Abstract
Background
A new generation of biologic and targeted agents may potentially replace traditional cytotoxic agents in lymphoma. Lenalidomide plus rituximab was felt to be a safe and promising backbone based on available data. Idelalisib is an oral PI3Kδ inhibitor approved for relapsed/refractory indolent lymphomas. The primary objective of these two trials was to determine the maximum tolerated dose of lenalidomide in combination with rituximab and idelalisib in relapsed follicular and mantle cell lymphoma.
Methods
A051201 (mantle cell lymphoma) and A051202 (follicular lymphoma) are phase I trials with a safety endpoint. Patients with histologically documented relapsed mantle cell lymphoma who had not received prior lenalidomide or idelalisib (A051201) started with oral lenalidomide 15 mg D1-21 q28d, oral idelalisib 150 mg BID with continuous 28-day cycles, and intravenous rituximab 375 mg/m2 weekly during cycle 1. Patients with histologically documented relapsed follicular lymphoma sensitive to prior rituximab (A051202) started with oral lenalidomide 10 mg D1-21 q28d and oral idelalisib 150 mg BID with continuous 28-day cycles, and intravenous rituximab 375 mg/m2 on C1D8, C1D15, C1D22 and C2D1.
Findings
All analyses are intent-to-treat. Eleven patients (3 mantle cell lymphoma, 8 follicular lymphoma) enrolled. Among the first 8 patients, four experienced unexpected dose-limiting toxicities: grade 4 sepsis syndrome, grade 4 hypotension with grade 3 rash and fevers, grade 4 transaminase elevation with fevers, and grade 3 pulmonary infection with grade 3 maculopapular rash. Symptom onset was 9–20 days after treatment initiation, coinciding with rituximab infusions. Both studies were amended to remove rituximab, but two of three additional patients had grade 3 rashes and one had grade 3 AST elevation. Both trials were permanently closed. The most common grade 3–4 adverse events were ALT elevation (2/3), and rash (2/3) for mantle cell patients and neutropenia (5/8) and rash (4/8) for follicular lymphoma patients. The primary endpoint of safety and tolerability was not met.
Interpretation
The combination of idelalisib, lenalidomide and rituximab in these trials is excessively toxic, and these trials serve as cautionary notes as new combinations are proposed. Off-target effects, drug-drug interactions, and emerging toxicities should be carefully evaluated when investigating biologic agents in combination and should never be done outside of a clinical trial setting.
Keywords: relapsed lymphoma, PI3K, immunomodulatory agents, rituximab, toxicity
INTRODUCTION
Follicular lymphoma and mantle cell lymphoma are incurable mature B-cell malignancies, affecting approximately 30,000 new patients annually. Both are historically managed with sequential cytotoxic combination regimens. The discovery of key survival pathways has prompted a new generation of oral targeted drugs with encouraging single agent activity. A rational strategy to systematically evaluate combinations of new agents for safety and efficacy to move away from chemotherapy has been a focus of the Cancer and Leukemia Group B (CALGB) and Alliance for Clinical Trials in Oncology (Alliance) lymphoma committee.
Lenalidomide, which has pleiotropic effects on malignant and microenvironmental cells, is active in both follicular lymphoma and mantle cell lymphoma.(1–3) The overall response rate with single agent lenalidomide is 20–50% in relapsed/refractory follicular lymphoma(2, 3) and 35% in relapsed/refractory mantle cell lymphoma(1) with median remission durations of approximately 16 months. When combined with rituximab, there is a suggestion of additive and possibly synergistic clinical activity; for example, in relapsed/refractory follicular lymphoma, lenalidomide plus rituximab (R-len) had significantly higher overall response rates (ORR) and complete response (CR) rates (75% ORR, 32% CR) versus lenalidomide alone (23% ORR, 7% CR).(4) In frontline follicular lymphoma, R-len achieved response in over 90% of patients with more than two-thirds achieving a complete remission.(5, 6) Similarly, R-len is highly active in recurrent mantle cell lymphoma,(7) and there are now impressive front-line data with the combination as well showing an overall response rate of 92%, complete remission rate of 64% and a 2-year overall survival of 97%.(8) R-lenalidomide is now being prospectively tested against chemoimmunotherapy (NCT01476787).
Idelalisib is an oral and specific inhibitor of the delta isoform of PI3K (phosphatidylinositol-4,5-bisphosphate 3-kinase). PI3K is both downstream of B-cell receptor signaling and upstream of other survival pathways, including Akt/mTOR. As monotherapy, idelalisib has activity in relapsed and highly refractory indolent lymphomas, with response rates over 50%, leading to Federal Drug Administration approval.(9) Common toxicities include fatigue, diarrhea, rash and pneumonia; grade 3 or higher toxicities are transaminase elevation and neutropenia in up to one-quarter of patients appears dose-independent.(9, 10) The majority of toxicities, including transaminase elevation, are usually reversible and may not recur with re-exposure. However, though less common, there are also observations of a delayed severe colitis that typically requires treatment cessation.(11) When combined with rituximab, idelalisib shows activity in relapsed/refractory chronic lymphocytic leukemia without unusual safety signals compared to rituximab alone.(12)
While single agent activity is an important advance, none of the new agents are curative and combination strategies testing safety and efficacy are needed. The CALGB/Alliance have conducted several trials with biologic doublets for both treatment-naïve and relapsed lymphomas(4, 13–15), with the overall goal of developing targeted regimens to replace cytotoxic therapy. Both A051201 (mantle cell lymphoma) and A051202 (follicular lymphoma) were designed to capitalize on clinical synergy of lenalidomide and rituximab observed in prior trials by adding the highly specific PI3Kδ inhibitor, idelalisib, in patients with relapsed mantle cell and follicular lymphoma.
PATIENTS AND METHODS
Eligibility Criteria
Eligibility criteria for A051201 included histologically documented mantle cell lymphoma (CD5+CD23−CD20+Cyclin D1+) from an initial or relapsed tissue specimen, at least one prior therapeutic regimen, no prior lenalidomide or idelalisib, Eastern Cooperative Oncology Group (ECOG) performance status 0–2, age ≥ 18, measurable disease ≥ 1 cm, and adequate organ function reflected by ANC ≥ 1000, platelets ≥ 75,000/mL, creatinine ≤1.5 × ULN, creatinine clearance ≥ 60 mL/min and total bilirubin ≤ 2 × ULN in the absence of Gilbert’s disease. Exclusion criteria included prior allogeneic stem cell transplant, central nervous system involvement, deep venous thrombosis or pulmonary embolism within three months of study entry, and hepatitis B or C infection. Eligibility criteria for A051202 were similar with the following differences: histologically documented follicular lymphoma grade 1–3a (documented CD20+), time to progression ≥ 6 months from last rituximab-containing regimen, and no radioimmunotherapy within 12 months of study entry.
Study Design and Objectives
The primary endpoints of the multicenter studies A051201 (mantle cell lymphoma) and A051202 (follicular lymphoma) were safety and tolerability combining idelalisib with lenalidomide and rituximab in patients with relapsed mantle cell lymphoma and relapsed follicular lymphoma, respectively, If the maximum tolerated doses (MTD) were established, a randomized phase II component was planned for A051201 evaluating lenalidomide plus rituximab with or without idelalisib (Figure 1). A051202 included an expansion cohort for an additional 10 patients to be treated at the MTD. Secondary endpoints included overall and complete response rates, progression-free survival (PFS) and overall survival (OS). Institutional review boards approved the protocol at each participating site, and written informed consent was obtained from all participating patients. The studies were registered at ClinicalTrials.gov (NCT01838434 and NCT01644799).
Figure 1.
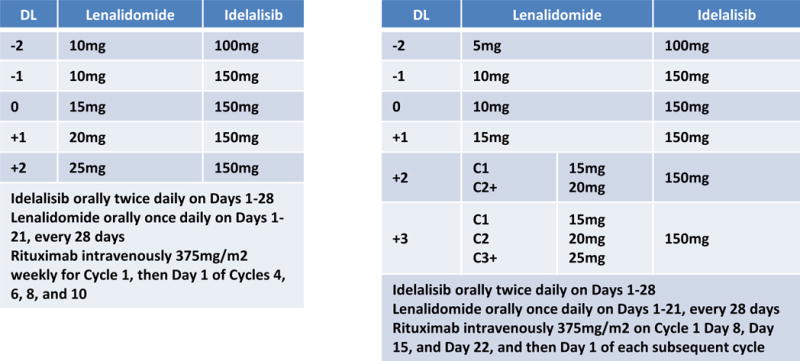
Initial treatment schemas for A051201 and A051202.
Treatment Plan
The therapeutic regimens are summarized in Figure 1. A051201 started at Dose Level 0 consisting of oral lenalidomide15 mg daily on Days 1–21, oral idelalisib 150 mg twice daily on days 1–28, and intravenous rituximab 375 mg/m2 weekly during Cycle 1, and then on Day 1 of each subsequent 28 day cycle. Patients completing 12 cycles were to be randomized to maintenance R-len or R-len plus idelalisib until progression, intolerance, or patient/physician discretion.
A051202 started at Dose Level 0 consisting of oral lenalidomide 10 mg daily on Days 1–21, oral idelalisib 150 mg twice daily on Days 1–21, and intravenous rituximab 375 mg/m2 on Day 8, 15, and 22 of Cycle 1, and then once more on Day 1 of Cycle 2. Each cycle length was 28 days. If it were feasible to escalate lenalidomide above 15 mg, there were plans for intra-patient dose escalation to 20 mg (Dose Level 3) or 25 mg (Dose Level 4) for patients treated beyond cycle 2. The treatment was scheduled for a total of 12 cycles of treatment without maintenance. If the MTD was established, an expansion cohort of ten additional patients was planned for further safety evaluation.
Statistical Design and Dose-Limiting Toxicity Definition
Both A051201 and A051202 were phase I trials utilizing a standard 3+3 design with the hypothesis that idelalisib, lenalidomide and rituximab would be a safe combination. The number of patients were determined by observed dose-limiting toxicity. Any patient receiving at least one dose of study treatment was included in the analysis. Dose-limiting toxicity assessment was based on Cycle 1 toxicities. Dose-limiting toxicity definition for A051201 included any grade 4 hematologic toxicity lasting longer than 7 days, any study drug-related grade 3 non-hematologic toxicity (except grade 3 fatigue, asymptomatic grade 3 AST/ALT elevation), or any study drug-related grade 4 non-hematologic toxicity. Specific dose modifications for hematologic toxicity, skin toxicity, venous thrombosis, renal dysfunction, hepatotoxicity and other non-hematologic toxicities were included. Dose-limiting toxicity definition for A051202 included any drug-related grade 3 non-hematologic toxicity (except fatigue, thrombosis, and asymptomatic grade 3 AST/ALT elevation), grade 4 hematologic toxicity lasting longer than 7 days, or any grade 4 non-hematologic toxicity.
Response and Toxicity Assessment Criteria
Although response was not the primary endpoint for the phase I portions of the studies, response assessments for both studies were based on Cheson 2007 criteria.16 The first restaging in A051201 was at 3 months, and the first restaging in A051202 was at 2 months.
Toxicity was reported using CTCAE v4.0 and reviewed during bi-weekly conference calls of the study team for each study separately. Once the nature of dose-limiting toxicities was emerging, enrollment was slowed to allow individual patients to complete one full cycle of therapy before enrolling additional subjects.
Statistical Analysis
Frequency tables were used to report patient characteristics, incidence of dose-limiting toxicity and observed responses [Complete Response (CR), Partial Response (PR), Stable Disease (SD) and Progressive Disease (PD)]. The Kaplan-Meier method was used to estimate PFS measured as the time from study entry until progression or death from any cause and overall survival measured as the time from study entry until death. All data was collected, monitored and reviewed by the Alliance Statistics and Data Center. The results presented in this report reflect data collected through August 1, 2016. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). The funding source (National Cancer Institute) had no role in the study design, collection, analysis or interpretation of data, or writing of the report.
RESULTS
Patient Characteristics
Between July 9, 2013 and September 30, 2014, eleven patients were enrolled and treated among four Alliance institutions before the trials were permanently halted; enrollment included 3 mantle cell lymphoma patients on A051201 and 8 follicular lymphoma patients on A051202 (Table 1). All consented patients are evaluable for the primary endpoint assessment of toxicity. There were no ineligible patients. There were three patients enrolled onto A051201 (mantle cell lymphoma) with two male, one female, median age 58 years (range, 53–65 years); all three patients had prior autologous stem cell transplant and rituximab treatment and the median prior regimens was two (range, 1–2). There were eight patients enrolled onto A051202 (follicular lymphoma) with five male, three female, median age 60 years (range, 47–77 years); all patients had prior rituximab and the median prior regimens was two (range, 1–7). All patients had a performance status 0–1.
Table 1.
Patient characteristics and outcomes. (AE, adverse event; DLT, dose-limiting toxicity; TX, treatment, MCL, mantle cell lymphoma, FL, follicular lymphoma; idela, idelalisib; len, lenalidomide, ritux, rituximab; AST, aspartate aminotransferase; ALT, alanine aminotransferase)
| Patient ID | Age, Gender | Diagnosis | Cumulative idelalisib dose | Cumulative lenalidomide dose | Cumulative rituximab dose | DLT | Off study for AE | Comments |
|---|---|---|---|---|---|---|---|---|
| 1-001 | 53 yo M | MCL | 6300mg | 300mg | 2910mg | YES | YES | Developed fevers and grade 4 AST and ALT elevation on Day 20 |
| 1-002 | 59 yo M | MCL | 4300mg | 630mg | – | NO | YES | Grade 3 maculopapular rash on Day 15 |
| 1-003 | 66 yo F | MCL, blastoid | 3650mg | 690mg | – | YES | YES | Grade 3 AST elevation and grade 3 maculopapular rash |
| 2-001 | 59 yo M | FL | 45,800mg | 1260mg | 3600mg | NO | YES | Grade 3 lung infection |
| 2-002 | 62 yo F | FL | 4950mg | 160mg | 1088mg | YES | YES | Grade 4 septic shock on Day 17 |
| 2-003 | 47 yo M | FL | 5000mg | 560mg | 2852mg | NO | NO | Had grade 3 maculopapular rash during cycle 1 but resumed treatment with reduction of both idelalisib and lenalidomide |
| 2-004 | 77 yo F | FL | 4650mg | 360mg | 2864mg | NO | YES | Grade 3 allergic reaction and maculopapular rash during Cycle 1. Rash recurred despite omission and reductions of lenalidomide and idelalisib. |
| 2-005 | 53 yo F | FL | 3000mg | 100mg | 667mg | YES | YES | Patient developed fevers, facial edema, total body coalescent rash, hypotension 24 hours after rituximab administration. Admitted to ICU and on pressors. |
| 2-006 | 59 yo M | FL | 33,600mg | 1260mg | 3116mg | NO | NO | |
| 2-007 | 61 yo M | FL | 4500mg | 150mg | 770mg | YES | YES | Grade 3 maculopapular rash and grade 3 lung infection on Day 15 |
| 2-008 | 65 yo M | FL | 50,400mg | 1050mg | – | NO | NO |
Treatment, Safety and Response
Patient details are presented in Table 1 and toxicity details in Table 2. Eight of eleven patients were removed from treatment due to an adverse event, and three patients required intensive care unit level care; two of these patients had hypotension requiring pressor support. There were no treatment related deaths.
Table 2.
Adverse Events, Serious or Life-threatening Adverse Events (grades 3/4), and Key Laboratory Abnormalities (n=11) occurring in at least two patients OR greater than grade 2 are included.
| A051202 | A051202 | |||||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Anemia | 2 (67%) | 0 | 0 | 6 (75%) | 0 | 0 |
| Lymphocyte count decreased | 2 (67%) | 0 | 0 | 2 (25%) | 5 (63%) | 1 (13%) |
| Neutrophil count decreased | 1 (33%) | 1 (33%) | 0 | 4 (50%) | 1 (13%) | 0 |
| Platelet count decreased | 3 (100%) | 0 | 0 | 3 (38%) | 1 (13%) | 0 |
| White blood cell decreased | 2 (67%) | 0 | 0 | 3 (38%) | 0 | 0 |
| Constipation | 0 | 0 | 0 | 3 (38%) | 1 (13%) | 0 |
| Diarrhea | 0 | 0 | 0 | 3 (38%) | 0 | 0 |
| Mucositis oral | 2 (67%) | 0 | 0 | 1 (13%) | 0 | 0 |
| Fatigue | 2 (67%) | 0 | 0 | 6 (75%) | 1 (13%) | 0 |
| Fever | 1 (33%) | 0 | 0 | 3 (38%) | 1 (13%) | 0 |
| Lung infection | 0 | 0 | 0 | 0 | 2 (25%) | 0 |
| Alanine Aminotransferase increased | 0 | 1 (33%) | 1 (33%) | 5 (63%) | 0 | 0 |
| Alkaline phosphatase increased | 0 | 0 | 0 | 2 (25%) | 0 | 0 |
| Aspartate aminotransferase increased | 1 (33%) | 0 | 1 (33%) | 3 (38%) | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 2 (25%) | 1 (13%) | 0 |
| Hyperuricemia | 0 | 0 | 0 | 2 (25%) | 0 | 0 |
| Hypoalbuminemia | 1 (33%) | 0 | 0 | 3 (38%) | 0 | 0 |
| Hypocalcemia | 2 (67%) | 0 | 0 | 1 (13%) | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 | 2 (25%) | 0 | 0 |
| Hypophosphatemia | 1 (33%) | 0 | 0 | 2 (25%) | 1 (13%) | 0 |
| Rash maculopapular | 0 | 2 (67%) | 0 | 1 (13%) | 4 (50%) | 0 |
For A051201, Patient 1-001 was registered and treated at Dose Level 1 (lenalidomide 15 mg, idelalisib 150 mg BID, rituximab 375 mg/m2) but developed grade 4 AST/ALT elevations (dose-limiting toxicity) and fevers on Day 20, was admitted to the intensive care unit, and was removed from treatment. The study was amended to remove rituximab due to toxicity observations on A051202 (see below), and two subsequent patients were enrolled. Patient 1-002 started at the amended doses (lenalidomide 15 mg, idelalisib 150 mg BID) but developed grade 3 maculopapular rash on Day 15. Both agents were held but the rash progressed and he was treated with oral steroids with eventual resolution of rash. Patient 1-003 started at the amended doses but developed a grade 3 maculopapular rash with the first cycle. Both agents were reduced to lenalidomide 10 mg/idelalisib 100 mg BID but she had grade 3 AST elevation and a grade 3 maculopapular rash despite the dose reduction and was removed from treatment.
In A051202, five patients were enrolled at Dose Level 0 (lenalidomide 10 mg, idelalisib 150 mg BID and rituximab 375 mg/m2 starting on Day 8). There were two dose-limiting toxicities in the first five patients including culture negative severe hypotension requiring pressors and grade 3 lung infection. The next two patients were enrolled at Dose Level -1 (lenalidomide 10mg, idelalisib 100mg BID, and rituximab 375mg/m2 starting on Day 8). The seventh patient experienced a grade 3 maculopapular rash and grade 3 pulmonary infection that started on Day 15. The study was amended to remove rituximab as the majority of the serious toxicities appeared to occur within 24 hours of a dose of rituximab, and one subsequent patient was enrolled. While this last patient tolerated treatment, patient 2-007 had grade 3 AST elevation and a grade 3 maculopapular rash despite omission of rituximab after the first week. Given the inability to deliver treatment due to toxicity, both studies were permanently closed. There were no grade 5 toxicities; two patients have died of progressive lymphoma.
There were no responders in A051201 (mantle cell lymphoma); one patient had stable disease and two patients had progressive disease. Among eight follicular lymphoma patients, there was one complete response and four partial responses (ORR 45%); one patient had stable disease and two had progressive disease. With a median follow-up of 7.4 months (range, 1.9–29.5 months), the median PFS for A051202 (follicular lymphoma) is 14.4 months (range, 5.7–29.5 months).
DISCUSSION
Despite encouraging single agent activity and clinical rationale to add idelalisib to a rituximab-lenalidomide (R-len) backbone, the triplet of idelalisib-lenalidomide-rituximab in Alliance A051201 (mantle cell lymphoma) and A051202 (follicular lymphoma) led to serious and unexpected toxicity consisting of culture negative sepsis syndrome, hypotension requiring pressor support, and grade 3 rash in four of the eventual eleven patients treated. The observation of four dose-limiting toxicities among the first eight enrolled patients is striking, particularly given the inflammatory nature of the toxicities suggestive of immune activation. The dose-limiting toxicities had a range of symptom onset 9–20 days after treatment initiation, and initially seemed to coincide with rituximab infusions. Despite elimination of rituximab, severe rashes and low grade fevers persisted, prompting dose reductions to likely inadequate levels of both lenalidomide and idelalisib. Both trials were halted given the inability to deliver the intended treatment safely. Overall, our brief experience underscores the limited knowledge regarding drug interactions and off-target effects, and serves as a cautionary note in developing biologic agents in combination and against ad hoc combinations outside of carefully monitored clinical trials.
It is noteworthy that the doublets, lenalidomide-rituximab (R-len) and idelalisib-rituximab (IR), have been safely combined and show significant efficacy in several clinical settings. In relapsed mantle cell lymphoma and indolent lymphomas, R-len is associated with neutropenia, thrombosis and fatigue; less than 10% of patients experience severe rash, fevers or pulmonary symptoms.(4, 6–8) Idelalisib as a single agent is known to cause significant, but transient, hepatotoxicity with transaminase elevation in a minority of patients, as well as a delayed colitis manifested by intractable diarrhea. The transaminase elevation typically responds to withholding idelalisib,(16) and it is safe and feasible in most cases to restart at a lower dose. When combined with rituximab in relapsed chronic lymphocytic leukemia patients, the frequency of severe adverse events of pyrexia, pneumonia, febrile neutropenia and diarrhea were all 4% or less.(12) Grade 3 or 4 transaminase elevation occurred in only 5% of patients. Lenalidomide and idelalisib have only been combined in one other published report, along with rituximab, and after enrolling seven patients, this trial closed prematurely mainly due to a high frequency of hepatotoxicity.(17)
There is increasing awareness that “chemotherapy-free” does not equate to “toxicity-free”, and this has challenged the design of trials with biologic agents.(18) A major issue is that the majority of new combinations are somewhat empiric, and based on rational but unproven hypotheses. Historically, new agents (which were overwhelmingly cytotoxic in mechanism) could be tested with the assumption that increased doses would be associated with predictable dose-related effects on organ function or marrow function. However, biologic agents often have pleiotropic effects that are independent of dose. Lenalidomide is an example of an agent where several common toxicities (rash, thromboembolic phenomena) have no apparent relationship with the administered dose. Thus, in hindsight, there are likely much better trial designs than the “3+3” method used here that would allow rapid assessment of toxicity and treatment modification(18).
In our series, we observed a constellation of fevers, hypotension and rash as well as hepatotoxicity and pulmonary infiltrates; these were unexpected, and are suggestive of immune activation and off-target effects. Immune activation is increasingly recognized in the context of PD-1 inhibitors or CAR-T cell therapy.(19, 20) While we cannot apply the clinical term of cytokine-release syndrome (CRS) to our experience, there are some notable similarities between the symptoms in patients treated with the triplet of idelalisib, lenalidomide and rituximab and CRS. These include frequent constitutional symptoms with severe rash, respiratory and cardiovascular compromise, and hepatic dysfunction occurring in an abrupt and fulminant manner. Given the life-threatening nature of CRS following immunotherapy, there are now grading systems in place that prompt specific interventions, including IL-6 blockade with tociluzimab. Our analysis did not collect serial samples to document cytokine elevation and therefore there is no confirmation that CRS occurred. The timing of clinically observed toxicities (range of time to onset 9–20 days) is unexplained, but consistent with an immune-mediated event or direct interaction of the study drugs on T-cells.
Mechanistically, both immunomodulatory agents and PI3K inhibitors impact T/NK-cell activity, and may have additive impact. Lenalidomide has direct effects on malignant B-cells, but much of its anti-neoplastic effect is attributed to proliferation and activation of normal T-cells and natural killer cells. For example, lenalidomide appears to “repair” the interaction between tumor infiltrating T-cells and follicular lymphoma cells, thus restoring and enhancing T-cell function.(21) Lenalidomide also directly stimulates T-cells via increased IL-2, IFN-γ and other inflammatory cytokine production. In vitro studies show that lenalidomide increases natural killer cell number, natural killer cell activity, and natural killer-mediated enhancement of antibody-dependent cellular toxicity (reviewed in (22)). The effect of PI3K inhibition on non-malignant immune cells is similarly complex. In murine models, inhibition of the delta isoform in regulatory T-cells facilitates activation of CD8+ cytotoxic T-cells and is associated with brisk tumor regression.(23) It is unclear if inhibition of the delta isoform is associated with increased expression of the gamma isoform of PI3K which is highly expressed in T-cells and could theoretically increase T-cell activity. The added effect of rituximab to an environment potentially full of primed T-cells is unclear, but enhanced antibody-dependent cell-mediated cytotoxicity could have contributed. We initially felt the timing of toxicity onset coincided with the administration of rituximab, but the persistence of significant rash, pulmonary and hepatotoxicity despite the elimination of rituximab suggests an interaction between the two oral agents was responsible for the toxicities. The rapid and fulminant timing of toxicity-onset may be related to the combined effect of each agent on T-cell activity, although this would need confirmation.
It is possible that both idelalisib and lenalidomide facilitated cytotoxic T-cell activation; however, the observation of severe toxicity in many but not all patients in our series suggests that there are other factors to be considered. In chronic lymphocytic leukemia, idelalisib-associated colitis and transaminase elevation may be associated with the number of prior immunosuppressive therapies; the authors of a study testing idelalisib and rituximab in treatment-naïve versus relapsed/refractory chronic lymphocytic leukemia patients observed that the incidence of grade 3 or 4 transaminase elevation was 23% versus 2% and diarrhea/colitis was 42% versus 6%, respectively.(24) In March 2016, an independent data and safety monitoring board found an excess of toxic deaths (mainly related to infection) in a combination study of idelalisib plus other agents, and with FDA support, halted studies in less heavily pre-treated lymphoma patients (http://www.fda.gov/Drugs/DrugSafety/ucm490618.htm).
In summary, the triplet regimen of idelalisib, lenalidomide and rituximab was too toxic, despite the safety of previously tested doublets and rationale to test the combination in patients. The nature of the toxicities supports an immune-activated state characterized by excessive inflammation. A more detailed evaluation of effects on cytokines, T-cell subsets, natural killer cells and clinical features predictive of toxicity and response should be included in any further testing of these classes of agents, and they should never be combined outside of a carefully designed and diligently monitored clinical trial setting.
Supplementary Material
Research in Context.
Evidence before this study
In 2012, we completed a literature search using the PubMed database to identify published studies on the use of combination treatment strategies in relapsed and refractory indolent lymphomas. We used the search terms “lenalidomide”, “rituximab”, “idelalisib”, “indolent lymphoma”, and “relapsed/refractory lymphoma”, with no date or language restrictions. At this time, there were no published studies using the triplet combination, lenalidomide, rituximab, and idelalisib, in recurrent indolent lymphomas or other hematologic malignancies. Several previous studies suggested impressive synergistic clinical activity of the doublet combination, rituximab and lenalidomide, in relapsed and refractory mantle cell and follicular lymphoma. In addition, idelalisib was known to have significant clinical activity as a monotherapy in refractory indolent lymphomas with a unique mechanism of action.
Added value of this study
There is a need to test novel, targeted drugs in combination regimens, in an effort to move away from traditional cytotoxic agents. In this study, the Alliance tested a new triplet combination, oral lenalidomide, oral idelalisib, and intravenous rituximab, in relapsed and refractory mantle cell (A051201) and follicular (A051202) lymphoma. Both trials were permanently halted due to serious toxicities including hypotension, sepsis syndrome, severe rash, and grade 3–4 transaminase elevation. It was determined from these two studies that the triplet combination of lenalidomide, idelalisib, and rituximab was too toxic. Our findings reveal the importance of studying proposed combinations of novel targeted drugs in well-monitored Phase I studies to clearly define any unexpected drug interactions and off-target effects.
Implications of all the available evidence
During the conduct of our studies (A051201 and A051202), a separate clinical trial was also in progress using the same triplet combination in relapsed and refractory indolent lymphoma (NCT01088048). This study found significant hepatotoxicity limiting further development of the triplet combination. The results of our study match this trial, confirming the unacceptable toxic effects of this triplet combination.
Acknowledgments
Funding: National Cancer Institute of the National Institutes of Health
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA007968, U10CA033601, U10CA041287, U10CA047559, U10CA077597, U10CA180833, U10CA180836, and U10CA180838. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutions participated in these studies: UNC Lineberger Comprehensive Cancer Center LAPS, Chapel Hill, NC, Thomas Shea, U10CA180838; University of Chicago Comprehensive Cancer Center LAPS, Chicago, IL, Hedy Kindler, U10CA180836; Washington University - Siteman Cancer Center LAPS, Saint Louis, MO, Nancy Bartlett, U10CA180833; and Weill Medical College of Cornell University New York, NY, Scott Tagawa.
Role of the Funding Source: The funding source (NCI) did not have any role in the study design, collection/analysis/interpretation of data, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
SMS has received research funding and consulting fees from Gilead and Celgene. NLB has received research funding from Gilead and Celgene. NWJ has received research funding from Celgene, has served on advisory boards for Gilead and Pharmacyclics, and is on the speaker’s bureau for Gilead. SIP has received research funding from Teva, Seattle Genetics, and Takeda, and has served on the advisory board for Teva. KLR has consulted for Celgene. AFC has received personal fees from Celgene and Gilead. BDC has received research funding from Celgene, Pharmacyclics, Genentech, Abbvie, Acerta, and Gilead; has consulted for Celgene, Pharmacyclics, Genentech, and Abbvie, and has served on advisory boards for Celgene, Pharmacyclics, Genentech, and Abbvie. EH has served on advisory boards for HTG Molecular Diagnostics, Alexion, and Seattle Genetics. JPL has received personal fees from Gilead, Celgene, and Genentech. AJ, BNP, SHJ and SES declare no conflicts of interest.
Presented in part at the American Society of Hematology Annual Meeting in 2014 (Blood, 124(21), 3091. Accessed September 17, 2016. Retrieved from http://www.bloodjournal.org/content/124/21/3091.)
Statement of Author Contribution:
|
| ||||
| Site | Site PI | A051201 | A051202 | Total |
|
| ||||
| UNC Lineberger CCC | Steven Park | 0 | 5 | 5 |
|
| ||||
| Wash U School of Medicine | Nancy L. Bartlett | 3 | 1 | 4 |
|
| ||||
| University of Chicago CCC | Sonali M. Smith | 0 | 1 | 1 |
|
| ||||
| Weill Medical College of Cornell | John P. Leonard | 0 | 1 | 1 |
|
| ||||
| Total | 3 | 8 | 11 | |
|
| ||||
References
- 1.Habermann TM, Lossos IS, Justice G, Vose JM, Wiernik PH, McBride K, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145(3):344–9. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 2.Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, Ramchandren R, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31(29):3688–95. doi: 10.1200/JCO.2013.49.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin’s Lymphoma. J Clin Oncol. 2009;27(32):5404–9. doi: 10.1200/JCO.2008.21.1169. [DOI] [PubMed] [Google Scholar]
- 4.Leonard JP, Jung SH, Johnson J, Pitcher BN, Bartlett NL, Blum KA, et al. Randomized Trial of Lenalidomide Alone Versus Lenalidomide Plus Rituximab in Patients With Recurrent Follicular Lymphoma: CALGB 50401 (Alliance) J Clin Oncol. 2015;33(31):3635–40. doi: 10.1200/JCO.2014.59.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin P, Jung SH, Johnson JL, Pitcher B, Elstrom R, Bartlett NL, et al. CALGB 50803 (Alliance): A phase II trial of lenalidomide plus rituximab in patients with previously untreated follicular lymphoma. J Clin Oncol. 2014;32:5s. (abstr 8521) [Google Scholar]
- 6.Fowler NH, Davis RE, Rawal S, Nastoupil L, Hagemeister FB, McLaughlin P, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15(12):1311–8. doi: 10.1016/S1470-2045(14)70455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Fayad L, Wagner-Bartak N, Zhang L, Hagemeister F, Neelapu SS, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13(7):716–23. doi: 10.1016/S1470-2045(12)70200-0. [DOI] [PubMed] [Google Scholar]
- 8.Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med. 2015;373(19):1835–44. doi: 10.1056/NEJMoa1505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–18. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flinn IW, Kahl BS, Leonard JP, Furman RR, Brown JR, Byrd JC, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123(22):3406–13. doi: 10.1182/blood-2013-11-538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weidner AS, Panarelli NC, Geyer JT, Bhavsar EB, Furman RR, Leonard JP, et al. Idelalisib-associated Colitis: Histologic Findings in 14 Patients. Am J Surg Pathol. 2015;39(12):1661–7. doi: 10.1097/PAS.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 12.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czuczman MS, Leonard JP, Jung S, Johnson JL, Hsi ED, Byrd JC, et al. Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus rituximab (CALGB 50402): Follicular Lymphoma International Prognostic Index (FLIPI) score is predictive of upfront immunotherapy responsiveness. Ann Oncol. 2012;23(9):2356–62. doi: 10.1093/annonc/mdr620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard JP, Friedberg JW, Younes A, Fisher D, Gordon LI, Moore J, et al. A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma. Ann Oncol. 2007;18(7):1216–23. doi: 10.1093/annonc/mdm114. [DOI] [PubMed] [Google Scholar]
- 15.Morrison VA, Jung SH, Johnson J, LaCasce A, Blum KA, Bartlett NL, et al. Therapy with bortezomib plus lenalidomide for relapsed/refractory mantle cell lymphoma: final results of a phase II trial (CALGB 50501) Leuk Lymphoma. 2015;56(4):958–64. doi: 10.3109/10428194.2014.938333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutre SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10):2779–86. doi: 10.3109/10428194.2015.1022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheah CY, Nastoupil LJ, Neelapu SS, Forbes SG, Oki Y, Fowler NH. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood. 2015;125(21):3357–9. doi: 10.1182/blood-2015-03-633156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD. Speed bumps on the road to a chemotherapy-free world for lymphoma patients. Blood. 2016;128(3):325–30. doi: 10.1182/blood-2016-04-709477. [DOI] [PubMed] [Google Scholar]
- 19.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–95. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer journal. 2014;20(2):119–22. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, Macdougall F, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114(21):4713–20. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gribben JG, Fowler N, Morschhauser F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J Clin Oncol. 2015;33(25):2803–11. doi: 10.1200/JCO.2014.59.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407–11. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–94. doi: 10.1182/blood-2015-03-630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


