Abstract
One of the major causes of decreased vision, irreversible vision loss and blindness worldwide is glaucoma. Increased intraocular pressure (IOP) is a major risk factor associated with glaucoma and its molecular mechanisms are not fully understood. The trabecular meshwork (TM) is the primary site of injury in glaucoma, and its dysfunction results in elevated IOP. The glaucomatous TM has increased extracellular matrix deposition as well as cytoskeletal rearrangements referred to as cross-linked actin networks (CLANs) that consist of dome like structures consisting of hubs and spokes. CLANs are thought to play a role in increased aqueous humor outflow resistance and increased IOP by creating stiffer TM cells and tissue. CLANs are inducible by glucocorticoids (GCs) and TGFβ2 in confluent TM cells and TM tissues. The signaling pathways of these induction agents give insight into the possible mechanisms of CLAN formation, but to date, the mechanism of CLANs regulation by these pathways has yet to be determined. Understanding the role CLANs play in IOP elevation and their mechanisms of induction and regulation may lead to novel treatment options to help prevent or intervene in glaucomatous damage to the trabecular meshwork.
Keywords: Cross-linked actin networks, trabecular meshwork, glaucoma, glucocorticoids, TGFβ2
I. Glaucoma
Glaucoma is a group of diseases characterized by optic neuropathy and retinopathy. Currently, nearly 3 million Americans and over 70 million individuals worldwide have glaucoma (Klein and Klein, 2013; Quigley, 1996). This makes glaucoma the most common neurodegenerative disease. Those over the age of 60 are six times more likely to develop glaucoma, and with our aging populations, it is projected that the number of people with glaucoma will increase to 76 million in 2020 and 111 million by 2040 (Tham et al., 2014).
Primary open angle glaucoma (POAG) is the most prevalent type of glaucoma and is characterized by an open iridocorneal angle with increased aqueous humor outflow resistance and optic nerve damage. Sustained increased intraocular pressure (IOP) is one of the major risk factors associated with POAG. This increased IOP is a result of increased aqueous humor drainage resistance at the trabecular meshwork (TM). The aqueous humor is secreted by the ciliary body and flows from the posterior to the anterior chamber, through the TM, through Schlemm’s canal (SC) into the collector channels and out through the aqueous veins. This conventional outflow pathway is the primary method for aqueous humor drainage. The molecular mechanisms associated with glaucomatous TM dysfunction are not fully understood and are a current focus in glaucoma research.
Changes in IOP (i.e. elevated IOP and IOP spikes) can cause chronic stress to the TM and the SC. This chronic stress alters the homeostasis of the TM inducing mechanical alterations to the composition and organization of the TM (Acott 2008, Fuchshofer 2009, Johnson 2006, Tamm 2007, Wordinger 1998, Clark 2005, Clark 1994, Read 2007), oxidative stress, and inhibition of the natural phagocytic activity of the TM cells (Liton and Gonzalez, 2008).
These glaucoma insults appear to precede IOP elevation. The alterations that occur to the TM may alter TM cell and tissue rigidity, since TM tissue from glaucomatous eyes is stiffer than TM tissue isolated from non-glaucomatous donor eyes (Last et al., 2011) and SC cells from glaucomatous eyes also are stiffer than control SC cells (Overby et al., 2014). Stiffening of the TM tissue causes an increase in aqueous humor outflow resistance and elevated IOP that stresses the optic nerve head (ONH). The glaucomatous ONH, like the glaucomatous TM (GTM), has increased extracellular matrix (ECM) deposition (Hernandez et al., 1990; Morrison et al., 1990; Zode et al., 2011) and reorganization of the actin cytoskeleton (Job et al., 2010). Cellular disruption in the ONH contributes to retinal ganglion cell (RGC) axon damage and apoptosis. Disruption of the ONH cells also leads to biomechanical changes to the lamina cribrosa (LC), which is the mesh-like structure where the RGC axons exit the eye to form the optic nerve.
Additionally, glucocorticoids (GCs) and transforming growth factor β2 (TGFβ2) are glaucoma-related insults that damage the TM. Glucocorticoid-induced ocular hypertension (GC-OHT) and glucocorticoid-induced glaucoma (GIG) have similar pathologies to POAG with elevated IOP and increased risk of optic nerve damage that leads to vision loss. GC-OHT occurs in 40% of the population undergoing prolonged systemic or ocular GC therapy (Armaly and Becker, 1965; Becker, 1965). In contrast, the vast majority of POAG patients develop GC-OHT and increased IOP after prolonged exposure to GCs (Armaly, 1963). GCs cause glaucoma-like insults to the TM that increase aqueous humor outflow resistance due to alterations to the TM, which include increased extracellular matrix deposition and reorganization of the actin cytoskeletal (Clark et al., 1994; Steely et al., 1992; Wilson et al., 1993; Zhou et al., 1998). GC effects on the TM are mediated by glucocorticoid receptor alpha (GRα), although expression of the dominant negative isoform GRβ in TM cells inhibits GRα mediated gene regulation and activities (Jain et al., 2014; Zhang et al., 2005; Zhang et al., 2007).
TGFβ2 expression is increased in the aqueous humor and TM of POAG eyes (Cousins et al., 1991; Inatani et al., 2001; Jampel et al., 1990; Tovar-Vidales et al., 2011; Tripathi et al., 1994). TGFβ2 causes similar alterations to the TM as those induced by GCs. TGFβ2 is a profibrotic cytokine that activates the TGFβ signaling pathway. In the Smad signaling pathway, TGFβ2 binds to the TGFβ receptor 2, which activates and binds to TGFβ receptor I and subsequently phosphorylates the Smad2/3 complex. Phosphorylated Smad2/3 then translocates into the nucleus and forms a Smad2/3/4 complex. This complex formation at the promoter region activates gene transcription and turns on the expression of ECM molecules such as TGM2, LOX and FN (Medina-Ortiz et al., 2013; Sethi et al., 2011; Tovar-Vidales et al., 2011). Alternatively, activation of the non-Smad pathways such as JNK, P38, or ERK 1/2 alter the expression of cell adhesion molecules (Wecker et al., 2013).
II. Role of cytoskeleton in cell/tissue functions
The cytoskeleton is found in the cytoplasm and nucleus and consists of three major classes: microtubules, intermediate filaments, and microfilaments. The major functions of the cytoskeleton are to give cells their shape, cohesiveness, and ability to sense and respond to their environment. One of the major classes of the cytoskeleton is the microfilaments, which are made of G-actin (globular actin) subunits and form bundles of F-actin (filamentous actin). The linear bundles of F-actin form flexible networks that regulate cell shape, phagocytosis, contraction, and motility. Bundles of F-actin fibers form contractile stress fibers.
The TM is the major site for drainage of the AH through the conventional pathway, and is the most important structure for the regulation of IOP. The TM is composed of multiple layers of collagenous beams including the uveal meshwork, corneoscleral meshwork, cribiform or juxtacanalicular (JCT) meshwork. The uveal and corneoscleral meshwork are made of TM beams lined with a continuous monolayer of TM cells (Acott and Kelley, 2008). The JCT cells are embedded in connective tissue, consisting of networks of irregularly oriented fibrils, which lack large fenestrated openings. The JCT is adjacent to endothelial cells lining the inner wall of SC, which is the primary exit site of AH from the eye. Microfilaments are heavily present in TM cells, the JCT, and the cells lining of the inner wall of SC, which are important for the contractile properties of TM tissues.
The AH outflow facility is highly influenced by the structure of the TM cytoskeleton (Peterson et al., 2000; Rao et al., 2001; Tian et al., 2000; Tian et al., 1998). The cytoskeleton is regulated by extracellular calcium, activation of specific small G-proteins, and hydrostatic pressure induced mechanical tension (Tian et al., 2000). Microfilaments structures in both the TM and SC are affected by mechanical factors (Ethier et al., 2004; Tumminia et al., 1998). The F-actin structures in non-glaucomatous TM (NTM) tissue have a more organized, linear appearance throughout the cytoskeleton compared to GTM. The disorganization of the GTM cytoskeleton has many actin tangles throughout the cells in the JCT (Read et al., 2007) and in the TM regions (Hoare et al., 2009). Similar alterations in the TM actin structure have been observed in response to mechanical stretch and sheer stress (Tumminia et al, 1998). This observation is in agreement with that seen in TM cell culture studies (Clark et al., 2005; Clark et al., 1995; Clark et al., 1994; Ethier et al., 2004), including those treated with dexamethasone (DEX; a glucocorticoid) and TGFβ2 (Clark et al., 1995; Clark et al., 1994; Filla et al., 2006; Fleenor et al., 2006; O’Reilly et al., 2011). These alterations to the actin structure are associated with increased outflow resistance, providing further evidence that microfilaments in the TM play an important role in AH outflow (Rao et al., 2005; Read et al., 2007).
The TM cells have some properties of myofibroblasts, expressing alpha-smooth muscle actin (αSMA) and smooth muscle myosin, which gives TM cells and tissues muscle-like contractile properties (Lepple-Wienhues et al., 1991). The contraction of the TM is regulated by myosin light chain and myosin light chain kinase (Nakajima et al., 2005; Wiederholt et al., 2000). If the tissue is in a prolonged or abnormal state of contraction, the cells ability to regulate cytoskeletal assembly and signal transduction is altered. These alterations may subsequently lead to TM rigidity and increased outflow resistance.
Similar to the TM, the ONH also undergoes mechanical and physiological changes in glaucoma pathogenesis (Job et al., 2010; Zode et al., 2011). The lamina cribrosa (LC) is the main structural tissue of the ONH, and like the TM it has a mesh-like structure. There are less axonal cytoskeletal proteins found in the retrolaminar region compared to the other laminar regions (Kang and Yu, 2015). The difference in cytoskeletal distribution may be due to the myelination that begins at the retrolaminar region compared to the increased need for scaffolding in the non-myelinated regions. In POAG, reactive optic nerve astrocytes express increased TGFβ2 (Pena et al., 1999; Zode et al., 2011). TGFβ2 can induce alterations of the cytoskeleton, increase ECM deposition and deform optic nerve axons (Fuchshofer and Tamm, 2012). These changes can impair axonal transport and neurotropic supply to the retinal ganglion cells. In a rat model of photocoagulation induced glaucoma, retinal whole-mounts in revealed severe loss of F-actin, microtubules and irregular F-actin structures prior to a decrease in retinal nerve fiber layer thickness (Huang et al., 2011). Disrupted axonal transport and damage to the axonal cytoskeleton were also found in a similar study (Chidlow et al., 2011). When elevated IOP is increased in porcine eyes, again axonal transport and the cytoskeleton are altered (Balaratnasingam et al., 2008; Balaratnasingam et al., 2007).
III. Initial discovery and cell biology of CLANs
The actin cytoskeleton is commonly arranged in organized linear fibers. In 1976, Lazarides described the formation of unique structures in the actin cytoskeleton of spreading non-muscle cells when first adhering to the culture plate, with foci containing actin and α-actinin, connected by actin-filaments and tropomyosin in a geodesic dome-like structure (Lazarides, 1976). These structures were later observed to have a three dimensional shape using stereo immunofluorescence microscopy (Osborn et al., 1978). Cytoskeletal rearrangements have been observed in various other cell types ((Barber et al., 2004; Entcheva and Bien, 2009; Meller and Theiss, 2006). These polygonal rearrangements are found during the cell attachment and spreading phases. They are thought to be a precursor for the organization and formation of stress fibers, responsible for stabilization of the cells during a highly dynamic process. The dome-like structures are transient and disappear once a cell flattens and spreads. Like the non-muscle cells that were studied in these experiments, attaching and spreading TM cells also form transient polygonal arrangement of actin microfilaments (Filla et al., 2011; Filla et al., 2009). In contrast to most other adherent cells, confluent TM cells can undergo cytoskeletal rearrangements from linear stress fibers to form distinct geodesic dome-like structures consisting of hubs (vertices) and spokes (connecting rods) known as cross-linked actin networks (CLANs) (Clark et al., 1994; Wilson et al., 1993) (Figure 1).
Figure 1. Representative image of CLANs.
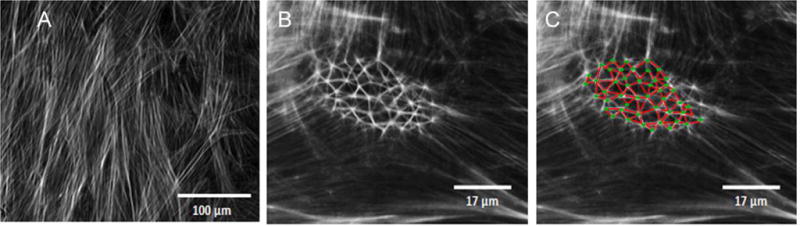
A) Linear actin cytoskeleton in confluent TM cells. B) CLANs formed in TM cells after treatment with TGFβ2 for 10 days C) Duplicated image of B with green dots to indicate hubs and red lines to show spokes.
IV. CLANs in TM
Formation of CLANs in the eye has been shown to be highly selective to the TM. The formation of these networks has also been found more commonly in the glaucomatous TM cells and tissues compared to the NTM (Clark et al., 1995; Hoare et al., 2009). Background CLANs occur in only 4% of confluent NTM cells, while approximately 25% of confluent cultured primary human GTM cells form CLANs (Clark and Wordinger, 2009). Treatment of NTM and GTM cells with GCs induce ultrastructure changes including a significant increase in CLAN formation, as well as cell and nuclear size, and increased extracellular matrix (ECM) (Clark et al, 1995; (Clark et al., 1994; Wilson et al., 1993). The induced formation of CLANs by prolonged (seven-ten days) DEX treatment is dose and time dependent, reversible upon DEX withdrawal, and mediated through the glucocorticoid receptor (GR) (Clark et al., 1994). Induction of CLANs with DEX leads to inhibition of TM cell proliferation, migration and phagocytosis (Clark et al., 1994; Matsumoto and Johnson, 1997; Zhang et al., 2007). Advances in the CLAN field have also attempted to inhibit CLAN formation. Tetrahydrocortisol (THF) is a cortisol metabolite that does not directly inhibit the glucocorticoid receptor, but it can inhibit and reverse DEX-induced CLAN formation (Clark et al., 1996).
Recently, Fujimoto and colleagues used DEX treated porcine TM cells to evaluate actin dynamics using live cell imaging (Fujimoto et al., 2016). A modified insect virus with an actin-GFP construct was used for cellular transduction, yielding approximately 23% of cells expressing the GFP-actin and approximately 28% of those cells revealed CLAN-like structures after 72 hours of DEX treatment. Similar to previous reports, they found that DEX treated cells were larger and migrated less than control cells. CLANs in porcine TM cells resolved after withdraw of DEX treatment. This study provides a dynamic model for future CLAN studies allowing us to better visualize the formation and dynamics of CLANs in confluent TM cells in real time.
To follow up on an initial report that DEX increased IOP in perfusion cultured human anterior segments (Clark et al., 1995), the same model was used to determine whether CLANs form in the TM tissue in situ (Clark et al., 2005). Since these studies showed that DEX induced CLAN formation as well as increased IOP, this led to our hypothesis that CLANs may increase TM stiffness resulting in increased aqueous outflow resistance. Ranghunathan et.al used atomic force microscopy to demonstrate that DEX treatment stiffens the TM in cultured human TM (HTM) cells and in rabbits treated with topical ocular DEX for 3 weeks (Raghunathan et al., 2015). Biomechanical computer modeling of CLANs has also been performed to calculate simulated force and distortion of CLANs (Zheng et al., 2014). In this study, bovine and HTM cells were treated with DEX and images of temporary arrangements of polygonal actin structures (spreading cells) and CLANs (confluent cells) were examined using ImageJ for CLAN size, circularity and dimensions of hubs and spokes dimension. Using this modeling system, the authors found that the size and the mechanical response of temporary arrangements of polygonal actin structures differ from CLANs in confluent cells. This type of modeling along with live cell imaging will provide valuable information about the potential role of CLANs in determining the rigidity of the TM in normal versus glaucomatous states.
In addition, TGFβ2 is also an inducer of CLANs in TM cells. We also know that TGFβ2 is elevated in the AH (Cousins et al., 1991; Inatani et al., 2001; Jampel et al., 1990; Tripathi et al., 1994) and TM cells (Tovar-Vidales 2011) of glaucoma patients. AH alone also is an inducer of CLAN formation (Inatani et al., 2001; O’Reilly et al., 2011).
Our recently submitted findings show that both the Smad and non-Smad TGFβ pathways are responsible for TGFβ2-induced CLAN formation. The non-Smad JNK and P38 pathways are only partly involved in this TGFβ2 mediated CLAN induction (Montecchi-Palmer et al. submitted for publication). Non-Smad Rho-associated protein kinase (ROCK) inhibitors are in clinical trials as IOP lowering agents for glaucoma therapy (Harrison et al., 2015). The effect of ROCK inhibitors on the TM is primarily due to relaxation of the TM by disruption of actin stress fibers and increased activation of myosin light chain phosphatase, resulting in decreased cellular contraction. ROCK inhibitors disrupt the actin cytoskeleton and thereby decrease TM stiffness (Kumar and Epstein, 2011; Murphy et al., 2014; Rao et al., 2001). ROCK inhibitors currently are in clinical development for lowering IOP via enhancing aqueous outflow through the TM (Wang and Chang, 2014). A ROCK inhibitor had variable effects on CLANs induced by TGFβ2. Treatment with TGFβ receptor, Smad, ERK and ROCK inhibitors showed complete or partial dissolution of already formed CLANs (Montecchi-Palmer et al. submitted for publication). The role of these pathways in CLAN formation and IOP regulation warrants further investigation.
In addition to these CLAN inhibition studies, a microarray gene expression profile of primary TM cells treated for 14 days with DEX revealed genes potentially involved in CLAN formation. Among these genes are encoded proteins that form or interact with the actin cytoskeleton, which include the actin genes smooth muscle aortic alpha-actin (ACTA2) and cardiac muscle actin (ACTC), filamins A, B and C, transgelin, nonmuscle heavy myosin, caldesmon 1, and tropomyosin (Rozsa et al., 2006). Using a method to isolate the TM cytoskeleton and analyze differential protein expression using 2D-DIGE, our lab identified the actin-associated proteins calponin, caldesmon, myosin light chain, and tropomyosin to be enriched in the cytoskeleton of TGFβ2 and DEX treated NTM cells, and these proteins co-localized with CLANs (Bermudez JY, 2016). However, the direct involvement of these genes and proteins in CLAN formation requires further investigation.
V. Signaling pathways involved in CLANs of spreading cells
All adherent cells have CLAN-like structures during the initial adherence and spreading phases on a culture dish. TM cells are unique in that CLANs occur in confluent cells isolated from glaucoma eyes or exposed to glaucoma insults, which is physiologically relevant to what is seen in TM tissues of glaucomatous eyes. There are differences in experimental methods used to study CLANs in the TM. Some studies use settling TM cells rather than confluent cell cultures. From these studies, the β-integrin pathway has been identified as an important pathway for transient formation of CLANs. β1 and β3 integrins work together to enhance CLANs that contain syndecan-4 (SDC4) in HTM cells (Filla et al., 2006). SDC4 is a plasma membrane proteoglycan that acts as a coreceptor to integrins making this protein directly involved in the β3 signaling pathway. β1 integrin utilizes phosphoinositide 3 kinase, while β3 uses Ras-related C3 botulinum toxin substrate 1 (Rac1) (Filla et al., 2009). Although β1 and β3 pathways may be involved in CLANs of spreading cells, their pathways are independent and converge to alter the cytoskeleton.
Moreover, CLANs of spreading DEX treated TM cells involve β3 integrin signaling and activation of ανβ3 integrin via a probable inside out signaling mechanism (Filla et al., 2011). SDC4, a coreceptor for ανβ3 integrin, is linked with Rho family GTPases by protein kinase C (PKC) including PKCε, and this signaling mechanism is thought to induce CLAN formation in HTM cells (Filla et al., 2014). In a genomic and proteomic study of DEX–treated TM cells, the Rho family GTPase effector protein binder of Rho GTPases 2 (Borg2) was upregulated along with PDZ and LIM Domain 1 (PDLIM1), a cytoskeletal adaptor protein with a PDZ binding domain for possible interaction with SDC4 (Clark et al., 2013). Although these findings suggest that these molecules are important in spreading cell CLAN formation, it is unknown whether the same signaling pathways work for CLAN formation in confluent TM cell cultures. TM cells in tissue are also confluent, so we suspect that the CLANs in confluent cultures would be more representative of CLANs in TM tissues, but further comparison studies are needed.
VI. CLANs in ONH cells
The LC in some ways is similar to TM tissues made of a series of mesh-like plates containing collagen, elastin, and other ECM molecules. The LC is found in the optic nerve head, where axons from the retinal ganglion cells converge to form the optic nerve. In addition to optic nerve head astrocytes, LC cells make ECM proteins (Hernandez et al., 1988; Kirwan et al., 2005; Zode et al., 2009) and are found within the laminar plates of the human ONH (Tovar-Vidales et al., 2016). The LC is the only other site in the eye were CLAN formation has been observed in cells and tissues (Job et al., 2010). Analogous to the TM, the glaucomatous LC cells in culture were larger and CLANs were more abundant compared to non-glaucomatous LC cells. Since CLANs have been found in the two main regions involved in glaucoma pathogenesis, there may be common pathways in both the glaucomatous TM and ONH that alter the biomechanical properties of these tissues. Evaluation of the molecular mechanisms involved in CLAN formation in these two tissues may lead to disease modifying therapies that can prevent the formation of CLANs in the anterior and posterior segments of the eye.
VII. Summary
Glaucoma pathogenesis alters trabecular meshwork structure and function. When the TM is damaged, AH no longer drains properly from the eye causing increased outflow resistance. Decreased aqueous humor outflow causes IOP elevation that results in damage to the ONH. The LC of the ONH is a tissue that is also multilayered like the TM. In open angle glaucoma eyes, the TM and LC have both been found to undergo cytoskeletal rearrangements forming CLANs. CLANs are thought to be a major contributing factor in creating tissue stiffness in the TM and LC. However, less is known about CLANs in the LC, and more research is required.
Glaucomatous TM cells and tissue undergo changes in the cytoskeleton and ECM, and both may contribute to AH outflow resistance (Acott and Kelley, 2008; Clark et al., 2005; Clark et al., 1995; Fuchshofer and Tamm, 2009; Johnson, 2006; Read et al., 2007; Tamm and Fuchshofer, 2007; Wordinger et al., 1998). The interaction of the ECM and cytoskeleton are critical for proper cell functions. When this interaction is altered either via cross-linking of the cytoskeleton or the ECM, TM stiffness may be altered. This change in tensile integrity plays a role in cell shape, mechanical responsiveness and signal transduction (Clark et al., 1994). Prolonged and abnormal contraction also contributes to the modification of the fenestrated structure of the TM, which leads to increased AH outflow resistance.
It is important to note that CLAN formation is associated with IOP elevation. Predictive mathematical models suggest how CLANs may increase stiffness of actin filaments and therefore overall cell stiffness (Gardel et al., 2004). Furthermore, glaucomatous TM tissue has a higher degree of stiffness compared to non-glaucomatous eyes (Last et al., 2011). To fully understand the role CLANs play in IOP elevation, it is important that we first determine the molecular mechanisms responsible for CLANs formation.
We currently know that DEX and TGFβ2 are inducers of CLANs in confluent TM cells and tissues (Clark et al., 1994; O’Reilly et al., 2011; Wilson et al., 1993). Figure 2 summarizes the role CLANs may play in glaucoma. Current cell, tissue, and mathematical modeling experiments have determined that CLAN formation is associated with IOP elevation. However, the exact effect of IOP elevation on CLANs is unknown. Our hypothesis is that IOP elevation is due to CLAN formation in the TM, but this requires additional studies. We know that inhibitors of the TGFβ2 Smad and non-Smad pathways, as well as ROCK inhibitors, can inhibit CLAN assembly, and resolve already formed CLANs.
Figure 2. Schematic of CLANs involvement in glaucoma.
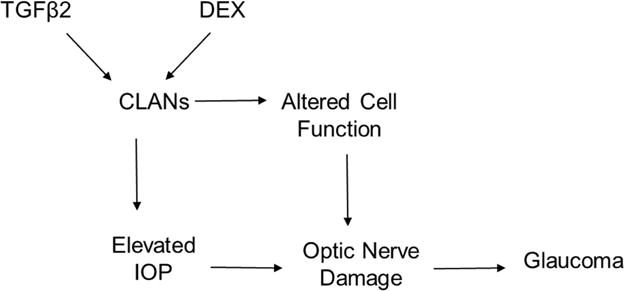
TGFβ2 and DEX can induce CLAN formation which leads to altered cell functions, optic nerve damage and eventually glaucoma. It is uncertain whether IOP elevation is the result or the cause of CLANs.
VIII. Future directions and remaining questions
Since the discovery of CLANs in the TM, we have gained an understanding of potential contributing factors to TM stiffness in glaucomatous individuals and a potential explanation as to how this rigidity contributes to IOP elevation. We have learned that CLANs are only found in the TM and LC tissues of the eye making these structures highly selective in tissues involved in glaucoma pathogenesis. Reports have begun to identify potential genes and encoded proteins that are associated with CLANs. However, we have yet to determine the exact role these genes and proteins play in CLAN formation and what are the exact molecular components that make up CLANs. Spreading TM cell studies have provided evidence for potential pathways involved in CLAN formation. Confluent TM cell studies indicate that both GC and TGFβ2 Smad and non-Smad pathways play a role in CLAN formation and stability. We still lack the knowledge about the pathways that are responsible for CLAN formation in glaucomatous TM and LC cells, although the responsible pathways may be very similar due to elevated expression of TGFβ2 in both of these tissues in POAG eyes.
In addition, we also still do not have answers to exactly how CLANs alter TM and ONH cellular functions. With the recent study of live cell imaging of CLANs, it is important that we continue to use such techniques to discover how CLANs contribute to TM cell/tissue reorganization and how this contributes to TM cell/tissue stiffness. It is also imperative that we determine if CLANs are directly responsible for glaucomatous as well as TGFβ2 and GC-induced IOP elevation.
Moreover, we have little understanding about CLANs in the ONH. Even though these CLANs are similar to those of the TM in appearance and likely alter certain cellular functions, we do not know whether the cellular pathways that regulate CLANs in the TM are the same as for CLANs in the LC. We also do not know if CLANs are directly involved in glaucomatous remodeling of the ONH. Future investigation of CLANs will enhance our knowledge of the molecular mechanisms that lead to glaucoma pathology and new potential targets for disease modifying therapies.
Highlights.
Cross-linked actin networks (CLANs) are more numerous and extensive in glaucomatous trabecular meshwork (TM) and lamina cribrosa (LC) cells and tissues compared to non-glaucomatous cells.
Two glaucoma associated factors, glucocorticoids and TGFβ2, also induce CLAN formation in TM cells.
CLANs may be responsible for altered TM and LC cell functions and increase cell stiffness in glaucoma
Acknowledgments
This work was supported by NIH grants R01EY016242 and R21EY023048.
Abbreviations
- AH
aqueous humor
- CLANs
cross-linked actin networks
- DEX
dexamethasone
- ECM
extracellular matrix
- GCs
glucocorticoids
- GC-OHT
glucocorticoid induced ocular hypertension
- GIG
glucocorticoid induced glaucoma
- HTM
human trabecular meshwork
- IOP
intraocular pressure
- JCT
juxtacanalicular
- LC
lamina cribrosa
- ONH
optic nerve head
- PDLIM1
PDZ and LIM Domain 1
- PKC
protein kinase C
- POAG
primary open angle glaucoma
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RGC
retina ganglion cell
- ROCK
Rho-associated protein kinase
- SC
Schlemm’s Canal
- SDC4
syndecan-4
- TGFβ2
transforming growth factor-beta 2
- THF
Tetrahydrocortisol
- TM
trabecular meshwork
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaly MF. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. Ii. The Effect of Dexamethasone in the Glaucomatous Eye. Arch Ophthalmol. 1963;70:492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- Armaly MF, Becker B. Intraocular pressure response to topical corticosteroids. Fed Proc. 1965;24:1274–1278. [PubMed] [Google Scholar]
- Balaratnasingam C, Morgan WH, Bass L, Cringle SJ, Yu DY. Time-dependent effects of elevated intraocular pressure on optic nerve head axonal transport and cytoskeleton proteins. Investigative ophthalmology & visual science. 2008;49:986–999. doi: 10.1167/iovs.07-1090. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Morgan WH, Bass L, Matich G, Cringle SJ, Yu DY. Axonal transport and cytoskeletal changes in the laminar regions after elevated intraocular pressure. Investigative ophthalmology & visual science. 2007;48:3632–3644. doi: 10.1167/iovs.06-1002. [DOI] [PubMed] [Google Scholar]
- Barber SC, Mellor H, Gampel A, Scolding NJ. S1P and LPA trigger Schwann cell actin changes and migration. Eur J Neurosci. 2004;19:3142–3150. doi: 10.1111/j.0953-816X.2004.03424.x. [DOI] [PubMed] [Google Scholar]
- Becker B. Intraocular Pressure Response to Topical Corticosteroids. Invest Ophthalmol. 1965;4:198–205. [PubMed] [Google Scholar]
- Bermudez JY,WH, Patel GC, Yan LY, Clark AF, et al. Two-Dimensional Differential In-Gel Electrophoresis (2D-DIGE) Reveals Proteins Associated with Cross-Linked Actin Networks in Human Trabecular Meshwork Cells. J Clin Exp Ophthalmol. 2016:584. [Google Scholar]
- Chidlow G, Ebneter A, Wood JP, Casson RJ. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol. 2011;121:737–751. doi: 10.1007/s00401-011-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AF, Brotchie D, Read AT, Hellberg P, English-Wright S, Pang IH, Ethier CR, Grierson I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- Clark AF, Lane D, Wilson K, Miggans ST, McCartney MD. Inhibition of dexamethasone-induced cytoskeletal changes in cultured human trabecular meshwork cells by tetrahydrocortisol. Investigative ophthalmology & visual science. 1996;37:805–813. [PubMed] [Google Scholar]
- Clark AF, Miggans S, Wilson K, Browder S, McCartney M. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J Glaucoma. 1995;4:183–188. [PubMed] [Google Scholar]
- Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Investigative ophthalmology & visual science. 1994;35:281–294. [PubMed] [Google Scholar]
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clark R, Nosie A, Walker T, Faralli JA, Filla MS, Barrett-Wilt G, Peters DM. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol Cell Proteomics. 2013;12:194–206. doi: 10.1074/mcp.M112.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Investigative ophthalmology & visual science. 1991;32:2201–2211. [PubMed] [Google Scholar]
- Entcheva E, Bien H. Mechanical and spatial determinants of cytoskeletal geodesic dome formation in cardiac fibroblasts. Integr Biol (Camb) 2009;1:212–219. doi: 10.1039/b818874b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier RC, Read TA, Chan DWH. Biomechanics of Schlemm’s canal endothelial cells: influence on F-actin architecture. Biophysical Journal. 2004;87:2828–2837. doi: 10.1529/biophysj.103.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla MS, Clark R, Peters DM. A syndecan-4 binding peptide derived from laminin 5 uses a novel PKCepsilon pathway to induce cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells. Exp Cell Res. 2014;327:171–182. doi: 10.1016/j.yexcr.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla MS, Schwinn MK, Nosie AK, Clark RW, Peters DM. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves beta3 integrin signaling. Investigative ophthalmology & visual science. 2011;52:2952–2959. doi: 10.1167/iovs.10-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla MS, Schwinn MK, Sheibani N, Kaufman PL, Peters DM. Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct beta1 and beta3 integrin pathways. Investigative ophthalmology & visual science. 2009;50:5723–5731. doi: 10.1167/iovs.08-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla MS, Woods A, Kaufman PL, Peters DM. Beta1 and beta3 integrins cooperate to induce syndecan-4-containing cross-linked actin networks in human trabecular meshwork cells. Investigative ophthalmology & visual science. 2006;47:1956–1967. doi: 10.1167/iovs.05-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor D, Shepard A, Hellberg P, Jacobson N, Pang IH, Clark FA. TGFB2-induced changes in human trabecular meshwork: Implications for intraocular pressure. Investigative ophthalmology & visual science. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm E. Modulation of extracellular matrix turnover in the trabecular meshwork. Experimental Eye Research. 2009;88:683–688. doi: 10.1016/j.exer.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Inoue T, inoue-Mochita M, Tanihara H. Live cell imaging of actin dynamics in dexamethasone-treated porcine trabecular meshwork cells. Exp Eye Res. 2016 doi: 10.1016/j.exer.2016.02.007. In press. [DOI] [PubMed] [Google Scholar]
- Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304:1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- Harrison BA, Almstead ZY, Burgoon H, Gardyan M, Goodwin NC, Healy J, Liu Y, Mabon R, Marinelli B, Samala L, Zhang Y, Stouch TR, Whitlock NA, Gopinathan S, McKnight B, Wang S, Patel N, Wilson AG, Hamman BD, Rice DS, Rawlins DB. Discovery and Development of LX7101, a Dual LIM-Kinase and ROCK Inhibitor for the Treatment of Glaucoma. ACS Med Chem Lett. 2015;6:84–88. doi: 10.1021/ml500367g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MR, Andrzejewska WM, Neufeld AH. Changes in the extracellular matrix of the human optic nerve head in primary open-angle glaucoma. Am J Ophthalmol. 1990;109:180–188. doi: 10.1016/s0002-9394(14)75984-7. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Igoe F, Neufeld AH. Cell culture of the human lamina cribrosa. Investigative ophthalmology & visual science. 1988;29:78–89. [PubMed] [Google Scholar]
- Hoare MJ, Grierson I, Brotchie D, Pollock N, Cracknell K, Clark AF. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Investigative ophthalmology & visual science. 2009;50:1255–1263. doi: 10.1167/iovs.08-2706. [DOI] [PubMed] [Google Scholar]
- Huang X, Kong W, Zhou Y, Gregori G. Distortion of axonal cytoskeleton: an early sign of glaucomatous damage. Investigative ophthalmology & visual science. 2011;52:2879–2888. doi: 10.1167/iovs.10-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- Jain A, Wordinger RJ, Yorio T, Clark AF. Role of the alternatively spliced glucocorticoid receptor isoform GRbeta in steroid responsiveness and glaucoma. J Ocul Pharmacol Ther. 2014;30:121–127. doi: 10.1089/jop.2013.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jampel HD, Leong KW, Dunkelburger GR, Quigley HA. Glaucoma filtration surgery in monkeys using 5-fluorouridine in polyanhydride disks. Arch Ophthalmol. 1990;108:430–435. doi: 10.1001/archopht.1990.01070050128046. [DOI] [PubMed] [Google Scholar]
- Job R, Raja V, Grierson I, Currie L, O’Reilly S, Pollock N, Knight E, Clark AF. Cross-linked actin networks (CLANs) are present in lamina cribrosa cells. The British journal of ophthalmology. 2010;94:1388–1392. doi: 10.1136/bjo.2009.176032. [DOI] [PubMed] [Google Scholar]
- Johnson M. What controls aqueous humor outflow resistance? Experimental Eye Research. 2006;82:545–557. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Yu DY. Distribution pattern of axonal cytoskeleton proteins in the human optic nerve head. Neural Regen Res. 2015;10:1198–1200. doi: 10.4103/1673-5374.162691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan RP, Fenerty CH, Crean J, Wordinger RJ, Clark AF, O’Brien CJ. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol Vis. 2005;11:798–810. [PubMed] [Google Scholar]
- Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Investigative ophthalmology & visual science. 2013;54:ORSF5–ORSF13. doi: 10.1167/iovs.13-12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Epstein DL. Rho GTPase-mediated cytoskeletal organization in Schlemm’s canal cells play a critical role in the regulation of aqueous humor outflow facility. J Cell Biochem. 2011;112:600–606. doi: 10.1002/jcb.22950. [DOI] [PubMed] [Google Scholar]
- Last JA, Pan T, Ding Y, Reilly C, Keller K, Acott T, Fautsch M, Murphy C, Russell P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Investigative ophthalmology & visual science. 2011;52:2147–2152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976;68:202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Stahl F, Weiderholt M. Differential smooth muscle-like contractile properties of trabecular meshwork and ciliary muscle. Experimental Eye Research. 1991;53:33–38. doi: 10.1016/0014-4835(91)90141-z. [DOI] [PubMed] [Google Scholar]
- Liton PB, Gonzalez P. Stress response of the trabecular meshwork. J Glaucoma. 2008;17:378–385. doi: 10.1097/IJG.0b013e31815f52a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Johnson DH. Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Investigative ophthalmology & visual science. 1997;38:1902–1907. [PubMed] [Google Scholar]
- Medina-Ortiz WE, Belmares R, Neubauer S, Wordinger RJ, Clark AF. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-beta2. Investigative ophthalmology & visual science. 2013;54:6779–6788. doi: 10.1167/iovs.13-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller K, Theiss C. Atomic force microscopy and confocal laser scanning microscopy on the cytoskeleton of permeabilised and embedded cells. Ultramicroscopy. 2006;106:320–325. doi: 10.1016/j.ultramic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Dorman-Pease ME, Dunkelberger GR, Quigley HA. Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch Ophthalmol. 1990;108:1020–1024. doi: 10.1001/archopht.1990.01070090122053. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Morgan JT, Wood JA, Sadeli A, Murphy CJ, Russell P. The formation of cortical actin arrays in human trabecular meshwork cells in response to cytoskeletal disruption. Exp Cell Res. 2014;328:164–171. doi: 10.1016/j.yexcr.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima E, Nakajima T, Minagawa Y, Shearer T, Azuma M. Contribution of ROCK in contraction of trabecular meshwork: proposed mechanism for regulating aqueous outlfow in monkey and human eyes. J of Pharm Sci. 2005;94:701–708. doi: 10.1002/jps.20285. [DOI] [PubMed] [Google Scholar]
- O’Reilly S, Pollock N, Currie L, Paraoan L, Clark AF, Grierson I. Inducers of cross-linked actin networks in trabecular meshwork cells. Investigative ophthalmology & visual science. 2011;52:7316–7324. doi: 10.1167/iovs.10-6692. [DOI] [PubMed] [Google Scholar]
- Osborn M, Born T, Koitsch HJ, Weber K. Stereo immunofluorescence microscopy: I. Three-dimensional arrangement of microfilaments, microtubules and tonofilaments. Cell. 1978;14:477–488. doi: 10.1016/0092-8674(78)90234-9. [DOI] [PubMed] [Google Scholar]
- Overby DR, Zhou EH, Vargas-Pinto R, Pedrigi RM, Fuchshofer R, Braakman ST, Gupta R, Perkumas KM, Sherwood JM, Vahabikashi A, Dang Q, Kim JH, Ethier CR, Stamer WD, Fredberg JJ, Johnson M. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc Natl Acad Sci U S A. 2014;111:13876–13881. doi: 10.1073/pnas.1410602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JD, Taylor AW, Ricard CS, Vidal I, Hernandez MR. Transforming growth factor beta isoforms in human optic nerve heads. The British journal of ophthalmology. 1999;83:209–218. doi: 10.1136/bjo.83.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70:307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Number of people with glaucoma worldwide. The British journal of ophthalmology. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investigative ophthalmology & visual science. 2015;56:4447–4459. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Investigative ophthalmology & visual science. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Rao V, Deng PF, Sasaki Y, Epstein D. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Experimental Eye Research. 2005;80:197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Read TA, Chan DWH, Ethier RC. Actin structure in the outflow tract of normal and glaucomatous eyes. Experimental Eye Research. 2007;84:214–226. doi: 10.1016/j.exer.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Rozsa FW, Reed DM, Scott KM, Pawar H, Moroi SE, Kijek TG, Krafchak CM, Othman MI, Vollrath D, Elner VM, Richards JE. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol Vis. 2006;12:125–141. [PubMed] [Google Scholar]
- Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Investigative ophthalmology & visual science. 2011;52:5240–5250. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Investigative ophthalmology & visual science. 1992;33:2242–2250. [PubMed] [Google Scholar]
- Tamm E, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Survey of Ophthalmology. 2007;52:S101–S104. doi: 10.1016/j.survophthal.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Investigative ophthalmology & visual science. 2000;41:619–623. [PubMed] [Google Scholar]
- Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol. 1998;116:633–643. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- Tovar-Vidales T, Clark AF, Wordinger RJ. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp Eye Res. 2011;93:442–451. doi: 10.1016/j.exer.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Vidales T, Wordinger RJ, Clark AF. Identification and localization of lamina cribrosa cells in the human optic nerve head. Exp Eye Res. 2016;147:94–97. doi: 10.1016/j.exer.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Tumminia S, Mitton K, Arora J, Zelenka P, Epstein D, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Investigative ophthalmology & visual science. 1998;39:1361–1371. [PubMed] [Google Scholar]
- Wang SK, Chang RT. An emerging treatment option for glaucoma: Rho kinase inhibitors. Clin Ophthalmol. 2014;8:883–890. doi: 10.2147/OPTH.S41000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecker T, Han H, Borner J, Grehn F, Schlunck G. Effects of TGF-B2 on cadherins and B-catenin in human trabecular meshwork cells. Investigative ophthalmology & visual science. 2013;54:6456–6462. doi: 10.1167/iovs.13-12669. [DOI] [PubMed] [Google Scholar]
- Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19:271–295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res. 1993;12:783–793. doi: 10.3109/02713689309020383. [DOI] [PubMed] [Google Scholar]
- Wordinger R, Clark AF, Agarwal R, Lambert W, McNatt L, Wilson SE, Qu Z, Fung BKK. Cultured human trabecular meshwork cells express functional growth factor receptors. Investigative ophthalmology & visual science. 1998;39:1575–1589. [PubMed] [Google Scholar]
- Zhang X, Clark AF, Yorio T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Investigative ophthalmology & visual science. 2005;46:4607–4616. doi: 10.1167/iovs.05-0571. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ognibene CM, Clark AF, Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res. 2007;84:275–284. doi: 10.1016/j.exer.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Currie L, Pollock N, Heath A, Sheridan C, Choudhary A, O’Reilly S, Grierson I. Measurement and computer modeling of temporary arrangements of polygonal actin structures in trabecular meshwork cells which consist of cross-linked actin networks and polygonal actin arrangements. J Ocul Pharmacol Ther. 2014;30:224–236. doi: 10.1089/jop.2013.0155. [DOI] [PubMed] [Google Scholar]
- Zhou L, Li Y, Yue BY. Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int J Mol Med. 1998;1:339–346. [PubMed] [Google Scholar]
- Zode GS, Clark AF, Wordinger RJ. Bone morphogenetic protein 4 inhibits TGF-beta2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. Glia. 2009;57:755–766. doi: 10.1002/glia.20803. [DOI] [PubMed] [Google Scholar]
- Zode GS, Sethi A, Brun-Zinkernagel AM, Chang IF, Clark AF, Wordinger RJ. Transforming growth factor-beta2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol Vis. 2011;17:1745–1758. [PMC free article] [PubMed] [Google Scholar]


