Abstract
Background
Temporomandibular disorder (TMD) incidences are believed to be related to parafunctional behaviors like teeth clenching.
Objectives
This pilot study aimed to (i) develop an automated clench-detection algorithm, and (ii) apply the algorithm to test for differences in nocturnal clenching in women with and without TMD.
Methods
Subjects gave informed consent to participate. Adult women were categorized using Diagnostic Criteria for TMD according to presence/absence (+/-) of both TM joint disc placement (DD) and chronic pain (P) into two groups (+DD+P, -DD-P) with 12 subjects each. Surface temporalis electromyography was recorded during oral tasks performed by subjects at two laboratory sessions. The data were used to characterize muscle activity per N of bite-force (μV/N) for each subject, develop the clench-detection algorithm and test its accuracy. Ambulatory surface temporalis electromyography was self-recorded by each subject over three nights and analyzed using the algorithm and bite-force (N) vs muscle activity μV/N calibrations. Bonferroni-adjusted homoscedastic t-tests assessed for significant between-group differences in clenching (p<0.05).
Results
Sensitivity, specificity, and accuracy of algorithm-detected laboratory clenches were all ≥96%. During self-recordings 95% of clenches had durations of <4 seconds and peak forces of <10 N in both groups. Mean clench durations were significantly longer (p=0.042) in +DD+P (1.9±0.8 seconds) than -DD-P subjects (1.4±0.4 seconds). Mean temporalis duty factors (%clench time/total recording time) were significantly larger (p=0.041) in +DD+P (0.47±0.34%) than -DD-P (0.26±0.22%) subjects.
Conclusions
Nocturnal temporalis muscle activities detected by a validated algorithm were longer per clench and recording time in +DD+P compared to -DD-P women.
Keywords: Electromyography, Masticatory muscles, Pattern recognition, Sleep bruxism, Temporomandibular joint disorders, Women
Background
Published literature suggests that the abnormal intensity and frequency of masticatory muscle activities associated with parafunctional jaw loading behaviors, such as teeth clenching and grinding, is a factor in the development of temporomandibular disorders (TMD).1-3 However, data from polysomnographic recordings of muscle behaviors in TMD and control subjects contradicts this association.4, 5 The inconsistency amongst findings may be due to differences in, and limitations of, study design, data collection and analytical methods.6-8 More specifically, previous reports characterizing parafunctional activity usually employed data collection methods of self-report questionnaires9 or ecological momentary assessments.10 Given the subjective influences of self-reporting methods,7 laboratory polysomnography (PSG) or ambulatory electromyography (EMG) may provide more objective and representative methods for assessing muscle activities associated with jaw loading behaviors.
The advantages of ambulatory EMG over PSG include greater ecological validity, lower costs, and ability to record awake- and sleep-state data. Recently published data,11 based on ambulatory EMG recordings, showed that during the day and night, subjects with and without TMD produced masticatory muscle activities predominantly at low intensities, associated with jaw loads in the range of 1-2 N. Subjects with TMD differed from control subjects by the amount of time their masticatory muscles were active at these low levels. However, the type of jaw loading behaviors which accounted for diurnal and nocturnal muscle activities were not determined.
Methods and criteria for computerized detection and characterization of the onset, duration, and intensity of jaw loading behaviors are limited.12 The most commonly reported computerized approach13 to identify onset and duration of behaviors recorded by surface EMG is a threshold-based estimation method, where one of three amplitude criteria are employed to detect events. The fixed amplitude criterion uses an a priori defined EMG threshold to identify onset and cessation of events but it does not customize the threshold for each individual, so it has been used less often. The peak amplitude or maximum voluntary contraction (MVC) criterion uses maximum recorded root-mean square (RMS) EMG values to express individual-specific EMG thresholds as percentages of MVC.2 However, this criterion is problematic given MVC is sensitive to training and visual feedback,14 and does not standardize load magnitude amongst subjects. The statistically-based amplitude criterion uses EMG recordings, first to identify resting-state baseline or background EMG activities and then expresses onset and duration of behaviors relative to specified standard deviations above resting-state data.13, 15 This criterion coupled with individual-specific EMG calibrations, such as jaw muscle activity per unit of bite-force (μV/N), enables unique characterization of a wider range of jaw loading behaviors.
The objectives of this pilot study were to: (1) record temporalis EMG from two clinically-defined diagnostic groups of women with/without (+/-) both TM joint (TMJ) disc placement (DD) and chronic pain (myalgia/arthralgia, P) in both laboratory and natural settings; (2) use laboratory recordings to develop and validate an automatic detection method for the onset and duration of sustained teeth clenching; (3) apply the automated clench-detection methodology to define nocturnal epochs of clenching behaviors in ambulatory temporalis EMG recordings; and (4) use the pilot data to determine the number of subjects required to detect diagnostic group differences in temporalis clenching behavior. Based on time-logs of each epoch, subject-specific bite force (N) vs muscle activation (μV/N) calibration data were used to estimate the magnitude (N) of the loading behavior per epoch. Statistical analyses tested the hypothesis that there were diagnostic group differences in frequency (total number, number/hour), duration (s), and intensity (N) of teeth clenching behaviors.
Methods
Subjects
This study was approved by the Institutional Review Boards of the University at Buffalo and University of Missouri-Kansas City. All participants volunteered for this pilot study. Subjects were recruited at the University at Buffalo School of Dental Medicine, and gave informed consent before enrolling. Given the higher incidence of TMD in women,16 this pilot project focused on night-time clenching behaviors in women.
Cone beam computed tomography (CBCT) and magnetic resonance (MR) images were used with Research Diagnostic Criteria for TMD by calibrated examiners to categorize subjects.17, 18 Subjects were excluded from participation if they reported a history of rheumatic diseases, presented with CBCT evidence of degenerative joint disease of the TMJ, had multiple missing teeth or large dental restorations, were pregnant, or were unable to perform the variety of tasks in the study protocol. This pilot investigation focused on equal numbers of age-matched women in two diagnostic groups: a +DD+P group containing subjects with both bilateral TMJ disc displacement and chronic pain, and a -DD-P group containing healthy control subjects without disc displacement and pain.
Laboratory EMG recording
Each subject presented for two laboratory EMG recording sessions, separated by a minimum of 3 nights during which ambulatory EMG recordings were completed. As previously described,11 at each laboratory session subjects had surface electrodes placed for bilateral EMG recording from the anterior temporalis muscles using standardized techniques. EMG outputs were amplified, filtered, and digitally recorded, along with outputs from a custom pre-calibrated force transducer. During each laboratory session, subjects performed static and dynamic biting tasks on the force transducer positioned between the molar teeth on one side at a time.11 RMS EMG values of temporalis muscle activities (μV) versus bite-force (N) from the biting tasks were plotted, linear regression applied and slopes (μV/N) calculated for each subject and session. Subjects then performed a set of 15 tasks (Table 1, Oral Task Collector, OTC) of common functional and parafunctional oral behaviors, over a period of approximately 20 minutes, following written and pictorial instructions via a laptop computer. These tasks were chosen to test the ability of an automated algorithm to detect clenching behavior from amongst 14 other behaviors that produced similar temporalis muscle EMG characteristics.
Table 1.
List of oral tasks subjects performed while EMG signals were recorded in each of the two laboratory sessions.
| Oral Task Collector | Subject# Session# | |
|---|---|---|
|
| ||
| Calibration Activity | Duration (s) | |
| 1 | Moderate sustained clench | 5 |
| 2 | Rhythmic clenches (∼1 Hz) | 15 |
| 3 | Molar tapping (∼1 Hz) | 15 |
| 4 | Static incisor bite on saliva ejector | 5 |
| 5 | Rhythmic incisor bites on saliva ejector (∼1 Hz) | 15 |
| 6 | Grind on right canine | 15 |
| 7 | Grind on left canine | 15 |
| 8 | Dynamic jaw play (right-left movement) | 15 |
| 9 | Smile | 5 |
| 10 | Right side dried meat chewing (∼1 Hz) | 15 |
| 11 | Left side dried meat chewing (∼1 Hz) | 15 |
| 12 | Right side gum chewing (∼1 Hz) | 15 |
| 13 | Left side gum chewing (∼1 Hz) | 15 |
| 14 | Dynamic right molar bites on saliva ejector | 15 |
| 15 | Maximum clench | 5 |
Ambulatory EMG recordings
At the first laboratory session, participants were trained to prepare their skin, position surface electrodes, and operate portable EMG recorders to collect right anterior temporalis EMG data as described previously.11 Briefly, EMG surface electrode signals were band pass filtered (20 – 1000 Hz) and amplified (5000×) by means of a custom built portable digital amplifier with input impedance of 250 MΩ, noise level of 0.7 μVolt, and common mode rejection ratio of 100 dB. Subjects were instructed to self-record in their natural environments for at least 5 hours for each of 3 nights and to keep a diary of start and stop times of the recording periods. Subjects returned equipment and data at the second laboratory session.
Clench detection algorithm
To extract data from each OTC EMG recording, the 15 predefined tasks (Table 1) and segments of background EMG activity signals were first identified visually (Fig. 1a) then concatenated to create an EMG event list data file (Fig. 2a). This was aided by hand-written timing logs and/or audio recordings of timing signals generated by the OTC program. The RMS EMG value of Task 15 (maximum clench) was used to normalize muscle activities, and the normalized RMS value of the background EMG activity (Fig. 2a) was calculated for the limited purpose of defining standard deviation (σ) thresholds.
Figure 1.
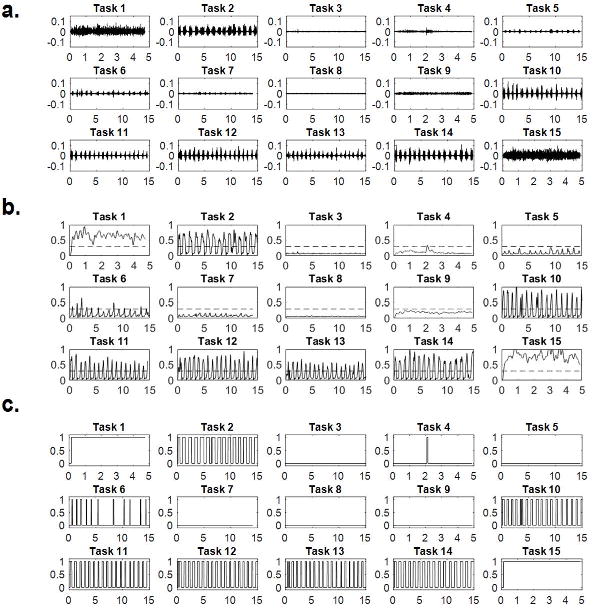
Steps to develop the clench detection algorithm: (a) EMG activity (mV) versus time (seconds) from the 15 predefined tasks performed by a subject during one laboratory session; (b) envelopes of each of the 15 tasks after normalization to RMS EMG of Task 15 (maximum clench), rectification and low-pass filtering with amplitude threshold (σ) shown in dashed lines; (c) square waves of each of the 15 tasks where values of 0 and 1 correspond to signals with envelopes smaller and larger, respectively, than the threshold.
Figure 2.
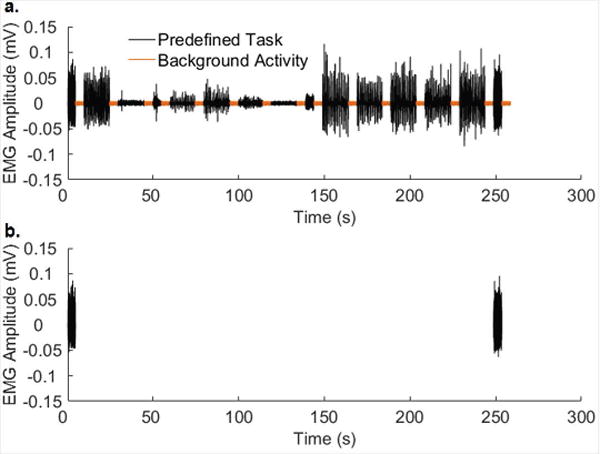
An event list data file. (a) The event list data file was constructed by concatenating the EMG recordings of 15 tasks (black) and background activity (orange). (b) Two detected clench events were identified.
A clench-detection algorithm was developed (MATLAB, MathWorks, Natick, MA) to identify and measure sustained clenches based on the event list. Steps for determination of sustained clenching included envelope calculation, square wave generation, and duration check. The envelope of each predefined task was calculated by rectifying and low-pass filtering the clench event signal (Fig. 1b). By setting the amplitude threshold to 4σ (Fig. 1b, orange dashed lines) and assigning 1 and 0 to the time samples whose envelope values were above and below 4σ, respectively, the square wave for each task was obtained (Fig. 1c). A sustained clench episode was defined as a signal whose square wave occurred for longer than 0.5 seconds. The start and stop times of each identified epoch of clenching behavior were logged to reference with the raw EMG signals, and subject-specific laboratory calibrations of temporalis muscle activity (μV) per N of bite-force were used to estimate magnitude of clenching load for each epoch.
Data analysis
The EMG data set for each subject consisted of 2 laboratory recordings and 3 night-time self-recordings. To validate the clench-detection algorithm, the algorithm was applied to the EMG event list data file from each OTC laboratory session to determine if all the arrhythmic (sustained) clench episodes could be recognized without referring to the corresponding laboratory log (Table 1) and to calculate the sensitivity, specificity, and accuracy of the detection algorithm. The number of detected sustained clench episodes (E1) and the number of tasks other than sustained clenching (E0) were used for the validation calculations. In each EMG event list data file, the first and last tasks were sustained clenches. Therefore, sensitivity was defined as number of correctly detected sustained clenches (E1) divided by two. Specificity was defined as number of correctly detected other oral tasks (E0) divided by 13. Accuracy was defined as the total number of correctly detected tasks (E1+E0) divided by 15.
The clench-detection algorithm was then applied via the customized computer program to analyze the night-time recordings of the temporalis muscle to determine the nocturnal clenching behavior of each subject. The parameters measured from each night-time recording were: the number of clench episodes, number of clench episodes per hour, mean clench duration, mean clench bite-force, and clench-related temporalis muscle duty factor. The clench-related temporalis muscle duty factor (%) was calculated as the sum of the clench episode durations divided by total recording time for a given night-time recording. Means and standard deviations of all parameters derived from the 3 nights of recording for each subject were determined.
Independent two-tailed homoscedastic t-tests with Bonferroni post-hoc corrections were used to determine differences between diagnostic groups (+DD+P vs -DD-P) for (i) number of clench episodes, (ii) number of clench episodes per hour, (iii) mean clench duration, (iv) mean clench bite-force, and (v) clench-related temporalis muscle duty factor. The average number of clench episodes were further analyzed with respect to duration (s) and bite-force (N). A Linear Mixed Effects Model was used to determine differences between diagnostic groups, restricted to levels including 95% of the data, for night-time average episode duration (s) and average episode force (N). Statistical differences are reported at p<0.05. Analyses were performed using commercially available software (SPSS, IBM SPSS Statistics, Version 23.0, IBM Corp., Armonk, NY; and Stata, StataCor LP. Stata Statistical Software: Release 14, 2015, College Station, TX).
Results
Twenty-four subjects met the inclusion criteria, with 12 women in each diagnostic group. The mean age (±standard deviation) of the +DD+P group was 37.4 (±14.9) years with a range of 21 to 62 years whereas, the mean age (±standard deviation) of the -DD-P group was 31.1(±8.7) years with a range of 24 to 56 years. The mean self-recording periods per night for +DD+P and –DD-P groups were 7.3 and 7.8 hours, respectively.
The two prescribed clenches were reliably recognized in the laboratory EMG event list data file by the clench-detection algorithm (Fig. 2b, Table 2) with excellent averaged sensitivity (97%), specificity (96%) and accuracy (97%) over the two laboratory sessions. Recognition rates did not vary significantly between laboratory sessions.
Table 2.
Clench detection algorithm validation: sensitivity, specificity and accuracy values from laboratory sessions in two groups of women with/without (+/-) both bilateral TMJ disc displacement (DD) and pain (P).
| Subject | Sensitivity[%] | Specificity [%] | Accuracy [%] |
|---|---|---|---|
| +DD+P1 | 100 | 100 | 100 |
| +DD+P2 | 100 | 100 | 100 |
| +DD+P3 | 100 | 92 | 93 |
| +DD+P4 | 100 | 100 | 100 |
| +DD+P5 | 100 | 100 | 100 |
| +DD+P6 | 100 | 100 | 100 |
| +DD+P7 | 100 | 100 | 100 |
| +DD+P8 | 100 | 100 | 100 |
| +DD+P9 | 100 | 100 | 100 |
| +DD+P10 | 100 | 100 | 100 |
| +DD+P11 | 50 | 100 | 93 |
| +DD+P12 | 100 | 100 | 100 |
|
| |||
| -DD-P1 | 100 | 92 | 93 |
| -DD-P2 | 100 | 100 | 100 |
| -DD-P3 | 100 | 92 | 93 |
| -DD-P4 | 100 | 92 | 93 |
| -DD-P5 | 100 | 100 | 100 |
| -DD-P6 | 100 | 92 | 93 |
| -DD-P7 | 100 | 100 | 100 |
| -DD-P8 | 100 | 100 | 100 |
| -DD-P9 | 100 | 85 | 87 |
| -DD-P10 | 100 | 100 | 100 |
| -DD-P11 | 100 | 92 | 93 |
| -DD-P12 | 100 | 92 | 93 |
|
| |||
| Mean | 97 | 96 | 97 |
The +DD+P group compared to -DD-P group showed (Table 3) significantly longer mean clench durations (1.9±0.8 s compared to 1.4±0.4 s; p=0.042) and higher clench-associated temporalis muscle duty factors (0.47±0.34% compared to 0.26±0.22%; p=0.042). There were no significant differences between diagnostic groups with respect to number of clench episodes per night (67.5±41.3 compared to 47.5±37.1), number of clench episodes per hour (9.1±5.5 compared to 6.3±5.2), or mean clench bite-force (6.9±6.0 N compared to 5.0±2.9 N).
Table 3.
Descriptive statistics for the detected clenches during three nights in two groups of women with/without (+/-) both bilateral TMJ disc displacement (DD) and pain (P). Independent two-tailed homoscedastic t-tests with Bonferroni post-hoc corrections were used to determine differences between diagnostic groups (-DD-P against +DD+P).
| Parameter | Mean ± SD | Bonferroni adjusted p-value |
|---|---|---|
| Number of clenches per night | 0.112 | |
| +DD+P | 67.5±41.3 | |
| -DD-P | 47.5±37.1 | |
| Number of clenches per hour | 0.106 | |
| +DD+P | 9.1±5.5 | |
| -DD-P | 6.3±5.2 | |
| Clench duration (second) | 0.042 | |
| +DD+P | 1.9±0.8 | |
| -DD-P | 1.4±0.4 | |
| Mean clench bite force (N) | 0.166 | |
| +DD+P | 6.9±6.0 | |
| -DD-P | 5.0±2.9 | |
| Duty factor (%clench time per total recording time) | 0.041 | |
| +DD+P | 0.47±0.34 | |
| -DD-P | 0.26±0.22 |
Analysis of the distribution of clench episodes by duration (Fig. 3a) and force (Fig. 3b) indicated than in both diagnostic groups, greater than 50% of detected clenches lasted for less than 1 s and were associated with jaw loads lower than 4 N. The majority (95%) of clenches lasted for less than 4 s and were associated with jaw loads lower than 10 N. Differences between diagnostic groups analyzed for episode duration at each level and episode force at each level (Fig. 3a and 3b) were not significant but night-time clenching where loads were maintained between 1-2 s and at 4-5 N were 1.5-fold and 1.9-fold, respectively, more prevalent in +DD+P than in - DD-P subjects. Power analyses of these pilot data using a Hedge's g medium effect size (1-2 s duration = 0.532, 4-5 N load = 0.482) determined that 69 subjects per diagnostic group would be required to demonstrate between-group differences in night-time clenching durations and loads.
Figure 3.
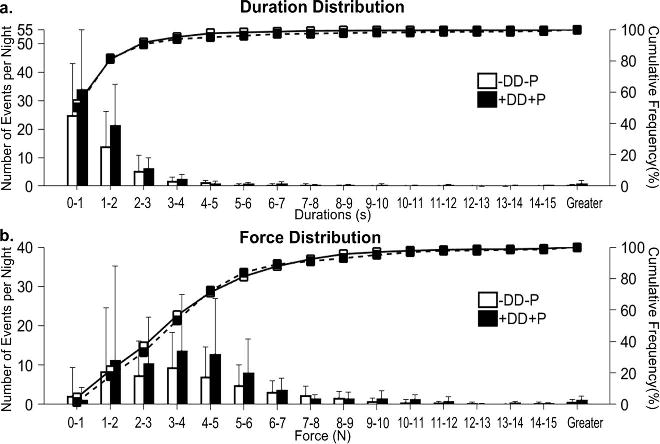
Distribution of number of clench episodes per night by (a) duration time and (b) bite-force.
Discussion and Conclusions
This pilot study developed and validated an automated clench-detection algorithm to investigate if sustained night-time clenching behavior was more prevalent in +DD+P subjects compared to control (-DD-P) subjects. The validated computer algorithm facilitated the detection and characterization of a wide range of sustained clenching behaviors from EMG signals of the anterior temporalis muscle. In all subjects, 95% of the temporalis muscle related clenches had durations less than 4 s, and were associated with bite-forces lower than 10 N. At night, durations of clenching were 1.4-fold longer and temporalis muscle duty factors were 1.8-fold higher in the +DD+P compared to -DD-P group.
The duration of night recording periods of the participating subjects was similar to other reports.5, 19-22 The numbers of clenches per night in each diagnostic group were similar to previous reports for bruxer and non-bruxer groups.20 Mean durations of clenching behavior in the current study was similar to some previously reported data8 but shorter compared to other reports.19, 21, 22 The latter differences may be attributed to different EMG amplitude threshold criteria used to define onset and duration of clenching. The results suggested that all subjects generally activated their temporalis muscles at low magnitudes at night, but average duration of activation was longer in +DD+P than -DD-P subjects.
Parafunctional loading of the mandible has been considered to have clinical relevance with respect to development of TMD-related myofascial pain.23 Until recently, the focus has been on the management of sleep bruxism.24 However, the current results are consistent with previous reports4, 5, 11 which suggested that sleep bruxism is an infrequent phenomenon compared to the predominant activities which were associated with low intensity jaw loading. That is, in the current study, 95% of night-time temporalis activities lasted less than 4 s and were associated with less than 10 N of bite-force. Similarly, a previous study has shown that low intensity jaw loading activities also predominate during awake-state ambulatory EMG recordings of the masticatory muscles, and furthermore, subjects with chronic TMD pain were found to engage more frequently in these low intensity jaw loading activities compared to control subjects.11
Sustained clenches of low intensity have been posited to contribute to jaw muscle fatigue and the development myofascial pain.23, 25 Alternatively, frequent low intensity muscle activity may be a sign of homeostatic dysregulation. This has been demonstrated recently by data from animal models showing that increased activity of the sympathetic nervous system resulted in increased trigeminal motor neuron excitability26 and glial cell-mediated neuroplasticity of trigeminal ganglia primary afferents27 and subnucleus caudalis interneurons,28 and showing associations between stress, autonomic nervous system (ANS) dysregulation, and masticatory muscle activity.29 In humans, increased masticatory muscle activities at low levels during sleep in subjects with self-reported anxiety and somatization compared to control subjects have been reported.6 These findings support an alternative hypothesis where the relatively frequent low intensity muscle activity shown by the +DD+P subjects may be a sign of ANS dysregulation that promotes central and peripheral neuroplastic changes leading to pain responses to non-noxious stimuli. Further research is required to address if homeostatic dysregulation is the basis of TMD-related chronic pain, rather than muscle activity per se.
With respect to TMJ disc displacement, frequent and prolonged low intensity jaw loading may have contributed to mechanical fatigue failure of the TMJ disc cartilage. Whether or not this is due to a classical mechanical fatigue mechanism or a combined mechanism which includes compromised disc cell nutrition30 due to duration of loading, remains to be determined.
From a technical perspective, a challenge remains to detect automatically and characterize fully clenching behaviors solely based on ambulatory EMG recordings. The computer-based algorithm had excellent sensitivity, specificity, and accuracy with very low variation to detect clenching episodes. However, the protocol required the additional calibration steps of determining subject-specific muscle activity per bite force (μV/N) from laboratory EMG recordings to facilitate characterization of the wide range of jaw loads associated with clenching. Other limitations of the current work include the small number of subjects and the lack of inclusion of men. As well, since TMD-related myofascial pain is not limited to the temporalis muscle, an analysis of masseter and lateral pterygoid activities during both day-time and night-time periods would be beneficial in future. It should also be noted that there were no recordings of EMG activities from subjects in the +DD-P and -DD+P diagnostic groups.
In conclusion, temporalis EMG was recorded from two diagnostic groups of women in the laboratory and natural settings and used to develop a computing recognition algorithm, based on statistical amplitude thresholds, that automatically and reliably detected sustained teeth clenching behaviors. At night, subjects in both diagnostic groups predominantly used their temporalis muscles for durations less than 4 s and at magnitudes lower than 10 N. Nocturnal temporalis muscle activities detected by the validated algorithm were significantly longer per clench by 1.8-fold and recording time by 1.4-fold in +DD+P compared to -DD-P women. A power analysis of these pilot data determined that 69 subjects per diagnostic group would be required to demonstrate a medium effect size for between-group differences in night-time clenching durations of 1-2 s and 4-5 N of load.
Acknowledgments
Mr. Kim Theesen helped in the production of the figures. This project was supported by NIH grants R01DE021134 (HY) and R01DE016417 (JCN) and a NIH T32 post-doctoral fellowship DE017551 (MCC). The authors thank the subjects for their contributions. At the University at Buffalo, Dr. Khawaja Nasir assisted in subject characterization and Dr. Heidi Crow provided general support.
Dr. Iwasaki reports grants from National Institutes of Health, during the conduct of the study.
Footnotes
Disclosures: The other authors have stated explicitly that there are no conflicts of interest in connection with this article.
References
- 1.Blanco Aguilera A, Gonzalez Lopez L, Blanco Aguilera E, De la Hoz Aizpurua JL, Rodriguez Torronteras A, Segura Saint-Gerons R, et al. Relationship between self-reported sleep bruxism and pain in patients with temporomandibular disorders. Journal of oral rehabilitation. 2014;41:564–572. doi: 10.1111/joor.12172. [DOI] [PubMed] [Google Scholar]
- 2.Fujisawa M, Kanemura K, Tanabe N, Gohdo Y, Watanabe A, Iizuka T, et al. Determination of daytime clenching events in subjects with and without self-reported clenching. Journal of oral rehabilitation. 2013;40:731–736. doi: 10.1111/joor.12087. [DOI] [PubMed] [Google Scholar]
- 3.Lavigne GJ, Rompre PH, Poirier G, Huard H, Kato T, Montplaisir JY. Rhythmic masticatory muscle activity during sleep in humans. Journal of dental research. 2001;80:443–448. doi: 10.1177/00220345010800020801. [DOI] [PubMed] [Google Scholar]
- 4.Raphael KG, Janal MN, Sirois DA, Dubrovsky B, Wigren PE, Klausner JJ, et al. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. Journal of oral rehabilitation. 2013;40:883–891. doi: 10.1111/joor.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raphael KG, Sirois DA, Janal MN, Wigren PE, Dubrovsky B, Nemelivsky LV, et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143:1223–1231. doi: 10.14219/jada.archive.2012.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khawaja SN, Nickel JC, Iwasaki LR, Crow HC, Gonzalez Y. Association between waking-state oral parafunctional behaviours and bio-psychosocial characteristics. Journal of oral rehabilitation. 2015 doi: 10.1111/joor.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2010;109:e26–50. doi: 10.1016/j.tripleo.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Schmitter M, Kares-Vrincianu A, Kares H, Bermejo JL, Schindler HJ. Sleep-associated aspects of myofascial pain in the orofacial area among Temporomandibular Disorder patients and controls. Sleep medicine. 2015;16:1056–1061. doi: 10.1016/j.sleep.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Michelotti A, Cioffi I, Festa P, Scala G, Farella M. Oral parafunctions as risk factors for diagnostic TMD subgroups. Journal of oral rehabilitation. 2010;37:157–162. doi: 10.1111/j.1365-2842.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan SE, Ohrbach R. Self-Report of Waking-State Oral Parafunctional Behaviors in the Natural Environment. Journal of oral & facial pain and headache. 2016;30:107–119. doi: 10.11607/ofph.1592. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki LR, Gonzalez YM, Liu H, Marx DB, Gallo LM, Nickel JC. A pilot study of ambulatory masticatory muscle activities in temporomandibular joint disorders diagnostic groups. Orthodontics & craniofacial research. 2015;18(Suppl 1):146–155. doi: 10.1111/ocr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaros AG, Williams K, Lausten L, Friesen LR. Tooth contact in patients with temporomandibular disorders. Cranio : the journal of craniomandibular practice. 2005;23:188–193. doi: 10.1179/crn.2005.027. [DOI] [PubMed] [Google Scholar]
- 13.Staude G, Flachenecker C, Daumer M, Wolf W. Onset Detection in Surface Electromyographic Signals: A Systematic Comparison of Methods. EURASIP Journal on Advances in Signal Processing. 2001;2001:67–81. [Google Scholar]
- 14.Lobbezoo F, van der Glas HW, van Kampen FM, Bosman F. The effect of an occlusal stabilization splint and the mode of visual feedback on the activity balance between jaw-elevator muscles during isometric contraction. Journal of dental research. 1993;72:876–882. doi: 10.1177/00220345930720050801. [DOI] [PubMed] [Google Scholar]
- 15.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101:511–519. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 16.Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14:T20–32. e21–23. doi: 10.1016/j.jpain.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2009;107:844–860. doi: 10.1016/j.tripleo.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. Journal of oral & facial pain and headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo LM, Gross SS, Palla S. Nocturnal masseter EMG activity of healthy subjects in a natural environment. Journal of dental research. 1999;78:1436–1444. doi: 10.1177/00220345990780080901. [DOI] [PubMed] [Google Scholar]
- 20.Lavigne GJ, Rompre PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. Journal of dental research. 1996;75:546–552. doi: 10.1177/00220345960750010601. [DOI] [PubMed] [Google Scholar]
- 21.Okeson JP, Phillips BA, Berry DT, Baldwin RM. Nocturnal bruxing events: a report of normative data and cardiovascular response. Journal of oral rehabilitation. 1994;21:623–630. doi: 10.1111/j.1365-2842.1994.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 22.Reding GR, Zepelin H, Robinson JE, Jr, Zimmerman SO, Smith VH. Nocturnal teeth-grinding: all-night psychophysiologic studies. Journal of dental research. 1968;47:786–797. doi: 10.1177/00220345680470052001. [DOI] [PubMed] [Google Scholar]
- 23.Glaros AG, Tabacchi KN, Glass EG. Effect of parafunctional clenching on TMD pain. Journal of orofacial pain. 1998;12:145–152. [PubMed] [Google Scholar]
- 24.McAuliffe P, Kim JH, Diamond D, Lau KT, O'Connell BC. A sleep bruxism detection system based on sensors in a splint - pilot clinical data. Journal of oral rehabilitation. 2015;42:34–39. doi: 10.1111/joor.12223. [DOI] [PubMed] [Google Scholar]
- 25.Svensson P, Burgaard A, Schlosser S. Fatigue and pain in human jaw muscles during a sustained, low-intensity clenching task. Archives of oral biology. 2001;46:773–777. doi: 10.1016/s0003-9969(01)00028-0. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz PB, Mir S, Peever JH. Noradrenergic modulation of masseter muscle activity during natural rapid eye movement sleep requires glutamatergic signalling at the trigeminal motor nucleus. The Journal of physiology. 2014;592:3597–3609. doi: 10.1113/jphysiol.2014.272633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnussen C, Hung SP, Ribeiro-da-Silva A. Novel expression pattern of neuropeptide Y immunoreactivity in the peripheral nervous system in a rat model of neuropathic pain. Mol Pain. 2015;11:31. doi: 10.1186/s12990-015-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goto T, Oh SB, Takeda M, Shinoda M, Sato T, Gunjikake KK, et al. Recent advances in basic research on the trigeminal ganglion. J Physiol Sci. 2016;66:381–386. doi: 10.1007/s12576-016-0448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song F, Li Q, Wan ZY, Zhao YJ, Huang F, Yang Q, et al. Lamotrigine reverses masseter overactivity caused by stress maybe via Glu suppression. Physiol Behav. 2014;137:25–32. doi: 10.1016/j.physbeh.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Wright GJ, Kuo J, Shi C, Bacro TR, Slate EH, Yao H. Effect of mechanical strain on solute diffusion in human TMJ discs: an electrical conductivity study. Annals of biomedical engineering. 2013;41:2349–2357. doi: 10.1007/s10439-013-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


