulmonary nodules on chest computed tomography (CT) after being treated for 6 months with ruxolitinib, an inhibitor of Janus kinase (JAK) 1 and 2, to treat primary myelofibrosis. We examined the lesions by bronchoscopy, and the biopsy specimen revealed fungus bodies of Cryptococcus with granulomatous inflammation. As a result, the patient was diagnosed with pulmonary cryptococcosis. The patient was treated with fluconazole (200 mg daily for 2 weeks) with concomitant ruxolitinib administration, but the pulmonary lesions progressed. Subsequently, the patient was treated with voriconazole (300 mg daily for 3 weeks), but the lesions worsened further. The administration of ruxolitinib was therefore discontinued, and the dosage of voriconazole was increased to 400 mg daily. Three months later, the pulmonary lesions diminished in size. The present case of pulmonary cryptococcosis occurred in a patient treated with ruxolitinib. Treatment of pulmonary cryptococcosis with concomitant JAK inhibitor administration may result in poor treatment efficacy. It might be better to stop administration of JAK inhibitors, if possible, in patients being treated for pulmonary cryptococcosis.
Keywords: Janus kinase inhibitor, Myelofibrosis, Pulmonary cryptococcosis, Pulmonary nodules, Ruxolitinib
Abbreviations: CT, computed tomography; IL, interleukin; JAK, Janus kinase; TNF, tumor necrosis factor
1. Introduction
Ruxolitinib, an inhibitor of Janus kinase (JAK) 1 and 2, has been approved for the treatment of myelofibrosis [1], [2], [3], [4] and polycythemia vera [5], [6]. JAK inhibitors exert immunosuppressive activities through the downregulation of several cytokines, such as interleukins (ILs), interferon-γ, and tumor necrosis factor-α (TNF-α) [7]. Pulmonary cryptococcosis is known to occur particularly frequently in immunocompromised hosts [8]. However, there are only a few reports of pulmonary cryptococcosis in patients treated with JAK inhibitors [9], [10]. Here, we report a case of pulmonary cryptococcosis in a ruxolitinib-treated patient with primary myelofibrosis.
2. Case report
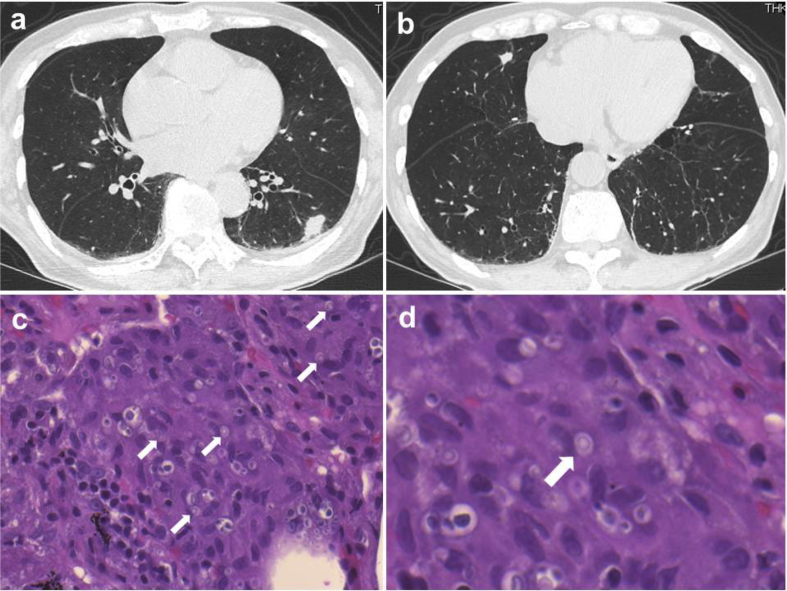
A 79-year-old man with primary myelofibrosis who had been treated for 6 months with ruxolitinib showed multiple pulmonary nodules on chest computed tomography (CT) (Fig. 1a and b). The lesions were found mainly in the subpleural regions, and the largest lesion was 18 mm in diameter. The patient had no respiratory symptoms.
Fig. 1.
Initial chest computed tomography and microscopic findings of the transbronchial lung biopsy specimen from the left lung (hematoxylin and eosin staining). a, b: After taking ruxolitinib for 6 months, multiple nodules were seen in both lungs. The patient was diagnosed as pulmonary cryptococcosis. c, d: The lung specimen shows fungus bodies of Cryptococcus (arrows) with granulomatous inflammation.
Ruxolitinib was initially started because of splenomegaly and fatigue, and these symptoms had improved gradually. He was retired and had lived in an urban apartment house. He had no smoking history and no contact with birds or other animals.
On physical examination, the patient was afebrile, his consciousness was clear, blood pressure was 144/71 mmHg, pulse rate was 71/min, and oxygen saturation was 97% on room air. Cardiopulmonary and neurological physical findings were normal.
On laboratory investigations, the findings from a complete blood cell count were almost normal except for anemia (hemoglobin 8.3 g/dl); nutrition, hepatic function, and renal function were normal; and C-reactive protein was mildly increased (0.55 mg/dl). Serum cryptococcal antigen was positive, with a titer of 1:8. The patient was seronegative for human immunodeficiency virus.
Subsequently, we examined the lesions by bronchoscopy. A biopsy targeting the largest lesion of the left lower lobe was performed. On a histopathological examination, the biopsy specimen revealed fungus bodies of Cryptococcus with granulomatous inflammation (Fig. 1c and d). Cryptococcus was not detected on bronchoalveolar lavage fluid culture. Spinal fluid obtained by lumbar puncture revealed no organisms by Gram staining, and it was also negative for cryptococcal antigen. The findings of ocular fundi were normal. Conclusively, the patient was diagnosed with pulmonary cryptococcosis.
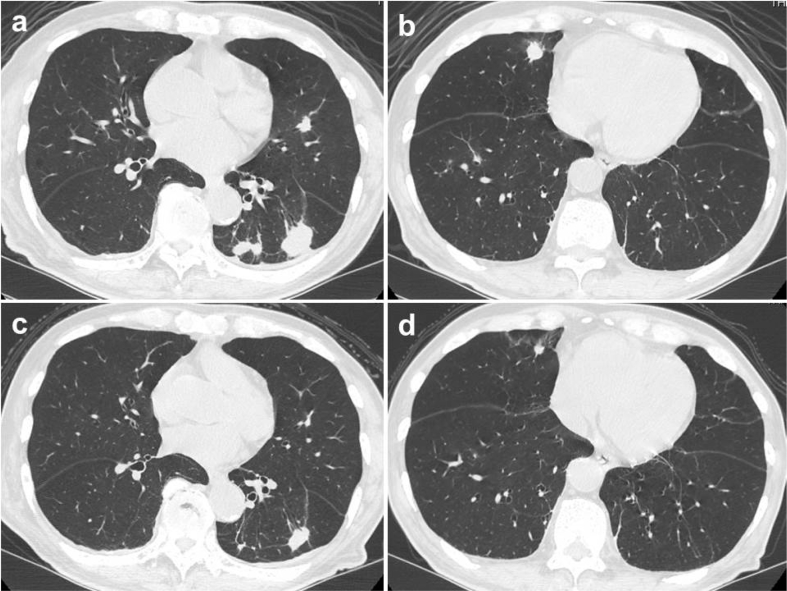
The patient was treated with fluconazole 200 mg daily for 2 weeks, but the findings of the chest CT examination worsened. Subsequently, the patient was treated with voriconazole 300 mg daily for 3 weeks, but the lesions worsened further (Fig. 2a and b). The administration of ruxolitinib was therefore discontinued, and the dosage of voriconazole was increased to 400 mg daily. Three months later, the pulmonary lesions diminished in size (Fig. 2c and d). To date, the patient has continued voriconazole for a total of 5 months without re-administration of ruxolitinib, and the lesions of pulmonary cryptococcosis have been improving. Fortunately, worsening of primary myelofibrosis has been in the range of tolerance.
Fig. 2.
Chest computed tomography after starting the treatment for pulmonary cryptococcosis. a, b: The findings of pulmonary cryptococcosis lesions after 2 weeks of fluconazole and subsequent 3 weeks of voriconazole concomitant with ruxolitinib administration. All pulmonary lesions progressed. c, d: The findings of pulmonary cryptococcosis lesions after 3 months of voriconazole without ruxolitinib administration. All pulmonary lesions diminished in size.
3. Discussion
In this case report, we showed two important clinical observations: (i) one patient who received treatment with a JAK inhibitor developed pulmonary cryptococcosis; and (ii) treatment of pulmonary cryptococcosis with concomitant JAK inhibitor administration may be poorly effective.
First, the present case of pulmonary cryptococcosis occurred in a patient treated with ruxolitinib. To our knowledge, only one case of pulmonary cryptococcosis in a ruxolitinib-treated patient has been reported previously [9], making this the second report of pulmonary cryptococcosis in a ruxolitinib-treated patient. Tuberculosis [4], [11], herpes zoster virus infection [3], [6], herpes simplex virus infection [12], toxoplasmosis retinitis [13], and cryptococcal meningoencephalitis [14] have occurred in patients treated with ruxolitinib. Infections such as tuberculosis, viral infections, and fungal infections are mainly controlled by cell-mediated immunity. It has been shown that ruxolitinib suppresses the cell-mediated immunity by inhibiting the Th1 response and reducing the production of interferon-γ [15]. In addition, studies in mice have shown that signal transducer and activator of transcription 1 (STAT1) and signaling through the JAK/STAT pathway play an important role in the protective response against cryptococcosis via STAT1-mediated classical macrophage activation [16], [17], [18]. Therefore, JAK inhibitors are expected to suppress STAT1-mediated signaling. In the present case, suppression of anti-cryptococcal responses may have induced the development of pulmonary cryptococcosis, just as in the previously reported case [9]. Regarding other JAK inhibitors, a tofacitinib-treated patient with pulmonary cryptococcosis has been reported [10]. That case of pulmonary cryptococcosis may also have been induced by suppressed anti-cryptococcal responses caused by JAK inhibitors.
Second, the treatment of pulmonary cryptococcosis with concomitant JAK inhibitor administration may be poorly effective. Of the two cases of pulmonary cryptococcosis previously reported, one was treated with concomitant JAK inhibitor administration [9], while the other was treated after discontinuation of JAK inhibitor administration [10]. Both cases recovered. The present case was initially treated with concomitant ruxolitinib administration, but the disease progressed. Suppressed anti-cryptococcal responses caused by JAK inhibitors hamper the treatment of pulmonary cryptococcosis. As such, JAK inhibitor administration should be discontinued if pulmonary cryptococcosis is severe.
In recent years, several biological agents, such as TNF-α inhibitors and IL-6 inhibitors, have been used to treat rheumatoid arthritis, Crohn's disease, and psoriasis. Treatments with these biological agents always carry a risk of infection. We previously reported a case of pulmonary cryptococcosis and pneumocystis pneumonia in an etanercept-treated patient with rheumatoid arthritis [19]. When administering a biological agent as treatment, careful follow-up of the patient is required.
4. Conclusion
The present case of pulmonary cryptococcosis occurred in a patient treated with ruxolitinib. Treatment of pulmonary cryptococcosis with concomitant JAK inhibitor administration may result in poor treatment efficacy. Further, suppressed anti-cryptococcal responses caused by JAK inhibitors hampers the treatment of pulmonary cryptococcosis. It might be better to stop administration of JAK inhibitors, if possible, in patients being treated for pulmonary cryptococcosis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Verstovsek S., Mesa R.A., Gotlib J. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison C., Kiladjian J.J., Al-Ali H.K. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 3.Verstovsek S., Mesa R.A., Gotlib J. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015;100(4):479–488. doi: 10.3324/haematol.2014.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervantes F., Vannucchi A.M., Kiladjian J.J. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- 5.Vannucchi A.M., Kiladjian J.J., Griesshammer M. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 2015;372(5):426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passamonti F., Griesshammer M., Palandri F. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 2017;18(1):88–99. doi: 10.1016/S1470-2045(16)30558-7. [DOI] [PubMed] [Google Scholar]
- 7.Quintás-Cardama A., Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin. Cancer Res. 2013;19(8):1933–1940. doi: 10.1158/1078-0432.CCR-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappas P.G., Perfect J.R., Cloud G.A. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 2001;33(5):690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 9.Wysham N.G., Sullivan D.R., Allada G. An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor. Chest. 2013;143(5):1478–1479. doi: 10.1378/chest.12-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seminario-Vidal L., Cantrell W., Elewski B.E. Pulmonary cryptococcosis in the setting of tofacitinib therapy for psoriasis. J. Drugs Dermatol. 2015;14(8):901–902. [PubMed] [Google Scholar]
- 11.Colomba C., Rubino R., Siracusa L. Disseminated tuberculosis in a patient treated with a JAK2 selective inhibitor: a case report. BMC Res. Notes. 2012;5:552. doi: 10.1186/1756-0500-5-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong L.X., Jackson J., Kerstetter J., Worswick S.D. Reactivation of herpes simplex virus infection in a patient undergoing ruxolitinib treatment. J. Am. Acad. Dermatol. 2014;70(3):e59–60. doi: 10.1016/j.jaad.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg R.A., Reichel E., Oshry L.J. Bilateral toxoplasmosis retinitis associated with ruxolitinib. N. Engl. J. Med. 2013;369(7):681–683. doi: 10.1056/NEJMc1302895. [DOI] [PubMed] [Google Scholar]
- 14.Chen C.C., Chen Y.Y., Huang C.E. Cryptococcal meningoencephalitis associated with the long-term use of ruxolitinib. Ann. Hematol. 2016;95(2):361–362. doi: 10.1007/s00277-015-2532-7. [DOI] [PubMed] [Google Scholar]
- 15.Ostojic A., Vrhovac R., Verstovsek S. Ruxolitinib for the treatment of myelofibrosis: its clinical potential. Ther. Clin. Risk Manag. 2012;8:95–103. doi: 10.2147/TCRM.S23277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardison S.E., Herrera G., Young M.L. Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J. Immunol. 2012;189:4060–4068. doi: 10.4049/jimmunol.1103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leopold Wager C.M., Hole C.R., Wozniak K.L. STAT1 signaling is essential for protection against Cryptococcus neoformans infection in mice. J. Immunol. 2014;193:4060–4071. doi: 10.4049/jimmunol.1400318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leopold Wager C.M., Hole C.R., Wozniak K.L. STAT1 signaling within macrophages is required for antifungal activity against Cryptococcus neoformans. Infect. Immun. 2015;83:4513–4527. doi: 10.1128/IAI.00935-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagasaka S., Yamasaki M., Saitoh N. A case of pulmonary cryptococcosis and pneumocystis pneumonia in a patient with rheumatoid arthritis. Jpn. J. Chest Dis. 2016;75(11):1299–1305. Japanese. [Google Scholar]