Abstract
Accumulation of toxic proteins in neurons has been linked with the onset of neurodegenerative diseases, which in many cases are characterized by altered neuronal function and synapse loss. Molecular chaperones help protein folding and the resolubilization of unfolded proteins, thereby reducing the protein aggregation stress. While most of the chaperones are expressed in neurons, their functional relevance remains largely unknown. Here, using bioinformatics analysis, we identified 95 Drosophila chaperones and classified them into seven different classes. Ubiquitous actin5C-Gal4-mediated RNAi knockdown revealed that ∼50% of the chaperones are essential in Drosophila. Knocking down these genes in eyes revealed that ∼30% of the essential chaperones are crucial for eye development. Using neuron-specific knockdown, immunocytochemistry, and robust behavioral assays, we identified a new set of chaperones that play critical roles in the regulation of Drosophila NMJ structural organization. Together, our data present the first classification and comprehensive analysis of Drosophila chaperones. Our screen identified a new set of chaperones that regulate eye and NMJ morphogenesis. The outcome of the screen reported here provides a useful resource for further elucidating the role of individual chaperones in Drosophila eye morphogenesis and synaptic development.
Keywords: Drosophila, chaperones, RNAi, eye morphogenesis, neuromuscular junction, Mutant Screen Report
Within cells, proteins fold into three-dimensional conformations to attain their native state in order to achieve functionality. However, under physiological or environmental stress, proteins undergo misfolding that can lead to nonnative protein interactions and aggregation (Tyedmers et al. 2010). Cells have therefore evolved an intrinsic network of protein quality control machinery that functions to balance protein folding, misfolding, aggregation, and degradation; thereby maintaining protein homeostasis (proteostasis). This protein quality control machinery involves molecular chaperones that act as the first line of defense and participate in the refolding or, alternatively, the degradation of misfolded proteins (Kim et al. 2013).
Chaperones constitute diverse group of proteins that assist the noncovalent assembly/disassembly of other macromolecular structures (Liberek et al. 2008). Some, but not all, chaperones are also stress or heat shock proteins (HSPs) as their functional relevance increases under stress conditions, which otherwise may cause proteins to unfold and aggregate (Feder and Hofmann 1999). Chaperones are often classified according to their molecular weight, and members include Hsp100, Hsp90, Hsp70, Hsp60, Hsp40 (DnaJ), prefoldins, and the small HSPs (sHSPs) (Gong et al. 2009). They regulate multiple aspects of cellular physiology. For instance, in addition to their fundamental roles in de novo protein folding, chaperones also regulate critical cellular processes such as exocytosis and endocytosis (Young et al. 2003), autophagy (Kaushik and Cuervo 2012), apoptosis (Soti et al. 2003), and proteasomal degradation (Hohfeld et al. 2001).
Postmitotic cells like neurons are particularly prone to detrimental effects of misfolded/aggregated proteins as they cannot dilute toxic protein aggregates by cell division, which may result in an accumulation of misfolded proteins (Muchowski and Wacker 2005). The disruption of neuronal proteostasis may lead to aberrantly folded proteins that typically lose their functions. The accumulation of misfolded and aggregated proteins is also cytotoxic and has been implicated in the pathogenesis of many neurodegenerative diseases (Ross and Poirier 2004).
It is widely appreciated that, when compromised, chaperone activity and proteasomal machinery fall short of compensating for the protein damage caused by misfolding and free radicals. Moreover, this leads to the accumulation of protein aggregates, a situation called “chaperone overload” (Soti et al. 2003). Studies in different organisms have reported a tight correlation between age-dependent protein misfolding and weakened proteasomal activity in neurons, which makes them vulnerable to protein aggregation (Morimoto 2008). Elevated heat shock response promotes longevity (Yokoyama et al. 2002; Vos et al. 2016; Morley and Morimoto 2004) whereas defective chaperone molecules along with proteotoxic stress result in early aging (Macario and Conway de Macario 2002).
HSPs have been reported to play a key role in neurogenesis (Calabrese et al. 2002) and are constitutively expressed in neurons (Brown and Rush 1990). Most HSPs show higher expression levels in neuronal tissues (D’Souza and Brown 1998). Interestingly, several reports suggest that a compromised chaperone activity (e.g., for Hsp40 and Hsp70) leads to impaired neurotransmission signifying their neuronal function (Bronk et al. 2001; Morgan et al. 2001). Chaperones are induced in various neuropathies, which points toward their neuroprotective role (Yenari et al. 1999). While chaperones are widely expressed in neurons, very little is known about their specific roles in the nervous system (Muchowski 2002).
In order to determine their functional relevance in vivo, we performed an RNAi-mediated targeted genetic screen for all Drosophila chaperone proteins identified using bioinformatics analysis. We identified several novel chaperones for which neither gene group membership nor protein family has been assigned in FlyBase. We classified Drosophila chaperones into seven different classes based on their domain organization. Next, we ubiquitously knocked-down 167 RNAi lines for a total of 95 Drosophila chaperones using actin5C-Gal4 and selected essential chaperones. To better understand the cellular functions of essential chaperones, we knocked-down these genes in eyes and identified several chaperones involved in eye morphogenesis and/or rhabdomere development. Finally, neuron-specific knockdown of RNAi lines corresponding to 42 essential chaperones identified several candidates that altered Drosophila NMJ development. We shortlisted nine candidates belonging to different chaperone families based on the severity of NMJ structural defects. Neuronal knockdown of some of these candidates resulted in a larval crawling defect as well as compromised adult climbing ability. We suggest that further analysis of the individual chaperones identified in this screen would help us better understand the molecular mechanisms and pathways that they regulate during Drosophila eye and NMJ morphogenesis.
Materials and Methods
In silico identification of Drosophila chaperones
Seven protein families (sHsps, Hsp40, prefoldins, Hsp60/Cpn60, Hsp70, Hsp90, and Hsp100) were considered as canonical chaperone families based on thorough literature analysis (Hartl and Hayer-Hartl 2002; Gong et al. 2009; Saibil 2013). The Saccharomyces cerevisiae “chaperome” is well characterized with the exact number of candidate proteins belonging to each chaperone family known, and thus the same was used as a template to identify the Drosophila “chaperome.” BLASTP searches were performed in FlyBase (http://flybase.org/blast/) and PSI-BLAST searches were conducted in the NCBI database targeted to Drosophila melanogaster (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using the protein sequence of each S. cerevisiae chaperone as a query. From the results of BLAST searches, nonredundant hits were listed. Each protein from the list was analyzed for domain organization using the SMART database (http://smart.embl-heidelberg.de/) (Schultz et al. 1998). Based on the domain organization and information available in FlyBase, Drosophila chaperones were classified into seven chaperone families (e.g., J-domain-containing chaperones were enlisted as members of the Hsp40 family) to make a comprehensive list of Drosophila chaperones. The chaperones for which neither gene group membership nor protein family was assigned in FlyBase were considered to be novel chaperones. Results were compared with the Heat Shock Protein Information Resource HSPIR database (http://pdslab.biochem.iisc.ernet.in/hspir/) (Ratheesh Kumar et al. 2012).
Fly strains and genetics
RNAi lines used in this study were procured from the Vienna Drosophila Resource Centre (VDRC) (Dietzl et al. 2007). Flies were cultured at 25° on a corn meal-agar medium containing yeast granules. A bipartite UAS/Gal4 system (Brand and Perrimon 1993) was used for tissue-specific knockdown of chaperones. The Gal4 driver lines used in this study were actin5C-Gal4 (BDSC-25374), ey-Gal4 (BDSC-5534), D42-Gal4 (BDSC-8816), and elavC155-Gal4 (BDSC-458). The white-eyed w1118 Drosophila strain was used as control except where indicated. All the RNAi knockdown experiments were performed at 29° under controlled humidity (60% RH) in an incubator. To screen for essential chaperones, actin5C-Gal4 without Dicer-2 was used. While Dicer-2 does enhance the efficiency of knockdown, it may also cause off-target knockdowns and lead to additional lethality (Dietzl et al. 2007). Hence, we chose not to use Dicer-2 for screening chaperones.
Bright-field imaging
Seven-day-old flies were anesthetized using ether and photomicrographs of eyes were taken using a color camera mounted on a Leica M205FA Stereo Zoom Microscope. All images were processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Antibodies and immunocytochemistry
Wandering third instar larvae were dissected on a sylgard dish in cold calcium-free HL3 saline (70 mM NaCl, 5 mM KCl, 20 mM MgCl2, 10 mM NaHCO3, 5 mM Trehalose, 115 mM sucrose, 5 mM HEPES, and 5 mM EGTA) and fixed in 4% paraformaldehyde in PBS for 30 min. Larval fillets were then washed in PBS containing 0.2% Triton X-100, blocked for 1 hr in 5% normal goat serum, and then incubated overnight at 4° with the primary antibody. Monoclonal antibodies (anti-CSP and mAb22C10) were obtained from the Developmental Studies Hybridoma Bank (University of Iowa) and were used at 1:50 dilution. Fluorophore-coupled secondary antibodies Alexa Fluor 488, Alexa Fluor 568, or Alexa Fluor 633 (Molecular Probes and Thermo Fisher Scientific) were used at 1:800 dilution. Alexa 488-conjugated anti-HRP was used at 1:800 dilution. Stained larval preparations were mounted in VECTASHIELD (Vector Laboratories) and imaged with a laser scanning confocal microscope (LSM 780; Carl Zeiss). All images were processed with Adobe Photoshop 7.0 (Adobe Systems).
Futsch loop quantification
Third instar larval fillets were double stained with HRP and mAb22C10. Confocal (LSM 780; Carl Zeiss) images of NMJ at muscle 6/7, A2 hemisegment were captured using a 63 × /1.4 NA objective. Images were digitally magnified using ImageJ (NIH) and the total number of boutons was first determined by manually counting the number of HRP-positive varicosities. This was followed by counting the number of complete looped structures that colocalized with HRP. Incomplete loops and loops with diffused or interrupted staining were not included in the count. The total number of loops was divided by the total bouton number to arrive at the percentage of boutons with complete Futsch positive loops (Roos et al. 2000; Hummel et al. 2000).
Locomotive behavioral assays
The third instar larval crawling and Rapid Iterative Negative Geotaxis assays with adult flies were performed as previously described (Nichols et al. 2012; Gargano et al. 2005). elavC155-Gal4/+ was used as control for these assays upon pan-neuronal knockdown and D42-Gal4/+ was used as control for behavioral assays upon motor neuron-specific knockdown. A Nikon COOLPIX P600 camera was used for video recording and imaging. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego).
Larval crawling assay:
Each vial harboring third instar larvae had 10 ml of 20% sucrose solution poured in and was left for 15 min to let the larvae float on top. Third instar larvae were gently collected using a 1.0 ml pipette with a cut tip and washed twice with deionized water. Ten larvae of each genotype were subsequently transferred to a 2% agarose gel in a Petri dish with gridline markings spaced at 0.5 cm. The larvae were allowed to acclimatize to the new environment before videotaping. The average distance crawled (in centimeters) by larvae was calculated based on the average number of gridlines passed by the posterior ends of the larvae in 30 sec. All statistical analysis conducted is based on one-way ANOVA with a post hoc Tukey’s test for multiple comparisons.
Climbing ability test:
Five-day-old flies of respective genotypes were collected in transparent 50 ml falcon tubes marked with a medial line. All the tubes were arranged in a holder and a camera was set at a 1 meter distance from the Falcon holder. Flies were allowed to settle at the bottom by gently tapping the Falcon holder thrice on the surface. Negative geotaxis of the flies was videotaped and the number of flies crossing the medial line in 5 sec was counted for each genotype. The assay was repeated 10 times, and the climbing ability was presented as the average percentage of flies crossing medial line in 5 sec. All the statistical analysis conducted is based on one-way ANOVA with a post hoc Tukey’s test for multiple comparisons.
Data availability
All supportive data and materials generated in this study will be made available upon request. Data supporting total bouton number and Futsch loop quantification are provided as Supplemental Material, Table S3.
Results
Identification and classification of Drosophila chaperones
Based on the published literature, sHsps, prefoldins, Hsp40, Hsp60, Hsp70, Hsp90, and Hsp100 are considered as canonical chaperone families. Members of these chaperone families interact with each other to form a complex network called as the “chaperome,” which monitors cellular proteostasis (Brehme et al. 2014). These chaperones are well characterized in S. cerevisiae and have been classified into different families (Gong et al. 2009). Thus, BLASTP searches were performed using sequences of S. cerevisiae chaperones to identify Drosophila counterparts, which were further classified into different chaperone families based on their domain organization. We report the most comprehensive list of Drosophila chaperones to date, with all details including their FlyBase ID, symbol, gene name, alternate name, gene group membership, and protein family (Table 1). The total number of chaperones in Drosophila is higher when compared with S cerevisiae. While 63 chaperones belonging to seven classes have been reported for S. cerevisiae (Gong et al. 2009), our analysis in Drosophila revealed a total of 95 chaperones, which we classified into seven families. Interestingly, Hsp40 outnumbers other classes of chaperones, as evident from our bioinformatics analysis (Table 1). Moreover, we identified seven novel chaperones (CG15676, CG15266, CG2911, CG1416, CG6355, CG7182, and CG4538) for which information regarding gene group membership and protein family has not been assigned in FlyBase (Table 1).
Table 1. Identification and classification of Drosophila chaperones.
| Sr. No. | Annotation | FlyBase ID | Gene Name | Symbol | Alternate Name | Gene Group Membership | Protein Family |
|---|---|---|---|---|---|---|---|
| Small heat shock proteins | |||||||
| 1 | CG4167 | FBgn0001227 | Heat shock gene 67Ba | Hsp67Ba | gene 1, gene1 | Small heat shock proteins | Small heat shock protein (HSP20) family |
| 2 | CG4183 | FBgn0001225 | Heat shock protein 26 | Hsp26 | DmHsp26, hsp26, 26 | Small heat shock proteins | Small heat shock protein (HSP20) family |
| 3 | CG4190 | FBgn0001229 | Heat shock gene 67Bc | Hsp67Bc | gene 3 | Small heat shock proteins | Small heat shock protein (HSP20) family |
| 4 | CG4460 | FBgn0001223 | Heat shock protein 22 | Hsp22 | DmHsp22, CG32041 | Small heat shock proteins | Small heat shock protein (HSP20) family |
| 5 | CG4461 | FBgn0035982 | CG4461 | Hsp20 | Small heat shock proteins | ||
| 6 | CG4463 | FBgn0001224 | Heat shock protein 23 | Hsp23 | DmHsp23, 23 | Small heat shock proteins | Small heat shock protein (HSP20) family |
| 7 | CG4466 | FBgn0001226 | Heat shock protein 27 | Hsp27 | Hsp28, DmHsp27, Dhsp27, hsp 27 | Small heat shock proteins | Small heat shock protein (HSP20) family |
| 8 | CG4533 | FBgn0011296 | Lethal (2) essential for life | l(2)efl | Cryab | Small heat shock proteins | Small heat shock protein (HSP20) family |
| 9 | CG7409 | FBgn0035817 | CG7409 | Small heat shock proteins | |||
| 10 | CG13133 | FBgn0032181 | CG13133 | Small heat shock proteins | |||
| 11 | CG14207 | FBgn0031037 | CG14207 | Small heat shock proteins | |||
| Prefoldins | |||||||
| 1 | CG6302 | FBgn0010741 | Prefoldin 2 | Pfdn2 | l(3)01239 | Prefoldin subunit β family | |
| 2 | CG6719 | FBgn0264694 | Merry-go-round | Mgr | Prefoldin subunit α family | ||
| 3 | CG7048 | FBgn0038976 | Prefoldin 5 | Pfdn5 | Prefoldin subunit α family | ||
| 4 | CG7770 | FBgn0036918 | Prefoldin 6 | Pfdn6 | Prefoldin subunit β family | ||
| 5 | CG10635 | FBgn0035603 | Prefoldin 4 | Pfdn4 | Prefoldin subunit β family | ||
| 6 | CG15266 | FBgn0259982 | Lethal (2) 35Cc | l(2)35Cc | |||
| 7 | CG15676 | FBgn0034651 | CG15676 | ||||
| Heat shock protein 40 kDa (HSP40) | |||||||
| 1 | CG1107 | FBgn0037218 | Auxilin | aux | dAux | Heat shock protein 40/DnaJ cochaperones | |
| 2 | CG1409 | FBgn0029964 | CG1409 | Heat shock protein 40/DnaJ cochaperones | |||
| 3 | CG1416 | FBgn0032961 | CG1416 | ||||
| 4 | CG2239 | FBgn0027654 | jdp | jdp | dJDP | Heat shock protein 40/DnaJ cochaperones | |
| 5 | CG2790 | FBgn0027599 | CG2790 | Heat shock protein 40/DnaJ cochaperones | |||
| 6 | CG2887 | FBgn0030207 | CG2887 | Heat shock protein 40/DnaJ cochaperones | |||
| 7 | CG2911 | FBgn0037350 | CG2911 | ||||
| 8 | CG3061 | FBgn0038195 | CG3061 | Heat shock protein 40/DnaJ cochaperones | |||
| 9 | CG4164 | FBgn0031256 | Shriveled | shv | Heat shock protein 40/DnaJ cochaperones | ||
| 10 | CG4599 | FBgn0032586 | Tetratricopeptide repeat protein 2 | Tpr2 | dTPR2 | Heat shock protein 40/DnaJ cochaperones | |
| 11 | CG5001 | FBgn0031322 | CG5001 | Heat shock protein 40/DnaJ cochaperones | |||
| 12 | CG5268 | FBgn0038387 | Black pearl | Blp | l(3)01618 | Heat shock protein 40/DnaJ cochaperones | Belongs to the TIM16/PAM16 family |
| 13 | CG5504 | FBgn0002174 | Lethal (2) tumorous imaginal discs | l(2)tid | Heat shock protein 40/DnaJ cochaperones | ||
| 14 | CG6395 | FBgn0004179 | Cysteine string protein | Csp | Dcsp, ab49 | Heat shock protein 40/DnaJ cochaperones | |
| 15 | CG6693 | FBgn0037878 | CG6693 | Heat shock protein 40/DnaJ cochaperones | |||
| 16 | CG7130 | FBgn0037151 | CG7130 | Heat shock protein 40/DnaJ cochaperones | |||
| 17 | CG7133 | FBgn0037150 | CG7133 | Heat shock protein 40/DnaJ cochaperones | |||
| 18 | CG7387 | FBgn0035852 | CG7387 | Heat shock protein 40/DnaJ cochaperones | |||
| 19 | CG7394 | FBgn0036173 | CG7394 | Heat shock protein 40/DnaJ cochaperones | Belongs to the TIM14 family | ||
| 20 | CG7556 | FBgn0030990 | CG7556 | Heat shock protein 40/DnaJ cochaperones | |||
| 21 | CG7872 | FBgn0030658 | CG7872 | Heat shock protein 40/DnaJ cochaperones | Belongs to the DNAJC25 family | ||
| 22 | CG8014 | FBgn0015477 | Receptor-mediated endocytosis 8 | Rme-8 | l(2)45Ba | Heat shock protein 40/DnaJ cochaperones | |
| 23 | CG8286 | FBgn0037718 | P58IPK | P58IPK | Heat shock protein 40/DnaJ cochaperones | ||
| 24 | CG8448 | FBgn0034091 | Mrj | Mrj | dMRJ | Heat shock protein 40/DnaJ cochaperones | |
| 25 | CG8476 | FBgn0038127 | CG8476 | Heat shock protein 40/DnaJ cochaperones | |||
| 26 | CG8531 | FBgn0033918 | CG8531 | Heat shock protein 40/DnaJ cochaperones | |||
| 27 | CG8583 | FBgn0035771 | Secretory 63 | Sec63 | Heat shock protein 40/DnaJ cochaperones | ||
| 28 | CG8863 | FBgn0038145 | DnaJ-like-2 | Droj2 | Heat shock protein 40/DnaJ cochaperones | ||
| 29 | CG9089 | FBgn0030805 | Wurst | Wus | Heat shock protein 40/DnaJ cochaperones | ||
| 30 | CG9828 | FBgn0032474 | DnaJ homolog | DnaJ-H | Heat shock protein 40/DnaJ cochaperones | ||
| 31 | CG10375 | FBgn0039116 | CG10375 | Heat shock protein 40/DnaJ cochaperones | |||
| 32 | CG10565 | FBgn0037051 | CG10565 | Heat shock protein 40/DnaJ cochaperones | |||
| 33 | CG10578 | FBgn0263106 | DnaJ-like-1 | DnaJ-1 | Hsp40, dHdj1, DnaJ1, droj1 | Heat shock protein 40/DnaJ cochaperones | |
| 34 | CG11035 | FBgn0037544 | CG11035 | Heat shock protein 40/DnaJ cochaperones | |||
| 35 | CG12020 | FBgn0035273 | CG12020 | Heat shock protein 40/DnaJ cochaperones | |||
| 36 | CG14650 | FBgn0037252 | CG14650 | Heat shock protein 40/DnaJ cochaperones | |||
| 37 | CG17187 | FBgn0037882 | CG17187 | Heat shock protein 40/DnaJ cochaperones | |||
| 38 | CG30156 | FBgn0050156 | CG30156 | Heat shock protein 40/DnaJ cochaperones | |||
| 39 | CG32640 | FBgn0052640 | CG32640 | Heat shock protein 40/DnaJ cochaperones | |||
| 40 | CG32641 | FBgn0052641 | CG32641 | Heat shock protein 40/DnaJ cochaperones | |||
| 41 | CG32727 | FBgn0265265 | CG32727 | Heat shock protein 40/DnaJ cochaperones | |||
| 42 | CG34246 | FBgn0263606 | Heat shock protein cognate 20 | Hsc20 | l(3)72Do | Heat shock protein 40/DnaJ cochaperones | |
| 43 | CG40178 | FBgn0058178 | CG40178 | Heat shock protein 40/DnaJ cochaperones | |||
| 44 | CG42567 | FBgn0260775 | DnaJ-like-60 | DnaJ-60 | DnaJ60 | Heat shock protein 40/DnaJ cochaperones | |
| 45 | CG43322 | FBgn0263027 | CG43322 | Heat shock protein 40/DnaJ cochaperones | |||
| Heat shock protein 60 kDa (HSP60) | |||||||
| 1 | CG2830 | FBgn0011244 | Heat shock protein 60B | Hsp60B | Hsp64 | Heat shock protein 60 chaperonins group i | Chaperonin (HSP60) family |
| 2 | CG5374 | FBgn0003676 | Chaperonin-containing TCP1 subunit 1 | CCT1 | T-cp1, T-cpl, tcp1, Tcp1-like, Tcp1-α | Heat shock protein 60 chaperonins group ii | TCP-1 chaperonin family |
| 3 | CG5525 | FBgn0032444 | Chaperonin-containing TCP1 subunit 4 | CCT4 | CCT4, Tcp1-δ | Heat shock protein 60 chaperonins group ii | |
| 4 | CG6355 | FBgn0028741 | Fab1 kinase | fab1 | |||
| 5 | CG7033 | FBgn0030086 | Chaperonin-containing TCP1 subunit 2 | CCT2 | CCT2, Tcp1-β | Heat shock protein 60 chaperonins group ii | |
| 6 | CG7235 | FBgn0031728 | Heat shock protein 60C | Hsp60C | Hsp64 | Heat shock protein 60 chaperonins group i | Chaperonin (HSP60) family |
| 7 | CG8231 | FBgn0027329 | Chaperonin-containing TCP1 subunit 6 | CCT6 | Tcp-1ζ, TCP-1ζ, l(1)G0022 | Heat shock protein 60 chaperonins group ii | |
| 8 | CG8258 | FBgn0284436 | Chaperonin-containing TCP1 subunit 8 | CCT8 | Tcp1-θ | Heat shock protein 60 chaperonins group ii | |
| 9 | CG8351 | FBgn0037632 | Chaperonin-containing TCP1 subunit 7 | CCT7 | tcp-1η, Cct7 | Heat shock protein 60 chaperonins group ii | |
| 10 | CG8439 | FBgn0010621 | Chaperonin-containing TCP1 subunit 5 | CCT5 | cct5, Tcp1-ɛ | Heat shock protein 60 chaperonins group ii | |
| 11 | CG8977 | FBgn0015019 | Chaperonin-containing TCP1 subunit 3 | CCT3 | Cctγ, Y, Cctg, cct-γ, TCPG_DROME, Tcp1-γ, Cctγ | Heat shock protein 60 chaperonins group ii | TCP-1 chaperonin family |
| 12 | CG12101 | FBgn0015245 | Heat shock protein 60A | Hsp60A | hsp60, l(1)BP5, Hsp60A, Dmhsp60, 12, Hsp64 | Heat shock protein 60 chaperonins group i | Chaperonin (HSP60) family |
| 13 | CG16954 | FBgn0032525 | Heat shock protein 60D | Hsp60D | Heat shock protein 60 chaperonins group i | ||
| Heat Shock Protein 70 kDa (HSP70) | |||||||
| 1 | CG2918 | FBgn0023529 | CG2918 | EG:25E8.1, GRP170 | Atypical heat shock protein 70 chaperones | ||
| 2 | CG4147 | FBgn0001218 | Heat shock 70-kDa protein cognate 3 | Hsc70-3 | Bip, HSC3, Hsc70, Grp78, Hsc-70-3, dBiP | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 3 | CG4264 | FBgn0266599 | Heat shock protein cognate 4 | Hsc70-4 | Hsc4, Hsc70, hsp70 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 4 | CG5436 | FBgn0001230 | Heat shock protein 68 | Hsp68 | 68 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 5 | CG5834 | FBgn0051354 | Heat shock protein 70Bbb | Hsp70Bbb | Hsp70, Hsp70B | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 6 | CG6489 | FBgn0013279 | Heat shock protein 70Bc | Hsp70Bc | Hsp70, Hsp70B, hsp-70, dhsp70 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 7 | CG6603 | FBgn0026418 | Hsc70Cb | Hsc70Cb | HSC70 | Atypical heat shock protein 70 chaperones | |
| 8 | CG7182 | FBgn0035878 | CG7182 | ||||
| 9 | CG7756 | FBgn0001217 | Heat shock protein cognate 2 | Hsc70-2 | Hsc70, HSC2 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 10 | CG8542 | FBgn0001220 | Heat shock protein cognate 5 | Hsc70-5 | Hsc70, Hsc5 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 11 | CG8937 | FBgn0001216 | Heat shock protein cognate 1 | Hsc70-1 | Hsc70 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 12 | CG18743 | FBgn0013276 | Heat shock protein 70Ab | Hsp70Ab | Hsp70, Hsp70A, hsp-70, dhsp70 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 13 | CG31359 | FBgn0013278 | Heat shock protein 70Bb | Hsp70Bb | Hsp70, Hsp70B, hsp-70, dhsp70 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 14 | CG31366 | FBgn0013275 | Heat shock protein 70Aa | Hsp70Aa | Hsp70, Hsp70A, hsp-70, dhsp70 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| 15 | CG31449 | FBgn0013277 | Heat shock protein 70Ba | Hsp70Ba | Hsp70, Hsp70B, hsp-70, dhsp70 | Heat shock protein 70 chaperones | Heat shock protein 70 family |
| Heat shock protein 90 kDa (HSP90) | |||||||
| 2 | CG1242 | FBgn0001233 | Heat shock protein 83 | Hsp83 | Hsp90, Hsp82, E(sina)2, 83 | Heat shock protein 90 chaperones | Heat shock protein 90 family |
| 1 | CG3152 | FBgn0026761 | Trap1 | Trap1 | Heat shock protein 90 chaperones | ||
| 3 | CG5520 | FBgn0039562 | Glycoprotein 93 | Gp93 | Heat shock protein 90 chaperones | ||
| Heat shock protein 100 kDa (HSP100) | |||||||
| 1 | CG4538 | FBgn0038745 | CG4538 | ||||
The comprehensive list of all Drosophila chaperones is obtained by using bioinformatics analysis. BLASTP searches in FlyBase (http://flybase.org/) and PSI-BLAST searches in NCBI using protein sequences of yeast chaperones as query sequences were performed. A comprehensive list from nonredundant hits of each BLASTP search was further analyzed for domain organization and the validated candidates are reported as a member of respective chaperone families in Drosophila based on their domain organization. In total, Drosophila genome contains 95 chaperones which we classify into seven families. The list of chaperones belonging to each class is shown in the table. There are several chaperones in our comprehensive list for which protein family or gene group membership has not been assigned in FlyBase. The chaperones with no data on gene family and gene group membership are considered as novel chaperones and highlighted in the table. Sr., serial number; No., number; ID, identifier.
Ubiquitous knockdown identified several essential chaperones in Drosophila
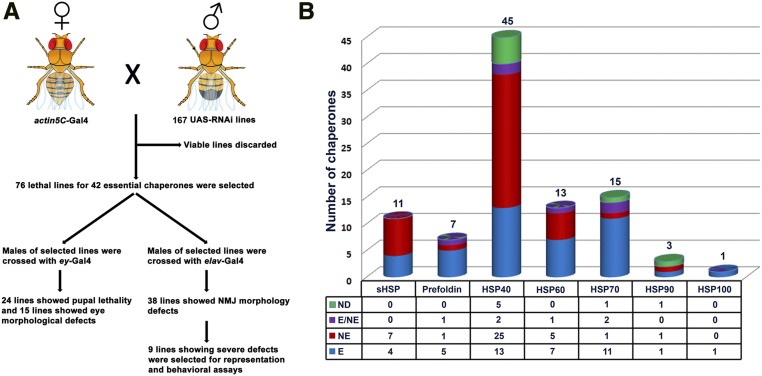
The RNAi technique has evolved to be a powerful approach to perform genome-wide or targeted reverse genetic screens to identify genes involved in various cellular pathways (Sharma and Rao 2009; Mohr 2014; Chen and Xu 2016; Agrawal and Hardin 2016; Vissers et al. 2016). For instance, such screens have identified novel genes involved in Drosophila nervous system development (Koizumi et al. 2007) and wound closure (Lesch et al. 2010). While most of the chaperones are expressed in the nervous system, their functional requirements in neurons remain largely unknown. To address this, we performed an RNAi screen to identify Drosophila chaperones with neuron-specific functions. We first identified the essential chaperones in Drosophila by actin5C-Gal4-mediated knockdown of 168 RNAi lines against 95 chaperone genes. Lines that did not produce viable F1 progeny were considered lethal. Since RNAi-mediated knockdown may cause off-target effects or inefficient knockdown (Dietzl et al. 2007), we used multiple RNAi lines for each chaperone gene subject to its availability at the VDRC. Chaperones for which the proportion of lethal lines was more than the viable lines were considered as essential chaperones (Figure 1). Due to this conservative approach, our list of 42 essential chaperones may be an underestimate in Drosophila. Alternatively, if at least one lethal line for an individual chaperone gene is considered to be a sign of its essential function, the list can be extended to 51 essential chaperones in Drosophila.
Figure 1.
Workflow for the RNAi screen and list of Drosophila chaperones. (A) Schematic representation of workflow for UAS-RNAi for identification of their neuronal function in Drosophila. A total of 167 RNAi lines corresponding to 95 chaperones were crossed with actin5C-Gal4 for ubiquitous knockdown. The candidate chaperones responsible for lethal events in the F1 generation were considered as essential. All the essential line were then crossed with either ey-Gal4 (to identify chaperones required in eye morphogenesis) or with pan-neuronal elavC155-Gal4 (to identify chaperones required for neuronal function for which NMJ morphology was used as readout). Detailed analysis is shown in Table S1 and Table S2. (B) Histogram showing seven families of chaperones in Drosophila. The number above the histogram represents the total number of chaperones in each family. The Hsp40 family dominated the list, while only one Hsp100 was identified in Drosophila. The table below the histograms shows the number of essential chaperones (E), nonessential chaperones (NE), chaperones for which 50% of lines were lethal (E/NE), and genes for which lines could not be procured (ND). Detailed analysis of actin5C- Gal4 mediated knockdown is shown in Table S1. HSP, heat shock protein; NMJ, neuromuscular junction; RNAi, RNA interference; sHSP, small HSP; UAS, upstream activation sequence.
Eye-specific knockdown identified several essential chaperones required for rhabdomere biogenesis
The Drosophila compound eye has been widely used as an excellent model system to screen for and identify new genes involved in development, eye physiology, and neurodegeneration (Bonini and Fortini 2002). The major advantage of using the Drosophila eye as a read out in a screen is that even the subtle morphological defects of eyes can be easily recognized. This allows rapid assessment of phenotype, which can be correlated to cellular defect, disease mechanism, and/or neurodegeneration (Prussing et al. 2013; Lu and Vogel 2009). Moreover, the Drosophila eye, being a nonvital organ, allows eye-specific expression of disease-related genes to better understand genetic interactions owing to disease onset and progression (Hirth 2010).
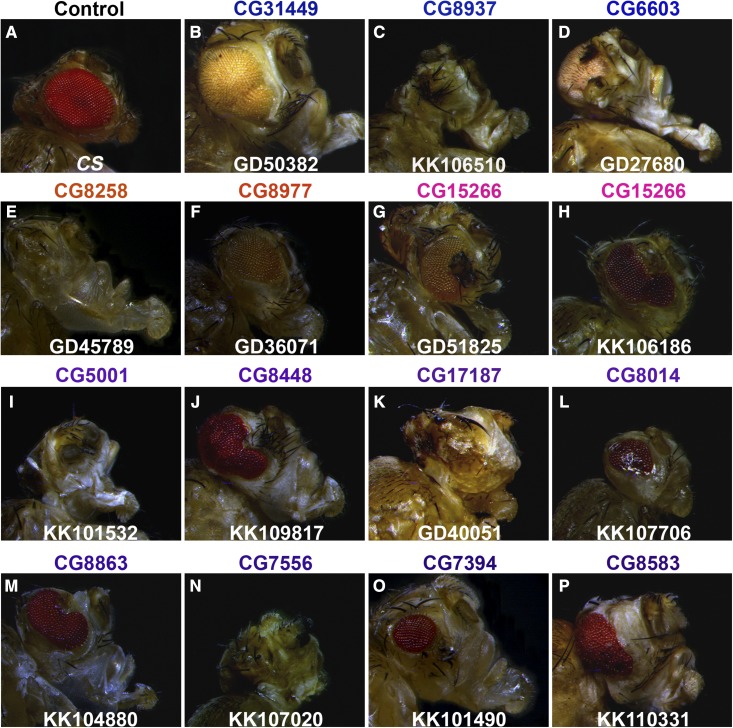
Hence, in order to further investigate their functional relevance, we knocked-down essential Drosophila chaperones in eyes using the ey-Gal4 driver. Seventy-six RNAi lines corresponding to 42 essential chaperones were crossed to the ey-Gal4 driver. The eyes of anesthetized F1-flies were assessed under a light microscope. Knockdown of several essential chaperones resulted in eye morphological defects suggesting that they are crucial for the development of proper rhabdomeres (Figure 2). The range of eye morphological defects and their penetrance is summarized in Table 2. For three of the chaperones (CG8014, CG7394, and CG8583), only one of the two independent RNAi lines resulted in eye morphological defects. This discrepancy could be due to variability in their knockdown efficiency. In addition, ey-Gal4-driven knockdown of 22 lines led to pupal lethality (Table S2) or headless pupae (data not shown). This suggests that ey-Gal4 expression is not tightly restricted to the Drosophila eyes. Taken together, we identified a set of Drosophila chaperones that regulate eye morphogenesis. However, the precise mechanisms by which these individual chaperones regulate eye morphology need to be further addressed.
Figure 2.
ey-Gal4-driven knockdown of candidate essential chaperones alters eye morphology. Photomicrograph of eyes of anesthetized 7-d-old F1 progeny, representing eye-specific knockdown of essential chaperone genes. Canton special (CS) flies crossed with ey-Gal4 are used as control (A). Knockdown of essential chaperones belonging to different families, Hsp70 (blue, B–D), Hsp60 (orange, E and F), Prefoldins (magenta, G and H), and Hsp40 (violet, I–P) exhibit a wide range of eye morphology defects.
Table 2. Eye-specific knockdown of several Drosophila chaperones result in eye morphological defects.
| Sr. No. | VDRC Line | Gene | Representative Eye Phenotype | % Flies Showing Eye Morphology Defects |
|---|---|---|---|---|
| Heat shock protein 70 chaperones | ||||
| 1 | GD50382 | CG31449 | Rough/large eye | 100 |
| 2 | KK106510 | CG8937 | Eyeless | 100 |
| 3 | GD27680 | CG6603 | Severely deformed eye | 40 |
| Heat shock protein 60 chaperonins | ||||
| 4 | GD45789 | CG8258 | Severely deformed eye | 80 |
| 5 | GD36071 | CG8977 | Rough eye | 100 |
| Prefoldin | ||||
| 6 | GD51825 | CG15266 | Ectopic bristles in eyes | 50 |
| 7 | KK106186 | CG15266 | Deformed eye | 100 |
| Heat shock protein40/DnaJ cochaperones | ||||
| 8 | KK101532 | CG5001 | Eyeless | 100 |
| 9 | KK109817 | CG8448 | Deformed eye | 90 |
| 10 | GD40051 | CG17187 | Severely deformed eye | 100 |
| 11 | KK107706 | CG8014 | Liquid facet-like phenotype | 100 |
| 12 | KK104880 | CG8863 | Deformed eye | 100 |
| 13 | KK107020 | CG7556 | Severely deformed eye | 90 |
| 14 | KK101490 | CG7394 | Small eye, reduced number of ommatidia | 100 |
| 15 | KK110331 | CG8583 | Rough and deformed eye | 100 |
ey-Gal4-driven knockdown identified essential chaperones required for regulating eye morphology and/or rhabdomere development. Knockdown of some of these candidate genes using ey-Gal4 also leads to partial pupal lethality. Sr., serial number; No., number; VDRC, Vienna Drosophila Resource Center.
Several essential chaperones regulate NMJ structural development
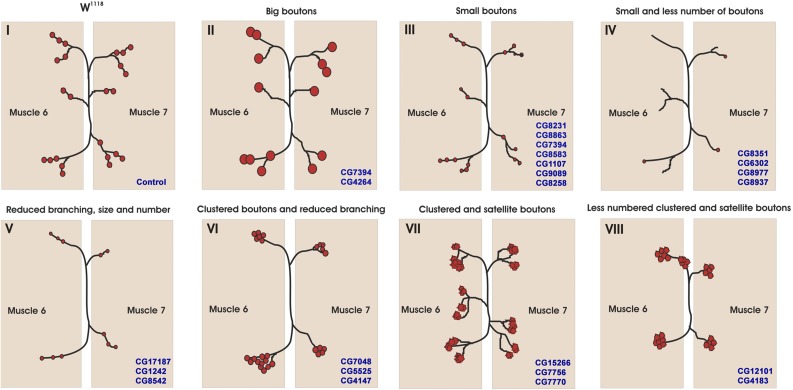
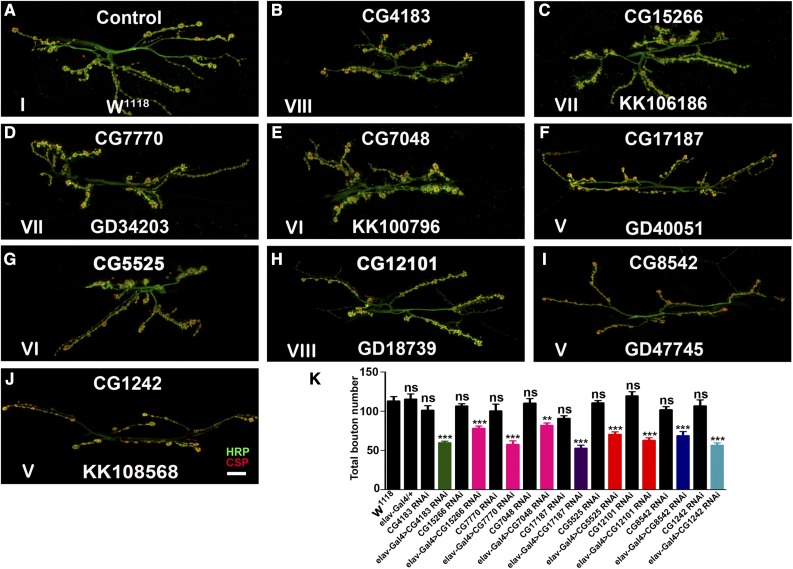
While most of the chaperones are expressed in the nervous system, their role in neuronal development and function remains largely unknown. Hence, to assess the requirement of Drosophila chaperones in neurons, we knocked-down essential chaperones using the pan-neuronal driver elavC155-Gal4 and analyzed the larval NMJ (a specialized synapse formed between motor neurons and muscles) morphology as a read out for possible defects. The NMJs are also among the earliest pathological targets at the onset of several neurological disorders including tauopathies, amyotrophic lateral sclerosis, and spinal muscular atrophy. Neuronal knockdown of these genes identified several chaperones that regulate NMJ structural development in Drosophila (Figure 3 and Table S2). We obtained a range of NMJ defects that are summarized in Table S2. All the lines tested for selected genes show more or less similar phenotypes for NMJ morphological defects. The data reported in Table S2 were single blinded, and at least 7 out of 10 NMJs in RNAi knockdown could be identified that differed from control NMJs. However, we have chosen lines that showed 100% penetrance in NMJ morphological defects for representation purpose in Figure 4. Nine of the essential chaperones gave severe NMJ morphological defects (Figure 4). We found that, as compared to the control synapse, neuronal knockdown of these essential chaperones resulted in a significant reduction in the number of boutons (Figure 4K). Taken together, these data suggest that many of the chaperones regulate NMJ morphology in Drosophila.
Figure 3.
Cartoon representation of the range of neuromuscular junction (NMJ) morphological defects observed due to knockdown of essential chaperones in neurons. Cartoon represents NMJ phenotypic classes upon neuronal depletion of essential chaperones in Drosophila. Various NMJ phenotypes were observed and classified into different classes (I–VIII). The NMJ with (I) control, (II) big boutons, (III) small boutons, (IV) small and less number of boutons, (V) reduced branching, size, and number, (VI) clustered boutons and reduced branching, (VII) clustered and satellite boutons, and (VIII) less numbered clustered and satellite boutons are represented.
Figure 4.
Neuronal depletion of candidate essential chaperones alter NMJ morphology in Drosophila. (A–J) Confocal images of NMJ synapses at muscle 6/7 of (A) control, elav-Gal4 driven (B) CG4183 RNAi, (C) CG15266 RNAi, (D) CG7770 RNAi, (E) CG7048 RNAi, (F) CG17187 RNAi, (G) CG5525 RNAi, (H) CG12101 RNAi, (I) CG8542 RNAi, and (J) CG1242 RNAi double immunolabeled with a presynaptic marker (CSP, red) and neuronal membrane marker (HRP, green) to reveal the bouton outline at the NMJs. Compared to the control NMJ, elav-Gal4-mediated depletion of the above-mentioned essential chaperones showed significantly altered NMJ morphology. The Roman numerals in the image correlate the NMJ morphological defect of each panel with the corresponding phenotypic class depicted in Figure 3. The bouton numbers were determined by manually counting the number of CSP-positive varicosities at the NMJs. Bar in (J) represents 20 µm. (K) Histogram showing average number of boutons at muscle 6/7 of A2 hemisegment in the control, elav-Gal4/+, CG4183 RNAi, elav-Gal4-driven CG4183 RNAi, CG15266 RNAi, elav-Gal4-driven CG15266 RNAi, CG7770 RNAi, elav-Gal4-driven CG7770 RNAi, CG7048 RNAi, elav-Gal4-driven CG7048 RNAi, CG17187 RNAi, elav-Gal4-driven CG17187 RNAi, CG5525 RNAi, elav-Gal4-driven CG5525 RNAi, CG12101 RNAi, elav-Gal4-driven CG12101 RNAi, CG8542 RNAi, elav-Gal4-driven CG8542 RNAi, CG1242 RNAi, and elav-Gal4-driven CG1242 RNAi. Histogram in black represents controls including w1118, elav-Gal4/+, and the parental lines. Total number of boutons upon pan-neuronal knockdown of sHSP (green), Prefoldins (magenta), Hsp40 (violet), Hsp60 (orange), Hsp70 (dark blue), and Hsp90 (light blue) have been represented. At least eight NMJ synapses of A2 hemisegments from four larvae of each genotype were used for bouton quantification. ** P < 0.001 and *** P < 0.0001. Error bars represent SEM (mean ± SEM). Statistical analysis based on one-way ANOVA with post hoc Tukey’s test for multiple comparisons. CSP, cysteine string protein; HRP, horseradish peroxidase; Hsp, heat shock protein; NMJ, neuromuscular junction; ns, not significant; RNAi, RNA interference; sHSP, small HSP.
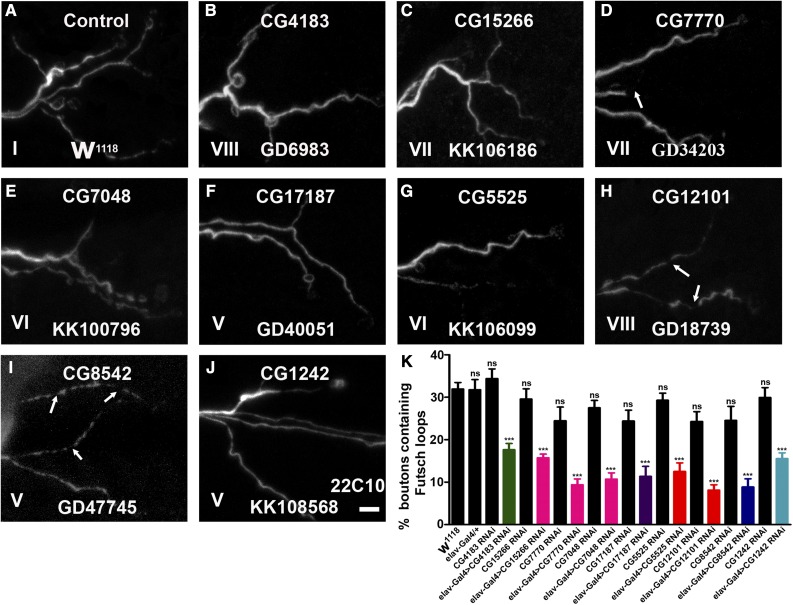
Microtubule cytoskeleton is disorganized in neuronally depleted essential chaperones
Previous reports suggest that synaptic growth is regulated by microtubule organization and that perturbation of the cytoskeleton affects synaptic growth (Pawson et al. 2008). Hence, in order to assess alterations in the microtubule cytoskeleton upon pan-neuronal reduction of essential chaperones, we labeled synapses with an anti-Futsch antibody that labels axonal and nerve terminal cytoskeleton (Roos et al. 2000). In the control boutons, the microtubule appeared continuous with periodic loop structures (Figure 5). However, the microtubules in the chaperone-depleted larvae showed significantly reduced microtubule loops (Figure 5K). Interestingly, neuronal reduction of some of the chaperones such as CG12101, CG7770, and CG8542 showed interrupted microtubule assembly at the presynapse. These data support the fact that the identified essential chaperones regulate NMJ structural morphology by regulating the cytoskeletal architecture.
Figure 5.
Neuronal knockdown of candidate essential chaperones cause disruption of the presynaptic cytoskeleton. (A–J) Representative images of third instar larval NMJs from muscle 6/7 of A2 hemisegment in (A) control or elav-Gal4-driven (B) CG4183 RNAi, (C) CG15266 RNAi, (D) CG7770 RNAi, (E) CG7048 RNAi, (F) CG17187 RNAi, (G) CG5525 RNAi, (H) CG12101 RNAi, (I) CG8542 RNAi, and (J) CG1242 RNAi animals labeled with mAb22C10. Compared to the control NMJ, elavC155-Gal4-driven knockdown of above essential chaperones shows a significant reduction in the number of Futsch-positive loops and, in some cases, synapses with broken Futsch loops were also seen (marked with arrows). The Roman numerals in the image correlate the NMJ morphological defect of each panel with the corresponding phenotypic class depicted in Figure 3. Bar in (J) represents 4 µm. (K) Histogram showing quantification of the percentage of boutons containing Futsch loops of control, elav-Gal4/+, CG4183 RNAi, elav-Gal4-driven CG4183 RNAi, CG15266 RNAi, elav-Gal4-driven CG15266 RNAi, CG7770 RNAi, elav-Gal4-driven CG7770 RNAi, CG7048 RNAi, elav-Gal4-driven CG7048 RNAi, CG17187 RNAi, elav-Gal4-driven CG17187 RNAi, CG5525 RNAi, elav-Gal4-driven CG5525 RNAi, CG12101 RNAi, elav-Gal4-driven CG12101 RNAi, CG8542 RNAi, elav-Gal4-driven CG8542 RNAi, CG1242 RNAi, and elav-Gal4-driven CG1242 RNAi. Histogram in black represents controls including w1118, elav-Gal4/+, and parental lines. Percentage of boutons containing Futsch loops upon pan-neuronal knockdown of sHSP (green), Prefoldins (magenta), Hsp40 (violet), Hsp60 (orange), Hsp70 (dark blue), and Hsp90 (light blue) have been represented. At least eight NMJ synapses from four larvae of each genotype were used for Futsch loop quantification. *** P < 0.0001. Error bars represent SEM (mean ± SEM). Statistical analysis based on one-way ANOVA with post hoc Tukey’s test for multiple comparisons. Hsp, heat shock protein; NMJ, neuromuscular junction; ns, not significant; RNAi, RNA interference; sHSP, small HSP.
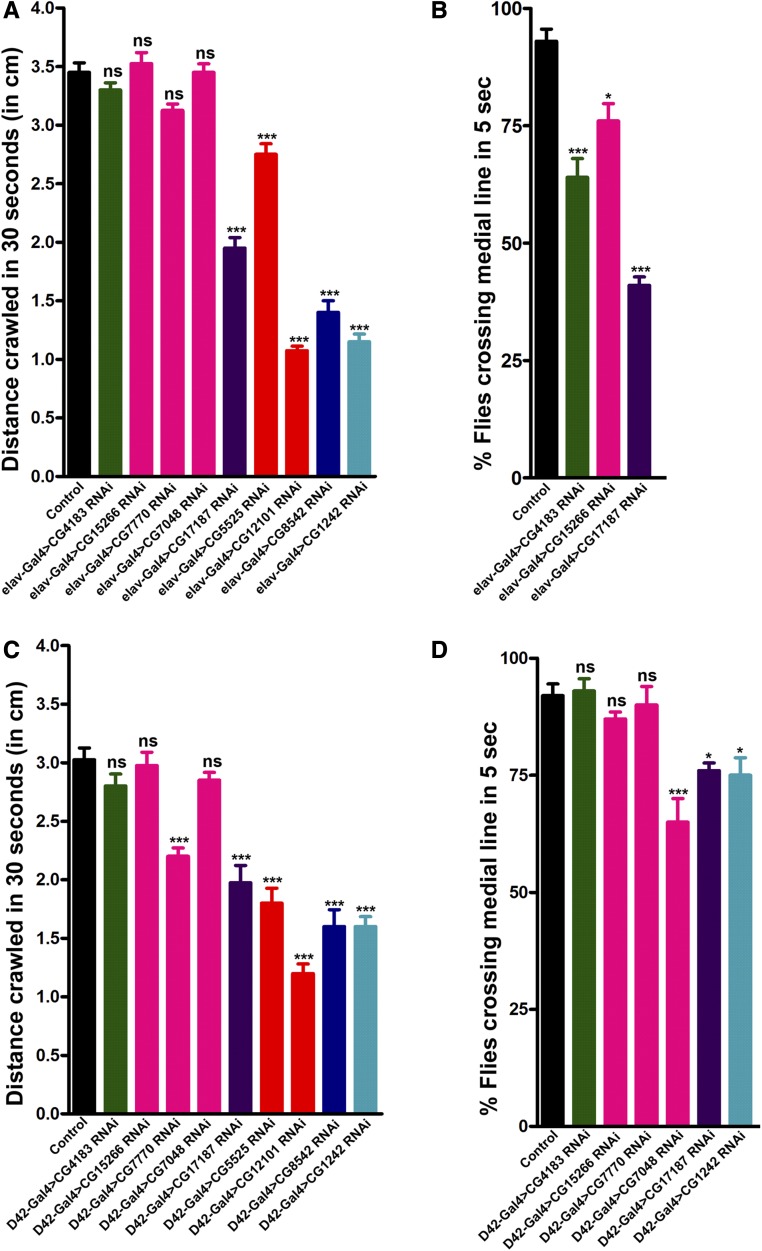
Neuronal depletion of candidate chaperones result in motor behavior deficits
From screening of pan-neuronal knockdown of all essential chaperones, nine candidate genes resulted in severe morphological defects at the NMJ. Aberrant neuronal communication and thus neuronal dysfunction is evident with such severe NMJ morphology defects. Since such defects often lead to compromised locomotor ability (Mudher et al. 2004; Mhatre et al. 2014), we next examined the effect of pan-neuronal knockdown of these candidate chaperones on locomotive behavior. We analyzed the larval crawling and climbing abilities of adults upon pan-neuronal knockdown of chaperones. Larval crawling ability was significantly reduced upon knockdown of five selected essential chaperones CG1242, CG12101, CG5525, CG8542, and CG17187 (Figure 6A). Compared to the control, we observed significant adult climbing defects upon knockdown of three of these genes (Figure 6B). The climbing ability test could only be performed for three chaperones (CG4183, CG15266, and CG17187) as pan-neuronal knockdown of the rest of the selected chaperones resulted in pupal lethality.
Figure 6.
Locomotive behavior affected by pan-neuronal knockdown of essential chaperones. (A) Pan-neuronal downregulation of several essential chaperones affect crawling ability of third instar larvae. Histogram showing average distance crawled in 30 sec by control larvae (3.45 ± 0.08) or larvae with pan-neuronal knockdown of CG4183 (3.3 ± 0.06), CG15266 (3.52 ± 0.09), CG7770 (3.12 ± 0.06), CG7048 (3.45 ± 0.07), CG17187 (1.95 ± 0.09), CG5525 (2.75 ± 0.09), CG12101 (1.08 ± 0.04), CG8542 (1.40 ± 0.10), and CG1242 (1.15 ± 0.06). n = 10, *** P < 0.0001. Error bars represent SEM (mean ± SEM). Statistical analysis based on one-way ANOVA with post hoc Tukey’s test for multiple comparisons. Distance crawled in 30 sec (cm) upon pan-neuronal knockdown of sHSP (green), Prefoldins (magenta), Hsp40 (violet), Hsp60 (orange), Hsp70 (dark blue), and Hsp90 (light blue) have been represented. (B) Pan-neuronal knockdown of essential chaperones affect climbing ability of adult flies. Histogram shows % flies crossing median line in 5 sec in control flies (93.00 ± 2.60) or flies with pan-neuronal knockdown of CG4183 (64.00 ± 4.00), CG15266 (76.00 ± 3.71), and CG17187 (41.00 ± 1.79). n = 10, *P < 0.01, ***P < 0.0001. Error bars represent SEM (mean ± SEM). Statistical analysis based on one-way ANOVA with post hoc Tukey’s test for multiple comparisons. Percentage of flies crossing the medial line upon pan-neuronal knockdown of sHSP (green), Prefoldins (magenta), and Hsp40 (violet) have been represented. (C) Motor neuron-specific downregulation of several essential chaperones affect crawling ability of third instar larvae. Histogram showing average distance crawled in 30 sec by control larvae (3.03 ± 0.10) or larvae with motor neuron-specific knockdown of CG4183 (2.80 ± 0.10), CG15266 (2.97 ± 0.11), CG7770 (2.20 ± 0.07), CG7048 (2.85 ± 0.07), CG17187 (1.98 ± 0.15), CG5525 (1.80 ± 0.13), CG12101 (1.20 ± 0.08), CG8542 (1.60 ± 0.15), and CG1242 (1.60 ± 0.08). n = 10, *** P < 0.0001. Error bars represent SEM (mean ± SEM). Statistical analysis based on one-way ANOVA with post hoc Tukey’s test for multiple comparisons. Distance crawled in 30 sec (cm) upon motor neuron specific knockdown of sHSP (green), Prefoldins (magenta), Hsp40 (violet), Hsp60 (orange), Hsp70 (dark blue), and Hsp90 (light blue) have been represented. (D) Motor neuron-specific downregulation of several essential chaperones affect climbing ability of adult flies. Histogram shows % flies crossing median line in 5 sec in control flies (92.00 ± 2.49) or flies with motor neuron specific knockdown of CG4183 (93.00 ± 2.60), CG15266 (87.00 ± 1.53), CG7770 (90.00 ± 3.94), CG7048 (65.00 ± 5.00), CG17187 (76.00 ± 1.63), and CG1242 (75.00 ± 3.73). n = 10, * P < 0.01 and *** P < 0.0001. Error bars represent SEM (mean ± SEM). Statistical analysis based on one-way ANOVA with post hoc Tukey’s test for multiple comparisons. Percentage of flies crossing the medial line upon motor neuron-specific knockdown of sHSP (green), Prefoldins (magenta), Hsp40 (violet), and Hsp90 (light blue) have been represented. Hsp, heat shock protein; ns, not significant; sHSP, small HSP.
Since pan-neuronal knockdown of most of the selected chaperones resulted in pupal lethality, we used the motor neuron-specific D42-Gal4 driver to expand our findings on adult behavior. We performed a larval crawling assay for D42-Gal4-driven knockdown of nine selected essential chaperones. Significant defects in crawling ability were observed upon knockdown of six of the nine chaperones (Figure 6C). The adult climbing assay could only be performed for six out of nine selected chaperones, as D42-Gal4-driven knockdown of three of the selected chaperones still resulted in pupal lethality (Figure 6D).
The behavioral defect upon neuronal knockdown of several candidate genes is consistent with the NMJ phenotype. It will be interesting to further investigate the functional role of these individual chaperones at the NMJ and to find out whether the behavioral deficit is coupled with their requirement in neurons.
Discussion
Processes regulating protein turnover and trafficking affect myriad functions in all cells. Given the undeniable role of molecular chaperones in maintaining cellular proteostasis, chaperones are likely to have important roles in neuromorphogenesis, both during development and synaptic plasticity. Consistent with this, several molecular chaperones are reported to play neuroprotective roles, have been linked to various neuropathies (Smith et al. 2015), and are considered to be potential therapeutic targets for neurodegenerative disorders (Ebrahimi-Fakhari et al. 2013). However, despite their importance in neuronal proteostasis, neurodegeneration, and therapeutics, very little is known about the specific functions of molecular chaperones in neurons. Hence, to elucidate their neuronal functions, we performed an RNAi screen of all canonical chaperones in Drosophila and identified several chaperones that play crucial roles in regulating eye morphogenesis and NMJ structural plasticity.
Although ubiquitous, molecular chaperones are best characterized in the unicellular eukaryote S. cerevisiae (Gong et al. 2009). Thus, using S. cerevisiae chaperone sequences as a template for bioinformatics analysis, we identified and classified all Drosophila chaperones and established a list of 95 candidates, the most comprehensive list in this organism to date. We followed an unbiased but conservative approach to identify essential chaperones and screened multiple RNAi lines for each chaperone to overcome the limitations of the RNAi approach. As evident from ubiquitous knockdown, there is a greater proportion of essential chaperones in Drosophila compared to that in S. cerevisiae. For further functional characterization of these essential chaperones, only lethal lines were tested to avoid cases such as inefficient knockdown and lack of remarkable phenotypes, an approach earlier mentioned in a similar screen (Liu et al. 2016). Using pan-neuronal and eye-specific Gal4 drivers, lethal lines were screened to identify essential chaperones having possible neuronal functions. Although we suggest that our list of essential chaperones is conservative, we do not rule out the possibility of off-target knockdown of the genes. Genes belonging to the same chaperone family are highly conserved at the nucleotide level. This may lead to the knockdown of multiple members of a chaperone family, in addition to the gene against which the RNAi line was used. Gene-specific knockout of these candidate chaperones would be a more appropriate way to assess their essential function.
We observed varying eye phenotypes upon eye-specific knockdown of almost one-third of essential chaperones. The majority of chaperones exhibiting eye morphological defects were annotated as Hsp40s (also called as J-proteins). Along with their Hsp70 partners, Hsp40s are reported to be involved in maintaining cellular proteostasis by regulating protein folding, protein turnover, and remodeling of macromolecular structures (Hartl et al. 2011). For example, HsJ1, a neuronal Hsp40, is required for the sorting of terminally misfolded proteins to the proteasome in Drosophila (Westhoff et al. 2005). Additionally, J-proteins and Hsp70 have been shown to combat protein aggregation and/or induce apoptosis in cultured neurons (Kobayashi et al. 2000), further linking the Hsp70:Hsp40 chaperone machinery with neuronal functions. This is specifically relevant to CG8863 and CG5001, whose yeast counterparts Ydj1 and Sis1 (cytosolic Hsp40s) play important roles in protein folding and clearance of protein aggregates.
Silencing of some of the Hsp70s, Cpn60s, and prefoldin family proteins also resulted in eye morphological defects suggesting important roles in rhabdomere biogenesis. In-depth analysis of the mechanism underlying eye morphological defects due to downregulation of these chaperones will help us better understand the eye-specific function of these essential chaperones. It is likely that de novo protein folding, protein turnover, or remodeling of the cytoskeleton may be regulated by these chaperones and that their perturbation causes eye defects. Eyeless also expresses in the brain and other parts of the nervous system (Callaerts et al. 2001). Consistent with this, our result showed that ∼50% of the essential chaperones resulted in lethality upon eyeless-Gal4 driven knockdown, some showing a headless phenotype (for example, CG5525 belonging to the Hsp60 family).
Interestingly, pan-neuronal silencing of many essential chaperones resulted in morphological defects at the third instar larval NMJ. Among these, nine essential chaperones belonging to different families exhibited dramatic changes in NMJ morphology, signifying their importance in synaptic development, growth, and plasticity. Knockdown of these essential chaperones underscores their requirements in possible de novo protein folding or their role in mitochondrial function, cytoskeleton organization, neurogenesis, and spliceosome remodeling, all of which may result in altered NMJ morphology. While an imbalance in cellular proteostasis has a marked effect on the NMJ phenotype, one pathway that has been tightly linked with altered NMJ morphology is perturbation of the cytoskeletal architecture (Bodaleo and Gonzalez-Billault 2016). Synaptic morphology is regulated by a neuronal cytoskeleton that maintains the growing end of the synapse. The microtubule-associated neuronal protein Futsch regulates synaptic microtubules, which is essential for synaptic growth at the Drosophila NMJ. Futsch loops were shown to be associated with stable synaptic boutons, any deregulation or alteration of which impairs microtubule organization (Roos et al. 2000). Molecular chaperones play an important role in cytoskeletal organization and/or cytoskeleton remodeling (Liang and MacRae 1997). Along the same lines, alteration of NMJ morphology was observed upon neuronal depletion of candidate chaperones as a consequence of a perturbed neuronal cytoskeleton. Consistent with the observed cytoskeletal defects, sHsps, prefoldins, and Hsp83 have been shown to either associate with cytoskeletal components or have cytoskeletal remodeling functions (Liang and MacRae 1997). Interestingly, some of these essential chaperones, including CG4183 (sHsp), CG12101 (Hsp60), and CG5525 (Hsp60) have been shown to be microtubule-associated proteins, and CG1242 (Hsp90) is a part of the actin cytoskeleton (Hughes et al. 2008; Kiger et al. 2003). Defects in NMJ morphology upon neuronal depletion of CG8542 (mortalin) and CG12101 (Hsp60), the mitochondrial Hsp70 and Hsp60, respectively, suggest that mitochondrial protein import and folding is somehow crucial for synaptic development. Perturbation of cellular protein folding and the remodeling of protein aggregates was found to have a severe impact on both eye development and synaptic plasticity. Several selected essential chaperones exhibited defects in locomotive behavior upon pan-neuronal as well as motor neuron-specific knockdown, suggesting their crucial neuron-specific function. One of the surprising candidates that showed significant eye and NMJ phenotypes was the S. cerevisiae Cwc23 ortholog (CG17187). RNAi against CG17187 resulted in defective rhabdomere biogenesis and altered NMJ morphology, as well as behavioral deficits. Thus, we surmise that, like Cwc23, CG17187 could also be involved in splicing (Sahi et al. 2010). It is likely that defective splicing of multiple genes involved in neuronal functions could lead to pleiotropic defects in eyes and at the NMJ.
Several studies have used the RNAi-mediated knockdown approach to assess the role of chaperones in various cellular contexts. For instance, a genome-wide RNAi screen in Drosophila identified Hsp70 as a regulator of intestinal stem cells (Zeng et al. 2015). Similarly, an RNAi screen identified Hsp60 and Hsc70 as novel modulators of mitochondrial function (Chen et al. 2008); Hsp40 was also identified as a modifier of Huntingtin aggregation (Doumanis et al. 2009). In a similar screen, almost 2000 Drosophila genes having close human orthologs were screened, which led to the identification of several genes (including chaperones) that affect NMJ growth and maintenance (Valakh et al. 2012). We found that out of the 95 chaperones on our list of total Drosophila chaperones, 20 were screened by Valakh et al. (2012). Thus, one of the reasons why we found a greater number of chaperones affecting NMJ morphology may be the fact that we started with a higher number of candidate chaperones. Moreover, out of nine Drosophila chaperones that showed 100% penetrance, six have been tested in a previous screen, and some of these chaperones were also found to be important for NMJ growth and development (Valakh et al. 2012). There are some discrepancies in the findings by Valakh et al. (2012) and our data. Although we used similar drivers and phenotypic assays, the observed discrepancies are due to different RNAi lines used in these two studies. In addition, Valakh et al. (2012) maintained all the lines at 25° for RNAi experiments, whereas we performed all RNAi knockdown experiments at 29°, which could have contributed toward the phenotypic variations between these studies.
To our knowledge, this is the first report of the identification and classification of the Drosophila “chaperome,” as well as the only comprehensive screen to identify all essential chaperones. In this screen, we have tried to shortlist the chaperones with neuron-specific function to be further characterized for their mechanism of action. Outcomes of this study and further analysis will provide valuable insights and resources for research on chaperones as possible therapeutic targets for neurodegenerative disease.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.041632/-/DC1.
Acknowledgments
We would like to thank the Vienna Drosophila Resource Center for fly stocks and the Developmental Studies Hybridoma Bank, University of Iowa for monoclonal antibodies. We thank members of the C.S. and V.K. laboratories for many useful comments on the manuscript. This work was supported by project grants from the Department of Biotechnology (BT/PR12149/BRB/10/1348/2014), to C.S. and Science and Engineering Research Board (SR/FT/LS-103/2010), Government of India to V.K. We thank the Institute of Science Education and Research Bhopal for intramural funds and the Central Instrumentation Facility.
Author contributions: C.S. and V.K. conceived the experiments. S.R., B.M., C.S., and V.K designed the experiments. S.R., B.M., A.P., and A.V. performed the experiments. S.R., B.M., C.S., and V.K. analyzed and wrote the manuscript with input from other authors. The authors declare no competing or financial interests.
Footnotes
Communicating editor: A. Bashirullah
Literature Cited
- Agrawal P., Hardin P. E., 2016. An RNAi screen to identify protein phosphatases that function within the Drosophila circadian clock. G3 (Bethesda) 6: 4227–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodaleo F. J., Gonzalez-Billault C., 2016. The presynaptic microtubule cytoskeleton in physiological and pathological conditions: lessons from Drosophila fragile X syndrome and hereditary spastic paraplegias. Front. Mol. Neurosci. 9: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini N. M., Fortini M. E., 2002. Applications of the Drosophila retina to human disease modeling. Results Probl. Cell Differ. 37: 257–275. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118(2): 401–415. [DOI] [PubMed] [Google Scholar]
- Brehme M., Voisine C., Rolland T., Wachi S., Soper J. H., et al. , 2014. A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 9(3): 1135–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk P., Wenniger J. J., Dawson-Scully K., Guo X., Hong S., et al. , 2001. Drosophila Hsc70–4 is critical for neurotransmitter exocytosis in vivo. Neuron 30(2): 475–488. [DOI] [PubMed] [Google Scholar]
- Brown I. R., Rush S. J., 1990. Expression of heat shock genes (hsp70) in the mammalian brain: distinguishing constitutively expressed and hyperthermia-inducible mRNA species. J. Neurosci. Res. 25(1): 14–19. [DOI] [PubMed] [Google Scholar]
- Calabrese V., Scapagnini G., Ravagna A., Giuffrida Stella A. M., Butterfield D. A., 2002. Molecular chaperones and their roles in neural cell differentiation. Dev. Neurosci. 24(1): 1–13. [DOI] [PubMed] [Google Scholar]
- Callaerts P., Leng S., Clements J., Benassayag C., Cribbs D., et al. , 2001. Drosophila Pax-6/eyeless is essential for normal adult brain structure and function. J. Neurobiol. 46(2): 73–88. [DOI] [PubMed] [Google Scholar]
- Chen J., Shi X., Padmanabhan R., Wang Q., Wu Z., et al. , 2008. Identification of novel modulators of mitochondrial function by a genome-wide RNAi screen in Drosophila melanogaster. Genome Res. 18(1): 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Xu L., 2016. Genome-wide RNAi screening to dissect the TGF-beta signal transduction pathway. Methods Mol. Biol. 1344: 365–377. [DOI] [PubMed] [Google Scholar]
- D’Souza S. M., Brown I. R., 1998. Constitutive expression of heat shock proteins Hsp90, Hsc70, Hsp70 and Hsp60 in neural and non-neural tissues of the rat during postnatal development. Cell Stress Chaperones 3(3): 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448(7150): 151–156. [DOI] [PubMed] [Google Scholar]
- Doumanis J., Wada K., Kino Y., Moore A. W., Nukina N., 2009. RNAi screening in Drosophila cells identifies new modifiers of mutant huntingtin aggregation. PLoS One 4(9): e7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D., Saidi L. J., Wahlster L., 2013. Molecular chaperones and protein folding as therapeutic targets in Parkinson’s disease and other synucleinopathies. Acta Neuropathol. Commun. 1: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder M. E., Hofmann G. E., 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61: 243–282. [DOI] [PubMed] [Google Scholar]
- Gargano J. W., Martin I., Bhandari P., Grotewiel M. S., 2005. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 40(5): 386–395. [DOI] [PubMed] [Google Scholar]
- Gong Y., Kakihara Y., Krogan N., Greenblatt J., Emili A., et al. , 2009. An atlas of chaperone-protein interactions in Saccharomyces cerevisiae: implications to protein folding pathways in the cell. Mol. Syst. Biol. 5: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Hayer-Hartl M., 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295(5561): 1852–1858. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Bracher A., Hayer-Hartl M., 2011. Molecular chaperones in protein folding and proteostasis. Nature 475(7356): 324–332. [DOI] [PubMed] [Google Scholar]
- Hirth F., 2010. Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol. Disord. Drug Targets 9(4): 504–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J., Cyr D. M., Patterson C., 2001. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2(10): 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. R., Meireles A. M., Fisher K. H., Garcia A., Antrobus P. R., et al. , 2008. A microtubule interactome: complexes with roles in cell cycle and mitosis. PLoS Biol. 6(4): e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T., Krukkert K., Roos J., Davis G., Klambt C., 2000. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron 26(2): 357–370. [DOI] [PubMed] [Google Scholar]
- Kaushik S., Cuervo A. M., 2012. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 22(8): 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger A. A., Baum B., Jones S., Jones M. R., Coulson A., et al. , 2003. A functional genomic analysis of cell morphology using RNA interference. J. Biol. 2(4): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. E., Hipp M. S., Bracher A., Hayer-Hartl M., Hartl F. U., 2013. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 82: 323–355. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kume A., Li M., Doyu M., Hata M., et al. , 2000. Chaperones Hsp70 and Hsp40 suppress aggregate formation and apoptosis in cultured neuronal cells expressing truncated androgen receptor protein with expanded polyglutamine tract. J. Biol. Chem. 275(12): 8772–8778. [DOI] [PubMed] [Google Scholar]
- Koizumi K., Higashida H., Yoo S., Islam M. S., Ivanov A. I., et al. , 2007. RNA interference screen to identify genes required for Drosophila embryonic nervous system development. Proc. Natl. Acad. Sci. USA 104(13): 5626–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch C., Jo J., Wu Y., Fish G. S., Galko M. J., 2010. A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics 186(3): 943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., MacRae T. H., 1997. Molecular chaperones and the cytoskeleton. J. Cell Sci. 110(Pt. 13): 1431–1440. [DOI] [PubMed] [Google Scholar]
- Liberek K., Lewandowska A., Zietkiewicz S., 2008. Chaperones in control of protein disaggregation. EMBO J. 27(2): 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ge Q., Chan B., Liu H., Singh S. R., et al. , 2016. Whole-animal genome-wide RNAi screen identifies networks regulating male germline stem cells in Drosophila. Nat. Commun. 7: 12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Vogel H., 2009. Drosophila models of neurodegenerative diseases. Annu. Rev. Pathol. 4: 315–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario A. J., Conway de Macario E., 2002. Sick chaperones and ageing: a perspective. Ageing Res. Rev. 1(2): 295–311. [DOI] [PubMed] [Google Scholar]
- Mhatre S. D., Satyasi V., Killen M., Paddock B. E., Moir R. D., et al. , 2014. Synaptic abnormalities in a Drosophila model of Alzheimer’s disease. Dis. Model. Mech. 7(3): 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S. E., 2014. RNAi screening in Drosophila cells and in vivo. Methods 68(1): 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. R., Prasad K., Jin S., Augustine G. J., Lafer E. M., 2001. Uncoating of clathrin-coated vesicles in presynaptic terminals: roles for Hsc70 and auxilin. Neuron 32(2): 289–300. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I., 2008. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 22(11): 1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J. F., Morimoto R. I., 2004. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell 15(2): 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski P. J., 2002. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron 35(1): 9–12. [DOI] [PubMed] [Google Scholar]
- Muchowski P. J., Wacker J. L., 2005. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 6(1): 11–22. [DOI] [PubMed] [Google Scholar]
- Mudher A., Shepherd D., Newman T. A., Mildren P., Jukes J. P., et al. , 2004. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol. Psychiatry 9(5): 522–530. [DOI] [PubMed] [Google Scholar]
- Nichols C. D., Becnel J., Pandey U. B., 2012. Methods to assay Drosophila behavior. J. Vis. Exp. 61: 3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson C., Eaton B. A., Davis G. W., 2008. Formin-dependent synaptic growth: evidence that Dlar signals via Diaphanous to modulate synaptic actin and dynamic pioneer microtubules. J. Neurosci. 28(44): 11111–11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussing K., Voigt A., Schulz J. B., 2013. Drosophila melanogaster as a model organism for Alzheimer’s disease. Mol. Neurodegener. 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheesh Kumar R., Nagarajan N. S., Arunraj S. P., Sinha D., Veedin Rajan V. B., et al. , 2012. HSPIR: a manually annotated heat shock protein information resource. Bioinformatics 28(21): 2853–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J., Hummel T., Ng N., Klambt C., Davis G. W., 2000. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron 26(2): 371–382. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Poirier M. A., 2004. Protein aggregation and neurodegenerative disease. Nat. Med. 10(7): S10–S17. [DOI] [PubMed] [Google Scholar]
- Sahi C., Lee T., Inada M., Pleiss J. A., Craig E. A., 2010. Cwc23, an essential J protein critical for pre-mRNA splicing with a dispensable J domain. Mol. Cell. Biol. 30(1): 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H., 2013. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 14(10): 630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J., Milpetz F., Bork P., Ponting C. P., 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95(11): 5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Rao A., 2009. RNAi screening: tips and techniques. Nat. Immunol. 10(8): 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. L., Li W., Cheetham M. E., 2015. Molecular chaperones and neuronal proteostasis. Semin. Cell Dev. Biol. 40: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soti C., Sreedhar A. S., Csermely P., 2003. Apoptosis, necrosis and cellular senescence: chaperone occupancy as a potential switch. Aging Cell 2(1): 39–45. [DOI] [PubMed] [Google Scholar]
- Tyedmers J., Mogk A., Bukau B., 2010. Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11(11): 777–788. [DOI] [PubMed] [Google Scholar]
- Valakh V., Naylor S. A., Berns D. S., DiAntonio A., 2012. A large-scale RNAi screen identifies functional classes of genes shaping synaptic development and maintenance. Dev. Biol. 366(2): 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers J. H., Manning S. A., Kulkarni A., Harvey K. F., 2016. A Drosophila RNAi library modulates Hippo pathway-dependent tissue growth. Nat. Commun. 7: 10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M. J., Carra S., Kanon B., Bosveld F., Klauke K., et al. , 2016. Specific protein homeostatic functions of small heat-shock proteins increase lifespan. Aging Cell 15(2): 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff B., Chapple J. P., van der Spuy J., Hohfeld J., Cheetham M. E., 2005. HSJ1 is a neuronal shuttling factor for the sorting of chaperone clients to the proteasome. Curr. Biol. 15(11): 1058–1064. [DOI] [PubMed] [Google Scholar]
- Yenari M. A., Giffard R. G., Sapolsky R. M., Steinberg G. K., 1999. The neuroprotective potential of heat shock protein 70 (HSP70). Mol. Med. Today 5(12): 525–531. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Fukumoto K., Murakami T., Harada S., Hosono R., et al. , 2002. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 516(1–3): 53–57. [DOI] [PubMed] [Google Scholar]
- Young J. C., Barral J. M., Ulrich Hartl F., 2003. More than folding: localized functions of cytosolic chaperones. Trends Biochem. Sci. 28(10): 541–547. [DOI] [PubMed] [Google Scholar]
- Zeng X., Han L., Singh S. R., Liu H., Neumuller R. A., et al. , 2015. Genome-wide RNAi screen identifies networks involved in intestinal stem cell regulation in Drosophila. Cell Rep. 10(7): 1226–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supportive data and materials generated in this study will be made available upon request. Data supporting total bouton number and Futsch loop quantification are provided as Supplemental Material, Table S3.