Abstract
Integrating diverse genomics data can provide a global view of the complex biological processes related to the human complex diseases. Although substantial efforts have been made to integrate different omics data, there are at least three challenges for multi-omics integration methods: (i) How to simultaneously consider the effects of various genomic factors, since these factors jointly influence the phenotypes; (ii) How to effectively incorporate the information from publicly accessible databases and omics datasets to fully capture the interactions among (epi)genomic factors from diverse omics data; and (iii) Until present, the combination of more than two omics datasets has been poorly explored. Current integration approaches are not sufficient to address all of these challenges together. We proposed a novel integrative analysis framework by incorporating sparse model, multivariate analysis, Gaussian graphical model, and network analysis to address these three challenges simultaneously. Based on this strategy, we performed a systemic analysis for glioblastoma multiforme (GBM) integrating genome-wide gene expression, DNA methylation, and miRNA expression data. We identified three regulatory modules of genomic factors associated with GBM survival time and revealed a global regulatory pattern for GBM by combining the three modules, with respect to the common regulatory factors. Our method can not only identify disease-associated dysregulated genomic factors from different omics, but more importantly, it can incorporate the information from publicly accessible databases and omics datasets to infer a comprehensive interaction map of all these dysregulated genomic factors. Our work represents an innovative approach to enhance our understanding of molecular genomic mechanisms underlying human complex diseases.
Keywords: integrative analysis, multi-omics data, sparse modeling, glioblastoma multiforme, network analysis
Human complex diseases (e.g., cancer) are induced by various genomic and epigenomic alterations in multiple biological processes (Wang et al. 2011). Studying a single type of biological data is generally insufficient to fully explore the underlying mechanisms of the human complex diseases. Recent advances in high-throughput technologies allow efficient investigation of various omics data, such as single nucleotide polymorphism (SNP), copy number variation (CNV), DNA methylation, and gene expression. Several pioneering studies have yielded genome-scale large datasets, including genomic, epigenomic, transcriptomic, and proteomic information, which are publicly accessible from large collaborative projects, including the ENCODE Project Consortium (2012), NIH Epigenomics Roadmap (Bernstein et al. 2010), and The Cancer Genome Atlas (TCGA) (Weinstein et al. 2013).
Concomitantly, the integration analyses of these diverse omics data are increasingly adopted to identify the potential causal (epi)genomic factors and ultimately provide the systematic view of fundamental insights into the complex mechanisms underlying the etiology of human diseases (Chen et al. 2014b; Hamed et al. 2015). For example, integrating genotype data with whole-genome gene expression data or DNA methylation data can identify the expression quantitative trait loci (eQTL) or the methylation quantitative trait loci (meQTL) (Shabalin 2012; Schadt et al. 2005). Methods for integrating gene expression data with miRNA, SNP, CNV, and DNA methylation data have been applied in cancer genomics (West et al. 2012; Taylor et al. 2009). Additionally, some latent variable models, such as canonical correlation analysis and partial least squares, were applied to identify the relationship between different omics datasets (Friedman et al. 2008; Zhao et al. 2012; Soneson et al. 2010). Recently, network analysis is increasingly gaining acceptance as a useful tool for data integration (Chen et al. 2014b; Kholodenko et al. 2012). Various network analysis methods have been developed to incorporate transcriptomic and proteomic data for computation of biological networks (Gosline et al. 2015; Wachter and Beissbarth 2015), to elucidate causality in biological networks (Gitter et al. 2011; Ourfali et al. 2007), and to integrate and visualize complex metabolomics results even in cases where biochemical domain knowledge or molecular annotations are unknown (Grapov et al. 2015; Karnovsky et al. 2012).
Although substantial efforts have been made to integrate different omics data, there are at least three challenges for multi-omics integration methods to overcome: (i) How to simultaneously consider the effects of all kinds of genomic and epigenomic factors, since these factors jointly influence the phenotypes (Lander 2011); (ii) How to effectively incorporate the information from publicly accessible databases and omics datasets to fully capture the interactions among (epi)genomic factors from diverse omics data sources; and (iii) Until present, the combination of more than two omics datasets has been poorly explored compared with those that intend to integrate two various omics datasets, such as those in eQTL and meQTL analyses (Pineda et al. 2015). Current integration approaches are not sufficient to address all of these challenges simultaneously in one analytical framework.
To address these three challenges in multi-omics integration analysis, we presented a novel integrative analysis framework that incorporated sparse model, multivariate analysis, Gaussian graphical model (GGM), and network analysis. Our method can not only simultaneously identify disease-associated (epi)genomic factors from diverse omics data, but also incorporate the information from publicly accessible databases and omics datasets to infer the regulatory modules of these (epi)genomic factors. By applying this strategy to systemically study the genome-wide gene expression, DNA methylation, and miRNA expression data of glioblastoma multiforme (GBM) samples, we identified three regulatory modules of dysregulated (epi)genomic factors associated with GBM patient survival time. By combining the three regulatory modules with respect to the common regulatory factors, we further revealed a promising global regulatory pattern critical for GBM survival. Our integrative analysis represents an innovative approach to enhance our comprehensive understanding of molecular genomic mechanisms underlying human complex diseases.
Methods
It is well known that genes usually jointly contribute to certain diseases and many epigenomic factors play an important role in the development of complex diseases by regulating gene expression. Our goal is to infer a comprehensive interaction map of all these dysregulated (epi)genomic factors.
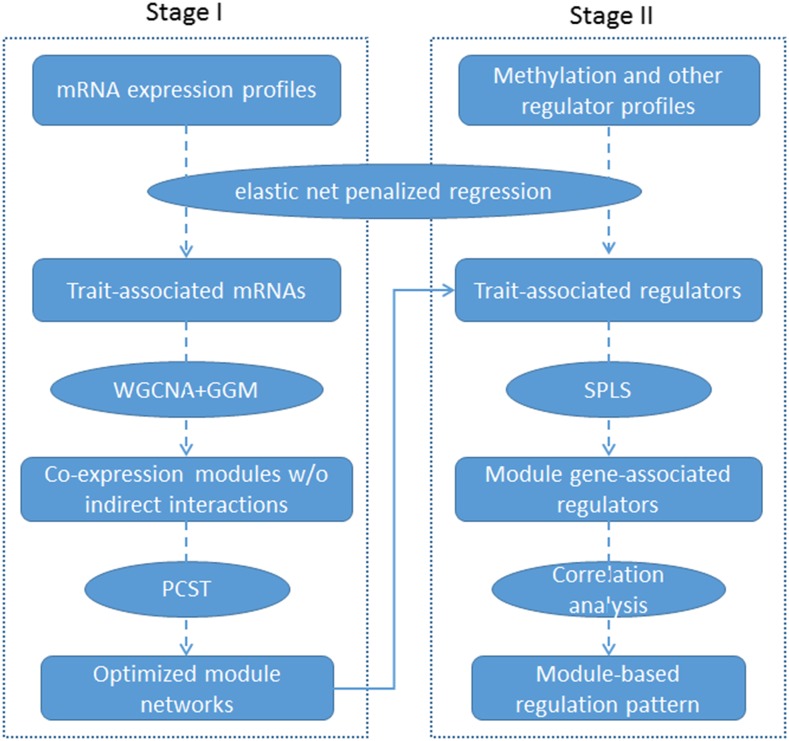
Thus, we divided our integrative analysis into two major stages as shown in Figure 1: we first identify the trait-related mRNAs and build optimized coexpression modules with these mRNAs, and then infer regulation pattern between the epigenomic (and/or other omics factors) and the identified coexpression module.
Figure 1.
Workflow of the integrative analysis of multi-omics data.
Stage 1: build optimized mRNA coexpression module networks associated with the trait of interest
Considering the different gene functional modules may contribute to certain diseases and the computational burden for the downstream analysis, we propose to identify trait-associated mRNAs and subsequently discover the coexpression modules with trait-associated mRNAs through coexpression network analysis.
First, we apply the elastic net penalized regression model to select a set of trait-associated mRNAs. The elastic net is particularly useful to handle the situation of small sample size and a large number of features. In addition, it encourages the selection of strongly correlated predictors in or out of the model together, which is helpful to preserve the information for the following module identification (Zou and Hastie 2005). The elastic net penalized regression model is illustrated as follows:
where the regularization parameter λ > 0 controls the overall strength of the penalty and 0 < α ≤ 1 bridges the gap between lasso (α = 1, the default) and ridge (α = 0) penalty. is the loss function given a fitted model, such as the residual sum of squares for the ordinary linear model or the negative log partial likelihood function for the Cox’s proportional hazards model. The optimal λ and α can be chosen by 10-fold cross-validation.
The trait-associated mRNAs are then subjected to weighted correlation network analysis (WGCNA) (Zhang and Horvath 2005) for the identification of high coexpression modules, denoted as where is the number of modules identified. Computations are carried out using the R package WGCNA (Zhang and Horvath 2005). The relationships stored in the coexpression modules include direct interactions, which connects one pair of genes directly, and indirect interactions, where two genes are connected due to a path with multiple edges (Poyatos 2011). We then remove the indirect interactions in the coexpression modules through a partial correlation analysis.
The GGM reveals direct associations with conditional independence/dependence among variables using partial correlation coeffcients (Mader et al. 2015). Given a coexpression module it is assumed the expression of the genes in follows a multivariate Gaussian distribution with mean μ and covariance matrix Σ. The conditional independence between gi and gj given the other genes g − {ij}, denoted by P(gi, gj|g−{ij}) = P(gi|g − {ij}) P(gj|g − {ij}), is equivalent to that the corresponding element in the precision matrix is zero (Wang and Huang 2014), i.e., ωij = 0. The precision matrix is the inverse of the covariance matrix of genes in denoted by Ω = (ωij) = Σ−1. A partial correlation P(gi, gj|g − {ij}) is formally written as with the property where , and are the estimators of , and (Wang et al. 2016). Only the edges with significantly different from zero will be preserved. An FDR of 0.05 is used as the cut-off for statistical significance to adjust for the multiple testing. The filtered modules are denoted by
After removing the indirect interactions, an optimal subnetwork is refined that which may play an important role on the trait of interest. Given one module is , with a node set as and direct interactions set as the Prize Collecting Steiner Tree (PCST) algorithm, which is able to reconstruct compact networks of the functionally relevant connections with control of the false positives in the network (Huang and Fraenkel 2009), is used to find a set of most confident interactions that connect the terminal genes in the network , using the following function that simultaneously minimizes the cost of edges included and the penalties of nodes excluded:
where the node prize bi is the weight of node i; ce is the cost of edges with k interaction evidence, rj indicates the score of the jth interaction evidence. The weight of node i is from the univariate mRNA trait regression analysis. We assign the absolute value of the regression coefficient of the node i to bi. The cost of edges is derived from two kinds of evidence: (i) information from publicly accessible databases and omics datasets, such as the Search Tool for the Retrieval of Interacting Genes (STRING) database (http://string-db.org/), or public datasets; and (ii) correlations between nodes in the local datasets. Instead of using the standard definition of we combine the two kinds of evidence into the edge weight using where
a naive Bayesian approach to measure the interaction evidence among nodes (Szklarczyk et al. 2011). is the subscore downloaded from STRING or the absolute value of the correlations from the local datasets. This method integrates the scores by multiplying the probabilities of associations not predicting a functional interaction while adjusting for the prior probability () for any two genes being linked, which is 0.063 according to the KEGG benchmark dataset (Franceschini et al. 2013). In the calculation, the prior corrected score is constrained to be within [0, 1] (see the source code online). Compared with the standard method, it yields higher confidence when more than one type of evidence supports a given interaction (von Mering et al. 2003).
Stage 2: infer module-based regulation pattern with epigenomic and/or other omics data
To identify DNA methylation sites and other factors that potentially regulate the optimized module subnetwork (such as miRNAs), we examine their associations with the genes within Let be the data matrix of derived in stage 1, where n is the number of patients with complete phenotypic and multi-omics data, and p is the number of mRNAs in the given module
First, we screened trait-associated methylation sites and other regulators by the elastic net penalized regression model as in the analyses of mRNAs in stage 1. Let be the combined data matrix for the q trait-associated regulators, which may include methylation sites and other factors. To deal with the large number of features and small sample size, we use the sparse partial least square (SPLS) regression model to identify the regulators that are correlated with the module (Meng et al. 2016):
where β are loading vectors for the latent vectors respectively. The sparse regularization function including L1 and L2 penalties is imposed on β: the L1 penalty is applied to set the coefficients of the irrelevant variables to 0; the L2 penalty is added to handle multicollinearity among covariates (Chun and Keles 2010; Chun et al. 2011). The optimal regularization parameter can be chosen by 10-fold cross-validation (Chun et al. 2011). The resulted regulators associated with the module are denoted by
To reveal the module-based regulation patterns, the Pearson’s correlation is used to detect correlations between the identified regulators and mRNAs within the module The cut-off of correlation test p value ≤0.05 (t-test) is applied to select those regulator mRNA pairs of potential interest. Those chosen pairs are further filtered by external databases if there are any, i.e., Exiqon miRSearch, TargetScan, and microRNA.org for miRNA–mRNA regulatory relationships (Chen and Rajewsky 2007; Akhtar et al. 2016).
Data availability
The source code in this study is publicly available at https://github.com/xu1912/SMON.git.
Results
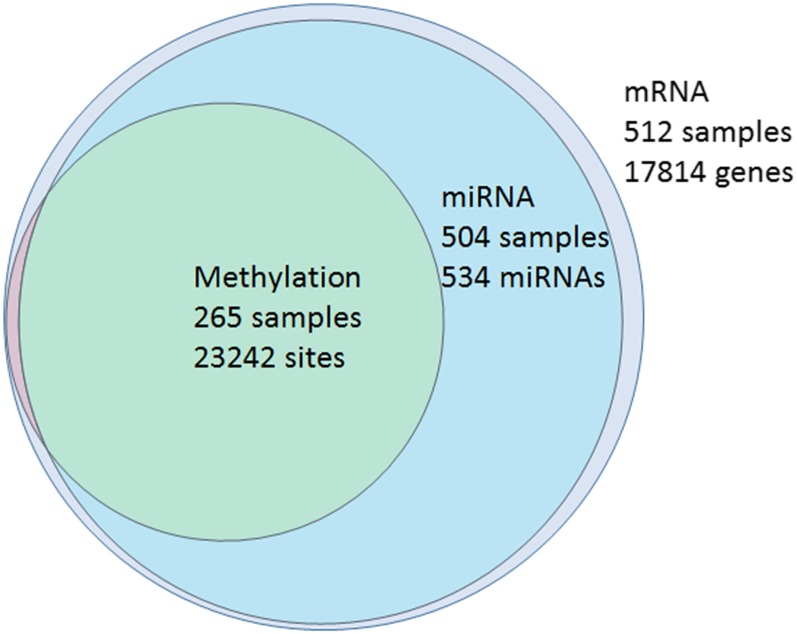
Here, we apply our framework to integrate three different omics datasets (mRNA expression, miRNA expression, and DNA methylation) from the GBM study. All normalized data (level 3) were collected from The Cancer Genome Atlas (TCGA) portal, and can be accessed from the TCGA-GBM project repository at National Cancer Institute Genomic Data Commons Data Portal (https://portal.gdc.cancer.gov/legacy-archive/search/f). To minimize the scale differences among different omics data, the features of the three omics datasets were standardized to have zero means and unit SDs. The clinical outcome of interest is the patient survival time. From the GBM datasets, we excluded those patients whose survival time was <30 d to remove short survival due to reasons such as postoperative complications from the surgery (Kim et al. 2010). Figure 2 presents the sample size of the three omics datasets and the overlapping samples among them.
Figure 2.
Venn diagram of the samples with different Omics data. Methylation ∩ miRNA = 261; methylation ∩ mRNA = 265; miRNA ∩ mRNA = 504.
Identifying trait-associated genes in mRNA data are the first step in reconstructing trait-associated coexpression modules. As detailed in the Methods section, the sparse Cox’s proportional hazards model was used to select genes associated with patients’ survival time, which can simultaneously incorporate thousands of genomic markers working collaboratively with joint effects on the trait of interest in a single statistical model. After 10-fold cross-validation, we identified 217 genes with 512 subjects in mRNA data significantly associated with GBM survival time, including numerous GBM-related genes such as FZD7 (Kierulf-Vieira et al. 2016), TPPP3 (Fomchenko et al. 2011), and LGALS3 (Ma et al. 2014). Using the Dynamic Tree Cut method in WGCNA, we identified three coexpression modules, which are composed of 50, 28, and 18 genes, respectively. There were 121 genes (55.76% in total identified genes) that were not grouped in any community. This was partially due to the minimum size of modules we set. In this study, the minimum size of modules was set as 15, which means those genes were not assigned in a module due to their size being <15, even if they were grouped.
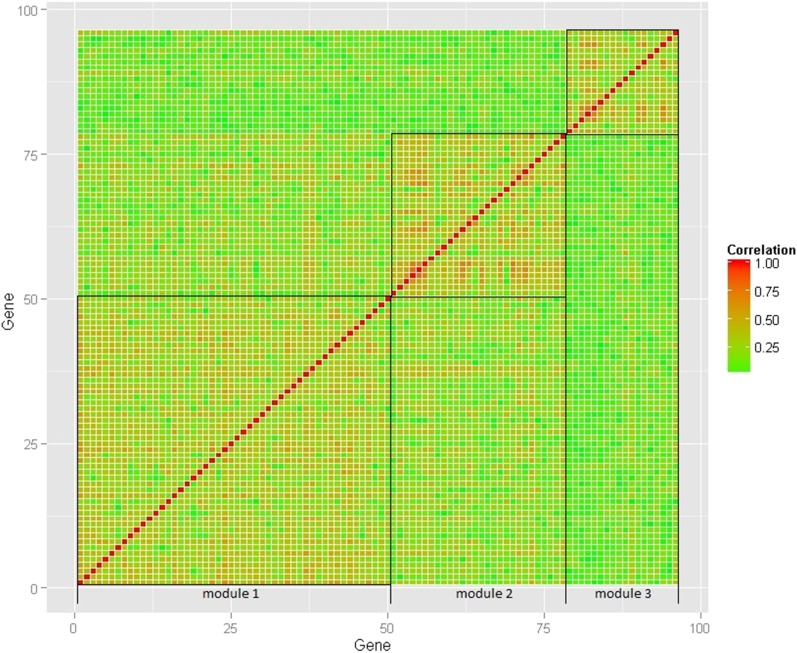
Figure 3 presents the correlation heat map of modules constructed through WGCNA. In the heat map, each row (column) corresponds to a gene. The independent modules are represented as isolated boxes along the diagonal. Inspecting the correlation between and within the module memberships, these genes within each module are found to be strongly connected (reflected by the majority of red blocks within each module in Figure 3), and the genes between modules show weak connections (reflected by the overall green block in the heat map in Figure 3). The weak interconnectivity between modules suggests that the three modules may function separately and affect patient survival time in relatively independent ways.
Figure 3.
Heat map of correlations between and within coexpression modules constructed by WGCNA. Each row/column represents a gene. Each cell element is the absolute value of correlation coefficient between two genes. The intensity of red coloring indicates the strength of correlation between pairs of genes, with green color corresponding to low correlation. The independent modules are represented as isolated boxes along the diagonal.
We estimated the partial correlations between genes in each module to trim indirect interactions. In each module, we selected significant gene pairs (FDR ≤ 0.05) for their partial correlations and reconstructed the coexpression networks using these significant gene pairs. As shown in Supplemental Material, Figure S1, in modules 1–3, we identified 272, 141, and 141 gene pairs with significant partial correlations, respectively, and trimmed 77.80, 62.70, and 7.84% of gene pairs as indirect interactions. In addition, by incorporating the information on protein–protein interactions (PPIs) from the STRING database, we further filtered the unreliable or indirect interactions in the modules by PCST. In total, 49 gene pairs from module 1, 27 pairs from module 2, and 17 pairs from module 3 were determined as the most reliable interactions (Figure S1) that had multiple lines of evidence supporting their potential functionality in the cell. We also performed pathway enrichment analysis on the genes collected from all the three modules with WEB-based GEne SeT AnaLysis Toolkit (Wang et al. 2013) to investigate how well the modules functioned in a GBM-related process, as annotated by KEGG database (Kanehisa et al. 2012). An additional file (Table S1) lists the nine most significant KEGG pathways with adjusted p values <0.05, including mRNA surveillance pathway, pathways in cancer, WNT signaling pathway, etc. Among these pathways, it is well known that WNT signaling pathway regulates proliferation, death, and migration and cell fate decision. Dysregulation of the WNT signaling pathway was associated with various cancers, including GBM (Lee et al. 2016). The members FZD7 and FZD10 in the WNT signaling pathway are important receptors. In many types of cancer, FZD2 expression was strongly correlated with poor prognosis (Mine et al. 2015). Therefore, our results may reveal the regulation pattern of FZD2 and FZD10 expression by network analysis, which could be utilized for epigenomic-based therapy for GBM.
To incorporate DNA methylation and miRNA data into the coexpression modules, we identified miRNAs and methylation sites that are associated with patient survival time, and then used SPLS regression to determine those miRNAs and DNA methylation sites that are also associated with the genes of the three coexpression modules in Table 1. Further, within each module, we determined genes with cis-correlated DNA methylation sites, as well as miRNA–mRNA pairs.
Table 1. The identified miRNAs and methylation sites for three modules by SPLS model.
| mRNAs | miRNAs | Methylation Sites | |
|---|---|---|---|
| Module 1 | 50 | 46 | 353 |
| Module 2 | 28 | 11 | 125 |
| Module 3 | 18 | 33 | 174 |
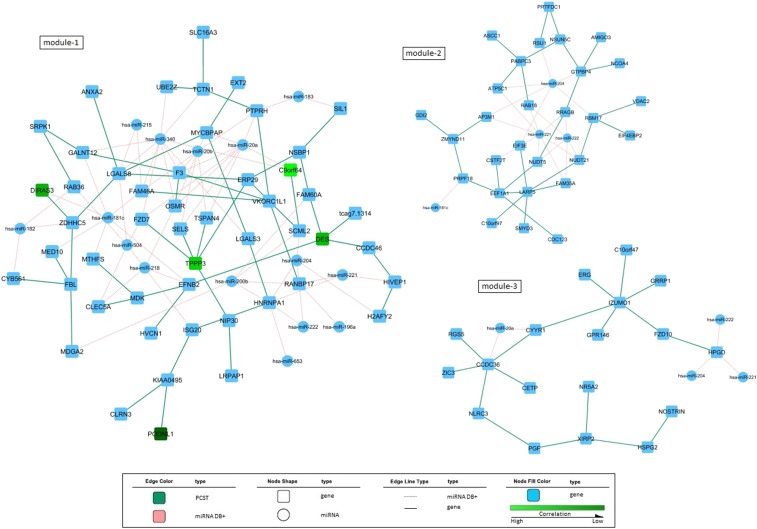
It is known that DNA methylation is an important epigenomic mechanism to regulate gene expression. If there is a significant association for one gene between its methylation level and expression level, it is called cis relationship; otherwise, it is defined as trans relationship (Smith and Meissner 2013). Since the reproducibility of trans relationships is still in debate, we focused on genes with cis relationships (van Nas et al. 2010; van Eijk et al. 2012). It can be seen that only four gene sites in module 1 have cis effects (Figure 4). The methylation levels of these four gene sites show negative correlations with the expression levels of corresponding genes. In modules 2 and 3, we did not identify the gene sites with cis effects on corresponding genes.
Figure 4.
The interaction modules incorporating information of miRNAs and methylation sites. Each rectangle represents a gene; the circle represents miRNA. The green rectangles are genes with cis effects, with brighter indicating higher. The pink dashed edges indicate miRNA–gene interactions annotated in previously mentioned miRNA databases. The green solid edges are gene–gene connections resulting from PCST.
miRNA is well known for the major function of cleaving transcripts of its target genes at the post-transcriptional level (He and Hannon 2004). Thus, we were most interested in a negative correlation between miRNA and gene expression. The external databases Exiqon miRSearch, TargetScan, and microRNA.org were used to filter the miRNA–mRNA pairs with miRNA–target regulatory relationships (Chen and Rajewsky 2007; Akhtar et al. 2016). Those miRNA–mRNA interactions with significant negative correlations and miRNA–target relationships are kept. As shown in Figure 4, we identified 15 miRNAs for module 1, four for module 2, and four for module 3.
Our results highlight a number of interesting regulatory mechanisms that may be critical for GBM development and progress. For example, our results suggest that miRNA-181c and the methylation level of DIRAS3 both contribute to the alteration of DIRAS3 expression (module 1 in Figure 4), which may, in turn, affect the GBM survival time. DIRAS3 (also known as ARHI) is a known tumor suppressor gene and overexpression of DIRAS3 resulted in suppression of glioma cell proliferation, arrest of cell-cycle progression, reduction in cell migration and invasion, and promotion of cell apoptosis (Chen et al. 2014a). In addition, miRNA-181c was reported as a tumor-related gene in glioma cells (Ruan et al. 2015). Thus, our results indicate one possible regulation mechanism of these tumor-related factors and may provide candidate targets for gene therapy of glioma.
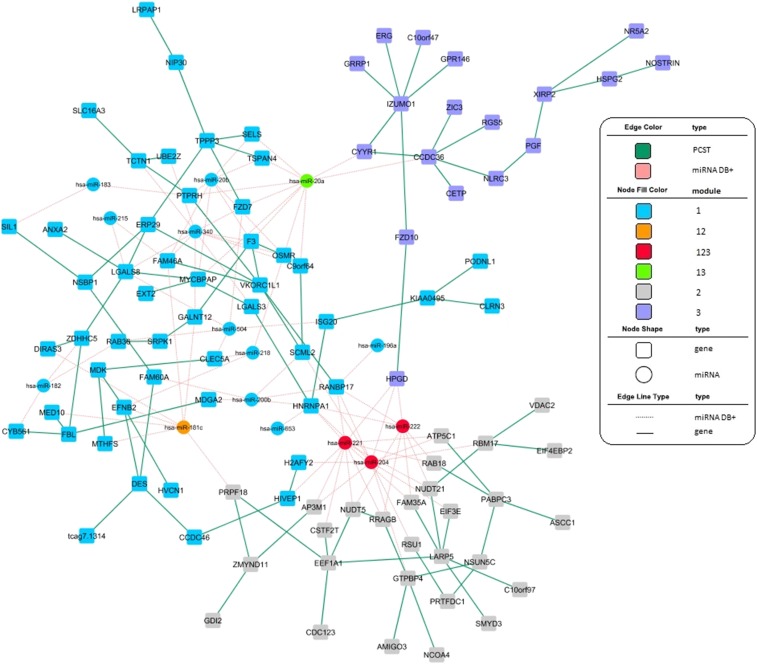
We combined the three modules to have a global view (Figure 5) of the regulatory networks contributing to GBM patient survival time. The top six miRNAs with most edges in the combined network were miRNA-221, miRNA-204, miRNA-20a, miRNA-340, miRNA-222, and miRNA-181c. Among these six miRNAs, miRNA-221, -204, and -222 were shared by three modules; miRNA-181c was shared by modules 1 and 2; miRNA-20a was shared by modules 1 and 3; and miRNA-340 was not shared among modules. These miRNAs may mediate cooperative regulation of different modules and thus may play particularly critical roles in regulating GBM development and progress. miRNA-221 and miRNA-222 are oncogenic miRNAs that have been studied in relation to a diverse list of cancers, including GBM. When overexpressed in vitro, both miRNA-221 and miRNA-222 potentiate classic cancer hallmarks, i.e., proliferation, angiogenesis, and invasion (Henriksen et al. 2014; Singh et al. 2012; Zhang et al. 2009). Due to their broader functional relevance, miRNA-20a, -204, -181c, and -340 also have been identified as oncogenic genes and may serve as targets for treatment of GBM (Wang et al. 2015; Wei et al. 2015; Xia et al. 2015; Huang et al. 2015; Ruan et al. 2015). Additionally, several target genes of these miRNAs have been validated in previous studies, e.g., RAB18, RSU1, GTPBP4, DIRAS3, and F3 (Behrends et al. 2003; Chunduru et al. 2002; Lee et al. 2007; Riemenschneider et al. 2008; Gessler et al. 2010). Taken together, our findings highlight several miRNAs that may regulate multiple signaling cascades crucial for gliomagenesis and therefore, these miRNAs could be therapeutically significant.
Figure 5.
Combined regulatory network with three identified modules. Each rectangle represents a gene; the circle represents miRNA. Different colors of the rectangles/circles indicate their belonging to a single module or overlap of the three modules. The pink dashed edges indicate miRNA–gene interactions annotated in previously mentioned miRNA databases. The green solid edges are gene–gene connections resulting from PCST.
Discussion
Single-omics studies (genome/transcriptome/epigenome/proteome) fall short of illuminating the underlying functional mechanisms and providing a comprehensive view of the regulatory patterns of genomic factors across multiple omics datasets for the etiology of human diseases (Farber and Lusis 2009). Integrating multi-omics datasets in network analysis may facilitate the discovery of novel susceptibility genes for human complex diseases, and more importantly, yield a comprehensive understanding of the complex regulatory mechanisms embedded in and across multi-omics data (Farber 2010). In this study, we proposed an integrative network analysis framework with epigenomic and transcriptomic data to identify regulatory patterns relevant to the trait of interest. The additional analysis in File S1 indicates that our framework can produce reliable results.
Our framework started with the coexpression network analysis to identify the coexpression modules based on the following two considerations: (i) due to the complexity of human diseases, it is highly likely that different gene functional modules may contribute to certain diseases, and since different modules tend to have different biological functions, it is reasonable to analyze each module separately in the sense that different biological functionalities should be considered separately (Ma et al. 2012); (ii) a benefit of coexpression network analysis is that it can greatly decrease the computational burden for the downstream analysis, e.g., partial correlation analysis and inference of optimal subnetworks. For example, if we have 300 trait-associated genes, there will be 44,850 gene pairs to be tested in partial correlation analysis, and this number will increase dramatically with the increase of the number of trait-associated genes, which will lead to heavy computation burden and decrease of power to identify gene pairs with significant partial correlations.
In the case of complex diseases (such as GBM), comprehensively identifying interactions among (epi)genomic factors is important to systematically dissect cellular roles of those (epi)genomic factors and to gain insights into metabolic pathways. With a coexpression network, the number of correlations is generally considerably high, suggesting a plethora of indirect interactions (Krumsiek et al. 2011). To remove indirect interactions among genomic factors and refer reliable regulation networks, our proposed method incorporated two kinds of analyses for a given coexpression module: partial correlation analysis and inference of optimal subnetworks. In partial correlation analysis, GGMs were applied to distinguish direct from indirect associations by estimating the conditional dependence between genes based on partial correlation coefficients. However, conditional independence by itself is insufficient to remove all indirect relationships. Thus, compiling the information from external public databases will be helpful to further prune those unlikely, indirect, and spurious interactions. We can retrieve various genomic interactions from many available public databases and multi-omics datasets, such as PPIs. In this study, we incorporated interaction information from PPIs and the results of the partial correlation analysis to compute a score as a confidence score for each interaction in the module. The most reliable interactions of each module were further inferred through searching optimal subnetwork for the given module.
Since our goal is to identify regulatory patterns relevant to the trait of interest from epigenomic and transcriptomic data, it is reasonable to only choose the trait-associated (epi)genomic factors from multi-omics data for the subsequent network analysis. This selection can not only remove noise but also decrease the computational cost in the network construction of multi-omics data. During the selection procedure, we adopted a sparse model using L1 and L2 penalties to identify the trait-associated genomic factors, which has the following specific advantages: (i) it accommodates tens of thousands of features at a time and identifies joint effects of a combination of trait-associated genomic factors, including those with small effect sizes; (ii) using both L1 and L2 penalties, it is able to select groups of correlated variables, which are very common in high-dimensional genomic data. The selection of relevant correlated genomic factors is essentially important for coexpression network analysis and discovery of regulation patterns. Thus, the sparse model using both L1 and L2 penalties demonstrates the efficiency in feature selection and captures informative genomic factors.
In summary, our method can not only identify disease-associated dysregulated genomic factors, but also, more importantly, construct a comprehensive map of interactions of all these dysregulated genomic factors implicated in a specific disease. It is essential to understand the intricacy of the genomic mechanisms behind complex diseases, and this may support the development of new therapeutics. However, it should be recognized that network representation of the complexity of biological systems is just the beginning. This study is expected to pioneer an innovative approach to comprehensively enhance our understanding of molecular genomic mechanism in human complex diseases.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.042408/-/DC1.
Acknowledgments
This study benefited from the support of the National Institutes of Health grants (R01-AR057049, R01-AR059781, D43-TW009107, P20-GM109036, R01-MH107354, R01-MH104680, and R01-GM109068) and the Edward G. Schlieder Endowment Fund (awarded to H.-W.D.). The authors declare that they have no conflict of interest.
Footnotes
Communicating editor: J. Prendergast
Literature Cited
- Akhtar M. M., Micolucci L., Islam M. S., Olivieri F., Procopio A. D., 2016. Bioinformatic tools for microRNA dissection. Nucleic Acids Res. 44(1): 24–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends U., Schneider I., Rossler S., Frauenknecht H., Golbeck A., et al. , 2003. Novel tumor antigens identified by autologous antibody screening of childhood medulloblastoma cDNA libraries. Int. J. Cancer 106(2): 244–251. [DOI] [PubMed] [Google Scholar]
- Bernstein B. E., Stamatoyannopoulos J. A., Costello J. F., Ren B., Milosavljevic A., et al. , 2010. The NIH roadmap epigenomics mapping consortium. Nat. Biotechnol. 28(10): 1045–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Shi S., Yang W., Chen C., 2014a Over-expression of ARHI decreases tumor growth, migration, and invasion in human glioma. Med. Oncol. 31(3): 846. [DOI] [PubMed] [Google Scholar]
- Chen J. C., Alvarez M. J., Talos F., Dhruv H., Rieckhof G. E., et al. , 2014b Identification of causal genetic drivers of human disease through systems-level analysis of regulatory networks. Cell 159(2): 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Rajewsky N., 2007. The evolution of gene regulation by transcription factors and microRNAs. Nat. Rev. Genet. 8(2): 93–103. [DOI] [PubMed] [Google Scholar]
- Chun H., Keles S., 2010. Sparse partial least squares regression for simultaneous dimension reduction and variable selection. J. R. Stat. Soc. Series B Stat. Methodol. 72(1): 3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H., Ballard D. H., Cho J., Zhao H., 2011. Identification of association between disease and multiple markers via sparse partial least-squares regression. Genet. Epidemiol. 35(6): 479–486. [DOI] [PubMed] [Google Scholar]
- Chunduru S., Kawami H., Gullick R., Monacci W. J., Dougherty G., et al. , 2002. Identification of an alternatively spliced RNA for the Ras suppressor RSU-1 in human gliomas. J. Neurooncol. 60(3): 201–211. [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium , 2012. An integrated encyclopedia of DNA elements in the human genome. Nature 489 (7414): 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber C. R., 2010. Identification of a gene module associated with BMD through the integration of network analysis and genome-wide association data. J. Bone Miner. Res. 25(11): 2359–2367. [DOI] [PubMed] [Google Scholar]
- Farber C. R., Lusis A. J., 2009. Future of osteoporosis genetics: enhancing genome-wide association studies. J. Bone Miner. Res. 24(12): 1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomchenko E. I., Dougherty J. D., Helmy K. Y., Katz A. M., Pietras A., et al. , 2011. Recruited cells can become transformed and overtake PDGF-induced murine gliomas in vivo during tumor progression. PLoS One 6(7): e20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., et al. , 2013. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41(Database issue): D808–D815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Hastie T., Tibshirani R., 2008. Sparse inverse covariance estimation with the graphical lasso. Biostatistics 9(3): 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler F., Voss V., Dutzmann S., Seifert V., Gerlach R., et al. , 2010. Inhibition of tissue factor/protease-activated receptor-2 signaling limits proliferation, migration and invasion of malignant glioma cells. Neuroscience 165(4): 1312–1322. [DOI] [PubMed] [Google Scholar]
- Gitter A., Klein-Seetharaman J., Gupta A., Bar-Joseph Z., 2011. Discovering pathways by orienting edges in protein interaction networks. Nucleic Acids Res. 39(4): e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosline S. J., Oh C., Fraenkel E., 2015. SAMNetWeb: identifying condition-specific networks linking signaling and transcription. Bioinformatics 31(7): 1124–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapov D., Wanichthanarak K., Fiehn O., 2015. MetaMapR: pathway independent metabolomic network analysis incorporating unknowns. Bioinformatics 31(16): 2757–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed M., Spaniol C., Zapp A., Helms V., 2015. Integrative network-based approach identifies key genetic elements in breast invasive carcinoma. BMC Genomics 16(Suppl. 5): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Hannon G. J., 2004. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5(7): 522–531. [DOI] [PubMed] [Google Scholar]
- Henriksen M., Johnsen K. B., Andersen H. H., Pilgaard L., Duroux M., 2014. MicroRNA expression signatures determine prognosis and survival in glioblastoma multiforme-a systematic overview. Mol. Neurobiol. 50(3): 896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Qiu S., Ge R., He L., Li M., et al. , 2015. miR-340 suppresses glioblastoma multiforme. Oncotarget 6(11): 9257–9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. S., Fraenkel E., 2009. Integrating proteomic, transcriptional, and interactome data reveals hidden components of signaling and regulatory networks. Sci. Signal. 2(81): ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S., Sato Y., Furumichi M., Tanabe M., 2012. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40(Database issue): D109–D114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky A., Weymouth T., Hull T., Tarcea V. G., Scardoni G., et al. , 2012. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics 28(3): 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko B., Yaffe M. B., Kolch W., 2012. Computational approaches for analyzing information flow in biological networks. Sci. Signal. 5(220): re1. [DOI] [PubMed] [Google Scholar]
- Kierulf-Vieira K. S., Sandberg C. J., Grieg Z., Gunther C. C., Langmoen I. A., et al. , 2016. Wnt inhibition is dysregulated in gliomas and its re-establishment inhibits proliferation and tumor sphere formation. Exp. Cell Res. 340(1): 53–61. [DOI] [PubMed] [Google Scholar]
- Kim H., Huang W., Jiang X., Pennicooke B., Park P. J., et al. , 2010. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc. Natl. Acad. Sci. USA 107(5): 2183–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumsiek J., Suhre K., Illig T., Adamski J., Theis F. J., 2011. Gaussian graphical modeling reconstructs pathway reactions from high-throughput metabolomics data. BMC Syst. Biol. 5: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., 2011. Initial impact of the sequencing of the human genome. Nature 470(7333): 187–197. [DOI] [PubMed] [Google Scholar]
- Lee H., Kim D., Dan H. C., Wu E. L., Gritsko T. M., et al. , 2007. Identification and characterization of putative tumor suppressor NGB, a GTP-binding protein that interacts with the neurofibromatosis 2 protein. Mol. Cell. Biol. 27(6): 2103–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lee J. K., Ahn S. H., Lee J., Nam D. H., 2016. WNT signaling in glioblastoma and therapeutic opportunities. Lab. Invest. 96(2): 137–150. [DOI] [PubMed] [Google Scholar]
- Ma J., Yao Y. L., Wang P., Liu Y. H., Zhao L. N., et al. , 2014. MiR-152 functions as a tumor suppressor in glioblastoma stem cells by targeting Kruppel-like factor 4. Cancer Lett. 355(1): 85–95. [DOI] [PubMed] [Google Scholar]
- Ma S., Huang Y., Huang J., Fang K., 2012. Gene network-based cancer prognosis analysis with sparse boosting. Genet. Res. 94(4): 205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader W., Mader M., Timmer J., Thiel M., Schelter B., 2015. Networks: on the relation of bi- and multivariate measures. Sci. Rep. 5: 10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng C., Zeleznik O. A., Thallinger G. G., Kuster B., Gholami A. M., et al. , 2016. Dimension reduction techniques for the integrative analysis of multi-omics data. Brief Bioinform. 17(4): 628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine M., Yamaguchi K., Sugiura T., Chigita S., Yoshihama N., et al. , 2015. miR-203 inhibits frizzled-2 expression via CD82/KAI1 expression in human lung carcinoma cells. PLoS One 10(7): e0131350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourfali O., Shlomi T., Ideker T., Ruppin E., Sharan R., 2007. SPINE: a framework for signaling-regulatory pathway inference from cause-effect experiments. Bioinformatics 23(13): i359–i366. [DOI] [PubMed] [Google Scholar]
- Pineda S., Real F. X., Kogevinas M., Carrato A., Chanock S. J., et al. , 2015. Integration analysis of three omics data using penalized regression methods: an application to bladder cancer. PLoS Genet. 11(12): e1005689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyatos J. F., 2011. The balance of weak and strong interactions in genetic networks. PLoS One 6(2): e14598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemenschneider M. J., Reifenberger J., Reifenberger G., 2008. Frequent biallelic inactivation and transcriptional silencing of the DIRAS3 gene at 1p31 in oligodendroglial tumors with 1p loss. Int. J. Cancer 122(11): 2503–2510. [DOI] [PubMed] [Google Scholar]
- Ruan J., Lou S., Dai Q., Mao D., Ji J., et al. , 2015. Tumor suppressor miR-181c attenuates proliferation, invasion, and self-renewal abilities in glioblastoma. Neuroreport 26(2): 66–73. [DOI] [PubMed] [Google Scholar]
- Schadt E. E., Lamb J., Yang X., Zhu J., Edwards S., et al. , 2005. An integrative genomics approach to infer causal associations between gene expression and disease. Nat. Genet. 37(7): 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalin A. A., 2012. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics 28(10): 1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K., Vartanian A., Burrell K., Zadeh G., 2012. A microRNA link to glioblastoma heterogeneity. Cancers (Basel) 4(3): 846–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Z. D., Meissner A., 2013. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 14(3): 204–220. [DOI] [PubMed] [Google Scholar]
- Soneson C., Lilljebjorn H., Fioretos T., Fontes M., 2010. Integrative analysis of gene expression and copy number alterations using canonical correlation analysis. BMC Bioinformatics 11: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Franceschini A., Kuhn M., Simonovic M., Roth A., et al. , 2011. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39(Database issue): D561–D568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. W., Linding R., Warde-Farley D., Liu Y., Pesquita C., et al. , 2009. Dynamic modularity in protein interaction networks predicts breast cancer outcome. Nat. Biotechnol. 27(2): 199–204. [DOI] [PubMed] [Google Scholar]
- van Eijk K. R., de Jong S., Boks M. P. M., Langeveld T., Colas F., et al. , 2012. Genetic analysis of DNA methylation and gene expression levels in whole blood of healthy human subjects. BMC Genomics 13: 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nas A., Ingram-Drake L., Sinsheimer J. S., Wang S. S., Schadt E. E., et al. , 2010. Expression quantitative trait loci: replication, tissue- and sex-specificity in mice. Genetics 185(3): 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mering C., Huynen M., Jaeggi D., Schmidt S., Bork P., et al. , 2003. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 31(1): 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter A., Beissbarth T., 2015. pwOmics: an R package for pathway-based integration of time-series omics data using public database knowledge. Bioinformatics 31(18): 3072–3074. [DOI] [PubMed] [Google Scholar]
- Wang, J., D. Duncan, Z. Shi, B. Zhang, 2013 WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 41 (Web server issue): W77–W83. [DOI] [PMC free article] [PubMed]
- Wang T., Ren Z., Ding Y., Fang Z., Sun Z., et al. , 2016. FastGGM: an efficient algorithm for the inference of gaussian graphical model in biological networks. PLOS Comput. Biol. 12(2): e1004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Gulbahce N., Yu H., 2011. Network-based methods for human disease gene prediction. Brief. Funct. Genomics 10(5): 280–293. [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Huang H., 2014. Review on statistical methods for gene network reconstruction using expression data. J. Theor. Biol. 362: 53–61. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wang B., Shi Y., Xu C., Xiao H. L., et al. , 2015. Oncogenic miR-20a and miR-106a enhance the invasiveness of human glioma stem cells by directly targeting TIMP-2. Oncogene 34(11): 1407–1419. [DOI] [PubMed] [Google Scholar]
- Wei J., Qi X., Zhan Q., Zhou D., Yan Q., et al. , 2015. miR-20a mediates temozolomide-resistance in glioblastoma cells via negatively regulating LRIG1 expression. Biomed. Pharmacother. 71: 112–118. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Collisson E. A., Mills G. B., Shaw K. R., Ozenberger B. A., et al. , 2013. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 45(10): 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J., Bianconi G., Severini S., Teschendorff A. E., 2012. Differential network entropy reveals cancer system hallmarks. Sci. Rep. 2: 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Liu F., Zhang J., Liu L., 2015. Decreased expression of MiRNA-204–5p contributes to glioma progression and promotes glioma cell growth, migration and invasion. PLoS One 10(7): e0132399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Horvath S., 2005. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 4: Article17. [DOI] [PubMed] [Google Scholar]
- Zhang C. Z., Kang C. S., You Y. P., Pu P. Y., Yang W. D., et al. , 2009. Co-suppression of miR-221/222 cluster suppresses human glioma cell growth by targeting p27(kip1) in vitro and in vivo. Int. J. Oncol. 34(6): 1653–1660. [DOI] [PubMed] [Google Scholar]
- Zhao T., Liu H., Roeder K., Lafferty J., Wasserman L., 2012. The huge package for high-dimensional undirected graph estimation in R. J. Mach. Learn. Res. 13: 1059–1062. [PMC free article] [PubMed] [Google Scholar]
- Zou H., Hastie T., 2005. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Series B Stat. Methodol. 67: 301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source code in this study is publicly available at https://github.com/xu1912/SMON.git.