Abstract
Mucor circinelloides is a human pathogen, biofuel producer, and model system that belongs to a basal fungal lineage; however, the genetics of this fungus are limited. In contrast to ascomycetes and basidiomycetes, basal fungal lineages have been understudied. This may be caused by a lack of attention given to these fungi, as well as limited tools for genetic analysis. Nonetheless, the importance of these fungi as pathogens and model systems has increased. M. circinelloides is one of a few genetically tractable organisms in the basal fungi, but it is far from a robust genetic system when compared to model fungi in the subkingdom Dikarya. One problem is the organism is resistant to drugs utilized to select for dominant markers in other fungal transformation systems. Thus, we developed a blaster recyclable marker system by using the pyrG gene (encoding an orotidine-5′-phosphate decarboxylase, ortholog of URA3 in Saccharomyces cerevisiae). A 237-bp fragment downstream of the pyrG gene was tandemly incorporated into the upstream region of the gene, resulting in construction of a pyrG-dpl237 marker. To test the functionality of the pyrG-dpl237 marker, we disrupted the carRP gene that is involved in carotenoid synthesis in pyrG− mutant background. The resulting carRP::pyrG-dpl237 mutants exhibit a white colony phenotype due to lack of carotene, whereas wild type displays yellowish colonies. The pyrG marker was then successfully excised, generating carRP-dpl237 on 5-FOA medium. The mutants became auxotrophic and required uridine for growth. We then disrupted the calcineurin B regulatory subunit cnbR gene in the carRP::dpl237 strain, generating mutants with the alleles carRP::dpl237 and cnbR::pyrG. These results demonstrate that the recyclable marker system is fully functional, and therefore the pyrG-dpl237 marker can be used for sequential gene deletions in M. circinelloides.
Keywords: Mucor circinelloides, URA blaster, pyrG blaster, recyclable genetic marker, mucormycosis
Mucor circinelloides is a basal fungus belonging to the phylum Mucoromycota (Spatafora et al. 2016). M. circinelloides is one of the etiological agents of mucormycosis. Mucormycosis is an opportunistic fungal infection recently recognized as an emerging infectious disease (Kauffman 2004; Brown 2005; Chayakulkeeree et al. 2006; Lanternier et al. 2012a). Fungi in the order Mucorales are the causal agents of mucormycosis, and include Mucor spp., Rhizopus spp., Lichtheimia (previously Absidia) spp., Apophysomyces spp., Cunninghamella spp., Rhizomucor spp., and others (Chayakulkeeree et al. 2006; Ibrahim and Spellberg 2006; Neblett Fanfair et al. 2012). Recent data indicates a significant increase in mucormycosis due to a rising number of immunocompromised patients with conditions such as diabetes, HIV/AIDS, hematologic malignancies, hematopoietic stem cell/solid organ transplantation, or trauma (Ribes et al. 2000; Marr et al. 2002; Kontoyiannis et al. 2005; Roden et al. 2005; Spellberg et al. 2005). Mucormycosis is the second most common mold infection among patients with hematological malignancies and transplants, and is associated with high mortality rates of ∼50% for all mucormycosis infections and >90% in disseminated infections (Ribes et al. 2000; Roden et al. 2005; Lanternier et al. 2012b). Even after recovery, patients often suffer from permanent disfiguration in the affected areas due to surgical debridement as a frequent treatment modality (Kwon-Chung and Bennet 1992; Kontoyiannis et al. 2005; Roden et al. 2005; Spellberg et al. 2005; Kontoyiannis and Lewis 2006).
M. circinelloides has served as a system to study virulence and pathogenesis of mucormycosis in animal host models. For example, various Mucor isolates produce spores of different sizes, and the size of spores contributes to virulence as larger spores are more virulent than smaller spores (Li et al. 2011). The difference in virulence may be due to a difference in the interactions of larger and smaller spores with macrophages whereby larger spores germinate inside macrophages, and smaller spores remain dormant inside macrophages (Li et al. 2011). The morphogenesis of M. circinelloides also contributes to virulence, in which the filamentous form of the fungus is more virulent than the yeast form (Lee et al. 2013). In addition, the different morphogenic states (spores/hyphae vs. yeast) result in different host-pathogen interactions (Lee et al. 2015). For example, Mucor spores arrest or delay phagosome maturation upon phagocytosis by macrophages, whereas Mucor yeast do not. Cytokine responses from immune cells differ between Mucor spores/hyphae and yeast. A recent study found that a phospholipase D and myosin V family protein are required for full virulence in Mucor through an RNAi-based genome screen (Trieu et al. 2017).
M. circinelloides is also able to contaminate and spoil foods (Lazar et al. 2014; Lee et al. 2014). M. circinelloides is known to produce the toxin 3-nitropropionic acid (3NP) (Hollmann et al. 2008), which makes this fungus a potential cause of food poisoning. 3NP inhibits mitochondrial succinate dehydrogenase, causing acute brain injury, methemoglobinemia, dystonia after ingestion, and symptoms similar to Huntington’s disease (Alston et al. 1977; Gabrielson et al. 2001; Brouillet et al. 2005). Interestingly, 3NP was responsible for 217 sugarcane poisoning outbreaks in China during the period 1972–1989, which involved 884 patients with 88 mortalities [reviewed in Magan and Olsen (2004)]. Therefore, it is apparent that Mucor poses a threat to public health.
M. circinelloides also serves as a model to study light sensing in fungi. Asexual sporulation is triggered by light. Blue light activates carotenoid biosynthesis and sporangiophore phototropism (Navarro et al. 1995; Silva et al. 2006). Mucor also encodes homologs of the Neurospora crassa white collar-1 (WC-1), which forms the white collar complex with other factors that entrain the circadian clock (Liu and Bell-Pedersen 2006). The functions of the three wc-1 genes have been elucidated in Mucor, in that they are all involved in light response although each of them has distinct light transduction pathways (Silva et al. 2006, 2008; Navarro et al. 2013).
M. circinelloides also has a conserved and active RNAi pathway that governs the expression of nonintegrated transgenes (Nicolás et al. 2003), and therefore serves as a model to study RNA silencing in fungi. Genetic analysis demonstrated that Dicer, Argonaute, and RNA-dependent RNA polymerase play major roles in this RNA silencing, and these three components are conserved in M. circinelloides (de Haro et al. 2009; Calo et al. 2012; Billmyre et al. 2013; Cervantes et al. 2013). Interestingly, M. circinelloides exhibits a novel mechanism of drug resistance evoked by RNAi-mediated silencing (Calo et al. 2014). In the presence of the calcineurin inhibitor FK506, the fungus can silence the target gene of FK506, which encodes FKBP12, to become drug resistant. However, without selective pressure, the fungus will eventually deactivate the silencing mechanism and become drug sensitive in an epigenetic RNAi mediated process (Calo et al. 2014). A recent study found that the noncanonical RdRP-dependent/Dicer-independent RNAi pathway indeed inhibits the canonical RNAi-mediated drug resistant pathways, suggesting a balance between two RNAi pathways in M. circinelloides (Calo et al. 2017).
The recent oil crisis and environmental conservation initiatives have prompted innovations in the production of biofuel. Research has focused on oilseed crops and plant-based carbohydrates that can be converted into fatty acid esters by microorganisms (Kalscheuer et al. 2006; Steen et al. 2010). An additional important biofuel source is lipid-accumulating microorganisms, such as oleaginous fungi and algae (Kosa and Ragauskas 2011). M. circinelloides is an oleaginous microorganism that accumulates lipids as a storage source (Vicente et al. 2009; Kosa and Ragauskas 2011; Rodríguez-Frómeta et al. 2013) and produces a high level of linoleic acid, which served as a basis for the first commercial microbial lipid (Ratledge 2004). Biodiesel produced by M. circinelloides satisfies the specifications set by American and most European standards (Vicente et al. 2009).
M. circinelloides is clearly a prominent organism in many aspects of biology. Unfortunately, the genetics of this fungus are limited. Resistance to drugs utilized to select for dominant markers in other fungal transformations, including neomycin, nourseothricin, hygromycin, zeomycin, carboxin, and glufosinate, is common (data not shown). This precludes the use of these common dominant drug resistance markers for Mucor. Only two auxotrophic markers (leuA− and pyrG−) in one strain (Nicolas et al. 2007) and one auxotrophic marker (Met−) in another strain (Anaya and Roncero 1991) are available in the background of the sequenced isolates (http://genome.jgi-psf.org/Mucci2/Mucci2.home.html), which significantly limits the ability to disrupt multiple genes for genetic analysis.
In this study, we developed a recyclable pyrG blaster marker for multiple gene deletions in M. circinelloides that is analogous to the URA3 blaster marker for Candida albicans (Wilson et al. 2000). A 237-bp fragment of the 3′-downstream of the pyrG gene was tandemly incorporated in the 5′-upstream of the pyrG to generate a pyrG-dpl237 (237 bp-pyrG-237 bp) marker. The marker was used to successfully disrupt the carRP gene to generate an albino mutant (carRPΔ::pyrG-dpl237). Spontaneous excision of the pyrG marker in the carRPΔ::pyrG-dpl237 allele occurred; resulting in generation of carRPΔ::dpl237 mutants. Additionally, the cnbR gene was disrupted by using pyrG gene as a marker, demonstrating the pyrG blaster marker is fully functional and can be used for multiple gene disruption.
Materials and Methods
Fungal strains and growth conditions
The strains and plasmids used in this study are listed in Table 1. For spore production, M. circinelloides strains were grown on yeast peptone glucose agar (YPG, 3 g/liter yeast extract, 10 g/liter peptone, 20 g/liter glucose, 2% agar, pH 4.5) media at 26° in the light for 4 d. To collect spores, deionized sterile water was added to the plates and the spores were gently scraped with a cell spreader. For further experiments, the number of spores was calculated with the automated cell counter TC20 (Bio-Rad) or a hemocytometer. YPD agar was used to compare the phenotypes of yeast-locked cnbRΔ mutants with wild type. For transformation, minimal media with casamino acids (MMC; pH 3.2, 10 g casamino acids, 0.5 g yeast nitrogen base without amino acids and ammonium sulfate, 20 g glucose, 1 mg niacin, 1 mg thiamine, and 15 g agar in 1 liter dH2O) media containing 0.5 M sorbitol was used.
Table 1. List of strains and plasmids used in this study.
| Name | Genotypes or Characteristics | Reference | |
|---|---|---|---|
| M. circinelloides strains | MU402 | leuA− pyrG− | Nicolas et al. (2007) |
| MSL7 | leuA− pyrG− cnbRΔ::pyrG | Lee et al. (2013) | |
| MSL27 | leuA− pyrG− carRPΔ::pyrG-dpl237 | This study | |
| MSL28 | leuA− pyrG− carRPΔ::dpl237 | This study | |
| MSL29 | leuA− pyrG− carRPΔ::dpl237 | This study | |
| MSL30 | leuA− pyrG− carRPΔ::dpl237 | This study | |
| MAG1 | leuA− pyrG− carRPΔ::dpl237 cnbRΔ::pyrG | This study | |
| MAG2 | leuA− pyrG− carRPΔ::dpl237 cnbRΔ::pyrG | This study | |
| Plasmids | pCR21-TOPO | AmpicillinR KanamycinR | Invitrogen |
| pTOPO-pyrG | 2002-bp pyrG fragment cloned into pCR21-TOPO | Lee et al. (2013) | |
| pCnbR-KO | cnbR disruption construct cloned into pCR21-TOPO | Lee et al. (2013) | |
| pSL13 | pyrG blaster marker in pCR21-TOPO | This study |
M. circinelloides spores are multinucleate, so vegetative selection was required to isolate homokaryotic transformants. The initial heterokaryotic transformants were subjected to vegetative rounds of growth on MMC media to generate homokaryotic transformants. If necessary, MMC (pH 4.5) media was supplemented with 0.61 g/liter of uridine and 0.56 g/liter uracil. To select mutants that spontaneously excise the pyrG gene, 5-FOA (2.5 mg/ml) was supplemented to MMC (pH 4.5) media.
Development of a recyclable pyrG marker
A 2002-bp DNA fragment containing the pyrG gene was amplified with M13 forward and reverse primers from the vector pTOPO-pyrG that we previously constructed (Lee et al. 2013). This plasmid was used as an initial plasmid to construct a 237 bp-pyrG-237 bp (or pyrG-dpl237) allele. The 237-bp DNA fragment located downstream of the pyrG gene was amplified with the primers SCL635 and SCL636 (Table 2), which contain overhanging XbaI or NotI restriction enzyme recognition sites on either side. The amplified 237-bp fragment was cloned into pPyrG-TOPO digested with XbaI and NotI, generating the pSL13 (Table 1), which harbors a DNA cassette with 237-bp direct repeats on both sides of the pyrG gene.
Table 2. Primers used in this study.
| Name | 5′ → 3′ Sequence | Remark |
|---|---|---|
| SCL639 | CACCTTAGCACGACGAATCA | Primer for carRP disruption, 5′ region |
| SCL640 | ACTGGCCGTCGTTTTACCGGTGTGGAGAAAGAGGTGT | Primer for carRP disruption, 5′ region |
| SCL641 | GTCATAGCTGTTTCCTGCACTATCCCAGCAGAGCACA | Primer for carRP disruption, 3′ region |
| SCL642 | GCCATTTGTGTCTTGGGTTT | Primer for carRP disruption, 3′ region |
| SCL643 | ATGCGCATCTTCTTGGTCTT | Nested forward primer for carRP disruption |
| SCL644 | GCTTCTTTCTGGGCATGTGT | Nested reverse primer for carRP disruption |
| SCL649 | GGGAGGCAACAGATTGTGAT | 5′ outside forward of carRP disruption confirmation |
| SCL650 | TCTTTTTGCTGTTGCTGTGC | 3′ outside reverse of carRP disruption confirmation |
| SCL566 | ACCCACTCACTTTCCATTCG | Primer for pyrG to determine insertion |
| SCL567 | TGCTTTTGTTGGCTGAGATG | Primer for pyrG to determine insertion |
| SCL635 | TCTAGA GGGAGTCAAGGCAGGTCTTT | pyrG blaster 237-bp fragment with XbaI overhang |
| SCL636 | GCGGCCGCTGATAACTGATGCGCTGCAT | pyrG blaster 237-bp fragment with NotI overhang |
| SCL286 | CGGCAAGTACTGTGTCCTCA | Forward primer for cnbR disruption |
| SCL287 | ATGGCAAAGTCGAAGAGGAA | Reverse primer for cnbR disruption |
| SL1 | TCCACCACAGTGACCTTGAA | Forward primer for Southern blotting probe for carRP |
| SL2 | GGGGAGTGATAGGGCAGAAT | Reverse primer for Southern blotting probe for carRP |
| JOHE22226 | TAAGGTCGATGTTGCCACTG | Forward primer for 5′ outside of cnbR disruption confirmation |
| JOHE22231 | CGAAAACAACAAACGCATTG | Reverse primer for 3′ outside of cnbR disruption confirmation |
Disruption of genes
To disrupt the carRP gene, we constructed a disruption cassette containing the pyrG blaster marker flanked by ∼1 kb of 5′ and 3′ of the up- and downstream regions of the carRP gene via overlap PCR. The 5′ region was amplified with primers SCL639 and SCL640, and the 3′ region was amplified with primers SCL641 and SCL642. Genomic DNA of strain MU402 served as template. The pyrG blaster (pyrG-dpl237) marker was amplified with M13 forward and reverse primers. The three fragments were then subjected to an overlap PCR with nested primers SCL643 and SCL644, to generate a disruption allele. A mixture of 75 ng of each fragment was used as template. To disrupt the cnbR gene in the carRPΔ mutant background, the cnbR disruption cassette was amplified from plasmid pCnbR-KO (Lee et al. 2013) via PCR using a pair of primers, SCL286 and SCL287, with the Thermo Scientific DreamTaq DNA polymerase. The cnbR disruption construct was introduced into the MSL29 (leuA− pyrG− carRPΔ::dpl237) strain via electroporation as described previously (Lee et al. 2013). In brief, 108 germinated spores were protoplasted in the presence of chitosanase (US Biological) and lysing enzyme (Sigma). The protoplasts were resuspended in 0.5 M sorbitol solution and mixed with 3 μg of the disruption cassette DNA. Electroporation was performed in 0.2-cm cuvettes (pulse at 0.8 kV, 25 μF, 400 ohm) and the protoplasts were spread onto selective MMC (pH 3.2) media supplemented with 0.5 M sorbitol. From 16 independent transformations, we obtained five mutants that exhibit the yeast-locked phenotype. Two independently obtained mutants, MAG1 and MAG2, were used in this study (Table 1).
Southern blotting analysis
Southern blotting was performed to ensure the absence of the carRP gene, the presence of carRP::pyrG-dpl237, and the excision of the pyrG gene from the carRP::pyrG-dpl237 allele. Genomic DNA (30 μg) of the wild type and carRP::pyrG-dpl237 and carRP::dpl237 mutants was digested using either EcoRI or BclI enzyme, or both. The digested genomic DNA fragments were separated in a 1% agarose gel. The gel was then rinsed in deionized distilled water and placed in a denaturing solution (1.5 M NaCl, 0.5 M NaOH). The gel was then rinsed again with deionized distilled water and placed in a neutralization solution [1.5 M NaCl, 0.5 M Tris-HCl (pH 7.2), 1 mM EDTA]. Capillary transfer of DNA into nylon membrane was performed for 18 hr. The membrane was then placed in a UV cross-linking chamber (Spectrolinker XL-1000 UV cross-linker with an autoexposure setting; Spectronics Co.) to cross-link DNA to the membrane. The probe was generated by PCR with primers SL1 and SL2. The probe was then labeled with biotin by using the Thermo Scientific Biotin DecaLabel DNA labeling Kit following the manufacturer’s instruction. The Thermo Scientific Biotin Chromogenic Detection Kit was used to detect the labeled probes on the membrane following the manufacturer’s instruction.
Excision of the pyrG gene from the pyrG blaster marker
Spores of the MSL27 (carRPΔ::pyrG-dpl237) strain were placed on MMC media containing 5-FOA (2.5 mg/ml), uridine (0.61 mg/ml), and uracil (0.56 mg/ml), to select mutants that still produced white colonies but required uridine to grow. The candidate mutants were passaged on MMC media containing uridine, uracil, and 5-FOA. Excision of the pyrG gene was verified by PCR and Southern blotting.
Data availability
All the strains and plasmids used in this study are available upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results and Discussion
Generation of pyrG blaster marker and disruption of the carRP gene
We developed a pyrG blaster recyclable marker system by using the pyrG gene (encoding orotidine-5′-phosphate decarboxylase, ortholog of URA3 in Saccharomyces cerevisiae). To construct a pyrG blaster DNA cassette that can be easily amplified by PCR, we adapted a 237-bp repeat as previously described in C. albicans (Wilson et al. 2000). In our previous study, we generated the pTOPO-pyrG plasmid harboring a 2002-bp DNA fragment containing the pyrG gene (Lee et al. 2013) (Figure 1A, top). The plasmid was used as an initial plasmid to construct a 237 bp-pyrG-237 bp (or pyrG-dpl237) allele. The 237-bp DNA fragment located at the downstream of the pyrG gene was amplified with primers that contain overhanging XbaI or NotI restriction enzyme recognition sites on each side. The amplified 237-bp fragment was cloned into pTOPO-pyrG digested with XbaI and NotI, generating the pSL13 (Figure 1A, bottom), which harbors a DNA cassette with 237-bp direct repeats on both sides of the pyrG gene. The tandem-repeated 237 bp on both side of the pyrG gene enhances a spontaneous homologous recombination between them, resulting in excision of the pyrG gene. The marker gene therefore can be used for another gene disruption.
Figure 1.
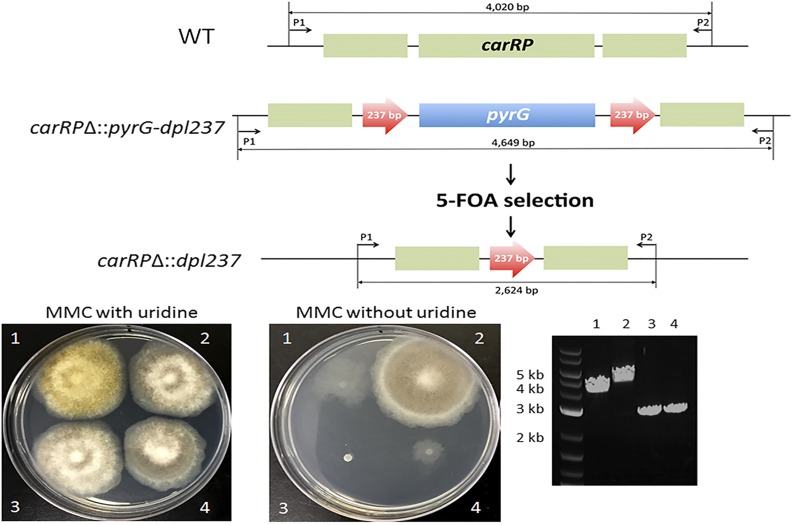
Development of a recyclable pyrG marker in M. circinelloides. (A) Construction of a pyrG blaster marker with repeats of 237 bp on 5′ and 3′ end. The short repeat (237 bp) in the 3′ end was tandemly incorporated to enhance the excision of the marker gene. The resulting pyrG blaster marker can be amplified by PCR with M13 forward and reverse primers. (B) Homologous replacement of the carRP gene with the pyrG blaster marker. The loss of the carRP gene results in a failure to produce carotenoids in M. circinelloides. Therefore, the carRPΔ mutants display white colonies as indicated by the red arrows on the plate, whereas wild-type cells form yellow colonies. Although the target gene is disrupted, only a portion of the nuclei contains the carRPΔ allele because the spores are multinucleated with aseptate hyphae. Transformants that stably form white colonies were selected to obtain the carRPΔ mutant with a homozygous karyotype. Gene size is not to scale.
To test the functionality of the pyrG blaster marker, we targeted the carRP gene, which encodes a protein with dual enzyme activities (lycopene cyclase and phytoene synthase) that are involved in carotenoid synthesis in M. circinelloides (Velayos et al. 1997) (Figure 1B). Complete loss of the carRP gene resulted in white colony formation due to the lack of accumulation of carotenoids, whereas the wild type displayed yellowish colonies. This characteristic of the carRP mutants enabled us to screen homokaryotic homologous recombinant transformants by evaluating the colony color (Rodríguez-Frómeta et al. 2013). We obtained seven transformants from eight independent transformations. Progeny from two transformants displayed both white and yellow colonies. The white and yellow mixed progeny indicate that the nuclei of the transformants are heterogeneous, containing nuclei with carRPΔ and wild-type carRP alleles. Mucor spores are multinucleate and their hyphae are coenocytic (aseptate), so cycles of vegetative passage under selective conditions are therefore required to enrich recombinant nuclei. The transformant MSL27 (carRPΔ::pyrG-dpl237) stably produced exclusively white colony progeny, and this strain was used to test the recyclable pyrG marker.
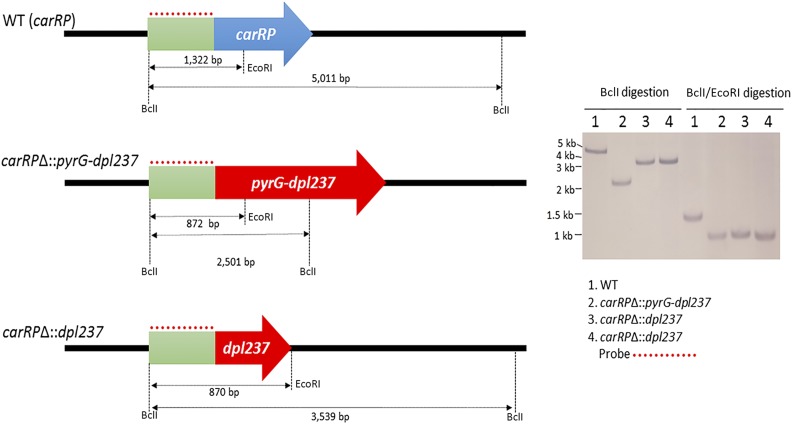
We further validated that spores of the MSL27 strain contain only the carRPΔ allele, and not the wild-type allele. The primers SCL649 (P1) and SCL650 (P2) outside of the disruption cassette were used to test the genotype of the progeny. A 4020-bp PCR fragment was produced from the wild-type carRP allele, and a 4649-bp fragment was produced from the carRPΔ allele, which confirms that the MSL27 strain does not contain the wild-type carRP allele (Figure 2). The disruption of the carRP gene was further verified by a Southern blotting analysis (Figure 3). A single digestion with BclI and a double digestion with BclI and EcoRI were performed. The probe for the 5′ upstream of the carRP gene recognized 5011-bp fragment when the wild-type genomic DNA was digested with BclI and 1322-bp fragment when double-digested with EcoRI and BclI. On the other hand, the replacement of the carRP gene with pyrG-dpl237 introduced additional EcoRI and BclI recognition sites; the probe then recognized 2501-bp fragment when the genomic DNA of MSL27 (carRPΔ::pyrG-dpl237) was single-digested with BclI, and an 872-bp fragment when double-digested with BclI and EcoRI.
Figure 2.
Confirmation of excision of the pyrG marker. The MSL27 (carRPΔ::pyrG-dpl237) strain was spot-inoculated on MMC media containing 5-FOA. After 3 d of incubation, spontaneous resistant mutants emerged forming a sector from colony (data not shown). The 5-FOA resistant mutants went through a vegetative cycle on MMC media containing 5-FOA. The obtained spores were subject to PCR analysis and auxotrophy tests. The excision of the pyrG marker from the carRPΔ::pyrG-dpl237 allele was confirmed by PCR (see text in the section of Excision of pyrG gene). The strains with the pyrG marker excised did not grow on media without uridine. Two independent carRPΔ::dpl237 strains were obtained. (1) MU402 (WT in carRP allele and pyrG−); (2) MSL27 (carRPΔ::pyrG-dpl237); (3) MSL28 (carRPΔ::dpl237); (4). MSL29 (carRPΔ::dpl237). Gene size is not to scale.
Figure 3.
Southern blot analysis confirms the deletion of the carRP gene and excision of the pyrG marker gene. Southern blotting was performed to ensure the deletion of the carRP gene and excision of the pyrG marker. Genomic DNA of the wild-type strain was digested with BclI and produced a 5011-bp fragment that was recognized by the probe. A double digestion with BclI and EcoRI resulted in a 1322-bp fragment that was recognized by the probe. The digestion of the genomic DNA of the carRPΔ::pyrG-dpl237 mutant with BclI produced a 2501-bp fragment that was recognized by the probe. The double digestion with the enzymes BclI and EcoRI produced an 872-bp fragment recognized by the probe. Finally, the carRPΔ::dpl237 mutant underwent the same digestions, where a 3539-bp fragment was produced after BclI enzyme digestion, and an 870-bp fragment was produced after BclI and EcoRI double digestion. Both fragments were detected during our hybridization procedure by our probe. Gene size is not to scale.
Excision of pyrG gene in the carRPΔ::pyrG-dpl237 mutant
To obtain spontaneous mutants that excised the pyrG marker, spores of the MSL27 (carRPΔ::pyrG-dpl237) strain were placed on MMC media containing 5-FOA (2.5 mg/ml), uridine (0.61 mg/ml), and uracil (0.56 mg/ml), to select mutants that still produced white colonies but required uridine to grow. The mutants that grow in the presence of 5-FOA are likely homokaryotic because resistance to 5-FOA is recessive. Two candidates (MSL28 and MSL29) were obtained (5-FOA resistance frequency at 5.2 ± 1.3 progeny/105 spores inoculated) and the absence of the pyrG marker gene was confirmed by PCR with the primers SCL649 (P1) and SCL650 (P2), in which a 2624-bp fragment was produced after excision of the pyrG gene, instead of the 4649-bp fragment from the carRPΔ::pyrG-dpl237 allele (Figure 2). We confirmed that the two carRPΔ::dpl237 strains, MSL28 and MSL29, produce white colonies (Figure 2). We also confirmed that the MSL28 and MSL29 (carRPΔ::dpl237) require uridine to grow on MMC media due to the excision of the functional pyrG gene. The excision of the pyrG gene was further verified by a Southern blotting analysis (Figure 3). When genomic DNAs of the two carRPΔ::dpl237 mutants were single-digested with BclI, the probe recognized a 3539-bp fragment, whereas in wild type and carRPΔ::pyrG-dpl237, the probe recognized a 5011-bp or a 2501-bp fragment, respectively. When double-digested with BclI and EcoRI, the probe recognized an 870-bp fragment in the carRPΔ::dpl237 mutants, whereas it recognized a 1322-bp or an 872-bp fragment in wild type or the carRPΔ::pyrG-dpl237 mutant, respectively (Figure 3). These results confirm the pyrG gene was successfully excised from the locus and the pyrG gene can be used to disrupt another gene.
Disruption of the cnbR gene in a carRPΔ::dpl237 mutant
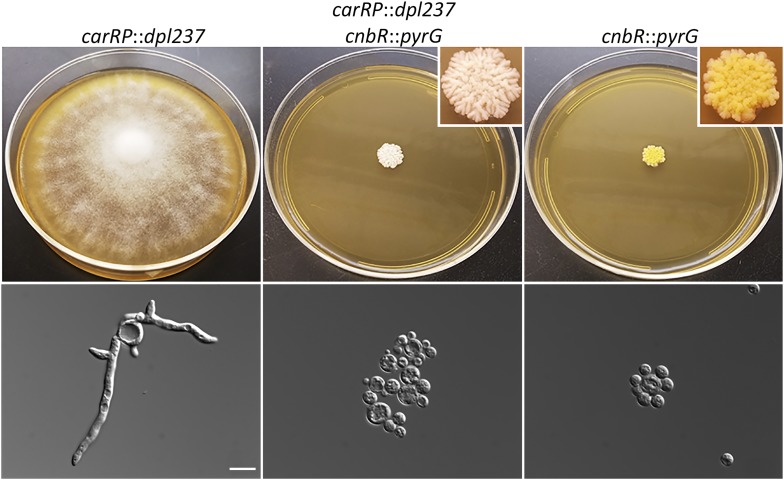
We proceeded to disrupt the calcineurin B regulatory subunit cnbR gene in the carRPΔ::dpl237 strain to verify that the recyclable marker system was fully functional. In our previous study, we found that the disruption of cnbR gene resulted in a yeast-locked phenotype, which makes screening for homologous recombinant progeny straightforward. The MSL29 strain (carRPΔ::dpl237) was subject to transformation to disrupt the cnbR gene. This resulted in 75 transformants from 16 independent transformations. We screened the transformants by PCR with the cnbR deletion-specific primers JOHE22226 and SCL566 (Table 2, P1 and P2 in Supplemental Material, Figure S1, respectively) as previously described (Lee et al. 2013). Five transformants were found to carry an allele with cnbR::pyrG (data not shown). Homokaryotic cnbR::pyrG mutants exhibit a yeast-locked phenotype (Lee et al. 2013). After two rounds of vegetative passage on selective media, all five of them produced yeast-locked colonies along with wild-type colonies that were heterokaryotic containing nuclei either with cnbR or cnbR::pyrG. The yeast-locked colonies were selected and it was confirmed that they carry cnbR::pyrG and carRP::dpl237 by PCR (Figure S1). A disruption in the cnbR gene in the carRPΔ::dpl237 strain resulted in progeny that were both yeast locked and white in color (Figure 4). The results verified that the recyclable pyrG blaster marker is fully functional and can be used for a series of gene deletions in M. circinelloides.
Figure 4.
Disruption of the cnbR gene in the carRPΔ::dpl237 mutant and phenotypes of the double mutants. The carRPΔ::dpl237 strain, MSL28, produces white colonies and requires uridine to grow. The cnbRΔ::pyrG strain, MSL7, produces a yeast-locked colony while still retaining the function of the carRP gene, and thus produces yellow yeast-locked progeny. The carRPΔ::dpl237 cnbRΔ::pyrG strain, MAG1, exhibits a yeast-locked phenotype and produces white colonies due to the disruption in both carRP and cnbR genes. Microscopic images were also taken to confirm the phenotype of each strain. Bar, 10 μm.
Conclusions
Compared to ascomycetes and basidiomycetes, basal fungal lineages have been understudied. This may be caused by a deficit in attention given to these fungi, as well as fastidious genetic analysis. However, the importance of these fungi as pathogens and model systems has increased. M. circinelloides is one of very few genetically amenable organisms belonging to the basal fungi, but it is far from a robust genetic system when compared to model fungi in the Dikarya. M. circinelloides is a human fungal pathogen of significant public health concern. The lack of robust genetic tools presents an obstacle for investigating the biology of this pathogen with an aim toward the development of effective antifungal drugs. This study generates the facile genetic tool, recyclable pyrG marker, which will drive fundamentally important biomedical research on this pathogen, while providing a broader and sustainable impact on the field by enabling detailed genetic analyses of related Mucoralean fungi of relevance to human health.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.041095/-/DC1.
Acknowledgments
We thank Anna Averette and Eun Young Huh for technical support. We are indebted to Yong-Sun Bahn, Katherine Mueller, and Alex Stevenson for discussions and critical reading. This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant R03 AI11917 and University of Texas at San Antonio research funds to S.C.L., and NIH/NIAID grants R38 AI39115-19 and R01 AI050113-13 to J.H. G.A. was supported by the Duke University Summer Research Opportunity Program.
Footnotes
Communicating editor: B. J. Andrews
Literature Cited
- Alston T. A., Mela L., Bright H. J., 1977. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc. Natl. Acad. Sci. USA 74: 3767–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaya N., Roncero M. I. G., 1991. Transformation of a methionine auxotrophic mutant of Mucor circinelloides by direct cloning of the corresponding wild type gene. Mol. Gen. Genet. 230: 449–455. [DOI] [PubMed] [Google Scholar]
- Billmyre R. B., Calo S., Feretzaki M., Wang X., Heitman J., 2013. RNAi function, diversity, and loss in the fungal kingdom. Chromosome Res. 21: 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet E., Jacquard C., Bizat N., Blum D., 2005. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J. Neurochem. 95: 1521–1540. [DOI] [PubMed] [Google Scholar]
- Brown J., 2005. Zygomycosis: an emerging fungal infection. Am. J. Health Syst. Pharm. 62: 2593–2596. [DOI] [PubMed] [Google Scholar]
- Calo S., Nicolás F. E., Vila A., Torres-Martínez S., Ruiz-Vázquez R. M., 2012. Two distinct RNA-dependent RNA polymerases are required for initiation and amplification of RNA silencing in the basal fungus Mucor circinelloides. Mol. Microbiol. 83: 379–394. [DOI] [PubMed] [Google Scholar]
- Calo S., Shertz-Wall C., Lee S. C., Bastidas R. J., Nicolas F. E., et al. , 2014. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature 513: 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo S., Nicolás F. E., Lee S. C., Vila A., Cervantes M., et al. , 2017. A non-canonical RNA degradation pathway suppresses RNAi-dependent epimutations in the human fungal pathogen Mucor circinelloides. PLoS Genet. 13: e1006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes M., Vila A., Nicolás F. E., Moxon S., de Haro J. P., et al. , 2013. A single Argonaute gene rarticipates in exogenous and endogenous RNAi and controls cellular functions in the basal fungus Mucor circinelloides. PLoS One 8: e69283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayakulkeeree M., Ghannoum M., Perfect J., 2006. Zygomycosis: the re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 25: 215–229. [DOI] [PubMed] [Google Scholar]
- de Haro J. P., Calo S., Cervantes M., Nicolás F. E., Torres-Martínez S., et al. , 2009. A single dicer gene is required for efficient gene silencing associated with two classes of small antisense RNAs in Mucor circinelloides. Eukaryot. Cell 8: 1486–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielson K. L., Hogue B. A., Bohr V. A., Cardounel A. J., Nakajima W., et al. , 2001. Mitochondrial toxin 3-nitropropionic acid induces cardiac and neurotoxicity differentially in mice. Am. J. Pathol. 159: 1507–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., Razzazi-Fazeli E., Grajewski J., Twaruzek M., Sulyok M., et al. , 2008. Detection of 3-nitropropionic acid and cytotoxicity in Mucor circinelloides. Mycotoxin Res. 24: 140–150. [DOI] [PubMed] [Google Scholar]
- Ibrahim A. S., Spellberg B., 2006. Zygomycetes as agents of infectious disease in humans, pp. 429–440 in Molecular Principles of Fungal Pathogenesis, edited by Heitman J., Filler S. G., Edwards J. E., Jr., Mitchell A. P. ASM Press, Washington, DC. [Google Scholar]
- Kalscheuer R., Stölting T., Steinbüchel A., 2006. Microdiesel: Escherichia coli engineered for fuel production. Microbiology 152: 2529–2536. [DOI] [PubMed] [Google Scholar]
- Kauffman C. A., 2004. Zygomycosis: reemergence of an old pathogen. Clin. Infect. Dis. 39: 588–590. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D. P., Lewis R. E., 2006. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect. Dis. Clin. North Am. 20: 581–607. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D. P., Lionakis M. S., Lewis R. E., Chamilos G., Healy M., et al. , 2005. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J. Infect. Dis. 191: 1350–1360. [DOI] [PubMed] [Google Scholar]
- Kosa M., Ragauskas A. J., 2011. Lipids from heterotrophic microbes: advances in metabolism research. Trends Biotechnol. 29: 53–61. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennet J. E., 1992. Mucormycosis, pp. 524–559 in Medical Mycology. Lea & Febiger, Philadelphia. [Google Scholar]
- Lanternier F., Dannaoui E., Morizot G., Elie C., Garcia-Hermoso D., et al. , 2012a A global analysis of mucormycosis in France: the RetroZygo study (2005–2007). Clin. Infect. Dis. 54: S35–S43. [DOI] [PubMed] [Google Scholar]
- Lanternier F., Sun H.-Y., Ribaud P., Singh N., Kontoyiannis D. P., et al. , 2012b Mucormycosis in organ and stem cell transplant recipients. Clin. Infect. Dis. 54: 1–8. [DOI] [PubMed] [Google Scholar]
- Lazar S. P., Lukaszewicz J. M., Persad K. A., Reinhardt J. F., 2014. Rhinocerebral Mucor circinelloides infection in immunocompromised patient following yogurt ingestion. Del. Med. J. 86: 245–248. [PubMed] [Google Scholar]
- Lee S. C., Li A., Calo S., Heitman J., 2013. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 9: e1003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Billmyre R. B., Li A., Carson S., Sykes S. M., et al. , 2014. Analysis of a foodborne fungal pathogen outbreak: virulence and genome of a Mucor circinelloides isolate from yogurt. mBio 5: e01390–e01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Li A., Calo S., Inoue M., Tonthat N. K., et al. , 2015. Calcineurin orchestrates dimorphic transitions, antifungal drug responses, and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 97: 844–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. H., Cervantes M., Springer D. J., Boekhout T., Ruiz-Vazquez R. M., et al. , 2011. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 7: e1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Bell-Pedersen D., 2006. Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot. Cell 5: 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magan N., Olsen M., 2004. Mycotoxins in Food: Detection and Control. Woodhead Publishing Ltd, Cambridge, UK. [Google Scholar]
- Marr K. A., Carter R. A., Crippa F., Wald A., Corey L., 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34: 909–917. [DOI] [PubMed] [Google Scholar]
- Navarro E., Sandmann G., Torres-Martinez S., 1995. Mutants of the carotenoid biosynthetic pathway of Mucor circinelloides. Exp. Mycol. 19: 186–190. [Google Scholar]
- Navarro E., Peñaranda A., Hansberg W., Torres-Martínez S., Garre V., 2013. A white collar 1-like protein mediates opposite regulatory functions in Mucor circinelloides. Fungal Genet. Biol. 52: 42–52. [DOI] [PubMed] [Google Scholar]
- Neblett Fanfair R., Benedict K., Bos J., Bennett S. D., Lo Y.-C., et al. , 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 367: 2214–2225. [DOI] [PubMed] [Google Scholar]
- Nicolás F. E., Torres-Martínez S., Ruiz-Vázquez R. M., 2003. Two classes of small antisense RNAs in fungal RNA silencing triggered by non‐integrative transgenes. EMBO J. 22: 3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas F. E., de Haro J. P., Torres-Martinez S., Ruiz-Vazquez R. M., 2007. Mutants defective in a Mucor circinelloides dicer-like gene are not compromised in siRNA silencing but display developmental defects. Fungal Genet. Biol. 44: 504–516. [DOI] [PubMed] [Google Scholar]
- Ratledge C., 2004. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86: 807–815. [DOI] [PubMed] [Google Scholar]
- Ribes J. A., Vanover-Sams C. L., Baker D. J., 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13: 236–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden M. M., Zaoutis T. E., Buchanan W. L., Knudsen T. A., Sarkisova T. A., et al. , 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41: 634–653. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Frómeta R. A., Gutiérrez A., Torres-Martínez S., Garre V., 2013. Malic enzyme activity is not the only bottleneck for lipid accumulation in the oleaginous fungus Mucor circinelloides. Appl. Microbiol. Biotechnol. 97: 3063–3072. [DOI] [PubMed] [Google Scholar]
- Silva F., Torres-Martínez S., Garre V., 2006. Distinct white collar-1 genes control specific light responses in Mucor circinelloides. Mol. Microbiol. 61: 1023–1037. [DOI] [PubMed] [Google Scholar]
- Silva F., Navarro E., Peñaranda A., Murcia-Flores L., Torres-Martínez S., et al. , 2008. A RING-finger protein regulates carotenogenesis via proteolysis-independent ubiquitylation of a white collar-1-like activator. Mol. Microbiol. 70: 1026–1036. [DOI] [PubMed] [Google Scholar]
- Spatafora J. W., Chang Y., Benny G. L., Lazarus K., Smith M. E., et al. , 2016. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108: 1028–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B., Edwards J., Jr., Ibrahim A., 2005. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 18: 556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen E. J., Kang Y., Bokinsky G., Hu Z., Schirmer A., et al. , 2010. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463: 559–562. [DOI] [PubMed] [Google Scholar]
- Trieu T. A., Navarro-Mendoza M. I., Pérez-Arques C., Sanchis M., Capilla J., et al. , 2017. RNAi-based functional genomics identifies new virulence determinants in mucormycosis. PLoS Pathog. 13: e1006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayos A., López-Matas M. A., Ruiz-Hidalgo M. J., Eslava A. P., 1997. Complementation analysis of carotenogenic mutants of Mucor circinelloides. Fungal Genet. Biol. 22: 19–27. [DOI] [PubMed] [Google Scholar]
- Vicente G., Bautista L. F., Rodríguez R., Gutiérrez F. J., Sádaba I., et al. , 2009. Biodiesel production from biomass of an oleaginous fungus. Biochem. Eng. J. 48: 22–27. [Google Scholar]
- Wilson R. B., Davis D., Enloe B. M., Mitchell A. P., 2000. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast 16: 65–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the strains and plasmids used in this study are available upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.