Abstract
Trastuzumab improves clinical outcomes in patients with human epidermal growth factor receptor-2-positive breast cancer. However, cardiotoxicity is a potentially important concern, and the long-term cardiac effects of trastuzumab therapy remain unclear. Although reduction of cardiac function by trastuzumab is mostly reversible, some patients, especially those with cardiac risk factors, may rarely experience chronic heart failure or prolonged left ventricular ejection fraction reduction. There have been no detailed published analyses of patients with such unfavorable clinical courses. We report the rare case of a metastatic breast cancer in a woman without cardiac risk factors who experienced long-term irreversible cardiotoxicity after discontinuation of trastuzumab therapy.
Keywords: breast cancer, trastuzumab, cardiotoxicity
INTRODUCTION
Trastuzumab therapy has led to meaningful improvements in clinical outcomes of patients with early-stage and metastatic breast cancer overexpressing human epidermal growth factor receptor-2 (HER2) [1, 2]. Trastuzumab is generally well tolerated, but it is associated with a potentially important clinical concern regarding cardiac dysfunction. Trastuzumab has been associated with Type II chemotherapy-related cardiac dysfunction, as opposed to cardiotoxicity related to anthracyclines (Type I chemotherapy-related cardiac dysfunction) [3]. Several factors such as previous anthracycline therapy, medication for hypertension, radiation treatment to the left chest, lower baseline left ventricular ejection fraction (LVEF) and older patient age are known to increase the risk of trastuzumab-induced cardiotoxicity [4–6]. Although the main cardiotoxicity of trastuzumab is only an asymptomatic decline in LVEF and is mostly reversible with interruption of trastuzumab or cardiac medication, some patients, especially those with cardiac risk factors, may rarely experience chronic heart failure or prolonged LVEF reduction. In addition, the long-term cardiac effects of trastuzumab therapy for such patients remain unclear, and there have been no detailed analyses of patients with such unfavorable clinical courses with prolonged cardiotoxicities resulting from treatment with trastuzumab.
Here, we present the rare case of a metastatic breast cancer in a postmenopausal woman without cardiac risk factors who experienced long-term irreversible trastuzumab cardiotoxicity despite discontinuation of therapy.
CASE REPORT
In August 2012, a 52-year-old woman presented to the Department of Breast Surgery at Osaka-Minami Medical Center with a complaint of a 1-year history of a left breast mass with bleeding tendency. The patient was in good health, having no comorbidities and taking no medications. On physical examination, an 8-cm hemorrhagic mass with skin invasion was noted in the upper inner quadrant of the breast. Several swollen lymph nodes were also palpable in the left axillary and supraclavicular regions. Tumor biopsy revealed invasive ductal carcinoma. The tumor was found to be estrogen receptor-positive (90%), progesterone receptor-negative (0%) and HER2-positive (3+) by immunohistochemical examination. Chest and abdominal computed tomography showed multiple lymph node metastases between the left axillary and periclavicular regions, as well as solitary liver metastasis. Bone scintigraphy revealed multiple bone metastases. The cancer was staged clinically as stage IV (T4b N3c M1).
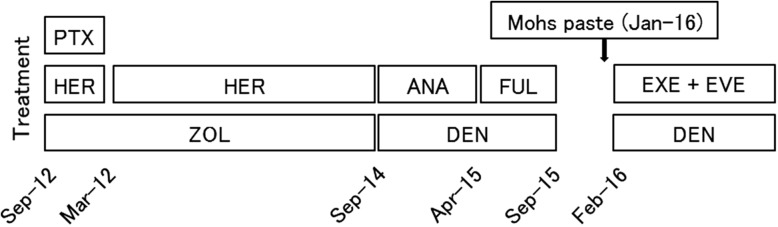
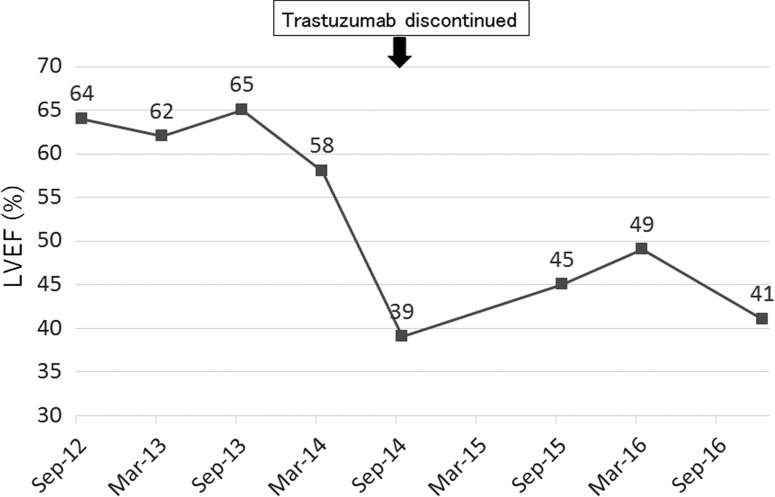
The patient was first treated with paclitaxel (90 mg/m2) plus trastuzumab (2 mg/kg) every week for 12 cycles from September to December 2012. After that, owing to grade 3 peripheral neuropathy induced by paclitaxel, she underwent treatment with trastuzumab (6 mg/kg) alone every 3 weeks until September 2014. Zoledronic acid (4 mg) every 4 weeks was also administered for bone metastasis from the beginning of therapy. These treatments were very effective, resulting in marked reduction in size of not only the breast tumor but also the metastatic lymph nodes and liver tumor. The patient’s cardiac function was evaluated by echocardiography every 6 months from the initiation of treatment. At the beginning of therapy, the LVEF as assessed by echocardiography was within normal limits (64%), but it demonstrated a decline to 39% in September 2014. Although, the patient reported no cardiac symptoms during this course of therapy, the decision was made, with reluctance, to discontinue the trastuzumab owing to cardiotoxicity. From October 2014 to April 2015, anastrozole (1 mg) as maintenance hormonal therapy plus monthly denosumab (120 mg) was administered, but the breast tumor increased in size. The patient was then treated with fulvestrant (500 mg) every 4 weeks for 5 months as second-line hormonal therapy. From October 2015, at the patient’s request, she was on a 3-month hiatus from treatment. The breast tumor alone continued to grow, with bleeding tendency, and the patient received Mohs paste therapy in January 2016. From the following month, exemestane (25 mg) plus everolimus (10 mg) was administered until December 2016, achieving stable disease. Over a period of more than 2 years after trastuzumab discontinuation, the patient’s LVEF did not recover to within normal limits. The time course of the patient’s treatment and the contrasting LVEF changes as assessed by echocardiography were summarized in Figs 1 and 2, respectively. After the initial LVEF decline, she regularly visited the cardiologist to undergo follow-up of cardiac function, without taking any medications because of the lack of cardiac symptoms.
Figure 1:
Time course of treatment. Abbreviations: PTX, paclitaxel; HER, trastuzumab; ANA, anastrozole; FUL, fulvestrant; EXE, exemestane; EVE, everolimus; ZOL, zoledronic acid; DEN, denosumab.
Figure 2:
Time course of left ventricular ejection fraction by echocardiography.
DISCUSSION
We report the rare case of a woman with metastatic breast cancer without any cardiac risk factors who experienced long-term irreversible trastuzumab cardiotoxicity despite discontinuation of the drug. To the best of our knowledge, there have been no previous detailed case reports that have described the same clinical course as that in this case.
The cardiotoxicities associated with trastuzumab were first reported in metastatic breast cancer cases, with ~22% of patients receiving trastuzumab experiencing symptomatic or asymptomatic cardiac dysfunction [1]. While the drug has significant therapeutic effect, the cardiotoxicity of trastuzumab, ranging from asymptomatic left ventricular dysfunction to congestive heart failure, has emerged as a critical concern in clinical practice [1, 2, 7]. Trastuzumab-related cardiotoxicity has been considered dose-independent and highly reversible even when the drug is continued, in contrast with anthracycline regimens [8]. Unlike the defined period of adjuvant therapy for early-stage breast cancer, the cardiac effects of prolonged trastuzumab administration in a metastatic setting have yet to be clarified, because there have been few studies, despite the increasing numbers of patients who have actually received trastuzumab for several years. In the MD Anderson Cancer Center cohort, the largest study of detailed cardiotoxicities of long-term trastuzumab therapy for metastatic breast cancer, the median cumulative time for trastuzumab administration was 21.3 months, and 49 patients (28% of the total cohort) experienced cardiac events. All but three patients experienced improved LVEF or symptoms of congestive heart failure with trastuzumab discontinuation and cardiovascular treatment. Unfortunately, details of the clinical course of the three patients who did not recover from the cardiotoxicities were not available [4].
Although several risk factors are considered to increase trastuzumab-induced cardiotoxicity [4–6], our patient did not have any significant risk factors, and pretreatment risk stratification is challenging in clinical practice. In terms of the efficacy of trastuzumab, considering the fact that an excellent anti-tumor effect was obtained during trastuzumab therapy and maintained after discontinuation of trastuzumab, except for the breast tumor, the breast cancer in this patient was suspected to be very sensitive to trastuzumab. Although there is neither a justifiable determinate pathogenesis nor clinical evidence, and no more than speculation, higher sensitivity to trastuzumab therapy might contribute to the onset and/or prolongation of the cardiotoxicity of the drug. In this case, trastuzumab was discontinued only after the LVEF decreased to 39% in September 2014. However, 6 months earlier, the LVEF had already shown a declining tendency, even though the decrease was within <10% from baseline (Fig. 2), so if trastuzumab had been withdrawn at that time, the patient’s cardiac function might have recovered earlier. As the patient had no cardiac risk factors, we evaluated cardiac function only every 6 months during trastuzumab administration, but it should be performed more frequently (e.g. every 3 months according to National Comprehensive Cancer Network (NCCN) guidelines [9]) to identify early changes in LVEF. In addition, echocardiographic strain imaging is considered to be useful for the detection of sub-clinical left ventricular dysfunction than LVEF by standard transthoracic echocardiography [10]. The clinical utility of cardiac markers such as troponin and B-type natriuretic peptide are controversial in the diagnosis or prognosis of trastuzumab-induced cardiotoxicity according to the conflicting results of recent clinical studies [11, 12].
Prophylactic administration of myocardial protective agents such as angiotensin receptor inhibitors and β-blockers, which are generally used for heart failure, may be useful for preventing cardiotoxicity associated with prolonged administration of trastuzumab. In the adjuvant setting, several clinical trials showed that these protective effects on cardiac functions had positive results in terms of maintaining LVEF at baseline levels [12, 13]. Hence, even without risk factors as in this case, for the patients with metastatic breast cancer, oncologists may consider prophylactic administration of these drugs during long-term (e.g. over 1 year) trastuzumab therapy in the collaboration with cardiologists.
In conclusion, although it is limited to a single case study, the present data can further highlight the potential irreversibility of trastuzumab-induced cardiotoxicity. Physicians must be careful to balance the benefits and harms of prolonged trastuzumab-based therapies, especially for patients with cardiovascular risk factors, such as older age and previous anthracycline exposure. Therefore, further studies with larger sample sizes will be required to provide a comprehensive understanding of cardiotoxicity associated with long-term trastuzumab administration.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
There was no funding for this report.
ETHICAL APPROVAL
No ethical approval required.
CONSENT
This report has been written with the approval of the patient. Informed written consent was obtained prior to submission.
GUARANTOR
Medical director, Satoru Tanaka.
REFERENCES
- 1. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- 2. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–72. [DOI] [PubMed] [Google Scholar]
- 3. Ewer MS1, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 2005;23:2900–2. [DOI] [PubMed] [Google Scholar]
- 4. Guarneri V, Lenihan DJ, Valero V, Durand JB, Broglio K, Hess KR, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. J Clin Oncol 2006;24:4107–15. [DOI] [PubMed] [Google Scholar]
- 5. Smith KL, Dang C, Seidman AD. Cardiac dysfunction associated with trastuzumab. Expert Opin Drug Saf 2006;5:619–29. [DOI] [PubMed] [Google Scholar]
- 6. Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007;25:3859–65. [DOI] [PubMed] [Google Scholar]
- 7. Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012;30:3792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beda M, Basso U, Ghiotto C, Monfardini S. When should trastuzumab be stopped after achieving complete response in HER2-positive metastatic breast cancer patients? Tumori 2007;93:491–2. [DOI] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Breast Cancer V.2.2016. www.nccn.org/professionals/physician_gls/pdf/breast.pdf (13 February 2017, date last accessed).
- 10. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911–39. [DOI] [PubMed] [Google Scholar]
- 11. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A, et al. Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol 2016;2:1030–7. [DOI] [PubMed] [Google Scholar]
- 13. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016;37:1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]