Abstract
In the yeast Saccharomyces cerevisiae, the genes encoding the metallothionein protein Cup1 are located in a tandem array on chromosome VIII. Using a diploid strain that is heterozygous for an insertion of a selectable marker (URA3) within this tandem array, and heterozygous for markers flanking the array, we measured interhomolog recombination and intra/sister chromatid exchange in the CUP1 locus. The rate of intra/sister chromatid recombination exceeded the rate of interhomolog recombination by >10-fold. Loss of the Rad51 and Rad52 proteins, required for most interhomolog recombination, led to a relatively small reduction of recombination in the CUP1 array. Although interhomolog mitotic recombination in the CUP1 locus is elevated relative to the average genomic region, we found that interhomolog meiotic recombination in the array is reduced compared to most regions. Lastly, we showed that high levels of copper (previously shown to elevate CUP1 transcription) lead to a substantial elevation in rate of both interhomolog and intra/sister chromatid recombination in the CUP1 array; recombination events that delete the URA3 insertion from the CUP1 array occur at a rate of >10−3/division in unselected cells. This rate is almost three orders of magnitude higher than observed for mitotic recombination events involving single-copy genes. In summary, our study shows that some of the basic properties of recombination differ considerably between single-copy and tandemly-repeated genes.
Keywords: mitotic recombination, meiotic recombination, sister chromatid crossovers, repeated genes
HOMOLOGOUS recombination (HR) is an important mechanism for the repair of double-stranded DNA breaks (DSBs) in yeast (Symington et al. 2014) and in higher eukaryotes (Liang et al. 1998). Saccharomyces cerevisiae strains that lack HR are very sensitive to DNA-damaging agents such as X-rays (Resnick and Martin 1976), and have a high rate of spontaneous chromosome loss (Song and Petes 2012). HR is essential for the survival of mammalian cells (Helleday 2003).
Despite the importance of HR in the repair of spontaneous and induced DNA damage, there are many details concerning mitotic recombination that are still unclear. One issue is the timing of the DNA lesions that induce spontaneous mitotic recombination events. Using genetic systems that monitor interhomolog recombination, we (Lee et al. 2009; St. Charles and Petes 2013) and others (Esposito 1978) concluded that most recombination events are initiated in G1 of the cell cycle. This conclusion was unexpected since Rad52p foci (indicative of DNA damage) are much more common in the S-period and G2 than in G1 (Lisby et al. 2001). In addition, repair of DSBs by HR is inefficient in G1 (Aylon et al. 2004). A simple model consistent with all of these observations is that most DSBs occur during the S-period, but these DSBs are repaired by sister chromatid recombination rather than interhomolog recombination (Kadyk and Hartwell 1992; Lee et al. 2009). DSBs that occur in G1 are likely not repaired in G1, but the broken chromosome is replicated to produce two sister chromatids that are broken at the same position. Since the DSBs are at the same position, the sister chromatid cannot be used as a repair template, and the breaks are repaired using the intact homolog (Lee et al. 2009).
One set of experiments with data supporting the model described above was performed by Kadyk and Hartwell (1992). Since sister chromatids have identical sequences, it has been challenging to determine the frequency of sister chromatid recombination by genetic approaches. Kadyk and Hartwell (1992) developed a diploid yeast strain in which both interhomolog and intersister recombination could be monitored. Interhomolog recombination was monitored by measuring the frequency of gene conversion between leu1 heteroalleles. Sister chromatid exchange was examined using two incomplete copies of the ADE3 gene separated by a wild-type URA3 gene, a method similar to that developed by Fasullo and Davis (1987). One copy of the gene was truncated at the 5′-end and the other at the 3′-end. The two copies shared ∼300 bp of homology, and unequal sister chromatid recombination within this duplication could produce an Ade+ derivative. Thus, in this strain, the rates of Leu+ and Ade+ derivatives reflected the rates of interhomolog and intersister chromatid events, respectively.
Kadyk and Hartwell (1992) examined recombination induced by X-rays in G1- and G2-synchronized cells. Consistent with previous studies by others (Fabre et al. 1984), they observed that interhomolog recombination was efficiently induced in G1 cells, but not in G2 cells. In contrast, sister chromatid exchange was induced more efficiently in G2 than in G1. Their interpretation of this result was that DSBs produced by irradiation in G2 were preferentially repaired by sister chromatid recombination, resulting in a decrease in the frequency of interhomolog recombination. They estimated that >90% of the DSBs induced by X-rays in G2 were repaired by sister chromatid exchange.
A variety of other systems for the analysis of sister chromatid mitotic recombination have been developed (Szostak and Wu 1980; Jackson and Fink 1981; Arbel et al. 1999; Gonzalez-Barrera et al. 2003; Mozlin et al. 2008). Most of these assays have been done in haploid strains or have utilized plasmids, preventing a direct comparison of intersister and interhomolog events. In addition, in studies done in diploid strains, intersister and interhomolog recombination were assayed using different genes located at different positions in the genome. Below, we describe a diploid yeast strain in which both interhomolog and intersister chromatid recombination within the CUP1 locus can be monitored.
Most strains of S. cerevisiae have between 2 and 20 tandem copies of CUP1, a gene encoding a copper-binding metallothionein, located on chromosome VIII (Zhao et al. 2014; Strope et al. 2015). The degree of resistance to copper is proportional to the number of CUP1 genes in the tandem array (Fogel and Welch 1982). As will be discussed in detail below, unequal sister chromatid recombination events within tandem arrays can be monitored using single-copy genes inserted within the array (Petes 1980; Szostak and Wu 1980). In addition, interhomolog recombination between CUP1 arrays can be measured by using selectable drug resistance markers flanking the array. Thus, a direct comparison of intersister and interhomolog events can be made.
We found that intersister recombination events are >10-fold more frequent than interhomolog events. Most of these intersister events do not reflect unequal sister chromatid recombination but likely occur by single-strand annealing (SSA), intersister gene conversion, or DNA polymerase slippage. We also found that, although Rad51 and Rad52 are required for most types of HR, the absence of these proteins has a relatively small effect on recombination within the CUP1 array.
Transcription of the CUP1 genes is induced by high levels of copper (Karin et al. 1984). We demonstrated that mitotic recombination within the CUP1 array is strongly induced by transcription. We were also able to compare the properties of mitotic and meiotic recombination within the CUP1 locus. In meiosis, unlike mitosis, recombination occurs less frequently than for an average genomic interval, and meiotic recombination is not elevated by high levels of copper.
Materials and Methods
Yeast medium
Standard media were used (Guthrie and Fink 1991) unless noted below. Copper-containing medium was made by adding an aqueous solution of copper sulfate to synthetic dextrose (SD)-complete medium. HYG + CAN medium contained 120 mg/liter L-canavanine sulfate (Can) and 300 mg/liter hygromycin B (Hyg) in SD-arginine omission medium. Before adding Hyg, the liquid medium was adjusted to a pH value of 5.5 by addition of sodium hydroxide. The medium was then filter-sterilized, and mixed with autoclave-sterilized agar. HYG and GEN medium was made by supplementing YPD medium with either 300 mg/liter Hyg or 200 mg/liter geneticin (Gen). CAN medium was made by adding 120 mg/liter Can to SD-arginine omission medium. 5-fluoroorotic acid (5-FOA)-containing medium was SD-complete (SD-complete) with the addition of 40 mg of uracil and 1 g of 5-FOA per liter.
Yeast strains and plasmids
The genotypes and additional details of the construction of all strains used in this study are shown in Supplemental Material, Table S1 in File S1. All haploid strains are isogenic derivatives of strains S1/S288c (Engel et al. 2014), W303-1A (Thomas and Rothstein 1989), or YJM789 (Wei et al. 2007). A complete list of primers used in strain construction or PCR analysis is provided in Table S2 in File S1. Plasmids used in our work are listed in Table S3 in File S1. The plasmids pFA6a-kanMX4 and pAG25 were described in Wach et al. (1994) and Goldstein and McCusker (1999), respectively. Haploid strains with inserted genetic markers and various gene deletions were constructed by transformation. All insertions and deletions were confirmed by PCR. In addition, the rad52∆::natMX4 strains YZ43, YZ44, and YZ113, and the rad1∆::natMX4 strains YZ49, YZ50, and YZ116, were confirmed by their sensitivity to 50 J/m2 of UV radiation. The cup2∆::natMX4 strains YZ51, YZ52, and YZ117 were confirmed by their sensitivity to growth in medium containing 0.2 mM copper sulfate.
The strain YZ18 was constructed by integrating the URA3 marker into one of the CUP1 repeats in the strain YZ15. Genomic DNA from the wild-type strain S1 was amplified using primers VIII214177::URA3 F and VIII214177::URA3 R. Genomic DNA of independent YZ18 isolates was isolated and digested with HindIII, which cuts once within the URA3 marker, and in the regions flanking the CUP1 cluster. In YZ18-10, the strain used in subsequent constructions, the URA3 insertion was in the middle of the CUP1 array that contained ∼18 CUP1 repeats.
Previously, we found that the CUP1 clusters in strains YJM789 and JSC19-1 contained seven copies of the 1.2-kb CUP1 repeats (Zhao et al. 2014). The strain YZ17 was derived by selecting JSC19-1 derivatives with enhanced copper resistance; a concentration of 0.4 mM copper in SD-complete medium was used for this selection. Southern analysis verified that YZ17 consists of an expanded CUP1 cluster, containing ∼22 repeats.
The 2-micron-based plasmid p425-GPD-CUP1 was constructed by amplifying genomic DNA of the strain S1 using the primers HindIII-CUP1 stop ligate and CUP1 start-BamHI ligate. The resulting fragment was treated with HindIII and BamHI, and inserted into HindIII-BamHI-treated p426-GPD (ATCC 87359). In this plasmid, the CUP1 gene is regulated by the strong constitutive GPD promoter (alternative name of the TDH3 promoter) (Mumberg et al. 1995). This plasmid also contains a LEU2 marker for the maintenance and selection of the plasmid.
The plasmid YIp5-CUP1 has one CUP1 repeat inserted in the URA3-containing YIp5-integrating vector (Stearns et al. 1990). The CUP1 repeat was amplified from genomic DNA of W303-1A using the primers F-CUP1-EcoRI and R-CUP1-HindIII. The resulting fragment was treated with the EcoRI and HindIII restriction enzymes, and ligated to YIp5 DNA that had been treated with the same enzymes.
The haploid strain YZ26 was constructed by switching the mating type of the strain JSC10-1 (St. Charles and Petes 2013), a W303-1A derivative with the genotype MATa leu2-3,112 his3-11,15 ura3-1 ade2-1 trp1-1 can1-100Δ::natMX RAD5. The plasmid pSR109 (pGAL-HO) (Herskowitz and Jensen 1991) was transformed into JSC10-1 in omission medium lacking uracil and containing 2% raffinose and 0.1% glucose. HO expression was transiently induced by incubating cells for 5 hr in medium containing 2% galactose and 0.1% glucose. After this treatment, cells were plated on rich growth medium (YPD) and allowed to form colonies. The mating type of each colony was scored using “lawns” of tester strains (YJM789 and YJM790, Table S1 in File S1). Colonies were also tested on omission medium lacking uracil to confirm the loss of pSR109.
Measurements of recombination rates
The diploid strains YZ103 and YZ104, and related derivatives are sensitive to 5-FOA and Can, and resistant to the drugs Hyg and Gen. As described in the Results, we measured interhomolog recombination rates in the CEN8-hphMX4, hphMX4-URA3, and URA3-CAN1/kanMX4 genetic intervals by measuring the rate of derivatives that were 5-FOAR HygS GenS CanR, 5-FOAR HygR GenS CanR, and 5-FOAS HygR GenS CanR, respectively. Derivatives resulting from intra/sister chromatid events have the phenotype 5-FOAR HygR GenR CanS. To determine the rates of these events shown in Table 1, for each strain, we allowed cells to form single colonies at 30° on rich growth medium (YPD). Each colony was suspended in water, and various dilutions were plated on SD-complete, 5-FOA-containing medium, and HYG + CAN medium. Colonies formed on 5-FOA plates were replica-plated to medium containing Hyg, Gen, and Can, and the numbers of colonies on each plate with the phenotypes described above were counted. Colonies formed on HYG + CAN medium were replica-plated to medium lacking uracil. For each experiment, we calculated these numbers for ∼20 independent colonies, and we performed experiments using two derivatives of each strain. For each strain, these values were converted to rate estimates using the method of the median (Lea and Coulson 1949).
Table 1. Interhomolog and intra/sister chromatid mitotic recombination rates in wild-type and mutant yeast strains.
| Strain name | Relevant genotype (background) | CEN8-hphMX4 interhomolog | hphMX4-URA3 interhomolog | URA3-kanMX4/CAN1 interhomolog | hphMX4-kanMX4/CAN1 interhomologa | Intra/sister chromatid |
|---|---|---|---|---|---|---|
| YZ103 | Wild-type (W303-1A × W303-1A) | 8.2 × 10−6 (6.4–14) [1] | 3.3 × 10−6 (3.0–3.8) [1] | 5.1 × 10−6 (3.9–5.8) [1] | 8.4 × 10−6 [1] | 8.3 × 10−5 (6.5–12) [1] |
| YZ104 | Wild-type (W303-1A × YJM789) | 4.6 × 10−6 (3.0–6.3) [0.56] | 2.8 × 10−6 (2.3–3.1) [0.85] | 3.2 × 10−6 (3.0–3.5) [0.62] | 6 × 10−6 [0.71] | 1.5 × 10−4 (1–1.9) [1.8] |
| YZ113 | rad52/rad52 (W303-1A × W303-1A) | 1.3 × 10−6 (0.9–1.9) [0.16] | 1.3 × 10−7 (1.0–1.8) [0.04] | 3.9 × 10−7 (3.0–4.9) [0.08] | 5.2 × 10−7 [0.06] | 3.8 × 10−5 (3.2–4.5) [0.46] |
| YZ114 | rad51/rad51 (W303-1A × W303-1A) | 4.5 × 10−8 (3.4–5.2) [0.01] | 4.5 × 10−7 (3.2–6.4) [0.14] | 1.0 × 10−6 (0.9–1.4) [0.20] | 1.5 × 10−6 [0.18] | 1.9 × 10−5 (1.7–2.2) [0.23] |
| YZ115 | mre11/mre11 (W303-1A × W303-1A) | 6.2 × 10−5 (5.7–7.4) [7.6] | 2.4 × 10−5 (2.0–3.2) [7.3] | 1.5 × 10−5 (1.2–1.9) [2.9] | 3.9 × 10−5 [4.6] | 1.1 × 10−4 (0.9–1.4) [1.3] |
| YZ116 | rad1/rad1 (W303-1A × W303-1A) | 1.8 × 10−5 (1.3–2.3) [2.2] | 3.8 × 10−6 (3.4–4.1) [1.2] | 4.0 × 10−6 (3.7–5.1) [0.78] | 7.8 × 10−6 [0.93] | 1.5 × 10−4 (1.3–1.8) [1.8] |
| HB9 | rad5/rad5 (W303-1A × W303-1A) | 5.3 × 10−5 (3.1–7.9) [6.5] | 3.3 × 10−5 (2.6–3.8) [10] | 2.8 × 10−5 (2.1–4.0) [5.5] | 6.0 × 10−5 [7.1] | 1.5 × 10−4 (1.0–2.0) [1.8] |
| HB13 | mms2/mms2 (W303-1A × W303-1A) | 1.8 × 10−5 (1.1–2.6) [2.2] | 2.9 × 10−5 (2.2–3.3) [8.8] | 1.8 × 10−5 (1.5–2.1) [3.5] | 4.2 × 10−5 [5.0] | 9.3 × 10−5 (7.2–12) [1.1] |
The rates represent the number of events per cell division as determined by fluctuation analysis, as described in the text. Numbers in parentheses represent 95% C.I. and bold-faced numbers in square brackets are the rates divided by the rate of the wild-type strain YZ103.
The rates in this column are the sum of the rates in the fourth and fifth columns, and are the rates of interhomolog recombination within the entire CUP1 array.
To examine the effects of copper on mitotic recombination in strains YZ118 and YZ103 (Table 2), the experiments were done somewhat differently. YZ118 contains a plasmid with a CUP1 gene regulated by the GPD promoter as well as a LEU2 gene. Colonies were grown on plates containing SD-complete medium with 1.4 mM copper sulfate. These colonies were suspended in water and plated to three types of medium (all containing 0.2 mM copper sulfate): SD-complete, 5-FOA, and HYG + CAN medium. Rate estimates were performed as described above. Copper was included in the diagnostic media to force retention of the plasmid. For reasons that are not clear, the YZ118 strain fails to form colonies on medium lacking leucine and containing copper. For the control experiments in the absence of added copper, cells of YZ118 were grown on SD omission medium lacking leucine; subsequent analysis was done using the diagnostic plates with 0.2 mM copper sulfate. For the YZ103 strain, cells were allowed to form colonies on YPD medium or SD-complete medium with 1.4 mM copper sulfate. The media for diagnosis were the same as those used in the analysis of YZ118.
Table 2. Rates of interhomolog and intra/sister chromatid in cells grown in medium without added copper, and in medium with 1.4 mM copper.
| Strain | Relevant genotype | Condition | CEN8-hphMX4 interhomolog | hphMX4-URA3 interhomolog | URA3-kanMX4/CAN1 interhomolog | hphMX4-kanMX4/CAN1 interhomolog | Intra/sister chromatid |
|---|---|---|---|---|---|---|---|
| YZ103 | Wild-type | No added copper | 1.1 × 10−5 (0.9–1.7) [1] | 5.1 × 10−6 (4.4–7.0) [1] | 6.0 × 10−6 (4.4–8.1) [1] | 1.1 × 10−5 [1] | 1.0 × 10−4 (0.7–1.2) [1] |
| YZ103 | Wild-type | 1.4 mM copper sulfate | 1.3 × 10−5 (1.1–1.6) [1.2] | 8.6 × 10−5 (7.4–11) [17] | 8.8 × 10−5 (7.5–11) [15] | 1.7 × 10−4 [15] | 2.3 × 10−3 (2.0–2.7) [23] |
| YZ117 | cup2/cup2 | No added copper | 8.8 × 10−6 (6.2–13) [0.8] | 3.6 × 10−6 (2.3–4.8) [0.71] | 4.2 × 10−6 (3.1–5.7) [0.7] | 7.8 × 10−6 [0.71] | 1.1 × 10−4 (0.7–1.4) [1.1] |
| YZ118 | cup2/cup2 + p425-GPD-CUP1 | No added copper | 6.8 × 10−6 (4.6–10) [0.62] | 4.3 × 10−6 (2.7–4.9) [0.84] | 4.1 × 10−6 (3.1–5.2) [0.68] | 8.4 × 10−6 [0.76] | 6.7 × 10−5 (5.0–7.7) [0.67] |
| YZ118 | cup2/cup2 + p425-GPD-CUP1 | 1.4 mM copper sulfate | 1.3 × 10−5 (0.9–2.5) [1.2] | 1.2 × 10−5 (1.1–1.6) [2.4] | 9.9 × 10−6 (6.9–11) [1.7] | 2.2 × 10−5 [2] | 8.4 × 10−5 (7.3–10) [0.84] |
Numbers in parentheses after the rate estimates are the 95% C.I. Numbers in square brackets in bold are the rates divided by the rate observed in YZ103 grown in medium without added copper. The number of cells per culture was determined using synthetic dextrose-complete medium containing 0.2 mM copper.
Although we expect that derivatives of YZ103 and YZ104 that have the phenotype 5-FOAR HygS GenS CanR primarily reflect crossovers between CEN8 and hphMX4, loss of the homolog that carries the hphMX4, URA3, and CAN1/kanMX markers could result in a strain with the same phenotype. In the diploid YZ104, which has 55,000 heterozygous single nucleotide polymorphisms (SNPs) (St. Charles and Petes 2013), loss of one homolog would result in loss of heterozygosity (LOH) for all polymorphisms on chromosome VIII. Consequently, in 20 independent 5-FOAR HygS GenS CanR derivatives of YZ104, we checked for LOH for a polymorphism located within 3 kb of CEN8. Using primers VIII 108799-F and VIII 109229-R (Table S2 in File S1), we amplified genomic DNA from the 20 derivatives. The resulting 430 bp fragment from the YJM789-derived homolog has an AseI site that the W303-1A-derived fragment lacks. Thus, when the PCR products from diploids heterozygous for this polymorphism were treated with AseI, we found three fragments of sizes 430, 260, and 170 bp. In strains in which the W303-1A-derived homolog was lost, we found only the 260 and 170 bp fragments. Of the 20 isolates, 18 retained heterozygosity for the centromere-linked polymorphism, demonstrating that most 5-FOAR HygS GenS CanR derivatives are a consequence of crossovers rather than chromosome loss.
We also examined 5-FOAR HygS GenS CanR derivatives of YZ113 to determine whether these strains were monosomic. Since both homologs of YZ113 were derived from W303-1A, we could not use the PCR-based diagnosis of LOH described above. We labeled genomic DNA from the experimental derivatives with Cy5-dUTP and DNA from a control strain (YZ104) that was generated in a cross of W303-1A and YJM789 with Cy3-dUTP. These samples are mixed together and hybridized to a microarray that contains W303-1A- and YJM789-specific SNPs. By comparing the amounts of hybridization of the experimental strain relative to the control strain, we determined whether the experimental strain was monosomic for chromosome VIII. Details of this type of analysis are described in St. Charles and Petes (2013). The sequences of the oligonucleotides in the microarray are on the Gene Expression Omnibus Website (https://www.ncbi.nlm.nih.gov/geo/) under the address GPL20944.
Southern analysis
Genomic DNA from different strains was isolated in plugs of low-melt agarose as described previously (McCulley and Petes 2010). The samples were treated overnight at 37° with a diagnostic restriction enzyme (HindIII or EcoRI). The resulting DNA fragments were separated by CHEF (contour-clamped homogeneous electric field) gel electrophoresis (McCulley and Petes 2010), followed by transfer of the separated fragments to nylon membranes. The hybridization probes were prepared using digoxygenin (DIG)-dUTP labeling (Roche); details of the hybridization conditions were described previously (Zhao et al. 2014). DIG-labeled CUP1 probes that hybridize to both 2- and 1.2-kb CUP1 repeats were synthesized by DIG-PCR labeling using primers CUP1 amp5 and CUP1 amp3 (Table S2 in File S1). Probes that hybridize specifically to the 2-kb CUP1 repeats were DIG-labeled using primers CUP1 W303 spec amp5 and CUP1 W303 spec amp3 (Table S2 in File S1). The sizes of the tandem arrays were estimated relative to DNA size standard (Bioline DNA Hyperladders I and VI).
We examined transformants with the plasmid YIp5-CUP1 by similar methods. The purpose of these experiments was to determine whether the YIp5-CUP1 plasmid was maintained extrachromosomally or was integrated into the CUP1 array. Genomic DNA from the transformants was treated with EcoRI, which cuts within the YIp5-CUP1 plasmid but not within the CUP1 repeats. The samples were analyzed using CHEF gels as described above. We generated CUP1-specific probes using the primers CUP1 amp5* and CUP1 amp3*; probes specific to the pBR322 portion of YIp5 were produced using the probes bla-F and bla-R. For transformants with an unintegrated plasmid, when the samples were hybridized to the CUP1-specific probe, we expected to detect a fragment of ∼7.5 kb (representing the plasmid) and a large fragment (>30 kb) representing the CUP1 array. For transformants with an integrated plasmid, we expected to detect two large fragments. When transformants are hybridized to a vector-specific probe, those transformants that had not integrated the plasmid should have a fragment of 7.5 kb, whereas those with an integrated plasmid should have two larger DNA fragments that hybridize to the CUP1 probe; one of these fragments should also hybridize to a plasmid-specific probe. By these criteria, of 17 transformants examined, all had an integrated plasmid.
Statistical analysis
We performed comparisons using χ2 test, Fisher exact, or the Mann–Whitney nonparametric tests, and the VassarStat website (http://vassarstats.net). Ninety-five percent C.I. limits on rate estimates were calculated as described in Yin and Petes (2014). For comparisons of rate measurements in different strains or strains grown in different conditions, we calculated rates for each culture using the method of the median (Lea and Coulson 1949). We then compared these rates using the Mann–Whitney nonparametric test.
Meiotic analysis
For meiotic studies, we constructed a derivative of YZ103 that was heterozygous for a trp1 mutation (details in Table S1 in File S1). This diploid (MD692) was sporulated at room temperature using three types of medium: standard sporulation medium (Guthrie and Fink 1991), sporulation medium with 1.4 mM copper sulfate, and sporulation medium with 5 mM nicotinamide. Tetrads were dissected and analyzed using standard methods.
Data availability
All strains and plasmids constructed for this study are available upon request. The genotypes and constructions for these strains are in Table S1 in File S1, and sequences of the primers used in strain construction or analysis are in Table S2 in File S1. The plasmids used in the study are described in Table S3 in File S1.
Results
Experimental rationale
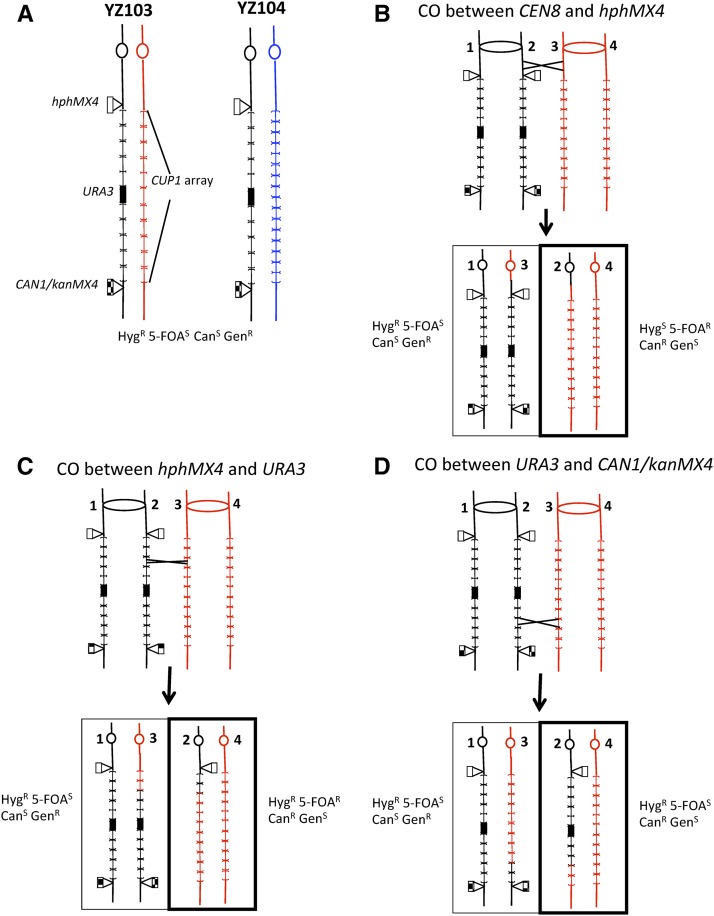
One goal of this project was to develop diploid yeast strains that would allow a comparison of mitotic recombination events between homologs with events that involve intrachromatid or sister chromatid interactions. Two diploids (YZ103 and YZ104) with similar arrangements of markers on chromosome VIII were constructed (Figure 1A). In both diploids, one homolog (derived from the haploid strain W303-1A) contained the hphMX4 gene (resulting in Hyg resistance) at the centromere-proximal end of the CUP1 tandem array, and a cassette containing the CAN1-kanMX4 genes (resulting in sensitivity to Can, and resistance to Gen) at the centromere-distal end of the cluster. In addition, this homolog has a copy of URA3 integrated within the CUP1 tandem array. In this homolog, there are 18 copies of a 2-kb CUP1 repeat, and the URA3 gene is integrated in the middle of the cluster (shown by Southern analysis, described in Materials and Methods). In the diploid YZ103, the other homolog is also derived from W303-1A but lacks the three insertions. In the diploid YZ104, the other homolog is derived from the haploid YJM789 (Wei et al. 2007). The CUP1 locus on this chromosome has 22 copies of a 1.2-kb repeat (Zhao et al. 2014). Both YZ103 and YZ104 are homozygous for can1 and ura3 mutations at their normal loci on chromosome V.
Figure 1.
Diploid strains (YZ103 and YZ104) used for the detection of interhomolog recombination between CEN8 and the CUP1 array, and within the CUP1 array. (A) Arrangement of markers on chromosome VIII in YZ103 and YZ104. The centromeres are shown as circles. In both diploids, one homolog has the marker hphMX4 inserted in single-copy sequences at the centromere-proximal end of the CUP1 array, an insertion of URA3 in the middle of the CUP1 cluster, and a cassette with CAN1/kanMX4 markers at the centromere-distal end of the array. Both homologs in YZ103 are derived from the haploid W303-1A and have 2.0-kb CUP1 repeats (Zhao et al. 2014); although there are 18 repeats in the CUP1 cluster of W303-1A, only 10 are shown, each in brackets. In YZ104, one homolog (shown in black) is derived from W303-1A, and the other (shown in blue) is derived from YJM789. Only 16 of the ∼22 1.2-kb CUP1 repeats of this homolog are shown. Both strains are phenotypically HygR 5-FOAS CanS GenR. (B) Detection of a crossover between CEN8 and hphMX4. After a crossover in this genetic interval (shown as an X), followed by cosegregation of chromatids 1 and 3 into one daughter cell and 2 and 4 in the other, one daughter cell (boxed in thin lines) would have the same phenotype as the parental strain, whereas the other daughter (boxed in thick lines) would be phenotypically distinct (HygS 5-FOAR CanR GenS). (C) Detection of a crossover between hphMX4 and URA3. A crossover in this interval would produce one daughter cell with the same phenotype as the starting diploid and a second daughter with the unique phenotype HygR 5-FOAR CanR GenS. (D) Detection of a crossover between URA3 and CAN1/kanMX4. One daughter cell would have the same phenotype as the starting strain, and the second daughter would have the phenotype HygR 5-FOAS CanR GenS. 5-FOA, 5-fluoroorotic acid; Can, L-canavanine; CO, crossover; Gen, geneticin; Hyg, hygromycin B; R, resistant; S, sensitive.
Interhomolog exchanges on chromosome VIII were monitored in three intervals as shown in Figure 1, B–D. Crossovers in the CEN8-hphMX4 interval (Figure 1B) can produce isolates that are Ura− (and, therefore, resistant to 5-FOA) HygS GenS CanR. A crossover in the hphMX4-URA3 interval (Figure 1C) results in 5-FOAR HygR GenS CanR isolates, and a crossover in the URA3-CAN1/kanMX4 interval (Figure 1D) results in 5-FOAS HygR GenS CanR isolates. We determined the frequencies of each of these phenotypic classes in multiple (>20) independent cultures, and converted these frequencies to rates using the method of the median as described in Materials and Methods.
Although we expected that most of the derivatives with the phenotype 5-FOAR HygS GenS CanR would represent crossovers (Figure 1B), loss of the homolog with the hphMX4, URA3, and CAN1/kanMX4 markers would produce the same phenotype. The diploid YZ104 is heterozygous for ∼55,000 SNPs distributed throughout the genome. To determine what fraction of the 5-FOAR HygS GenS CanR derivatives were crossovers, we examined 20 independent 5-FOAR HygS CanR GenS derivatives for LOH for a SNP located near CEN8. This diagnosis was done using primers flanking the polymorphism, followed by digestion of the product using an enzyme that cuts one allele but not the other; the details of this method are described in Materials and Methods. In the wild-type strain YZ104, 18 of 20 of the isolates retained heterozygosity for the centromere-linked marker, indicating that these derivatives reflected crossovers rather than chromosome loss.
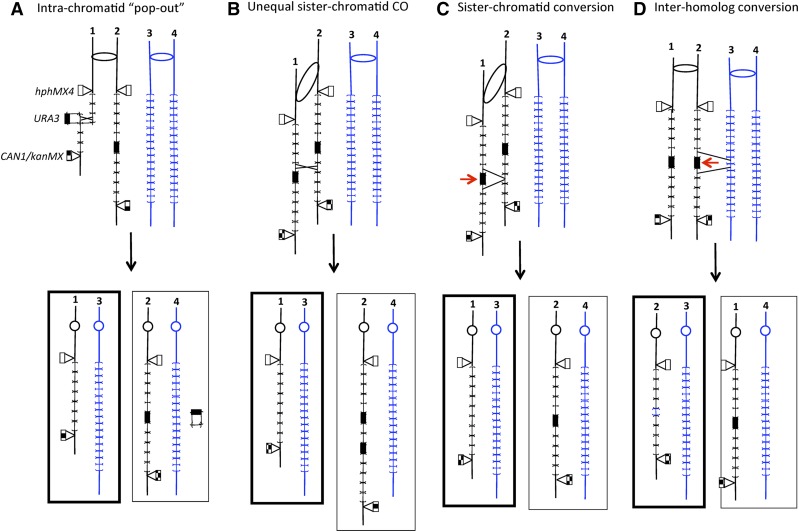
In addition to those recombination events involving the two homologs, we observed a high frequency of events in which the URA3 gene was lost and the flanking hphMX4 and CAN1/kanMX4 markers were retained. Strains of this genotype have the phenotype 5-FOAR HygR GenR CanS. A variety of different recombination mechanisms can generate this genotype, including intrachromatid “pop-out” recombination (Figure 2A), unequal sister chromatid recombination (Figure 2B), intersister chromatid gene conversion (Figure 2C), and interhomolog gene conversion (Figure 2D). In addition, the phenotype could reflect SSA (Figure 3, A and B) or DNA polymerase realignment/slippage (Figure 3C). By the experiments described below, we eliminated some of these possibilities. In the discussion below, all of the mechanisms except interhomolog gene conversion will be described as intra/intersister chromatid events.
Figure 2.
Mitotic recombination events leading to loss of URA3 marker and retention of the flanking hphMX4 and CAN1/kanMX4 markers. The chromosomes are shown following DNA replication, and the two homologs are shown in different colors; in this depiction, one homolog has 2-kb CUP1 repeats and the other 1.2-kb repeats. CUP1 repeats are indicated by brackets. The chromosomes of 5-FOAR daughter cells are outlined by thick lines. (A) Intrachromatid “pop-out” recombination. A crossover occurs within a chromatid, producing a shorter CUP1 array and a plasmid with the URA3 gene and three CUP1 repeats. The cell with the chromosomes outlined with thick lines would be 5-FOAR. Since each CUP1 repeat has an ARS element, the URA3-containing plasmid would be capable of autonomous replication. It is shown segregated into the daughter cell that also contains an integrated URA3 gene. (B) Unequal sister chromatid crossover. As a consequence of this event, the 5-FOAR daughter cell would contain a shorter CUP1 array, and the Ura+ daughter cell would contain a longer array with two URA3 insertions. (C) Intersister chromatid gene conversion. A DSB (shown as a red arrow) occurs near the URA3 insertion in one chromatid, and is repaired using the sister chromatid as a template. The net result of this event would be a loss of URA3 and one or more CUP1 repeats in one daughter cell with no alteration in the second daughter. (D) Interhomolog gene conversion. As in (C), this event is initiated with a DSB near or within the URA3 insertion. The repair template, however, is a chromatid of the other homolog instead of the sister chromatid. Associated with the loss of URA3 and the loss of some of the 2.0-kb CUP1 repeats, one would expect insertion of one or more 1.2-kb CUP1 repeats derived from the other homolog. 5-FOA, 5-fluoroorotic acid; CO, crossover; DSB, double-stranded DNA breaks; R, resistant; Ura+, uracil+.
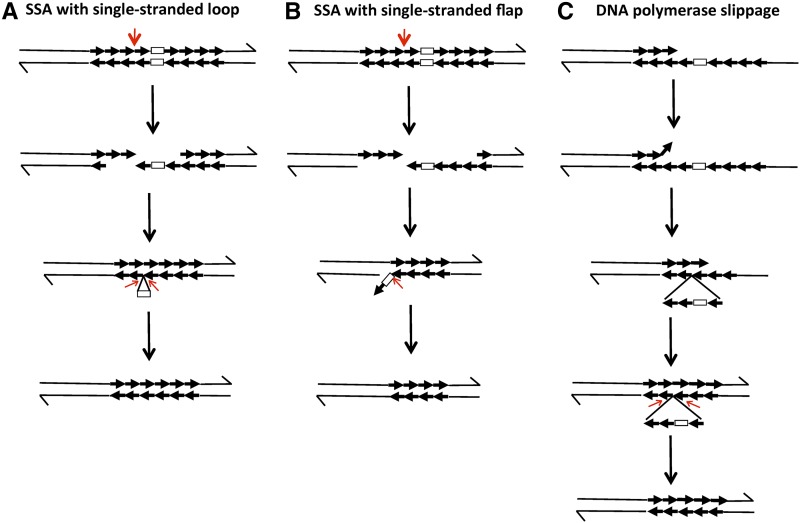
Figure 3.
Loss of the URA3 insertion by SSA or DNA polymerase slippage. In this diagram, we show the chromatid as double-stranded DNA molecules with the 3′-ends marked by arrows. CUP1 repeats are shown as thick arrows and the URA3 insertion is indicated by a rectangle. (A) SSA resulting in a single-stranded DNA loop. Following a DSB (shown with a thick red arrow), the broken ends are processed by 5′–3′ degradation. The single-stranded CUP1 repeat at the terminus at the right broken end anneals with repeats on the top strand of the left broken end. The resulting intermediate has a single-stranded loop containing the URA3 gene. This loop could be removed by cellular exonucleases (shown with thin red arrows) or the resulting DNA molecule could be replicated without removing the loop. The latter event would result in one daughter molecule that retains the URA3 insertion but has fewer CUP1 repeats, and a second molecule that loses the insertion and several CUP1 repeats. (B) SSA resulting in a single-stranded flap. This mechanism is very similar to that of (A) except for the amount of processing and the pattern of annealing. The resulting intermediate has a single-stranded flap that contains both the URA3 gene and one repeat. Removal of the flap would result in a shorter array that lacks the URA3 insertion. (C) DNA polymerase slippage. During DNA replication, the primer strand (the top strand) dissociates from the template and reassociates beyond the position of the URA3 insertion. The resulting intermediate has a single-stranded loop with the URA3 marker and several CUP1 repeats. Removal of the loop or replication of the intermediate would result in a daughter molecule that lacks the URA3 insertion and several CUP1 repeats. DSB, double-stranded DNA breaks; SSA, single-strand annealing.
Rates of interhomolog crossovers within the CUP1 array and in the CEN8-hphMX4 interval in YZ103 and YZ104
By fluctuation analysis (described in Materials and Methods), we measured the rates of mitotic recombination in the CEN8-hphMX4, the hphMX4-URA3, and the URA3-kanMX4/CAN1 intervals as 8.2 × 10−6, 3.3 × 10−6, and 5.1 × 10−6 per cell division, respectively (Table 1). Since the relative sizes of the CEN8-hphMX4 and CUP1 arrays are ∼107 and 30 kb, respectively, we expect the recombination rate in the CEN8-hphMX4 interval to be about fourfold higher than the rate for the CUP1 cluster (hphMX4-URA3 added to URA3-kanMX4/CAN1). The observed rate for the CEN8-hphMX4 interval was about the same as the rate within the cluster, indicating that the cluster is about fourfold “hotter” for interhomolog exchange than the CEN8-CUP1 interval. A similar comparison of the frequency of interhomolog recombination within the CUP1 locus to the frequency of recombination on the 1 Mb right arm of chromosome IV (St. Charles and Petes 2013) indicates that the CUP1 cluster is about eightfold elevated for mitotic recombination compared to the genomic average.
We also examined interhomolog crossovers in YZ104, in which the CUP1 clusters on the two homologs have different repeat lengths (Zhao et al. 2014). The interhomolog recombination rates in YZ103 and YZ104 are similar for all intervals, although the rates are slightly lower (25–50% lower) in YZ104. This difference may reflect the sequence heterogeneity between the interacting homologs or small differences in the enzymes that catalyze mitotic recombination in the hybrid background. With regard to the second point, Mre11p and Rad50p are different in amino acid sequence by 4 and 8 aa, respectively, between the strains W303-1A and YJM789. The activities of these proteins could be slightly different and/or since Mre11p/Rad50p/Xrs2p form complexes, the complexes formed by components derived from different genetic backgrounds could be suboptimal.
Rates of intra/sister chromatid events in YZ103 and YZ104
Most of the events that produce 5-FOAR HygR GenR CanS derivatives are likely to involve intra- or intersister chromatid recombination (Figure 2 and Figure 3). The rates with which the 5-FOAR HygR GenR CanS genotype is produced are 8.3 × 10−5/division in YZ103 and 1.5 × 10−4/division in YZ104. These rates are 10-fold (YZ103) and 26-fold (YZ104) higher than the rates of interhomolog recombination within the CUP1 cluster for each strain. These observations are consistent with the conclusion of Kadyk and Hartwell (1992) that sister chromatids are preferred over homologs as the substrate for the repair of DNA lesions.
Rate of interhomolog gene conversion in YZ104
As described above, derivatives of YZ103 or YZ104 with the 5-FOAR HygR GenR CanS could reflect various types of intra/intersister chromatid events or interhomolog gene conversion unassociated with crossing-over (Figure 2D). Such events can be detected in the YZ104 diploid. Loss of the URA3 gene, which is inserted in the 2-kb CUP1 repeats characteristic of the W303-1A-derived homolog, by gene conversion will result in insertion of one or more 1.2-kb CUP1 repeats from the YJM789-derived homolog. To restrict our analysis to the W303-1A-derived homolog, we sporulated strains with the 5-FOAR HygR GenR CanS phenotype, and identified spores that were HygR GenR CanS.
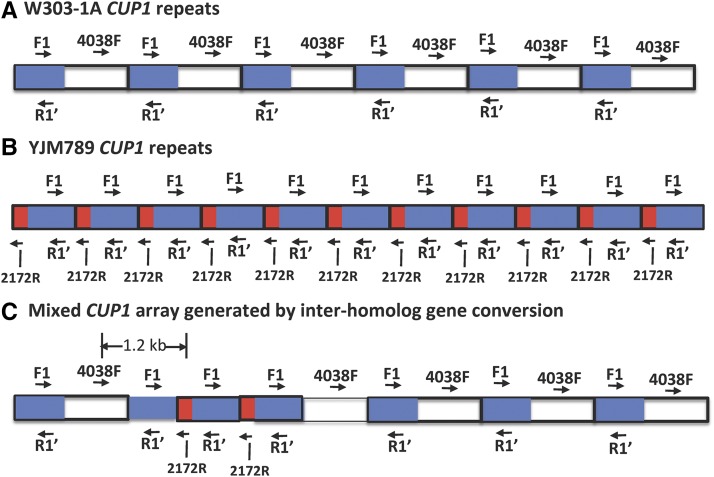
We did two types of PCR analysis to determine if there were 1.2-kb CUP1 repeats within the cluster of 2.0-kb repeats (Figure 4 and Figure S1 in File S1). The primers F1 and R1’ hybridize to both the 2- and 1.2-kb repeats, and PCR amplification with these primers leads to 2- and 1.2-kb products, respectively. Among 42 independent haploid isolates examined, none contained a mixture of both types of repeats. To confirm this result, we used primers 4038F (specific for the 2-kb repeats) and 2172R (specific for the 1.2-kb repeats). Arrays with a mixture of these two types of repeats should produce a 1.2-kb PCR product. None of the 42 isolates had such a product. We conclude that interhomolog gene conversion is not responsible for most of the 5-FOAR HygR GenR CanS strains.
Figure 4.
Detection of interhomolog gene conversion by PCR. CUP1 repeats are outlined by thick black lines. Sequences in common between the 2.0- and 1.2-kb CUP1 repeats are in blue, sequences unique to the 2.0-kb repeat are in white, and those unique to the 1.2-kb repeat are in red. Primers F1 and R1′ amplify both the 2.0- and 1.2-kb repeats whereas the 4038F and 2172R primers are specific for the 2.0- and 1.2-kb repeats, respectively. (A) 2.0-kb repeat array of W303-1A. Only six repeats of the 18-repeat array are shown. (B) 1.2-kb repeat array of YJM789. Only 11 repeats of the 22-repeat array are shown. (C) An array containing both 2.0- and 1.2-kb repeats. As an example of an interhomolog conversion, we show a 2.0-kb array with an insertion of two 1.2-kb repeats. A PCR reaction with primers 4038F and 2172R would produce a unique 1.2-kb band.
Loss of CUP1 repeats in YZ104 strains that have intra/sister chromatid recombination events
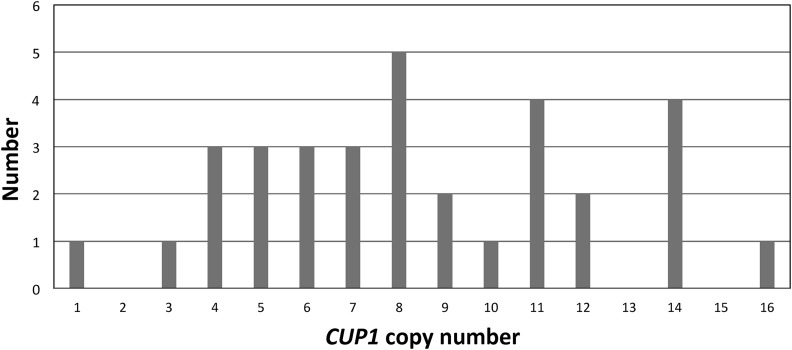
In most of the models shown in Figure 2 and Figure 3, one would expect loss of one or more CUP1 repeats in the 5-FOAR HygR GenR CanS strains that have lost the URA3 gene. To determine the number of CUP1 repeats that were lost, we examined the sizes of the CUP1 clusters on the W303-1A-derived homolog in 33 independent 5-FOAR HygR GenR CanS derivatives of YZ104. Genomic DNA was digested with EcoRI (which does not cut the repeats), and Southern analysis was conducted with a probe that hybridized specifically to the 2-kb CUP1 repeats (details in Materials and Methods). These data are summarized in Figure 5. The starting CUP1 copy number is 18, and copy numbers in the resulting 5-FOAR HygR GenR CanS daughter cells range from 1 to 16. The median number of retained repeats was eight.
Figure 5.
Numbers of remaining CUP1 repeats associated with loss of URA3 on the W303-1A-derived chromosome VIII homolog of YZ104; the starting strain had 18 repeats on this homolog. We isolated 33 independent spontaneous 5-FOAR HygR GenR CanS derivatives. CUP1 copy numbers were determined by measuring the size of CUP1 cluster by Southern analysis of EcoRI-digested genomic DNA. A hybridization probe specific for the W303-1A type of CUP1 repeat was used. 5-fluoroorotic acid; Can, L-canavanine; Gen, geneticin; Hyg, hygromycin B; R, resistant; S, sensitive.
Stimulation of mitotic recombination within the CUP1 cluster by addition of copper to the growth medium
The transcription of the CUP1 genes is greatly elevated (about 20-fold) by the addition of 1 mM CuSO4 to the medium (Karin et al. 1984). To determine if copper also leads to an elevated mitotic recombination rate, we grew the YZ103 strain in medium containing 1.4 mM CuSO4. The amount of CuSO4 in synthetic medium unsupplemented with extra copper is ∼0.25 µM (http://www.sigmaaldrich.com/catalog/product/sigma/y1251?lang=en®ion=US). The rates of recombination in various genetic intervals were measured as described above. The crossover rate in the CEN8-hphMX4 interval was similar in the absence or presence of added copper (Table 2). In contrast, the rate of interhomolog crossovers in the CUP1 array was elevated ∼15-fold in high-copper medium. Similarly, the rate of intra/intersister chromatid events was elevated 23-fold by growth in high-copper medium.
Since the stimulation of mitotic recombination by copper primarily elevates recombination in the CUP1 locus and since transcription of the CUP1 repeats is induced by copper, we tested whether the recombinogenic effect of copper was dependent on CUP1 transcription. We constructed a strain that was isogenic with YZ103 (YZ117) but was also homozygous for a deletion of CUP2. The Cup2p binds upstream of CUP1 to elevate its transcription in response to high levels of copper (Buchman et al. 1989; Huibregtse et al. 1989; Szczypka and Thiele 1989; Welch et al. 1989). The cup2 diploid YZ117 was unable to grow in concentrations of copper of 0.2 mM whereas the isogenic YZ103 strain could grow in concentrations ten-fold higher. In the absence of copper, YZ117 has similar crossover rates and intra/intersister chromatid events to YZ103 (Table 2).
Since YZ117 is very sensitive to copper, to determine whether the recombinogenic effects of copper required copper-stimulated transcription of the CUP1 array, we constructed a derivative of YZ117 (YZ118) that contained a high-copy number plasmid (p426-GPD-CUP1) in which transcription of the plasmid-borne CUP1 gene was regulated by the GPD (glceraldehyde-3-phosphate dehydrogenase) promoter; details of this construction are described in Materials and Methods. The plasmid also contained a wild-type LEU2 gene. The YZ118 strain grew in medium with high levels of copper sulfate (1.4 mM) but failed to grow in medium lacking leucine with high levels of copper. Therefore, in all experiments involving YZ118, to force retention of the plasmid, all diagnostic media contained 0.2 mM copper sulfate. In the experiments summarized in Table 2, the control strain YZ103 was grown and analyzed under the same conditions as YZ118.
As shown in Table 2, in YZ118, unlike YZ103, the presence of high levels of copper has little effect on interhomolog or on intra/sister chromatid recombination in the CUP1 array. This result argues strongly that the elevated rate of CUP1 recombination induced by copper in YZ103 is a consequence of elevated levels of CUP1 transcription.
Loss of the URA3 gene from the CUP1 tandem array in YZ103 is not a consequence of unequal sister chromatid exchange
As shown in Figure 2, although most of the events resulting in loss of URA3 in 5-FOAR HygR GenR CanS derivatives also lead to loss of CUP1 repeats in one daughter cell, the models differ in predictions about repeats in the other daughter cell. More specifically, if URA3 loss is a consequence of unequal sister chromatid crossovers, the Ura+ daughter cells would be expected to have additional CUP1 repeats and two copies of URA3 within one array (Figure 2B). In addition, “pop-out” recombination might produce a Ura+ daughter cell with one URA3 gene within the CUP1 array and one plasmid-borne URA3 gene (Figure 2A). Such plasmids would be expected to be capable of autonomous replication because the CUP1 repeats of W303-1A contain ARS elements (Zhao et al. 2014). For most of the other models shown in Figure 2 and Figure 3, in the Ura+ sector, we would expect only one copy of the URA3 gene in the array and no change in the number of CUP1 repeats per array.
To determine copy number for the URA3 and CUP1 genes in the Ura+ daughter cell that was produced in the same cell division as the Ura− cell, we used the haploid strain YZ18-10, one of the parental strains of YZ103. This strain has URA3 integrated into the middle of the CUP1 array, and has the hphMX4 and kanMX4/CAN1 genes flanking the array. The strain was grown overnight in medium lacking copper, and then grown for 6 hr in medium with 1.4 mM copper sulfate to induce recombination. The cells were then plated on rich growth medium and allowed to form colonies. The colonies were replica-plated to medium lacking uracil. About 1% of the colonies had approximately equal-sized Ura+/Ura− sectors; in such colonies, the Ura+ sector should represent the daughter cell produced in the same event as the Ura− daughter.
Genomic DNA derived from the Ura+ was isolated from 17 independent sectored colonies. The DNA was treated with HindIII, a restriction enzyme that cuts within the URA3 gene and sequences that flank the array, but does not cut within the CUP1 repeat. The resulting fragments were examined by Southern analysis using CUP1 sequences as the hybridization probe. If the tandem array in the Ura+ had two nontandem URA3 genes, as expected for an unequal crossover event, we would expect to detect three hybridizing bands. All samples had only two, excluding a 6.4-kb HindIII fragment reflecting nonspecific hybridization to the ribosomal RNA (rRNA) repeats (Figure S2 in File S1). This observation indicates that loss of URA3 is not a consequence of unequal sister-strand crossing over.
If loss of URA3 was a consequence of “pop-out” recombination, we would expect that the Ura+ sector might contain a plasmid containing CUP1 sequences and the URA3 gene. Cells with such plasmids would also produce three CUP1-hybridizing DNA fragments. To determine whether such a plasmid would be stable, we constructed a derivative of the URA3-containing YIp5 integrating vector that had one CUP1 repeat (YIp5-CUP1). By Southern analysis of 17 independent derivatives transformed with this plasmid, we showed that YIp5-CUP1 was not maintained as an extrachromosomal molecule, but was integrated into the CUP1 locus (details in Materials and Methods). Although this observation suggests that the plasmid produced by “pop-out” recombination could have reintegrated into the CUP1 array of the Ura+ cells, our observation that the Ura+ sector has only two CUP1-hybridizing DNA fragments rules out this possibility. Therefore, either loss of URA3 does not occur by “pop-out” recombination or the plasmid product is lost very quickly from the cells of the Ura+ sector.
Analysis of the genetic regulation of mitotic recombination within the CUP1 array
There are a large number of proteins required for wild-type levels of mitotic recombination in yeast (Symington et al. 2014). We constructed diploids that were isogenic with YZ103 except for homozygous mutations affecting various genes involved in recombination, and determined the effect of these mutations on interhomolog and intra/sister chromatid exchange.
The diploid YZ114 lacks Rad51p, a RecA-related protein required to form a filament on single-stranded DNA required for strand invasion. Rad51p is required for interhomolog recombination in most assays (Paques and Haber 1999; Symington et al. 2014). As expected, the rad51 mutation greatly (>100-fold) reduced the frequency of crossovers in the CEN8-hphMX4 interval (Table 1). Interhomolog recombination within the CUP1 cluster and intra/sister chromatid events were much less affected with reductions of four- to eightfold compared to the wild-type strain.
The strain YZ113 was homozygous for the rad52 mutation. The Rad52 protein, which aids in the loading of Rad51p onto single-stranded DNA coated with RPA, is required for most types of HR involving nonrepeated sequences (Symington et al. 2014). Loss of Rad52p reduced the rate of interhomolog events at the CUP1 locus ∼10-fold, and had no significant effect on the rate of intra/sister chromatid events. Surprisingly, the rate of interhomolog crossovers in the CEN8-hphMX4 interval was also decreased only sixfold, considerably less than the reduction observed for the rad51/rad51 strain. As described previously, there are two ways of producing 5-FOAR HygS CanR GenS derivatives of YZ113 and related strains: a crossover in the CEN8-hphMX4 interval, and loss of the homolog containing the hphMX4, URA3, and CAN1-kanMX4 markers. Since rad52/rad52 diploids have a very high rate of chromosome loss (Song and Petes 2012), it is possible that the many of the 5-FOAR HygS CanR GenS derivatives of YZ113 are a consequence of chromosome loss rather than mitotic recombination. We examined this possibility by a comparative genome hybridization experiment (details in Materials and Methods). Of eight independent 5-FOAR HygS CanR GenS derivatives examined, none had lost the homolog with the hphMX4, URA3, and CAN1-kanMX4 markers. Thus, we conclude that the rad52 mutation has a relatively modest effect on the rate of recombination in the CEN8-hphMX4 interval. Loss of Rad52 reduced the rate of interhomolog recombination within the CUP1 cluster ∼10- to 20-fold, but reduced intra/sister chromatid recombination only twofold (Table 1).
We examined recombination in the homozygous rad1/rad1 strain YZ116. The Rad1 endonuclease, in addition to its role in nucleotide excision repair (Tomkinson et al. 1993), is involved in processing the single-stranded branches that are intermediates in the SSA pathway of HR (Ivanov et al. 1996). Loss of Rad1 had no effect on interhomolog recombination within the CUP1 array, and resulted in a small (twofold) increase in recombination between CEN8 and the hphMX4 marker. Intra/sister chromatid recombination was also relatively unaffected (twofold increase).
We also measured recombination rates in YZ115, an mre11/mre11 strain. Mre11p, acting in a complex with Rad50p and Xrs2p (the MRX complex), has multiple cellular roles. The complex is involved in telomere length regulation (Kironmai and Muniyappa 1997), processing of broken DNA molecules (Mimitou and Symington 2009), and promoting postreplicative cohesion assembly (Unal et al. 2004). In assays of interhomolog mitotic recombination, mutants lacking any of the MRX proteins are hyper-Rec, and it has been suggested that the absence of damage-induced cohesin might reduce the efficiency of sister chromatid recombination, thereby elevating the frequency of interhomolog recombination [reviewed by Symington et al. (2014)]. We found an approximately fivefold elevation in interhomolog events, both in the CEN8-hphMX4 interval and within the CUP1 array (Table 1). In contrast, the rate of intra/sister chromatid events elevated only slightly (1.3-fold).
Lastly, we measured the rates of recombination in strains homozygous for rad5 (HB9) or mms2 (HB13) mutations. The Rad5 and Mms2 proteins have been implicated in error-free bypass of DNA damage, although Rad5 may also have a role in translesion synthesis (Boiteux and Jinks-Robertson 2013). Since error-free bypass involves a template switch to the sister chromatid, one might expect that mms2 and rad5 mutants would have a reduced rate of sister chromatid exchange and an elevated rate of interhomolog recombination. As expected, interhomolog exchange is elevated in both mutants by about fivefold, but sister chromatid exchange is not substantially reduced (Table 1).
In summary, both the interhomolog and intra/sister chromatid recombination events within the CUP1 cluster are unusual relative to most HR events involving nonrepeated genes. More specifically, although some reduction in recombination rates is observed in the rad51 and rad52 strains, the degree of this reduction is small. In contrast, interhomolog recombination in the CEN8-hphMX4 interval is strongly reduced in the rad51 strain, as expected from previous studies. We will discuss these results as well as our interpretation of the results obtained in rad1, mre11, rad5, and mms2 strains in detail below.
Meiotic recombination in the CUP1 array
We examined meiotic recombination within the CUP1 array in the diploid MD692 (isogenic with YZ103 except for being heterozygous for the centromere-linked trp1 marker). In the hphMX4-CAN1/kanMX4 interval (the markers that span the CUP1 locus), we observed 355 parental ditype, one nonparental ditype, and 44 tetratype tetrads. Applying the standard mapping equation (Perkins 1949) to these data, we calculate that the CUP1 locus is ∼6 cM (Table 3). Based on the physical length of the yeast genome [excluding ribosomal DNA (rDNA)] and the number of crossovers per meiosis (Mancera et al. 2008), we calculate that the genomic average is ∼2.7 kb/cM. Since the CUP1 locus is 30 kb in MD692, the expected map distance for the CUP1 locus is ∼11 cM. Thus, there is significant (P < 0.001, χ2 test) suppression of meiotic recombination within the CUP1 locus. These results are consistent with the low frequency of Spo11-induced DSBs at the CUP1 locus (Pan et al. 2011), although a direct comparison is difficult because the strain used for mapping (SK1) contains only one copy of CUP1.
Table 3. Meiotic recombination rates in the wild-type diploid strain MD692 sporulated under various conditions.
| Sporulation condition | Numbers of PD, NPD, and TT tetrads for the hphMX4-kanMX4/CAN1 interval | Total # of tetrads | hphMX4-kanMX4/CAN1 distance (cM) | % URA3 gene conversion events (#/total)a | |||
|---|---|---|---|---|---|---|---|
| # PD | # NPD | # TT | |||||
| Standard medium | 355 | 1 | 44 | 400 | 6 | 1.3 (5/400) | |
| Standard medium + 1.4 mM copper sulfate | 214 | 0 | 20 | 234 | 4.2 | 0.4 (1/234) | |
| Standard medium + 5 mM nicotinamide | 377 | 1 | 60 | 438 | 7.5 | 0.2 (1/438) | |
By tetrad analysis, we determined the meiotic distance between the hphMX4 and the kanMX4/CAN1 markers that flank the CUP1 cluster. Cells were sporulated in standard medium, standard medium with 1.4 mM copper sulfate, or standard medium with 5 mM nicotinamide. The crossover and conversion frequencies were not significantly affected by copper or nicotinamide. #, number; PD, parental ditype; NPD, nonparental ditype; TT, tetratype tetrads.
Of the five events observed in the strain sporulated under standard conditions, four were 1+:3− events and one was a 3+:1− event. Of the conversion events in the strain sporulated in the presence of copper or nicotinamide, both were 1+:3−.
Because copper induces mitotic recombination, we also sporulated MD692 in medium containing 1.4 mM copper sulfate to determine whether there was a similar stimulation of meiotic exchange. No significant increase in crossovers was observed (P > 0.05; χ2 test; Table 3). Meiotic recombination within the tandemly repeated rRNA gene cluster is strongly suppressed (Petes and Botstein 1977), and this suppression is dependent on the Sir2p histone deacetylase (Gottlieb and Esposito 1989). Nicotinamide, a negative regulator of Sir2p, also relieves the suppression of meiotic recombination in the rDNA (Bitterman et al. 2002). Consequently, we sporulated MD692 in medium containing 5 mM nicotinamide. The crossover frequency was not significantly elevated relative to that observed for MD692 sporulated under standard conditions (P > 0.05; χ2 test; Table 3).
In addition to crossovers in the CUP1 array, we found a small number of tetrads in which the URA3 marker showed non-Mendelian segregation (Table 3). These events, which could reflect conversions involving sister chromatids or homologs, or unequal sister chromatid crossovers, were not significantly different for any of the sporulation conditions examined.
In summary, our analysis indicates that the low level of meiotic recombination observed in the CUP1 array is not a consequence of the same silencing mechanism that is operative in the rRNA gene cluster, the telomeres, or the silent mating type cassettes. The lack of meiotic exchange at the CUP1 locus may reflect a novel mechanism of silencing or simply a lack of chromatin structural motifs associated with meiotic recombination hotspots. A number of studies have shown that recombination hotspots in yeast are associated with particular chromatin structures, although the nature of these structures is incompletely understood (Petes 2001; Lam and Keeney 2014).
Discussion
Our current study reveals several important features of mitotic and meiotic recombination of tandemly-repeated genes including: (1) for spontaneous mitotic recombination events involving the CUP1 locus, intra/sister chromatid events occur much more frequently than interhomolog events; (2) unequal sister chromatid recombination within the CUP1 array does not contribute substantially to loss of inserted markers; (3) interhomolog CUP1 mitotic recombination is elevated relative to average genomic intervals; (4) both interhomolog and sister chromatid recombination within the CUP1 array is strongly induced by high levels of CUP1 transcription; and (5) in contrast to mitotic recombination, meiotic recombination within the CUP1 array occurs at relatively low levels.
Relative frequency of interhomolog and intra/sister chromatid events
There are three methods that have been used to measure intra/sister chromatid recombination events in yeast: recombination between two tandem heteroallelic genes (usually done in haploid strains) (Jackson and Fink 1981), loss of a marker located within a tandem array of repeats (Petes 1980; Szostak and Wu 1980), and formation of a dimeric circle from a monomeric circular chromosome or plasmid (Game et al. 1989; Gonzalez-Barrera et al. 2003). The most direct comparison of interhomolog and intra/sister chromatid events was done by Kadyk and Hartwell (1992) in a diploid with heteroalleles on different homologs to monitor interhomolog exchange and different tandem heteroalleles to monitor intra/sister chromatid recombination. Our system has the advantage of measuring both types of recombination at the same genetic locus. Using X-rays to stimulate recombination, Kadyk and Hartwell concluded that intra/sister chromatids were the preferred substrate (relative to the homolog) for DSBs generated in G2 recombination by a factor of ∼20. Our finding that spontaneous mitotic intra/sister chromatid events are about 10–20-fold more frequent than interhomolog events at the CUP1 locus is in good agreement with this conclusion. Since our assay detects only those intra/sister chromatid events that are associated with loss of the URA3 gene, our measurement is an underestimate of the true rate of intra/sister chromatid exchanges.
Mechanism of intra/sister chromatid events
The loss of the URA3 gene within the CUP1 array was not usually a consequence of interhomolog recombination. Figure 2 and Figure 3 show some of the mechanisms that could result in loss of URA3 by intrachromatid or sister chromatid interactions. In studies in which a selectable marker was integrated in the rRNA genes, loss of the marker was shown to occur by both unequal crossing over (Szostak and Wu 1980; Zamb and Petes 1981) and gene conversion between sister chromatids (Gangloff et al. 1996). Since marker loss from the rDNA is independent of Rad52 (Zamb and Petes 1981; Ozenberger and Roeder 1991), it was argued that marker loss sometimes reflected SSA rather than “classic” crossovers or gene conversions (Ozenberger and Roeder 1991). In addition, since extrachromosomal plasmids containing rRNA genes are observed in some yeast strains (Larionov et al. 1980), “pop-out” recombination also occurs.
Similar to the rRNA genes, marker loss from the CUP1 array is likely to occur through >1 mechanism. However, from our analysis of the Ura+ sector of Ura−/Ura+ sectored colonies, we argue that reciprocal unequal crossover events (Figure 2B) are not common modes of marker loss, since we failed to detect the expected products of this class. This observation has the caveat that we examined sectored colonies generated by the hyper-Rec condition of high copper in the medium. We can also rule out interhomolog gene conversion unassociated with crossovers (Figure 2D) as a common mechanism for marker loss. As described above, none of 42 independent derivatives that lost URA3 had undergone gene conversion with the other homolog.
A model that we cannot exclude, but that appears unlikely, is DNA polymerase template switching (Figure 3C). In this mechanism, during DNA replication, the replicating strand dissociates from one repeat and reassociates with another. Although such events have been observed in Escherichia coli as RecA-independent interactions between repeats, these exchanges usually involve short (<200 bp) repeats (Lovett et al. 2002); above that threshold, RecA-dependent exchanges predominate. Second, deletions formed by template switching might be expected to involve preferentially adjacent or close repeats. The median number of repeats associated with loss of the URA3 marker was eight. Lastly, as discussed below, high levels of copper in the medium elevate interhomolog recombination and intra/sister chromatid events to approximately the same extent. If these types of events proceed by fundamentally different mechanisms, this congruence is surprising.
Although we cannot rule out the “pop-out” model shown in Figure 2A, we do not favor this model for several reasons. First, recombination events between tandemly-duplicated direct repeats produce the plasmid expected from “pop-out” recombination much less frequently than other types of recombination products (Schiestl et al. 1988). Second, extrachromosomal rRNA circles are usually monomeric (Meyerink et al. 1979), and the observed deletions in the CUP1 locus have a wide distribution centered around a loss of eight repeats (Figure 5).
The two mechanisms that fit our observations best are unequal intersister chromatid gene conversion (Figure 2C) and SSA (Figure 3, A and B). Both of these mechanisms have been invoked to explain loss of markers within the rDNA (Ozenberger and Roeder 1991; Gangloff et al. 1996). In current models of recombination, gene conversion unassociated with crossovers involves synthesis-dependent strand annealing. One of the ends resulting from a DSB invades an intact template, and the invading end primes DNA synthesis. Subsequently, this end disengages from the template and reattaches to the other broken end. If the URA3 marker is within the region of heteroduplex, the resulting single-stranded loop could be removed by cellular endonucleases; heteroduplexes that include large heterologies and the processing of large single-stranded loops have been detected during meiotic recombination in yeast (Kearney et al. 2001). An alternative possibility is that the URA3 marker is removed by double-stranded degradation of broken ends, leading to a gap. Although broken ends are usually processed by 5′–3′ processing of only one strand, degradation of both strands has been observed producing a double-stranded gap (Zierhut and Diffley 2008), and such gaps are readily repaired by HR (Orr-Weaver et al. 1981).
In general, recombination events requiring strand invasion would be expected to require the RecA-homolog Rad51p and the Rad51p-mediator/strand-exchange protein Rad52p (Symington et al. 2014). In our experiments, although loss of the Rad51 protein substantially reduced the rate of recombination in the interval between CEN8 and the CUP1 locus, intra/sister chromatid events were reduced only two- to fivefold (Table 1). One interpretation of this result is that loss of the URA3 marker occurs by SSA (discussed below) rather than intersister conversion. A complication of this interpretation is that interhomolog recombination within the CUP1 locus is also reduced by only 5- to 10-fold in the rad51 and rad52 strains, and such events are not explicable by SSA. It is possible that the absence of Rad51p or Rad52p greatly reduces the probability of a successful strand invasion, but this reduction is partly balanced by templates that contain many sites at which strand invasion can occur. More specifically, for single-copy sequences, there is only one position on an intact template molecule that has homology to a broken end. However, In the CUP1 array, each repeat in the intact template has homology to the DNA ends generated by DSBs in the CUP1 array.
The second plausible model consistent with our results is SSA (Figure 3, A and B). Most studies of SSA are performed in strains in which direct repeats of heteroallelic genes flank an intervening marker. Often, researchers select cells in which gene conversion produces a wild-type allele from the heteroalleles, and the presence or absence of the intervening marker is scored. In most such experiments, gene conversion events are reduced in rad51 strains, whereas deletions of the intervening marker occur at near wild-type levels (Prado et al. 2003; Symington et al. 2014). The frequency of deletion events involving direct repeats in rad52 strains relative to the wild-type frequency varies from 1% (1-kb repeats) to nearly 100% (>10 kb) (Paques and Haber 1999). In a previous study of HO-induced DSBs within the CUP1 locus, Ozenberger and Roeder (1991) found efficient Rad52p-independent repair. Thus, our observation that marker loss is relatively unaffected by rad51 or rad52 mutations is roughly in agreement with previous studies of SSA.
“Classical” SSA usually requires the Rad1p for efficient removal of the single-stranded branches generated when heteroduplexes are formed between two repeats that contain intervening heterology (Sugawara et al. 1997), although exceptions have been observed (McDonald and Rothstein 1994; Nag et al. 2005). In our study, loss of the URA3 marker was not substantially affected by the rad1 mutation (Table 1). Depending on the extent of processing of the broken ends and how the broken ends reanneal, SSA at the CUP1 locus may be associated with single-stranded loops (Figure 3A) or with single-stranded flaps. Although the role of Rad1p in the removal of flaps has been demonstrated, the removal of loops by Rad1p has not been examined.
Although it is possible that the URA3 marker is excised from the intermediates shown in Figure 3 by cellular endonucleases, two other mechanisms could be responsible for marker loss. First, replication of the intermediates shown in Figure 3 without removal of the loops would result in loss of URA3 and CUP1 repeats from one of the resulting DNA molecules. Second, as discussed above, double-stranded degradation of the broken molecules could result in loss of URA3 and CUP1 repeats unassociated with formation of single-stranded loops or branches.
By either the intersister chromatid conversion or SSA models, to have deletions involving large numbers of repeats, the broken DNA ends would have to be extensively processed by excising one or both strands of the broken ends. In HO-induced recombination in the CUP1 array, Ozenberger and Roeder (1991) found a similar distribution of deletions to one observed in our study (Figure 5). Previously, we showed that some interhomolog mitotic gene conversions that include a heterozygous Ty element were very long with a median size of >50 kb (Yim et al. 2014). Thus, the yeast cell has the capacity to generate conversion events and single-stranded annealing events that are large enough to explain our deletions.
The mre11 mutation elevated interhomolog recombination and had no substantial effect on intra/sister chromatid exchanges. Since Mre11p is required for DNA damage-induced sister chromatid cohesion (Unal et al. 2004), it is possible that mre11 mutants channel the repair of DNA damage from the sister chromatid to the homolog. By this model, we expect to observe reduced sister chromatid exchange in addition to elevated interhomolog recombination. Although no such reduction was detectable in our experiments, even a relatively small change in intra/sister chromatid recombination (which is much more frequent than interhomolog exchange) could significantly elevate interhomolog recombination. Alternatively, the mre11 mutants may have an elevated level of DSBs in addition to altering the ratio of interhomolog to intra/sister chromatid recombination in favor of interhomolog exchange.
The mms2 and rad5 mutations had effects that were qualitatively similar to the mre11 mutation: elevated recombination between homologs with little effect on intra/sister chromatid recombination. The ratio of intra/sister chromatid CUP1 exchanges to interhomolog CUP1 exchanges was reduced from the wild-type ratio of 10 –20 to ∼2 in mms2 and rad5 strains. This observation is consistent with a redirection of recombination events to the homolog from the sister, as expected if Mms2 and Rad5 are involved in a template switch to the sister chromatid. However, since we did not observe a reduction of intra/sister chromatid recombination, this possibility is only partially supported by the data.
Stimulation of CUP1 recombination by transcription
The phenomenon of transcription-associated recombination (TAR) was reported in yeast ∼30 years ago [Voelkel-Meiman et al. 1987; Thomas and Rothstein 1989; reviewed by Kim and Jinks-Robertson (2012) and Aguilera and Garcia-Muse (2013)]. TAR likely results from a number of different mechanisms including head-on collisions between the replication fork and the transcriptional machinery, DNA secondary structures (for example, hairpins) formed in single-stranded DNA resulting from transcription, accumulated supercoils in transcribed DNA, R-loop accumulation, damage induced on single-stranded DNA resulting from R-loop formation and/or the transcription “bubble,” and positioning of highly-transcribed genes at the nuclear pores (Aguilera and Garcia-Muse 2013). Since each CUP1 repeat has a bidirectional origin and a promoter, there will be head-on collisions between the replication and transcription proteins. However, the other models are also plausible.
An important point is that the copper-induced elevations in the levels of recombination are similar for the interhomolog CUP1 genes (15-fold) and the intra/sister chromatid CUP1 events (23-fold) (Table 2). The simplest interpretation of this result is that high levels of transcription elevate the frequency of recombinogenic DNA lesions, but do not affect the ratio of interhomolog to intra/sister chromatid events. This observation is more difficult to explain if interhomolog events are a consequence of HR, whereas intra/sister chromatid events reflect DNA polymerase slippage.
The high levels of recombination induced by copper may be evolutionarily advantageous. Growth of yeast cells in high copper selects for derivatives with longer CUP1 arrays (Fogel and Welch 1982). Since these derivatives likely arise by unequal crossovers, an elevation in the rate of recombination (hyper-Rec) will result in an increased rate of formation of these copper-resistant strains.
Meiotic recombination
In contrast to the hyper-Rec phenotype associated with mitotic recombination in the CUP1 cluster, meiotic recombination is about twofold lower than the average genomic interval. Unlike mitotic exchange, this level is unaffected by high levels of copper in the medium. Interhomolog meiotic recombination within the rRNA genes is much more strongly suppressed (>100-fold; Petes and Botstein 1977), although unequal meiotic recombination occurs frequently, in ∼10% of unselected tetrads (Petes 1980). This suppression is dependent on Sir2p (Gottlieb and Esposito 1989). Our observation that nicotinamide-containing sporulation medium does not elevate interhomolog mitotic recombination in the CUP1 array suggests a different mechanism for the modest reduction observed in our study. It is possible that the CUP1 sequences are simply not a good substrate for Spo11p-mediated DSB formation.
In our analysis, we observed 10 1+:3− and one 3+:1− segregation events for the URA3 marker in a total of 1072 tetrads. This frequency of ∼1% is consistent with the low level of meiotic crossovers. In contrast, in a study in which meiotic recombination events in the CUP1 array were detected by Southern analysis of the length of the array, Welch et al. (1990, 1991) found that 10–20% of the tetrads contained one or more spores with an altered CUP1 array. In most of these events, only a single spore of the four had an altered array, indicating that the alteration likely reflected SSA or sister chromatid gene conversion. This result argues that our analysis detects only a small fraction of the meiotic intra/sister chromatid events, although our study should detect all of the interhomolog exchanges. In summary, the CUP1 array has a twofold reduced rate of interhomolog exchange but a higher proportion of sister-strand recombination than observed for most meiotic events for which there is a strong interhomolog bias (Hunter 2015).
Summary
We described a diploid strain that can be used to monitor interhomolog and intra/sister chromatid recombination events at the CUP1 locus, and demonstrated that spontaneous intra/sister chromatid events are at least 10-fold more frequent than interhomolog events. The rates of both types of recombination are elevated by high concentrations of copper in the medium, and this effect is dependent on the Cup2 transcription factor. In contrast to mitotic recombination, meiotic recombination is suppressed at the CUP1 locus and is unaffected by high concentrations of copper.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.201285/-/DC1.
Acknowledgments
We thank S. Jinks-Robertson, L. Symington, and D. Thiel for suggestions. The research was supported by National Institutes of Health grants GM24110, GM52319, and R35-GM118020 to T.D.P., Y.Z., and A.P., who were also supported by a grant from the Army Research Office (SPS #200531).
Footnotes
Communicating editor: J. A. Nickoloff
Literature Cited
- Aguilera A., Garcia-Muse T., 2013. Causes of genome instability. Annu. Rev. Genet. 47: 1–32. [DOI] [PubMed] [Google Scholar]
- Arbel A., Zenvirth D., Simchen G., 1999. Sister chromatid-based DNA repair is mediated by RAD54, not by DMC1 or TID1. EMBO J. 18: 2648–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y., Liefshitz B., Kupiec M., 2004. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 23: 4868–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman K. J., Anderson R. M., Cohen H. Y., Latorre-Esteves M., Sinclair D. A., 2002. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 277: 45099–45107. [DOI] [PubMed] [Google Scholar]
- Boiteux S., Jinks-Robertson S., 2013. DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae. Genetics 193: 1025–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman C., Skroch P., Welch J., Fogel S., Karin M., 1989. The CUP2 gene product, regulator of yeast metallothionein expression, is a copper-activated DNA-binding protein. Mol. Cell. Biol. 9: 4091–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S. R., Dietrich F. S., Fisk D. G., Binkley G., Balakrishnan R., et al. , 2014. The reference genome sequence of Saccharomyces cerevisiae: then and now. G3 4: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., 1978. Evidence that spontaneous mitotic recombination occurs at the two-strand stage. Proc. Natl. Acad. Sci. USA 75: 4436–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre F., Boulet A., Roman H., 1984. Gene conversion at different points in the mitotic cycle of Saccharomyces cerevisiae. Mol. Gen. Genet. 195: 139–143. [DOI] [PubMed] [Google Scholar]
- Fasullo M. T., Davis R. W., 1987. Recombinational substrates designed to study recombination between unique and repetitive sequences in vivo. Proc. Natl. Acad. Sci. USA 84: 6215–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S., Welch J. W., 1982. Tandem gene amplification mediates copper resistance in yeast. Proc. Natl. Acad. Sci. USA 79: 5342–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game J. C., Sitney K. C., Cook V. E., Mortimer R. K., 1989. Use of a ring chromosome and pulsed-field gels to study interhomolog recombination, double-strand DNA breaks and sister-chromatid exchange in yeast. Genetics 123: 695–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff S., Zou H., Rothstein R., 1996. Gene conversion plays the major role in controlling the stability of large tandem repeats in yeast. EMBO J. 15: 1715–1725. [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1543. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barrera S., Cortes-Ledesma F., Wellinger R. E., Aguilera A., 2003. Equal sister chromatid exchange is a major mechanism of double-strand break repair in yeast. Mol. Cell 11: 1661–1671. [DOI] [PubMed] [Google Scholar]
- Gottlieb S., Esposito R. E., 1989. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56: 771–776. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Fink G. R., 1991. Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego. [Google Scholar]
- Helleday T., 2003. Pathways for mitotic homologous recombination in mammalian cells. Mutat. Res. 532: 103–115. [DOI] [PubMed] [Google Scholar]
- Herskowitz I., Jensen R. E., 1991. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 194: 132–146. [DOI] [PubMed] [Google Scholar]
- Huibregtse J. M., Engelke D. R., Thiele D. J., 1989. Copper-induced binding of cellular factors to yeast metallothionein upstream activation sequences. Proc. Natl. Acad. Sci. USA 86: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N., 2015. Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 7: a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov E. L., Sugawara N., Fishman-Lobell J., Haber J. E., 1996. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics 142: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R., 1981. Gene conversion between duplicated genetic elements in yeast. Nature 292: 306–311. [DOI] [PubMed] [Google Scholar]
- Kadyk L. C., Hartwell L. H., 1992. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics 132: 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Najarian R., Haslinger A., Valenzuela P., Welch J., et al. , 1984. Primary structure and transcription of an amplified genetic locus: the CUP1 locus of yeast. Proc. Natl. Acad. Sci. USA 81: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney H. M., Kirkpatrick D. T., Gerton J. L., Petes T. D., 2001. Meiotic recombination involving heterozygous large insertions in Saccharomyces cerevisiae: formation and repair of large unpaired DNA loops. Genetics 158: 1457–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N., Jinks-Robertson S., 2012. Transcription as a source of genome instability. Nat. Rev. Genet. 13: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kironmai K. M., Muniyappa K., 1997. Alteration of telomeric sequences and senescence caused by mutations in RAD50 of Saccharomyces cerevisiae. Genes Cells 2: 443–455. [DOI] [PubMed] [Google Scholar]
- Lam I., Keeney S., 2014. Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb. Perspect. Biol. 7: a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larionov V. L., Grishin A. V., Smirnov M. N., 1980. 3 μm DNA — an extrachromosomal ribosomal DNA in the yeast Saccharomyces cerevisiae. Gene 12: 41–49. [DOI] [PubMed] [Google Scholar]
- Lea D. E., Coulson C. A., 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49: 264–285. [DOI] [PubMed] [Google Scholar]
- Lee P. S., Greenwell P. W., Dominska M., Gawel M., Hamilton M., et al. , 2009. A fine-structure map of spontaneous mitotic crossovers in the yeast Saccharomyces cerevisiae. PLoS Genet. 5: e1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F., Han M., Romanienko P. J., Jasin M., 1998. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc. Natl. Acad. Sci. USA 95: 5172–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M., Rothstein R., Mortensen U. H., 2001. Rad52 forms DNA repair and recombination centers during S phase. Proc. Natl. Acad. Sci. USA 98: 8276–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett S. T., Hurley R. L., Sutera V. A., Jr., Aubuchon R. H., Lebedeva M. A., 2002. Crossing over between regions of limited homology in Escherichia coli. RecA-dependent and RecA-independent pathways. Genetics 160: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancera E., Bourgon R., Brozzi A., Huber W., Steinmetz L. M., 2008. High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 454: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley J. L., Petes T. D., 2010. Chromosome rearrangements and aneuploidy in yeast strains lacking both Tel1p and Mec1p reflect deficiencies in two different mechanisms. Proc. Natl. Acad. Sci. USA 107: 11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. P., Rothstein R., 1994. Unrepaired heteroduplex DNA in Saccharomyces cerevisiae is decreased in RAD1 RAD52-independent recombination. Genetics 137: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerink J. H., Klootwijk J., Planta R. J., van der Ende A., van Bruggen E. F., 1979. Extrachromosomal circular ribosomal DNA in the yeast Saccharomyces carlsbergensis. Nucleic Acids Res. 7: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou E. P., Symington L. S., 2009. DNA end resection: many nucleases make light work. DNA Repair (Amst.) 8: 983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozlin A. M., Fung C. W., Symington L. S., 2008. Role of the Saccharomyces cerevisiae Rad51 paralogs in sister chromatid recombination. Genetics 178: 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D., Muller R., Funk M., 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156: 119–122. [DOI] [PubMed] [Google Scholar]
- Nag D. K., Fasullo M., Dong Z., Tronnes A., 2005. Inverted repeat-stimulated sister-chromatid exchange events are RAD1-independent but reduced in a msh2 mutant. Nucleic Acids Res. 33: 5243–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J., 1981. Yeast transformation: a model system for the study of recombination. Proc. Natl. Acad. Sci. USA 78: 6354–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozenberger B. A., Roeder G. S., 1991. A unique pathway of double-strand break repair operates in tandemly repeated genes. Mol. Cell. Biol. 11: 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Sasaki M., Kniewel R., Murakami H., Blitzblau H. G., et al. , 2011. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell 144: 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques F., Haber J. E., 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63: 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D., 1949. Biochemical mutants in the smut fungus Ustilago maydis. Genetics 34: 607–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., 1980. Unequal meiotic recombination within tandem arrays of yeast ribosomal DNA genes. Cell 19: 765–774. [DOI] [PubMed] [Google Scholar]
- Petes T. D., 2001. Meiotic recombination hot spots and cold spots. Nat. Rev. Genet. 2: 360–369. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Botstein D., 1977. Simple Mendelian inheritance of the reiterated ribosomal DNA of yeast. Proc. Natl. Acad. Sci. USA 74: 5091–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F., Cortes-Lesesma F., Huertas P., Aguilera A., 2003. Mitotic recombination in Saccharomyces cerevisiae. Curr. Genet. 42: 185–198. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Martin P., 1976. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol. Gen. Genet. 143: 119–129. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Igarashi S., Hastings P. J., 1988. Analysis of the mechanism for reversion of a disrupted gene. Genetics 119: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Petes T. D., 2012. Haploidization in Saccharomyces cerevisiae induced by a deficiency in homologous recombination. Genetics 191: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Charles J., Petes T. D., 2013. High-resolution mapping of spontaneous mitotic recombination hotspots on the 1.1 Mb left arm of yeast chromosome IV. PLoS Genet. 9: e1003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Ma H., Botstein D., 1990. Manipulating yeast genome using plasmid vectors. Methods Enzymol. 185: 289–297. [DOI] [PubMed] [Google Scholar]
- Strope P. K., Skelly D. A., Kozmin S. G., Mahadevan G., Stone E. A., et al. , 2015. The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res. 25: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N., Paques F., Colaiacovo M., Haber J. E., 1997. Role of Saccharomyces cerevisiae Msh2 and Msh3 repair proteins in double-strand break-induced recombination. Proc. Natl. Acad. Sci. USA 94: 9214–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington L. S., Rothstein R., Lisby M., 2014. Mechanisms and regulation of mitotic recombination in Saccharomyces cerevisiae. Genetics 198: 795–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka M. S., Thiele D. J., 1989. A cysteine-rich nuclear protein activates yeast metallothionein gene transcription. Mol. Cell. Biol. 9: 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Wu R., 1980. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature 284: 426–430. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R., 1989. Elevated recombination rates in transcriptionally active DNA. Cell 56: 619–630. [DOI] [PubMed] [Google Scholar]
- Tomkinson A. E., Bardwell A. J., Bardwell L., Tappe N. J., Friedberg E. C., 1993. Yeast DNA repair and recombination proteins Rad1 and Rad10 constitute a single-stranded-DNA endonuclease. Nature 362: 860–862. [DOI] [PubMed] [Google Scholar]
- Unal E., Arbel-Eden A., Sattler U., Shroff R., Lichten M., et al. , 2004. DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Mol. Cell 16: 991–1002. [DOI] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S., 1987. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell 48: 1071–1079. [DOI] [PubMed] [Google Scholar]
- Wach A., Brachat A., Pohlmann R., Philippsen P., 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10: 1793–1808. [DOI] [PubMed] [Google Scholar]
- Wei W., McCusker J. H., Hyman R. W., Jones T., Ning Y., et al. , 2007. Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc. Natl. Acad. Sci. USA 104: 12825–12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch J., Fogel S., Buchman C., Karin M., 1989. The CUP2 gene product regulates the expression of the CUP1 gene, coding for yeast metallothionein. EMBO J. 8: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch J. W., Maloney D. H., Fogel S., 1990. Unequal crossing-over and gene conversion at the amplified CUP1 locus of yeast. Mol. Gen. Genet. 222: 304–310. [DOI] [PubMed] [Google Scholar]
- Welch J. W., Maloney D. H., Fogel S., 1991. Gene conversions within the Cup1r region from heterologous crosses in Saccharomyces cerevisiae. Mol. Gen. Genet. 229: 261–266. [DOI] [PubMed] [Google Scholar]
- Yim E., O’Connell K. E., St. Charles J., Petes T. D., 2014. High-resolution mapping of two types of spontaneous mitotic gene conversion events in Saccharomyces cerevisiae. Genetics 198: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Petes T. D., 2014. The role of Exo1p exonuclease in DNA end resection to generate gene conversion tracts in Saccharomyces cerevisiae. Genetics 197: 1097–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamb T. J., Petes T. D., 1981. Unequal sister-strand recombination within yeast ribosomal DNA does not require the RAD52 gene product. Curr. Genet. 3: 125–132. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Strope P. K., Kozmin S. G., McCusker J. H., Dietrich F. S., et al. , 2014. Structures of naturally-evolved CUP1 tandem arrays in yeast indicate that these arrays are generated by unequal nonhomologous recombination. G3 4: 2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierhut C., Diffley J. F., 2008. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 27: 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All strains and plasmids constructed for this study are available upon request. The genotypes and constructions for these strains are in Table S1 in File S1, and sequences of the primers used in strain construction or analysis are in Table S2 in File S1. The plasmids used in the study are described in Table S3 in File S1.