Abstract
Objectives To evaluate the risk of all cause mortality associated with initiating compared with not initiating benzodiazepines in adults, and to address potential treatment barriers and confounding related to the use of a non-active comparator group.
Design Retrospective cohort study.
Setting Large de-identified US commercial healthcare database (Optum Clinformatics Datamart).
Participants 1:1 high dimensional propensity score matched cohort of benzodiazepine initiators, and randomly selected benzodiazepine non-initiators with a medical visit within 14 days of the start of benzodiazepine treatment (n=1 252 988), between July 2004 and December 2013. To address treatment barriers and confounding, patients were required to have filled one or more prescriptions for any medication in the 90 days and 91-180 days before the index date (ie, the date of starting benzodiazepine treatment for initiators and the date of the selected medical visit for benzodiazepine non-initiators) and the high dimensional propensity score was estimated on the basis of more than 300 covariates.
Main outcome measure All cause mortality, determined by linkage with the Social Security Administration Death Master File.
Results Over a six month follow-up period, 5061 and 4691 deaths occurred among high dimensional propensity score matched benzodiazepine initiators versus non-initiators (9.3 v 9.4 events per 1000 person years; hazard ratio 1.00, 95% confidence interval 0.96 to 1.04). A 4% (95% confidence interval 1% to 8%) to 9% (2% to 7%) increase in mortality risk was observed associated with the start of benzodiazepine treatment for follow-ups of 12 and 48 months and in subgroups of younger patients and patients initiating short acting agents. In secondary analyses comparing 1:1 high dimensional propensity score matched patients initiating benzodiazepines with an active comparator, ie, patients starting treatment with selective serotonin reuptake inhibitor antidepressants, benzodiazepine use was associated with a 9% (95% confidence interval 3% to 16%) increased risk.
Conclusions This large population based cohort study suggests either no increase or at most a minor increase in risk of all cause mortality associated with benzodiazepine initiation. If a detrimental effect exists, it is likely to be much smaller than previously stated and to have uncertain clinical relevance. Residual confounding likely explains at least part of the small increase in mortality risk observed in selected analyses.
Introduction
Benzodiazepines are one of the most commonly prescribed classes of psychotropic drugs in developed countries.1 In 2008, approximately 5.2% of US adults aged 18 to 80 years used benzodiazepines in an outpatient setting,2 with use increasing from 4.1% in 1996 to 5.6% in 2013.3 Similarly, an estimated 8.4% of the population in British Columbia, Canada used a benzodiazepine in 2006, and 5.8% up to 16.3% used benzodiazepines across several European countries in 2008.4 Use seems to increase with age, with a higher proportion of any and long term use among patients aged more than 50 years.2 Because of their established efficacy,5 6 7 benzodiazepines are widely used in the treatment of anxiety and sleep disorders,1 8 9 which together with mood disorders have been found to be the most common indications for a prescription for benzodiazepines by the US Medical Expenditure Panel Survey in 2013.3
Despite earlier mixed results about a possible association between benzodiazepines and all cause mortality,10 and mostly no indication of an increased risk among patients aged 65 years or older beyond falls and fractures related mortality,11 12 13 14 more recent evidence has reported a threefold or higher increase in the risk of all cause mortality among adult populations using benzodiazepines,15 16 even for durations shorter than one month. Moreover, several studies conducted in adult or young adult populations have not provided support for a specific effect of benzodiazepines that could explain the increased risk in all cause mortality but have rather suggested associations with a wide range of causes of death, including cardiovascular disease,17 18 19 20 cancer,15 18 19 20 21 respiratory disease,20 and suicide.18 19 22 Although these studies included large populations and yielded precise estimates in a few instances, several concerns remain, such as lack of specificity of the effect and strong associations across a spectrum of different outcomes, unclear biological mechanisms, and several potentially important study design limitations, including confounding and selection bias.
Given the large number of people who use benzodiazepines and the severity of the outcome, the posited strong associations between benzodiazepines and mortality have important public health implications. We therefore evaluated the risk of all cause mortality associated with use of benzodiazepines in a large commercial US health insurance database, mitigating the impact of confounding through specific study design and analytic strategies.
Methods
Sources of data
We collected data from Optum Clinformatics Datamart (OptumInsight, Eden Prairie, MN), a large US commercial insurance database covering more than 14 million people annually from all 50 US states. For each participant, the dataset contains demographic information, health plan enrollment status, inpatient and outpatient medical encounters (coded using the international classification of diseases, ninth revision, clinical modification, and current procedural terminology, fourth edition), and drugs filled on an outpatient basis, including the national drug code numbers, quantity dispensed, and days’ supply. Data were available from January 2004 through December 2013.
Study population, medication use, and outcome definition
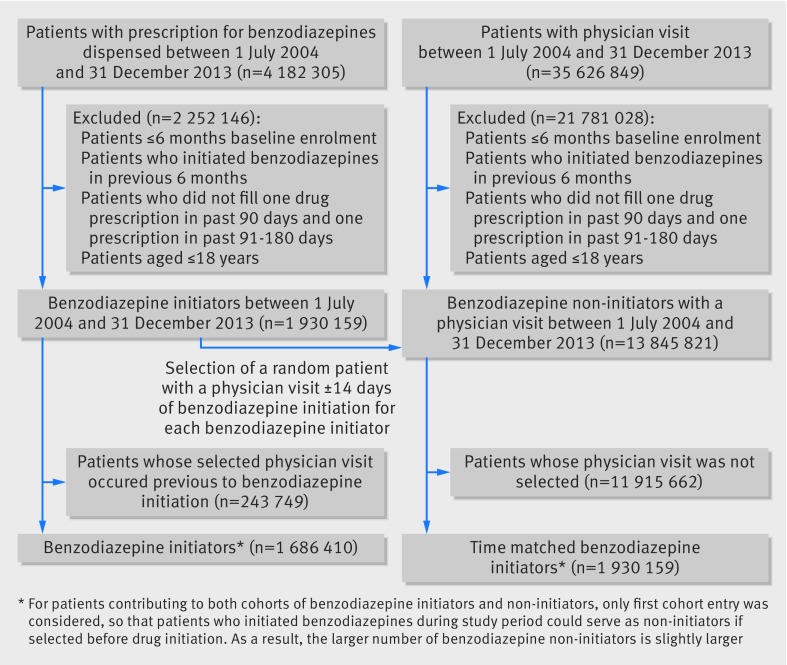
The exposed study population included patients aged 18 years and older who started a benzodiazepine (see eTable 1 in supplementary appendix for a list of benzodiazepines) between 1 July 2004 and 31 December 2013, and had six months of continuous health plan enrollment before drug initiation—that is, the index date for patients who start benzodiazepines (fig 1). We defined benzodiazepine initiators as those who had no use of any benzodiazepine in the previous six months. Patients were not allowed to enter the study cohort more than once. We opted for a primary non-active comparator group because the entire benzodiazepine class has been previously associated with an increased mortality risk, which precludes the use of a within class comparator, and because benzodiazepines have a wide range of indications and uses, which makes the selection of an appropriate out-of-class comparator group challenging. Non-users can have quite different characteristics from benzodiazepine initiators, such as a lower burden of comorbidities, and thus a lower mortality risk, or conversely higher barriers to treatment and surveillance for comorbidities, and thus a higher mortality risk23; both scenarios will need to be accounted for through proper study design or analysis choices, or both. To select patients who did not start benzodiazepine treatment with similar opportunity to be evaluated and treated by a physician as patients who started benzodiazepines and within a similar time window, for each benzodiazepine user we identified a random patient who had a medical visit within 14 days either way of the treatment start date for the corresponding benzodiazepine user and fulfilled the same inclusion criteria as benzodiazepine new users—that is, six months of continuous health plan enrollment before the selected medical visit and no use of any benzodiazepine in the six months before and including the date of the visit (ie, the index date for patients who did not start benzodiazepines). We also required patients who either did or did not start benzodiazepines to have at least one filled prescription in the 90 days before the index date and at least one filled prescription in the previous 91 through 180 days, to balance surveillance between groups and to make sure that patients in both groups were in contact with the healthcare system and had equal treatment opportunity. Starting from the patients with the earliest date for starting benzodiazepines in the study period, we repeated these steps for each patient who started benzodiazepines throughout the study period until the last such patient was identified. For patients contributing to both cohorts of users and non-users of benzodiazepines, we only considered the first cohort entry (see supplementary appendix eFigure 1 for study diagram).
Fig 1 Flowchart of study cohort
Follow-up began on the day after the index date for both cohorts. Patients were followed in an intention-to-treat approach until the occurrence of death, nursing home admission, end of continuous health plan enrollment, end of the study period (31 December 2013), or end of the observation period, whichever came first. Consistent with an intention-to-treat approach, we disregarded treatment variations occurring during the follow-up for both cohorts. We considered a six month observation period (180 days) in the main analysis and observation periods of 12 and 48 months of follow-up in sensitivity analyses. A 180 day observation period was chosen for the primary analysis as we empirically observed that in routine care approximately two thirds of patients who start benzodiazepines stop treatment within the first two months.
The study outcome was all cause mortality identified by linkage of the Optum Clinformatics Datamart database with the Social Security Administration Death Master File.24 The specificity of this master file for detecting mortality status has been estimated to range between 97% and 100%.25 26
Characteristics of patients
Patient characteristics were identified during the six months preceding and including the index date and included demographic information (age, sex), calendar year, comorbidities and lifestyle factors, use of medications, and indicators of healthcare utilization. We purposely considered a large number of patient characteristics to capture both overt risk factors for mortality and variables that may be proxies of risk factors that were unmeasured or incompletely measured in our dataset. Supplementary appendix eTable 2 shows the complete list of patient characteristics.
Statistical analysis
We cross tabulated patient baseline characteristics by those who started or did not start benzodiazepines, and in both groups we calculated number of mortality events and incidence rates. Cox proportional hazards analyses were used to estimate hazard ratios with 95% confidence intervals for the association between benzodiazepines and all cause mortality. Results are presented for three levels of adjustment: unadjusted, adjusted using 1:1 propensity score matching, to control for measured confounders27; and adjusted using 1:1 high dimensional propensity score matching, to further control for potential residual confounding.28 Exposure propensity scores were derived from the predicted probability of benzodiazepine treatment estimated in a logistic regression model that contained all patient characteristics (see supplementary eTable 2) without additional variable selection. Groups were 1:1 matched on their propensity score using a nearest neighbour matching algorithm without replacement.29 We calculated absolute standardized differences to assess covariate balance before and after propensity score matching.30 Through the high dimensional propensity score algorithm, an automated technique that identifies and prioritizes covariates that may serve as proxies for unmeasured confounders in large electronic healthcare databases,28 we selected a total of 200 empirically identified confounders and combined these with the investigator identified covariates (see supplementary appendix eTable 2) to estimate an empirically enriched propensity score. Stratification on the propensity score matched and high dimensional propensity score matched pair in Cox proportional hazards models to account for potential informative censoring did not change the point estimates appreciably, so this was omitted from all the adjusted analyses.
To test the robustness of our primary findings we performed subgroup and sensitivity analyses. Firstly, we varied the definition of exposure risk window and extended the observation period to 12 and 48 months of follow-up. Secondly, as greater risks for outcomes associated with the hypnotic effects of benzodiazepines have been found among older patients31 and possibly among users of benzodiazepines with longer half lives,32 33 we tested the potential for effect modification of benzodiazepines on mortality according to age categories (<65 years and ≥65 years) and duration of action (short acting and long acting, ie, <24 hours half life and ≥24 hours half life) (see supplementary appendix eTable 3).34 For these analyses, we estimated separate propensity scores for each subgroup. Thirdly, to address potential residual confounding associated with the use of a non-active comparator, we explored the use of an active comparator and performed analyses comparing initiators of benzodiazepines with initiators of selective serotonin reuptake inhibitor (SSRI) antidepressants for risk of all cause mortality. We chose SSRIs as the active comparator group because benzodiazepines and SSRIs are both psychotropic drugs and have some overlap in underlying indications, and because SSRIs have no known association with mortality, except for a possible association with increased suicidality in children and young adults.35 36
All analyses were done using SAS 9.3 Statistical Software (SAS Institute, Cary, NC).
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Study cohort and patient characteristics
Overall, 4 182 305 patients had filled a prescription for a benzodiazepine and 35 626 849 patients had a physician visit between July 2004 and December 2013 in the study database. After application of inclusion and exclusion criteria and random selection of comparison patients with a physician visit within 14 days either way of starting a benzodiazepine, our analytic sample included 1 686 410 patients who had started a benzodiazepine and 1 930 159 patients who had not but underwent a medical visit (fig 1). A total of 243 749 patients who had started benzodiazepines were excluded because they were selected as patients who had not started treatment before they started benzodiazepine. Short acting benzodiazepines were more frequently prescribed than long acting benzodiazepines, representing approximately 75% of filled prescriptions. Alprazolam was the most commonly prescribed agent among short acting benzodiazepines (47.2%), and diazepam was the most commonly prescribed agent among long acting benzodiazepines (87.7%).
Table 1 presents selected patient characteristics (see supplementary appendix eTable 2 for a complete list). We describe the covariates that are either more likely important risk factors for mortality or characterised by large imbalances (absolute standardized difference >0.1) between patients who initiated and did not initiate benzodiazepines. Compared with patients who did not initiate benzodiazepines, those who did were older, more often female, and had generally a higher burden of comorbidities, as measured by the Charlson comorbidity score.37 Benzodiazepine initiators were more likely than non-initiators to have a history of hypertension, ischemic heart disease, heart failure, stroke, hyperlipidemia, diabetes, kidney disease, and cancer, and they were more often characterised by having painful conditions and psychiatric disorders, including anxiety, insomnia, depression, and drug or alcohol misuse. Benzodiazepine initiators were also more likely to use other hypnotic drugs, opioids, and antidepressants and to utilize healthcare more frequently. The prevalence of most characteristics, including the ones with small imbalances across exposure groups (absolute standardized difference <0.1) before adjustment for high dimensional propensity score, was consistently higher among benzodiazepine initiators than non-initiators. After propensity score matching, all patient characteristics between benzodiazepine initiators and non-initiators were well balanced (table 1), as assessed by absolute standardized differences <0.1.30
Table 1.
Selected baseline characteristics of study participants before and after high dimensional propensity score matching. Values are percentages unless stated otherwise
| Characteristics | Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|---|
| Benzodiazepine initiators (n=1 686 410) | Benzodiazepine non-initiators (n=1 930 159) | Absolute standardized differences | Benzodiazepine initiators (n=1 252 988) | Benzodiazepine non-initiators (n=1 252 988) | Absolute standardized differences | ||
| Demographics: | |||||||
| Mean (SD) age (years) | 46.4 (13.8) | 43.6 (14.6) | 0.20 | 46.0 (13.8) | 45.2 (14.6) | 0.06 | |
| Men | 534 592 (35.1) | 770 133 (34.6) | 0.17 | 439 799 (35.1) | 433 534 (34.6) | 0.01 | |
| Comorbidities and life style factors: | |||||||
| Mean (SD) Charlson comorbidity score | 0.5 (1.2) | 0.4 (0.9) | 0.18 | 0.5 (1.1) | 0.4 (1.0) | 0.01 | |
| Smoking | 94 439 (5.6) | 71 416 (3.7) | 0.09 | 56 384 (4.5) | 56 384 (4.5) | 0.00 | |
| Obesity or overweight | 77 575 (4.6) | 75 276 (3.9) | 0.03 | 53 878 (4.3) | 52 625 (4.2) | 0.00 | |
| Hypertension | 468 822 (27.8) | 424 635 (22) | 0.13 | 320 765 (25.6) | 325 777 (26) | 0.01 | |
| Congestive heart failure | 28 669 (1.7) | 21 232 (1.1) | 0.05 | 17 542 (1.4) | 17 542 (1.4) | 0.00 | |
| Ischemic heart disease | 94 439 (5.6) | 75 276 (3.9) | 0.08 | 61 396 (4.9) | 62 649 (5) | 0.00 | |
| Cerebrovascular disease | 32 042 (1.9) | 21 232 (1.1) | 0.07 | 13 783 (1.1) | 13 783 (1.1) | 0.00 | |
| Other cardiovascular diseases | 102 871 (6.1) | 79 137 (4.1) | 0.09 | 63 902 (5.1) | 63 902 (5.1) | 0.00 | |
| Hyperlipidemia | 441 839 (26.2) | 426 565 (22.1) | 0.10 | 310 741 (24.8) | 315 753 (25.2) | 0.01 | |
| Diabetes | 163 582 (9.7) | 171 784 (8.9) | 0.03 | 120 287 (9.6) | 121 540 (9.7) | 0.00 | |
| Chronic obstructive pulmonary disease | 55 652 (3.3) | 36 673 (1.9) | 0.09 | 32 578 (2.6) | 31 325 (2.5) | 0.01 | |
| Asthma | 101 185 (6) | 84 927 (4.4) | 0.07 | 65 155 (5.2) | 65 155 (5.2) | 0.00 | |
| Pneumonia | 32 042 (1.9) | 25 092 (1.3) | 0.05 | 20 048 (1.6) | 20 048 (1.6) | 0.00 | |
| Osteoarthritis | 118 049 (7) | 94 578 (4.9) | 0.09 | 75 179 (6) | 76 432 (6.1) | 0.00 | |
| Other osteoarthritis and musculoskeletal disorders | 489 059 (29) | 440 076 (22.8) | 0.14 | 324 524 (25.9) | 324 524 (25.9) | 0.00 | |
| Neuropathic pain | 202 369 (12) | 142 832 (7.4) | 0.16 | 119 034 (9.5) | 119 034 (9.5) | 0.00 | |
| Back pain | 382 815 (22.7) | 285 664 (14.8) | 0.20 | 233 056 (18.6) | 233 056 (18.6) | 0.00 | |
| Fractures | 45 533 (2.7) | 46 324 (2.4) | 0.02 | 31 325 (2.5) | 30 072 (2.4) | 0.01 | |
| Falls | 13 491 (0.8) | 11 581 (0.6) | 0.02 | 8771 (0.7) | 8771 (0.7) | 0.00 | |
| Kidney disease | 57 338 (3.4) | 46 324 (2.4) | 0.06 | 36 337 (2.9) | 36 337 (2.9) | 0.00 | |
| Epilepsy or convulsions | 25 296 (1.5) | 15 441 (0.8) | 0.07 | 13 783 (1.1) | 12 530 (1) | 0.01 | |
| Cancer | 192 251 (11.4) | 146 692 (7.6) | 0.13 | 119 034 (9.5) | 120 287 (9.6) | 0.00 | |
| Anxiety | 241 157 (14.3) | 90 717 (4.7) | 0.33 | 98 986 (7.9) | 87 709 (7) | 0.03 | |
| Insomnia | 96 125 (5.7) | 40 533 (2.1) | 0.19 | 43 855 (3.5) | 38 843 (3.1) | 0.02 | |
| Other sleep disorders | 89 380 (5.3) | 65 625 (3.4) | 0.09 | 55 131 (4.4) | 52 625 (4.2) | 0.01 | |
| Depression | 274 885 (16.3) | 154 413 (8) | 0.26 | 1 44 094 (11.5) | 139 082 (11.1) | 0.01 | |
| Bipolar disorder | 38 787 (2.3) | 17 371 (0.9) | 0.11 | 17 542 (1.4) | 16 289 (1.3) | 0.01 | |
| Psychosis | 11 805 (0.7) | 5790 (0.3) | 0.06 | 5012 (0.4) | 5012 (0.4) | 0.00 | |
| Delirium | 11 805 (0.7) | 5790 (0.3) | 0.06 | 6265 (0.5) | 5012 (0.4) | 0.01 | |
| Drug or alcohol misuse | 37 101 (2.2) | 23 162 (1.2) | 0.08 | 20 048 (1.6) | 18 795 (1.5) | 0.01 | |
| Medications: | |||||||
| ACE inhibitors | 207 428 (12.3) | 218 108 (11.3) | 0.03 | 154 118 (12.3) | 156 624 (12.5) | 0.01 | |
| Angiotensin II receptor blockers | 134 913 (8) | 121 600 (6.3) | 0.07 | 95 227 (7.6) | 95 227 (7.6) | 0.00 | |
| β blockers | 237 784 (14.1) | 202 667 (10.5) | 0.11 | 162 888 (13) | 161 635 (12.9) | 0.00 | |
| Calcium channel blockers | 145 031 (8.6) | 202 667 (10.5) | 0.06 | 101 492 (8.1) | 105 251 (8.4) | 0.01 | |
| Thiazide diuretics | 82 634 (4.9) | 77 206 (4) | 0.04 | 57 637 (4.6) | 62 649 (5) | 0.02 | |
| Digoxin | 11 805 (0.7) | 9651 (0.5) | 0.03 | 7518 (0.6) | 7518 (0.6) | 0.00 | |
| Anticoagulants | 38 787 (2.3) | 34 743 (1.8) | 0.04 | 26 313 (2.1) | 26 313 (2.1) | 0.00 | |
| Antiplatelets | 38 787 (2.3) | 32 813 (1.7) | 0.04 | 26 313 (2.1) | 27 566 (2.2) | 0.01 | |
| Non-insulin glucose lowering agents | 119 735 (7.1) | 133 181 (6.9) | 0.01 | 88 962 (7.1) | 97 733 (7.8) | 0.03 | |
| Insulin | 35 415 (2.1) | 36 673 (1.9) | 0.01 | 25 060 (2) | 27 566 (2.2) | 0.01 | |
| Lipid lowering agents | 352 460 (20.9) | 351 289 (18.2) | 0.07 | 253 104 (20.2) | 261 874 (20.9) | 0.02 | |
| Oral corticosteroids | 227 665 (13.5) | 185 295 (9.6) | 0.12 | 142 841 (11.4) | 146 600 (11.7) | 0.01 | |
| NSAIDs | 298 495 (17.7) | 287 594 (14.9) | 0.08 | 199 225 (15.9) | 214 261 (17.1) | 0.03 | |
| Opioids | 590 244 (35) | 463 238 (24) | 0.24 | 377 149 (30.1) | 360 861 (28.8) | 0.03 | |
| Anticonvulsants | 148 404 (8.8) | 81 067 (4.2) | 0.19 | 76 432 (6.1) | 73 926 (5.9) | 0.01 | |
| SSRIs | 377 756 (22.4) | 223 898 (11.6) | 0.29 | 239 321 (19.1) | 189 201 (15.1) | 0.11 | |
| Other hypnotics | 195 624 (11.6) | 82 997 (4.3) | 0.27 | 100 239 (8) | 76 432 (6.1) | 0.07 | |
| Other anxiolytics | 37 101 (2.2) | 17 371 (0.9) | 0.11 | 20 048 (1.6) | 15 036 (1.2) | 0.03 | |
| Antipsychotics | 45 533 (2.7) | 21 232 (1.1) | 0.12 | 21 301 (1.7) | 20 048 (1.6) | 0.01 | |
| Barbiturates | 38 787 (2.3) | 21 232 (1.1) | 0.09 | 22 554 (1.8) | 17 542 (1.4) | 0.03 | |
| Indicators of healthcare utilization: | |||||||
| Any hospitalization | 175 387 (10.4) | 133 181 (6.9) | 0.12 | 101 492 (8.1) | 98 986 (7.9) | 0.01 | |
| Mean (SD) No of hospital days | 0.8 (4.2) | 0.5 (3.1) | 0.09 | 0.6 (3.4) | 0.6 (3.6) | 0.00 | |
| Mean (SD) No of physician visits | 4.0 (3.6) | 2.9 (2.9) | 0.31 | 3.4 (3.2) | 3.4 (3.1) | 0.00 | |
| Mean (SD) No of prescription drugs | 5.9 (4.0) | 4.3 (3.1) | 0.43 | 5.1 (3.6) | 5.1 (3.4) | 0.01 | |
| Psychiatric visit | 382 815 (22.7) | 210 387 (10.9) | 0.32 | 199 225 (15.9) | 182 936 (14.6) | 0.04 | |
ACE=angiotensin converting enzyme; NSAIDs=non-steroidal anti-inflammatory drugs; SSRIs=selective serotonin reuptake inhibitors.
In the primary analysis, the overall mean follow-up was 159 (SD 46) days for benzodiazepine initiators and 146 (56) days for non-initiators and the median was 180 days for both groups. Most patients were censored because of the end of the six month follow-up period (77.4% for benzodiazepine initiators and 65.7% for non-initiators), followed by the end of continuous health plan enrollment (18.6% and 30.4%, respectively).
Absolute and relative hazards
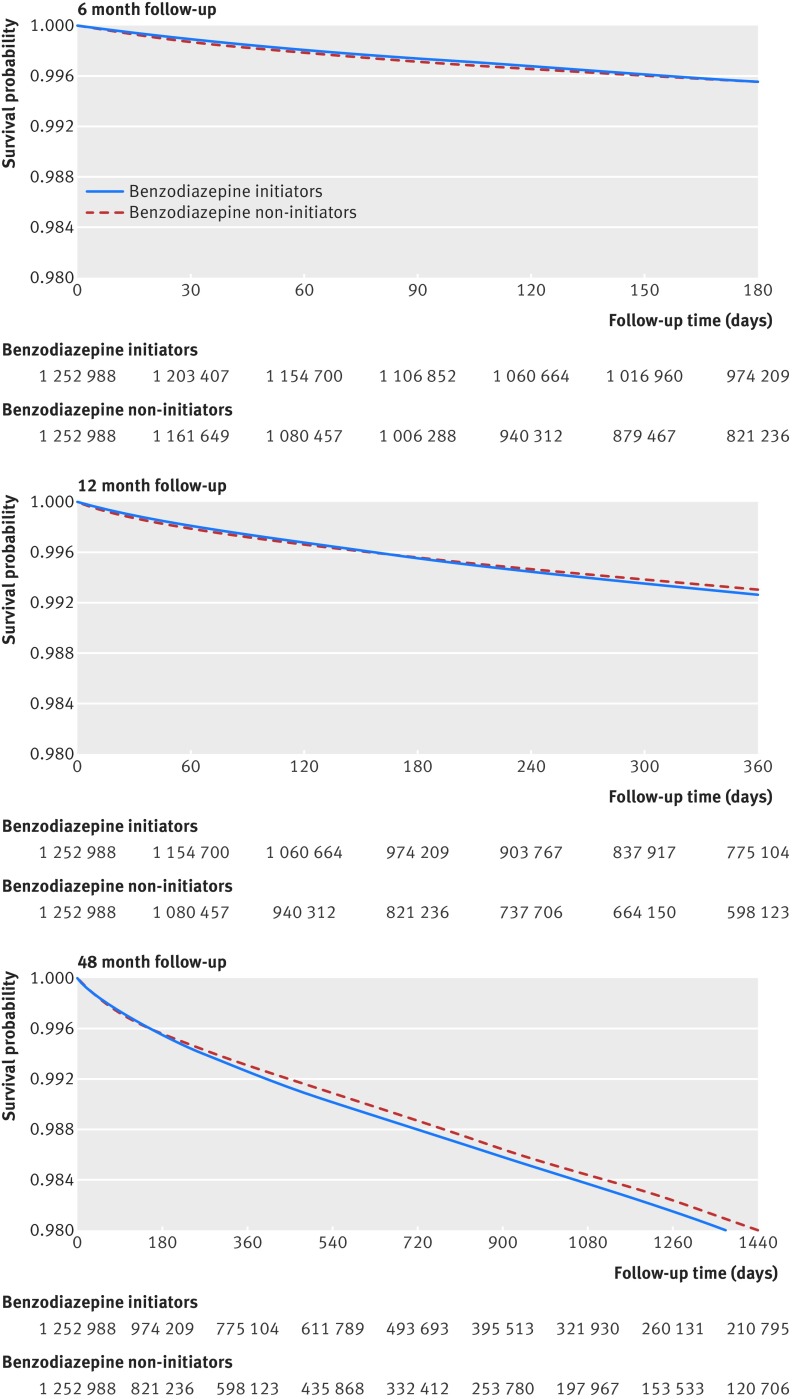
Overall, 8945 deaths occurred among benzodiazepine initiators (12.2 events per 1000 person years) and 5347 deaths among non-initiators (6.9 events per 1000 person years) within a six month observation period (table 2). Results from the unadjusted Cox regression analysis indicated that benzodiazepine initiators had a 79% increased risk of mortality compared with non-initiators (hazard ratio 1.79, 95% confidence interval 1.73 to 1.85). However, after 1:1 propensity score matching, the hazard ratio became consistent with no increased risk of death associated with benzodiazepine initiation compared with non-initiation (0.89, 0.85 to 0.93) and moved to 1.00 (0.96 to 1.04) in the 1:1 high dimensional propensity score matched population (table 2). High dimensional propensity score matched Kaplan-Meier curves comparing the survival probability between benzodiazepine users and non-users were consistent with our findings (fig 2, upper panel).
Table 2.
Risk of mortality associated with benzodiazepine initiation versus non-initiation in unadjusted, propensity score matched and high dimensional propensity score matched analyses within six months’ follow-up
| Analysis | Benzodiazepine initiators | Benzodiazepine non-initiators | Hazard ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | No of events | Person years | Incidence/1000 person years | No of patients | No of events | Person years | Incidence/1000 person years | |||
| Unadjusted | 1 686 410 | 8945 | 733 918 | 12.2 | 1 930 159 | 5347 | 772 958 | 6.9 | 1.78 (1.73 to 1.85) | |
| 1:1 propensity score matched | 1 256 630 | 4622 | 547 803 | 8.4 | 1 256 630 | 4839 | 503 208 | 9.6 | 0.89 (0.85 to 0.93) | |
| 1:1 high dimensional propensity score matched | 1 252 988 | 5061 | 546 435 | 9.3 | 1 252 988 | 4691 | 500 932 | 9.4 | 1.00 (0.96 to 1.04) | |
Fig 2 High dimensional propensity score matched Kaplan-Meier curves for survival probability since start of follow-up, and number of patients at risk
Sensitivity and subgroup analyses
When we varied the definition for exposure risk window and extended the observation period to 12 and 48 months of follow-up, we identified a small statistically significant increased risk of mortality among benzodiazepine initiators compared with non-initiators (hazard ratio 1.04, 95% confidence interval 1.01 to 1.08 and 1.05, 1.02 to 1.07, respectively, table 3). The middle and lower panels in figure 2 display the Kaplan-Meier curves comparing the survival probability between the high dimensional propensity score matched benzodiazepine initiators versus non-initiators over 12 and 48 months of follow-up.
Table 3.
Risk of mortality associated with benzodiazepine initiation versus non-initiation in high dimensional propensity score matched sensitivity and subgroup analyses
| Analysis | Benzodiazepine initiators | Benzodiazepine non-initiators | Hazard ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | No of Events | Person years | Incidence/1000 person years | No of patients | No of events | Person years | Incidence/1000 person years | |||
| Follow-up duration (months): | ||||||||||
| 12 | 1 252 988 | 7671 | 986 396 | 7.8 | 1 252 988 | 6552 | 855 316 | 7.7 | 1.04 (1.01 to 1.08) | |
| 48 | 1 252 988 | 13 532 | 2 241 015 | 6.0 | 1 252 988 | 10 299 | 1 696 159 | 6.1 | 1.05 (1.02 to 1.07) | |
| Patient age (years): | ||||||||||
| <65 | 1 156 209 | 2160 | 504 932 | 4.3 | 1 156 209 | 1843 | 462 811 | 4.0 | 1.09 (1.02 to 1.15) | |
| ≥65 | 92 273 | 2599 | 39 716 | 65.4 | 92 273 | 2708 | 36 438 | 74.3 | 0.89 (0.85 to 0.94) | |
| Drug duration of action: | ||||||||||
| Short acting | 1 011 732 | 4973 | 440 142 | 11.3 | 1 011 732 | 4370 | 403 954 | 10.8 | 1.06 (1.02 to 1.10) | |
| Long acting | 412 976 | 869 | 181 483 | 4.8 | 412 976 | 1341 | 165 450 | 8.1 | 0.60 (0.55 to 0.65) | |
Stratified analyses by age and by benzodiazepine duration of action showed no increased risk of all cause mortality associated with benzodiazepine initiation among older patients or those initiating benzodiazepines with a longer half life (table 3). However, younger patients and patients using short acting benzodiazepines had a statistically significant, small increase in risk of all cause mortality compared with those who did not initiate benzodiazepines (1.09, 1.02 to 1.15 and 1.06, 1.02 to 1.10, respectively).
When SSRIs were used as the active comparator to benzodiazepines, results from the unadjusted Cox regression analysis indicated that benzodiazepine initiators had a 62% increased risk of mortality compared with non-initiators (1.61, 1.53 to 1.68). The hazard ratio moved to 1.09 (1.03 to 1.16) after 1:1 high dimensional propensity score matching (table 4).
Table 4.
Risk of mortality associated with benzodiazepine initiation versus selective serotonin reuptake inhibitors (SSRIs) initiation in unadjusted, propensity score and high dimensional propensity score matched analyses
| Analysis | Benzodiazepine initiators | SSRI initiators | Hazard ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | No of Events | Person years | Incidence/1000 person years | No of patients | No of events | Person years | Incidence/1000 person years | |||
| Unadjusted | 1 063 845 | 6008 | 465 228 | 12.9 | 649 400 | 2276 | 282 646 | 8.1 | 1.61 (1.53 to 1.68) | |
| 1:1 propensity score matched | 563 088 | 2076 | 245 380 | 8.5 | 563 088 | 2096 | 245 252 | 8.5 | 0.99 (0.93 to 1.05) | |
| 1:1 high dimensional propensity score matched | 549 022 | 2213 | 239 380 | 9.2 | 549 022 | 2032 | 239 261 | 8.5 | 1.09 (1.03 to 1.16) | |
Discussion
Using a propensity score matched and high dimensional propensity score matched cohort that included more than 2 500 000 patients from a large commercial US health insurance database, we found no statistically significant difference in the occurrence of all cause mortality among patients who initiated benzodiazepines compared with non-initiators within a six month follow-up. We observed small, but statistically significant (owing to the large size of the cohort) increases in mortality risk associated with the initiation of benzodiazepines for follow-ups longer than six months, in younger patients, in patients initiating short acting drugs, and in the active comparison with selective serotonin reuptake inhibitor (SSRI) antidepressants. Although these results do not rule out the possibility of a small detrimental effect associated with benzodiazepines, this effect is likely to be much lower than previously proposed.15 16 Moreover, the direction and extent of the attenuation of the point estimates with high dimensional propensity score adjustment in both the comparisons with benzodiazepine non-initiators and SSRI initiators, and the tight confidence intervals driven by the large size of our study, suggest that residual confounding—rather than a true effect of benzodiazepine drugs—could drive the small increase in mortality risk observed in selected analyses.
Whether there is an effect on the risk of all cause mortality associated with the use of benzodiazepines has been an area of much discussion. The biological mechanism through which benzodiazepines would lead to an increased risk of all cause mortality is unclear. Benzodiazepines confer their effects through their action on γ-amino-butyric acid (GABA) type A receptors in the central nervous system, which are molecular substrates for the regulation of vigilance, anxiety, muscle tension, epileptogenic activity, and memory functions.38 Because of their psychotropic action, benzodiazepines have been associated with hypnotic related side effects such as daytime sleepiness, impairment of psychomotor and cognitive functioning, increased risk of motor vehicle collisions, and increased risk of falls and fractures,39 40 41 42 in particular among older patients,31 and with possible greater risks for benzodiazepines with a longer half life.32 33 Benzodiazepines have also been associated with an increased risk for development of dependence and misuse. However, this risk is not as substantial as with older sedatives and other recognised drugs of abuse,43 and overdose with a benzodiazepine rarely causes severe cardiovascular or respiratory depression and death.34 Yet several studies performed in adult populations have reported strong associations between benzodiazepines and all cause mortality, even for short durations of treatment,15 16 44 as well as associations with a wide range of causes of deaths, including cardiovascular disease17 18 19 20 and cancer,15 18 19 20 21 22 for which the biological mechanism leading to an increased risk remains unclear. The lack of specificity of the effect identified in these investigations coexists with several potentially important limitations at the study design and analysis level, the most important being confounding.11 45 46 More specifically, it has being suggested that benzodiazepine treatment could be a “marker” for unmeasured underlying conditions resulting in death, rather than being the cause of death in itself.14 47 In support of this theory, several investigations have shown that health status and a history of multiple chronic conditions are independent predictors of benzodiazepine use.1 8 9 In line with this theory, we observed a consistently higher prevalence of mortality risk factors among benzodiazepine initiators compared with non-initiators or SSRI initiators, and large attenuations in the point estimates after high dimensional propensity score adjustment for both comparisons. The remaining small increase in risk is likely due to residual confounding. Residual confounding rather than true effect modification may also explain the moderate discrepancy in the risk of all cause mortality between patients aged less than and more than 65 years and between those using short acting and long acting benzodiazepines. In addition, the lack of an increased risk in all cause mortality among benzodiazepine initiators aged more than 65 years and among those initiators long acting agents further suggests that the hypnotic effects of benzodiazepines are likely not responsible for the increased risk observed in previous investigations.
Strengths and limitations of this study
Our study exhibits several strengths. In this large population based study, which included more than 1 250 000 high dimensional propensity score matched benzodiazepine initiators, we were able to evaluate the association between benzodiazepines and the risk of all cause mortality among adult patients as treated in a routine care setting. Through the use of a large, nationwide US commercial healthcare database (Optum Clinformatics Datamart), we had access to detailed patient information, including patient’s diagnoses, use of medications, and measures of healthcare utilization. The linkage with the Social Security Administration Death Master File ensured that mortality was captured with high specificity, thus minimizing possible bias in the relative risk estimates. To minimize the potential for residual confounding, we employed multiple approaches in the study design and analysis, including the identification of benzodiazepine non-initiators who had similar patterns of healthcare utilization and treatment opportunity as benzodiazepine initiators, and the use of high dimensional propensity score analysis and an active comparator group to address potential unmeasured confounding. We also investigated the potential for effect modification in subgroup analyses by age and patients initiators benzodiazepines with shorter and longer half lives. The focus of the study on a middle aged, employed population, and the exclusion of frail participants who are not employed and not privately insured, may be one of the main advantages of this study as it is likely to control for a great deal of the confounding that may have been present in other studies.
Regardless, the main limitation to be considered remains confounding. Although the Optum Clinformatics Datamart database provides detailed patient information, certain relevant clinical information might not have been completely captured, because it is either not fully or not directly measured in administrative data, and this may have led to residual confounding. We adjusted for confounding by comparing benzodiazepine initiators with non-initiators who had evidence of other medication use before cohort entry to reduce the risk of treatment barriers among non-initiators, and by using high dimensional propensity score methodology and an active comparator group to address potential unmeasured confounding through proxy identification. Despite these efforts, the potential for some residual confounding remains, which may have driven the positive associations observed in selected analyses.
Conclusions
Results from this large cohort study based on an intention-to-treat approach suggest either no increase or a small increase in the risk of all cause mortality associated with benzodiazepine initiation. If a detrimental effect on all cause mortality exists, it is likely to be much lower than previously stated and to have only modest clinical relevance, given its magnitude from both an absolute and a relative perspective. The direction and the extent of the attenuation in the point estimates with increasing levels of adjustment, suggest that residual confounding likely explains at least part of the small increase in mortality risk observed in selected analyses.
What is already known on this topic
Because of their established efficacy for the treatment of anxiety and insomnia, benzodiazepines are one of the most commonly prescribed classes of psychotropic drugs in developed countries
Evidence has suggested a threefold or higher increase in the risk of all cause mortality among adults who use benzodiazepines, even for short durations
Although these findings are supported by large studies yielding precise estimates in a few instances, several concerns remain, including a lack of specificity of the effect, unclear biological mechanism, and several potentially important study design limitations
What this study adds
This study found either no increase or at most a minor increase in all cause mortality risk associated with benzodiazepine initiation
If a detrimental effect exists, it is likely to be much smaller than previously reported and to have uncertain clinical relevance
Web extra.
Extra material supplied by authors
Supplementary material: eTables 1-3 and eFigure 1
Contributors: EP and KFH were involved in all parts of the study. RL and MPL were involved in data analysis and revising the manuscript. RJG was involved in designing the study and revising the manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. EP and KFH are the guarantors.
Funding: This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. KFH was supported by a career development grant (K01MH099141) from the National Institute of Mental Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: EP reports receiving research funding from GSK and Boehringer-Ingelheim, outside the submitted work; RJG reports grants from AstraZeneca and Novartis outside the submitted work; KFH reports grants from Eli Lilly, Pfizer, Boehringer-Ingelheim, and GSK outside the submitted work.
Ethical approval: The use of this dataset for research was approved by the institutional review board (No 2013P000573) of the Brigham and Women’s Hospital, Boston, MA and a data use agreement was in place.
Data sharing: No additional data available.
Transparency: The manuscript’s guarantors (EP and KFH) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Cunningham CM, Hanley GE, Morgan S. Patterns in the use of benzodiazepines in British Columbia: examining the impact of increasing research and guideline cautions against long-term use. Health Policy 2010;97:122-9. 10.1016/j.healthpol.2010.03.008 pmid:20413177. [DOI] [PubMed] [Google Scholar]
- 2.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015;72:136-42. 10.1001/jamapsychiatry.2014.1763 pmid:25517224. [DOI] [PubMed] [Google Scholar]
- 3.Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing Benzodiazepine Prescriptions and Overdose Mortality in the United States. 1996-2013. Am J Public Health 2016;106:686-8. 10.2105/AJPH.2016.303061 pmid:26890165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huerta C, Abbing-Karahagopian V, Requena G, et al. Exposure to benzodiazepines (anxiolytics, hypnotics and related drugs) in seven European electronic healthcare databases: a cross-national descriptive study from the PROTECT-EU Project. Pharmacoepidemiol Drug Saf 2016;25 suppl 1:56-65. 10.1002/pds.3825. pmid:26149383. [DOI] [PubMed] [Google Scholar]
- 5.Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007;21:774-82. 10.1177/0269881107077355 pmid:17881433. [DOI] [PubMed] [Google Scholar]
- 6.van Balkom AJ, Bakker A, Spinhoven P, Blaauw BM, Smeenk S, Ruesink B. A meta-analysis of the treatment of panic disorder with or without agoraphobia: a comparison of psychopharmacological, cognitive-behavioral, and combination treatments. J Nerv Ment Dis 1997;185:510-6. 10.1097/00005053-199708000-00006 pmid:9284865. [DOI] [PubMed] [Google Scholar]
- 7.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007;22:1335-50. 10.1007/s11606-007-0251-z pmid:17619935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnenberg CM, Bierman EJM, Deeg DJH, Comijs HC, van Tilburg W, Beekman AT. Ten-year trends in benzodiazepine use in the Dutch population. Soc Psychiatry Psychiatr Epidemiol 2012;47:293-301. 10.1007/s00127-011-0344-1 pmid:21258999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fourrier A, Letenneur L, Dartigues JF, Moore N, Bégaud B. Benzodiazepine use in an elderly community-dwelling population. Characteristics of users and factors associated with subsequent use. Eur J Clin Pharmacol 2001;57:419-25. 10.1007/s002280100326 pmid:11599660. [DOI] [PubMed] [Google Scholar]
- 10.Charlson F, Degenhardt L, McLaren J, Hall W, Lynskey M. A systematic review of research examining benzodiazepine-related mortality. Pharmacoepidemiol Drug Saf 2009;18:93-103. 10.1002/pds.1694 pmid:19125401. [DOI] [PubMed] [Google Scholar]
- 11.Jaussent I, Ancelin ML, Berr C, et al. Hypnotics and mortality in an elderly general population: a 12-year prospective study. BMC Med 2013;11:212 10.1186/1741-7015-11-212 pmid:24070457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinkers DJ, Gussekloo J, van der Mast RC, Zitman FG, Westendorp RGJ. Benzodiazepine use and risk of mortality in individuals aged 85 years or older. JAMA 2003;290:2942-3. 10.1001/jama.290.22.2942 pmid:14665654. [DOI] [PubMed] [Google Scholar]
- 13.Hogan DB, Maxwell CJ, Fung TS, Ebly EM. Canadian Study of Health and Aging. Prevalence and potential consequences of benzodiazepine use in senior citizens: results from the Canadian Study of Health and Aging. Can J Clin Pharmacol 2003;10:72-7.pmid:12879145. [PubMed] [Google Scholar]
- 14.Rumble R, Morgan K. Hypnotics, sleep, and mortality in elderly people. J Am Geriatr Soc 1992;40:787-91. 10.1111/j.1532-5415.1992.tb01850.x pmid:1634722. [DOI] [PubMed] [Google Scholar]
- 15.Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open 2012;2:e000850 10.1136/bmjopen-2012-000850 pmid:22371848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weich S, Pearce HL, Croft P, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ 2014;348:g1996 10.1136/bmj.g1996 pmid:24647164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorogood M, Cowen P, Mann J, Murphy M, Vessey M. Fatal myocardial infarction and use of psychotropic drugs in young women. Lancet 1992;340:1067-8. 10.1016/0140-6736(92)93081-W pmid:1357456. [DOI] [PubMed] [Google Scholar]
- 18.Kripke DF, Klauber MR, Wingard DL, Fell RL, Assmus JD, Garfinkel L. Mortality hazard associated with prescription hypnotics. Biol Psychiatry 1998;43:687-93. 10.1016/S0006-3223(97)00292-8 pmid:9583003. [DOI] [PubMed] [Google Scholar]
- 19.Mallon L, Broman JE, Hetta J. Is usage of hypnotics associated with mortality?Sleep Med 2009;10:279-86. 10.1016/j.sleep.2008.12.004 pmid:19269892. [DOI] [PubMed] [Google Scholar]
- 20.Belleville G. Mortality hazard associated with anxiolytic and hypnotic drug use in the National Population Health Survey. Can J Psychiatry 2010;55:558-67. 10.1177/070674371005500904 pmid:20840803. [DOI] [PubMed] [Google Scholar]
- 21.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med 2002;251:207-16. 10.1046/j.1365-2796.2002.00941.x pmid:11886479. [DOI] [PubMed] [Google Scholar]
- 22.Tiihonen J, Suokas JT, Suvisaari JM, Haukka J, Korhonen P. Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry 2012;69:476-83. 10.1001/archgenpsychiatry.2011.1532 pmid:22566579. [DOI] [PubMed] [Google Scholar]
- 23.Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology 2001;12:682-9. 10.1097/00001648-200111000-00017 pmid:11679797. [DOI] [PubMed] [Google Scholar]
- 24.Social Security Administration. Social Security’s Death Master File. 2013. https://www.ssa.gov/dataexchange/request_dmf.html Accessed November 18, 2015.
- 25.Hermansen SW, Leitzmann MF, Schatzkin A. The impact on National Death Index ascertainment of limiting submissions to Social Security Administration Death Master File matches in epidemiologic studies of mortality. Am J Epidemiol 2009;169:901-8. 10.1093/aje/kwn404 pmid:19251755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr 2004;2:2 10.1186/1478-7954-2-2 pmid:15003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757-63. 10.7326/0003-4819-127-8_Part_2-199710151-00064 pmid:9382394. [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512-22. 10.1097/EDE.0b013e3181a663cc pmid:19487948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rassen JA, Glynn RJ, Brookhart MA, Schneeweiss S. Covariate selection in high-dimensional propensity score analyses of treatment effects in small samples. Am J Epidemiol 2011;173:1404-13. 10.1093/aje/kwr001 pmid:21602301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. 10.1002/sim.3697 pmid:19757444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madhusoodanan S, Bogunovic OJ. Safety of benzodiazepines in the geriatric population. Expert Opin Drug Saf 2004;3:485-93. 10.1517/14740338.3.5.485 pmid:15335303. [DOI] [PubMed] [Google Scholar]
- 32.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA 1989;262:3303-7. 10.1001/jama.1989.03430230088031 pmid:2573741. [DOI] [PubMed] [Google Scholar]
- 33.de Vries OJ, Peeters G, Elders P, et al. The elimination half-life of benzodiazepines and fall risk: two prospective observational studies. Age Ageing 2013;42:764-70. 10.1093/ageing/aft089 pmid:23900130. [DOI] [PubMed] [Google Scholar]
- 34.Mihic S, Harris R. Hypnotics and Sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12e. McGraw-Hill, 2011, http://accessmedicine.mhmedical.com.ezp-prod1.hul.harvard.edu/content.aspx?bookid=374&Sectionid=41266223, Accessed November 17, 2015. [Google Scholar]
- 35.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332-9. 10.1001/archpsyc.63.3.332 pmid:16520440. [DOI] [PubMed] [Google Scholar]
- 36.Stone M, Laughren T, Jones ML, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ 2009;339:b2880 10.1136/bmj.b2880 pmid:19671933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075-9, discussion 1081-90. 10.1016/0895-4356(93)90103-8 pmid:8410092. [DOI] [PubMed] [Google Scholar]
- 38.Rudolph U, Crestani F, Benke D, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature 1999;401:796-800. 10.1038/44579 pmid:10548105. [DOI] [PubMed] [Google Scholar]
- 39.Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs 2004;18:297-328. 10.2165/00023210-200418050-00003 pmid:15089115. [DOI] [PubMed] [Google Scholar]
- 40.Hansen RN, Boudreau DM, Ebel BE, Grossman DC, Sullivan SD. Sedative Hypnotic Medication Use and the Risk of Motor Vehicle Crash. Am J Public Health 2015;105:e64-9. 10.2105/AJPH.2015.302723 pmid:26066943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner AK, Zhang F, Soumerai SB, et al. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk?Arch Intern Med 2004;164:1567-72. 10.1001/archinte.164.14.1567 pmid:15277291. [DOI] [PubMed] [Google Scholar]
- 42.Requena G, Huerta C, Gardarsdottir H, et al. Hip/femur fractures associated with the use of benzodiazepines (anxiolytics, hypnotics and related drugs): a methodological approach to assess consistencies across databases from the PROTECT-EU project. Pharmacoepidemiol Drug Saf 2016;25:66-78. 10.1002/pds.3816. pmid:26100105. [DOI] [PubMed] [Google Scholar]
- 43.Uhlenhuth EH, Balter MB, Ban TA, Yang K. International study of expert judgment on therapeutic use of benzodiazepines and other psychotherapeutic medications: IV. Therapeutic dose dependence and abuse liability of benzodiazepines in the long-term treatment of anxiety disorders. J Clin Psychopharmacol 1999;19(Suppl 2):23S-9S. 10.1097/00004714-199912002-00005 pmid:10587281. [DOI] [PubMed] [Google Scholar]
- 44.Palmaro A, Dupouy J, Lapeyre-Mestre M. Benzodiazepines and risk of death: Results from two large cohort studies in France and UK. Eur Neuropsychopharmacol 2015;25:1566-77. 10.1016/j.euroneuro.2015.07.006 pmid:26256008. [DOI] [PubMed] [Google Scholar]
- 45.Hausken AM, Skurtveit S, Tverdal A. Use of anxiolytic or hypnotic drugs and total mortality in a general middle-aged population. Pharmacoepidemiol Drug Saf 2007;16:913-8. 10.1002/pds.1417 pmid:17486666. [DOI] [PubMed] [Google Scholar]
- 46.Kriegbaum M, Hendriksen C, Vass M, Mortensen EL, Osler M. Hypnotics and mortality--partial confounding by disease, substance abuse and socioeconomic factors?Pharmacoepidemiol Drug Saf 2015;24:779-83. 10.1002/pds.3745 pmid:25693746. [DOI] [PubMed] [Google Scholar]
- 47.Neutel CI, Johansen HL. Association between hypnotics use and increased mortality: causation or confounding?Eur J Clin Pharmacol 2015;71:637-42. 10.1007/s00228-015-1841-z pmid:25845656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: eTables 1-3 and eFigure 1