In the title compound, the central CoII atom is coordinated by four pyrrole N atoms of the porphyrin core and one O atom of the crown ether. Intramolecular N—H⋯O and intermolecular C—H⋯π interactions are observed
Keywords: crystal structure, crown ether-porphyrin, cobalt(II), hydrogen bonding
Abstract
In the title compound, [Co(C57H52N6O6)], the central CoII atom is coordinated by four pyrrole N atoms of the porphyrin core and one O atom of the crown ether. The complex has a distorted porphyrin core, with mean absolute core-atom displacements of 0.14 (10) (C a), 0.20 (10) (C b), 0.24 (4) (C m) and 0.18 (10) Å (C av), respectively. The axial Co—O bond length is 2.3380 (15) and the average Co—Np bond length is 1.968 (5) Å. Intramolecular N—H⋯O and intermolecular C—H⋯π interactions are observed.
Chemical context
Crown ether-porphyrinates have been developed to mimic the active site of the cytochrome c oxidase. There have been some reports on the single-crystal structures of crown ether-porphyrinates, including chlorido[52-N-(4-aza-18-crown-6)methyl-54,104,154,204-tetra-tert-butyl-56-methyl-5,10,15,20-tetraphenylporphyrinato]iron(III) (Dürr et al., 2007 ▸), 5,15-{2,2′-[3,3′-(1,4,10,13-tetraoxa-7,16- diazacyclooctadecan-7,16-diyl)dipropionamido]phenyl}-2,8,12,18-tetraethyl-3,7,13,17-tetramethylporphyrin and the corresponding zinc(II) compounds (Comte et al., 1998 ▸), 1,4,10,13-tetraoxa-7,16-diaza-cyclooctadecane-7,16-dicarboxylic acid{2,20-[10,20-bis-(3,5-dimethoxyphenyl)porphyrin-α-5,15-diyl]diphenyl}diamide and the corresponding zinc(II) and lead(II) compounds (Halime et al., 2007 ▸), aqua{5,15,10,20-bis[bis(2-(1,10-diaza-18-crown-6-1,10-diyl)carbonylaminophenyl]porphyrinato}zinc(II) (Michaudet et al., 2000 ▸). Herein, the crystal structure of a cobalt(II) porphyrin complex, (5-{3-[(1,4,7,10,13-pentaoxa-16-azacyclooctadecan-16-yl)carbonylamino]phenyl}-10,15,20-triphenylporphyrinato)cobalt(II), is reported.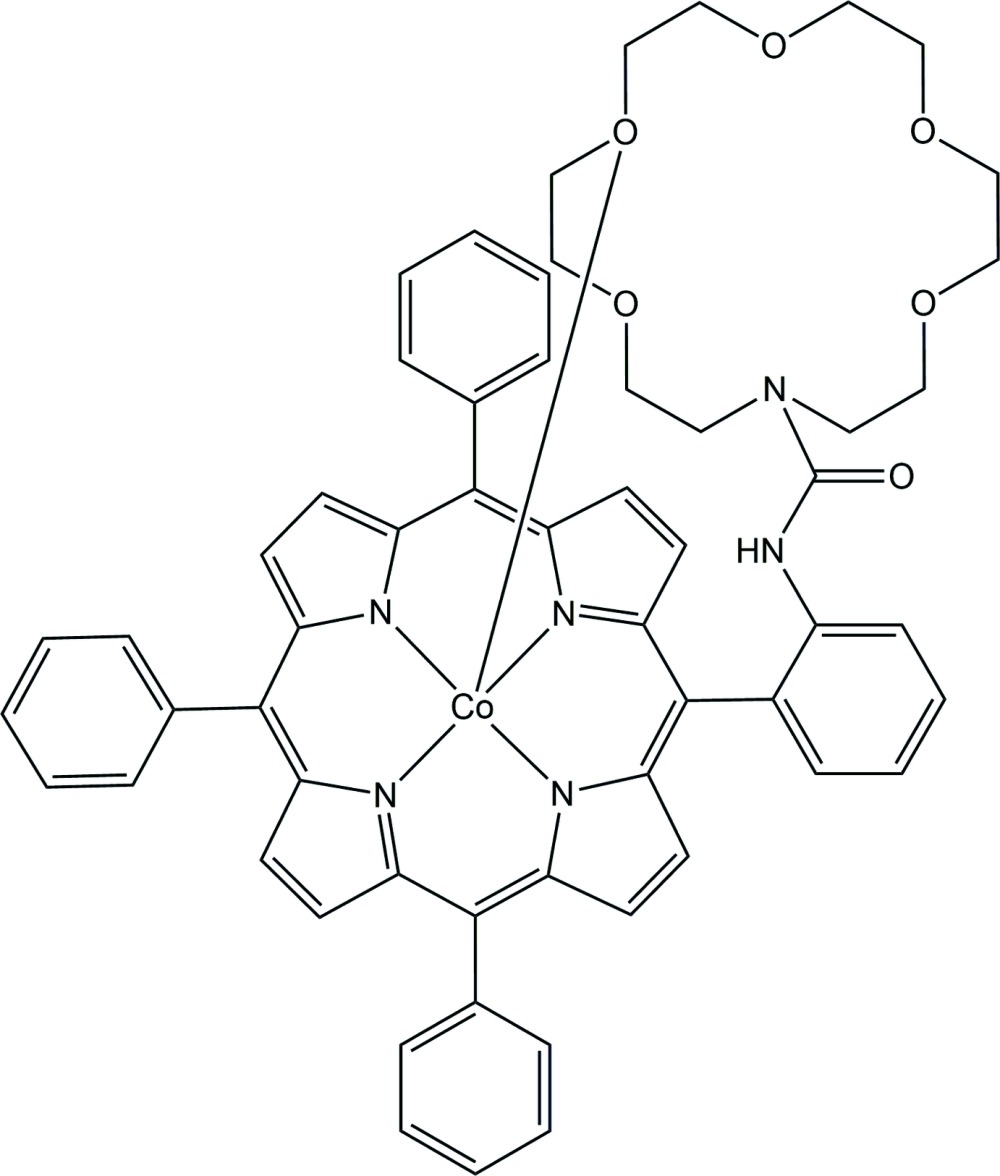
Structural commentary
In the crystal of the title compound (Fig. 1 ▸), the asymmetric unit contains one five-coordinate single-crowned porphyrin in which the oxygen atom (O3) of the crown ether ligates to the central cobalt(II) atom. Additional quantitative information on the structure is given in Fig. 2 ▸, which displays the detailed displacement of each porphyrin core atom (in units of 0.01 Å) from the 24-atom mean plane. Averaged values of the chemically unique bond lengths (in Å) and angles (in °) are also shown. The average Co—Np (Np is the porphyrin nitrogen atom) bond length is 1.968 (5), in the narrow range of 1.958 (2)–1.969 (2) Å reported by Dey & Rath (2014 ▸). The axial Co—O (O is the crown ether oxygen atom) bond length is 2.3380 (15) Å, slightly longer than the values of 2.230 (5) and 2.2724 (7) Å found in the structures of [CoII(TDPMP)(CH3OH)] [TDPMP = 5,10,15,20-tetrakis(diphenylmethyl)porphyrin; Runge et al., 1999 ▸] and [CoII(amtpp)]2 (amtpp = 52-amidato-5,10,15,20-tetraphenylporphyrin; Yamanishi et al., 2011 ▸), respectively.
Figure 1.
The molecular structure of the title compound, with displacement ellipsoids drawn at the 50% probability level.
Figure 2.
Diagrams of the porphyrin core of the title compound. Averaged values of the chemically unique bond lengths (in Å) and angles (in °) are shown. The numbers in parentheses are the s.u. values calculated on the assumption that the averaged values are all drawn from the same population. The perpendicular displacements (in units of 0.01 Å) of the porphyrin core atoms from the 24-atom mean plane are also displayed. Positive values of the displacements are towards the oxygen atom as the axial ligand.
The cobalt(II) cation is displaced slightly from the porphyrin core to the axial ligand, as illustrated by the displacement of the metal atom from the 24-atom mean plane (Δ24 = 0.06 Å). The title compound shows a distorted porphyrin core conformation. The mean absolute core-atom displacements C a, C b, C m and C av are 0.14 (10), 0.20 (10), 0.24 (4) and 0.18 (10) Å, respectively.
An intramolecular N—H⋯O interaction is found between one of the oxygen atoms (O2) of the crown ether and the nitrogen atom (N5) of the amide linker. The distance between O2 and N5 is 2.886 (2) (Table 1 ▸), consistent with the range (2.70–3.05 Å) suggested for the existence of N—H⋯O hydrogen bonding (Bertolasi et al., 1995 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N5—H5B⋯O2 | 0.93 (3) | 1.99 (3) | 2.866 (2) | 156 (2) |
Supramolecular features
In the title compound, as seen in Fig. 3 ▸, the distances between the hydrogen atoms (H30A, H31A, H32A, H33A) of the crown ether and the plane of the neighbouring porphyrin core are 2.52, 2.57, 2.71 and 2.34 Å, all of which are smaller than 2.9 Å, a limit suggested for the existence of C—H⋯π interactions (Takahashi et al., 2001 ▸). The molecular packing is shown in Fig. 4 ▸.
Figure 3.
The C—H⋯π interactions in the title compound. Dashed lines show the distances between hydrogen atoms of the crown ether and the porphyrin core plane. Other atoms have been omitted for clarity.
Figure 4.
A view of the molecular packing of the title compound in the crystal structure. H atoms have been omitted for clarity.
Synthesis and crystallization
General procedure: All reactions were carried out using standard Schlenk techniques under argon unless otherwise noted. Tetrahydrofuran (THF) was distilled over sodium/benzophenone, hexanes over potassium-sodium alloy and dichloromethane (CH2Cl2) over calcium hydride. 52-Aminophenyl-5,10,15,20-tetraphenylporphyrin was prepared according to the reported method (Lembo et al., 2009 ▸).
Synthesis of 5-{3-[(1,4,7,10,13-pentaoxa-16-azacyclooctadecan-16-yl)carbonylamino]phenyl}-10,15,20-triphenylporphyrin
5-{3-[(1,4,7,10,13-pentaoxa-16-azacyclooctadecan-16-yl)carbonylamino]phenyl}-10,15,20-triphenylporphyrin was prepared according to a modification of the reported methods (Wu & Starnes, 2012 ▸; Collman et al., 1998 ▸).
Triphosgene (220 mg, 0.74 mmol) was added to a THF (150 mL) solution of 52-aminophenyl-5,10,15,20-tetraphenylporphyrin (1.472 g, 2.3 mmol) and triethylamine (Et3N, 0.7 mL) at 273 K. The mixture was stirred for 1 h and evaporated to dryness under vacuum. A CH2Cl2 (150 mL) solution of 1-aza-18-crown-6 (0.66 g, 2.5 mmol) and Et3N (0.3 mL) was added to the resulting solid stepwise. After overnight stirring, the solution was evaporated. The porphyrin product (1.48 g, 70%) was obtained by chromatography on a silica gel column (CH2Cl2).
Synthesis of (5-{3-[(1,4,7,10,13-pentaoxa-16-azacyclooctadecan-16-yl)carbonylamino]phenyl}-10,15,20-triphenylporphyrinato)cobalt(II)
(5-{3-[(1,4,7,10,13-Pentaoxa-16-azacyclooctadecan-16-yl)carbonylamino]phenyl}-10,15,20-triphenylporphyrinato)cobalt(II) was prepared according to a modification of the reported method (Adler et al., 1970 ▸).
Dried CoCl2 (1.68 g, 12.9 mmol) was added to a THF (150 mL) solution of 52-N-(4-aza-18-crown-6)acylamino-5,10,15,20-tetraphenylporphyrin (0.6 g, 0.65 mmol). The mixture was refluxed for 3 h until the reaction was complete (monitored by TLC). The solution was extracted with CH2Cl2, washed with distilled water 2–3 times. After drying over Na2SO4 and filtration, the solvent was removed by rotoevaporation. The cobalt porphyrin product (0.52 g, 92%) was obtained by chromatography on a silica gel column (chloroform: methanol; 20:1). The title crystal was obtained in a THF solution with hexanes as non-solvent.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The hydrogen atoms attached to the nitrogen atom (N5) of the amide linker and the carbon atoms (C30, C31, C32, C33) of the crown ether were placed in the locations derived from a difference map, while others were placed in calculated positions (C—H = 0.95, 0.99 Å for aryl and methine H atoms, respectively). Hydrogen atoms were refined using a riding model with fixed isotropic displacement parameters of U iso(H) = 1.2U eq(C). One outlier was omitted in the last cycles of refinement.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Co(C57H52N6O6)] |
| M r | 975.97 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 100 |
| a, b, c (Å) | 17.2445 (6), 14.1398 (5), 19.6452 (7) |
| β (°) | 93.3307 (12) |
| V (Å3) | 4782.1 (3) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.42 |
| Crystal size (mm) | 0.37 × 0.20 × 0.06 |
| Data collection | |
| Diffractometer | Bruker D8 QUEST System |
| Absorption correction | Multi-scan (SADABS; Bruker, 2013 ▸) |
| T min, T max | 0.904, 0.975 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 70434, 10590, 8774 |
| R int | 0.062 |
| (sin θ/λ)max (Å−1) | 0.643 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.043, 0.106, 1.06 |
| No. of reflections | 10590 |
| No. of parameters | 667 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.69, −0.43 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989017007745/qm2115sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017007745/qm2115Isup2.hkl
CCDC reference: 1552184
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank the CAS Hundred Talent Program and the National Natural Science Foundation of China (grant No. 21371167, to JL).
supplementary crystallographic information
Crystal data
| [Co(C57H52N6O6)] | F(000) = 2044 |
| Mr = 975.97 | Dx = 1.356 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 17.2445 (6) Å | Cell parameters from 9294 reflections |
| b = 14.1398 (5) Å | θ = 2.7–27.2° |
| c = 19.6452 (7) Å | µ = 0.42 mm−1 |
| β = 93.3307 (12)° | T = 100 K |
| V = 4782.1 (3) Å3 | Block, black |
| Z = 4 | 0.37 × 0.20 × 0.06 mm |
Data collection
| Bruker D8 QUEST System diffractometer | 8774 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.062 |
| φ and ω scans | θmax = 27.2°, θmin = 2.7° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2008) | h = −22→22 |
| Tmin = 0.904, Tmax = 0.975 | k = −18→18 |
| 70434 measured reflections | l = −25→25 |
| 10590 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.043 | Hydrogen site location: mixed |
| wR(F2) = 0.106 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0377P)2 + 5.0698P] where P = (Fo2 + 2Fc2)/3 |
| 10590 reflections | (Δ/σ)max = 0.001 |
| 667 parameters | Δρmax = 0.69 e Å−3 |
| 0 restraints | Δρmin = −0.43 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Co1 | 0.77071 (2) | 0.20877 (2) | 0.81653 (2) | 0.01301 (7) | |
| N1 | 0.82538 (9) | 0.21197 (11) | 0.90768 (8) | 0.0141 (3) | |
| N2 | 0.67472 (9) | 0.16966 (11) | 0.85777 (8) | 0.0145 (3) | |
| N3 | 0.71366 (9) | 0.21920 (11) | 0.72734 (8) | 0.0141 (3) | |
| N4 | 0.86690 (9) | 0.24988 (11) | 0.77621 (8) | 0.0152 (3) | |
| C101 | 0.89945 (11) | 0.24414 (14) | 0.92504 (9) | 0.0166 (4) | |
| C102 | 0.79657 (11) | 0.18437 (14) | 0.96887 (9) | 0.0159 (4) | |
| C103 | 0.66772 (11) | 0.13693 (13) | 0.92345 (9) | 0.0151 (4) | |
| C104 | 0.60177 (11) | 0.15961 (13) | 0.82619 (9) | 0.0155 (4) | |
| C105 | 0.63444 (11) | 0.21165 (13) | 0.71265 (9) | 0.0154 (4) | |
| C106 | 0.74468 (11) | 0.23415 (13) | 0.66505 (9) | 0.0149 (4) | |
| C107 | 0.87938 (11) | 0.25847 (14) | 0.70782 (9) | 0.0161 (4) | |
| C108 | 0.93724 (11) | 0.27208 (14) | 0.80922 (10) | 0.0165 (4) | |
| C201 | 0.91561 (12) | 0.23959 (16) | 0.99755 (10) | 0.0217 (4) | |
| H(BA | 0.9622 | 0.2589 | 1.0218 | 0.026* | |
| C202 | 0.85233 (12) | 0.20276 (15) | 1.02461 (10) | 0.0214 (4) | |
| H(BB | 0.8457 | 0.1911 | 1.0716 | 0.026* | |
| C203 | 0.59031 (11) | 0.10269 (14) | 0.93144 (10) | 0.0189 (4) | |
| H(BC | 0.5713 | 0.0753 | 0.9714 | 0.023* | |
| C204 | 0.54965 (11) | 0.11668 (14) | 0.87155 (10) | 0.0180 (4) | |
| H(BD | 0.4967 | 0.1011 | 0.8613 | 0.022* | |
| C205 | 0.61636 (11) | 0.22583 (14) | 0.64096 (9) | 0.0183 (4) | |
| H(BE | 0.5660 | 0.2266 | 0.6186 | 0.022* | |
| C206 | 0.68428 (11) | 0.23789 (14) | 0.61139 (9) | 0.0176 (4) | |
| H(BF | 0.6910 | 0.2471 | 0.5642 | 0.021* | |
| C207 | 0.95868 (11) | 0.28399 (15) | 0.69821 (10) | 0.0218 (4) | |
| H(BG | 0.9818 | 0.2918 | 0.6558 | 0.026* | |
| C208 | 0.99406 (12) | 0.29471 (16) | 0.76082 (10) | 0.0221 (4) | |
| H(BH | 1.0463 | 0.3135 | 0.7710 | 0.027* | |
| C301 | 0.95317 (11) | 0.27274 (14) | 0.87929 (9) | 0.0166 (4) | |
| C302 | 0.72418 (11) | 0.14436 (13) | 0.97660 (9) | 0.0154 (4) | |
| C303 | 0.58063 (11) | 0.18478 (13) | 0.75899 (9) | 0.0156 (4) | |
| C304 | 0.82307 (11) | 0.24882 (13) | 0.65463 (9) | 0.0158 (4) | |
| C1 | 1.03134 (11) | 0.30727 (15) | 0.90615 (9) | 0.0190 (4) | |
| C2 | 1.03696 (12) | 0.39518 (16) | 0.93759 (10) | 0.0241 (4) | |
| H2A | 0.9909 | 0.4297 | 0.9449 | 0.029* | |
| C3 | 1.10865 (13) | 0.43372 (16) | 0.95862 (11) | 0.0265 (5) | |
| H3A | 1.1116 | 0.4936 | 0.9806 | 0.032* | |
| C4 | 1.17555 (12) | 0.38379 (16) | 0.94717 (11) | 0.0255 (5) | |
| H4A | 1.2248 | 0.4107 | 0.9597 | 0.031* | |
| C5 | 1.17140 (12) | 0.29514 (16) | 0.91776 (10) | 0.0228 (4) | |
| H5A | 1.2177 | 0.2609 | 0.9112 | 0.027* | |
| C6 | 1.09946 (11) | 0.25552 (15) | 0.89764 (10) | 0.0193 (4) | |
| C7 | 0.70432 (11) | 0.11388 (15) | 1.04666 (9) | 0.0182 (4) | |
| C8 | 0.67899 (13) | 0.18032 (17) | 1.09286 (11) | 0.0291 (5) | |
| H8A | 0.6739 | 0.2448 | 1.0797 | 0.035* | |
| C9 | 0.66109 (14) | 0.15283 (19) | 1.15802 (11) | 0.0335 (5) | |
| H9A | 0.6451 | 0.1988 | 1.1896 | 0.040* | |
| C10 | 0.66645 (13) | 0.05914 (19) | 1.17709 (11) | 0.0313 (5) | |
| H10A | 0.6531 | 0.0405 | 1.2213 | 0.038* | |
| C11 | 0.69126 (13) | −0.00749 (18) | 1.13190 (11) | 0.0299 (5) | |
| H11A | 0.6950 | −0.0721 | 1.1450 | 0.036* | |
| C12 | 0.71088 (12) | 0.02048 (16) | 1.06662 (10) | 0.0236 (4) | |
| H12A | 0.7289 | −0.0253 | 1.0358 | 0.028* | |
| C13 | 0.49628 (11) | 0.17977 (14) | 0.73654 (9) | 0.0173 (4) | |
| C14 | 0.44431 (12) | 0.24220 (15) | 0.76426 (10) | 0.0213 (4) | |
| H14A | 0.4630 | 0.2885 | 0.7962 | 0.026* | |
| C15 | 0.36532 (12) | 0.23753 (16) | 0.74581 (11) | 0.0253 (5) | |
| H15A | 0.3302 | 0.2794 | 0.7660 | 0.030* | |
| C16 | 0.33789 (13) | 0.17188 (18) | 0.69811 (11) | 0.0290 (5) | |
| H16A | 0.2842 | 0.1699 | 0.6843 | 0.035* | |
| C17 | 0.38857 (13) | 0.10962 (19) | 0.67089 (12) | 0.0337 (5) | |
| H17A | 0.3696 | 0.0639 | 0.6386 | 0.040* | |
| C18 | 0.46763 (12) | 0.11270 (17) | 0.69006 (11) | 0.0271 (5) | |
| H18A | 0.5021 | 0.0687 | 0.6712 | 0.033* | |
| C19 | 0.84853 (11) | 0.25600 (14) | 0.58351 (9) | 0.0162 (4) | |
| C20 | 0.87286 (12) | 0.34129 (15) | 0.55670 (10) | 0.0233 (4) | |
| H20A | 0.8704 | 0.3977 | 0.5828 | 0.028* | |
| C21 | 0.90072 (13) | 0.34417 (17) | 0.49178 (11) | 0.0277 (5) | |
| H21A | 0.9180 | 0.4024 | 0.4739 | 0.033* | |
| C22 | 0.90336 (13) | 0.26276 (17) | 0.45314 (11) | 0.0281 (5) | |
| H22A | 0.9221 | 0.2652 | 0.4086 | 0.034* | |
| C23 | 0.87903 (12) | 0.17848 (17) | 0.47881 (11) | 0.0273 (5) | |
| H23A | 0.8808 | 0.1226 | 0.4521 | 0.033* | |
| C24 | 0.85176 (12) | 0.17490 (15) | 0.54403 (10) | 0.0212 (4) | |
| H24A | 0.8351 | 0.1162 | 0.5617 | 0.025* | |
| N5 | 1.09580 (10) | 0.16211 (13) | 0.87059 (9) | 0.0219 (4) | |
| H5B | 1.0535 (17) | 0.124 (2) | 0.8796 (14) | 0.043 (8)* | |
| C25 | 1.13590 (11) | 0.13717 (15) | 0.81420 (10) | 0.0209 (4) | |
| O1 | 1.17389 (8) | 0.19470 (11) | 0.78285 (8) | 0.0257 (3) | |
| N6 | 1.12918 (10) | 0.04417 (13) | 0.79513 (9) | 0.0226 (4) | |
| O2 | 0.98449 (8) | 0.01154 (11) | 0.87015 (8) | 0.0262 (3) | |
| O3 | 0.79383 (8) | 0.05063 (10) | 0.78948 (8) | 0.0253 (3) | |
| O4 | 0.76136 (9) | −0.07052 (11) | 0.66442 (8) | 0.0298 (4) | |
| O5 | 0.87241 (9) | −0.04949 (11) | 0.55450 (8) | 0.0309 (4) | |
| O6 | 1.04706 (9) | −0.02582 (11) | 0.66271 (8) | 0.0288 (3) | |
| C26 | 1.11134 (12) | −0.03147 (15) | 0.84266 (11) | 0.0248 (4) | |
| H26A | 1.1258 | −0.0100 | 0.8897 | 0.030* | |
| H26B | 1.1438 | −0.0872 | 0.8333 | 0.030* | |
| C27 | 1.02716 (12) | −0.06149 (15) | 0.83898 (12) | 0.0261 (5) | |
| H27A | 1.0080 | −0.0701 | 0.7909 | 0.031* | |
| H27B | 1.0212 | −0.1220 | 0.8634 | 0.031* | |
| C28 | 0.90267 (12) | −0.00076 (16) | 0.86277 (11) | 0.0251 (5) | |
| H28A | 0.8774 | 0.0433 | 0.8937 | 0.030* | |
| H28B | 0.8894 | −0.0661 | 0.8760 | 0.030* | |
| C29 | 0.87176 (12) | 0.01736 (16) | 0.78963 (11) | 0.0265 (5) | |
| H29A | 0.9046 | 0.0650 | 0.7682 | 0.032* | |
| H29B | 0.8736 | −0.0419 | 0.7629 | 0.032* | |
| C30 | 0.73435 (13) | −0.02000 (16) | 0.77704 (13) | 0.0302 (5) | |
| H30A | 0.7553 (14) | −0.0834 (19) | 0.7934 (12) | 0.031 (7)* | |
| H30B | 0.6907 (16) | −0.0014 (19) | 0.8086 (14) | 0.040 (7)* | |
| C31 | 0.70500 (14) | −0.02511 (17) | 0.70410 (14) | 0.0331 (5) | |
| H31A | 0.6524 (17) | −0.067 (2) | 0.7009 (14) | 0.049 (8)* | |
| H31B | 0.6940 (17) | 0.043 (2) | 0.6862 (14) | 0.046 (8)* | |
| C32 | 0.74101 (14) | −0.06236 (17) | 0.59341 (13) | 0.0305 (5) | |
| H32A | 0.6896 (15) | −0.0967 (19) | 0.5824 (13) | 0.034 (7)* | |
| H32B | 0.7317 (14) | 0.0051 (19) | 0.5820 (13) | 0.031 (7)* | |
| C33 | 0.80347 (15) | −0.10470 (17) | 0.55274 (12) | 0.0315 (5) | |
| H33A | 0.8161 (14) | −0.1692 (19) | 0.5687 (12) | 0.030 (6)* | |
| H33B | 0.7829 (16) | −0.105 (2) | 0.5014 (14) | 0.044 (8)* | |
| C34 | 0.93009 (14) | −0.07851 (16) | 0.60548 (12) | 0.0294 (5) | |
| H34A | 0.9554 | −0.1377 | 0.5915 | 0.035* | |
| H34B | 0.9062 | −0.0896 | 0.6494 | 0.035* | |
| C35 | 0.98875 (14) | 0.00081 (17) | 0.61245 (12) | 0.0316 (5) | |
| H35A | 1.0121 | 0.0120 | 0.5683 | 0.038* | |
| H35B | 0.9631 | 0.0599 | 0.6262 | 0.038* | |
| C36 | 1.10801 (13) | 0.04208 (16) | 0.66854 (11) | 0.0287 (5) | |
| H36A | 1.0860 | 0.1062 | 0.6736 | 0.034* | |
| H36B | 1.1377 | 0.0412 | 0.6269 | 0.034* | |
| C37 | 1.16125 (13) | 0.01862 (16) | 0.73010 (11) | 0.0262 (5) | |
| H37A | 1.1722 | −0.0501 | 0.7301 | 0.031* | |
| H37B | 1.2112 | 0.0522 | 0.7262 | 0.031* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Co1 | 0.01207 (12) | 0.01794 (13) | 0.00923 (12) | −0.00057 (10) | 0.00228 (9) | 0.00073 (10) |
| N1 | 0.0128 (7) | 0.0173 (8) | 0.0125 (7) | 0.0002 (6) | 0.0035 (6) | 0.0008 (6) |
| N2 | 0.0147 (8) | 0.0176 (8) | 0.0116 (7) | 0.0007 (6) | 0.0030 (6) | 0.0007 (6) |
| N3 | 0.0147 (7) | 0.0165 (8) | 0.0114 (7) | −0.0013 (6) | 0.0034 (6) | −0.0003 (6) |
| N4 | 0.0156 (8) | 0.0185 (8) | 0.0115 (7) | −0.0008 (6) | 0.0018 (6) | 0.0011 (6) |
| C101 | 0.0145 (9) | 0.0210 (10) | 0.0142 (9) | 0.0012 (7) | 0.0006 (7) | 0.0012 (7) |
| C102 | 0.0158 (9) | 0.0190 (9) | 0.0133 (8) | 0.0031 (7) | 0.0026 (7) | 0.0012 (7) |
| C103 | 0.0156 (9) | 0.0174 (9) | 0.0129 (8) | 0.0006 (7) | 0.0049 (7) | 0.0008 (7) |
| C104 | 0.0135 (9) | 0.0184 (9) | 0.0149 (9) | −0.0005 (7) | 0.0030 (7) | −0.0014 (7) |
| C105 | 0.0158 (9) | 0.0170 (9) | 0.0132 (8) | −0.0003 (7) | 0.0010 (7) | −0.0012 (7) |
| C106 | 0.0194 (9) | 0.0149 (9) | 0.0104 (8) | −0.0010 (7) | 0.0021 (7) | 0.0001 (7) |
| C107 | 0.0156 (9) | 0.0191 (9) | 0.0139 (9) | −0.0012 (7) | 0.0044 (7) | 0.0011 (7) |
| C108 | 0.0136 (9) | 0.0206 (10) | 0.0156 (9) | −0.0018 (7) | 0.0027 (7) | 0.0019 (7) |
| C201 | 0.0181 (10) | 0.0327 (12) | 0.0138 (9) | −0.0012 (8) | −0.0026 (7) | 0.0020 (8) |
| C202 | 0.0200 (10) | 0.0322 (11) | 0.0120 (9) | 0.0005 (8) | 0.0002 (7) | 0.0033 (8) |
| C203 | 0.0174 (9) | 0.0234 (10) | 0.0162 (9) | −0.0008 (8) | 0.0051 (7) | 0.0042 (8) |
| C204 | 0.0149 (9) | 0.0223 (10) | 0.0172 (9) | −0.0011 (8) | 0.0037 (7) | 0.0022 (8) |
| C205 | 0.0184 (9) | 0.0226 (10) | 0.0137 (9) | −0.0001 (8) | −0.0002 (7) | −0.0003 (7) |
| C206 | 0.0214 (10) | 0.0208 (10) | 0.0107 (8) | −0.0016 (8) | 0.0012 (7) | −0.0002 (7) |
| C207 | 0.0187 (10) | 0.0310 (11) | 0.0161 (9) | −0.0029 (8) | 0.0047 (7) | 0.0039 (8) |
| C208 | 0.0151 (9) | 0.0332 (12) | 0.0184 (9) | −0.0045 (8) | 0.0036 (7) | 0.0053 (9) |
| C301 | 0.0138 (9) | 0.0205 (10) | 0.0156 (9) | −0.0005 (7) | 0.0003 (7) | 0.0008 (7) |
| C302 | 0.0169 (9) | 0.0173 (9) | 0.0123 (8) | 0.0022 (7) | 0.0033 (7) | 0.0012 (7) |
| C303 | 0.0140 (9) | 0.0175 (9) | 0.0155 (9) | 0.0002 (7) | 0.0015 (7) | −0.0012 (7) |
| C304 | 0.0197 (9) | 0.0164 (9) | 0.0115 (8) | −0.0012 (7) | 0.0044 (7) | 0.0007 (7) |
| C1 | 0.0169 (9) | 0.0278 (11) | 0.0124 (9) | −0.0030 (8) | 0.0007 (7) | 0.0041 (8) |
| C2 | 0.0183 (10) | 0.0320 (12) | 0.0218 (10) | 0.0000 (9) | 0.0009 (8) | 0.0007 (9) |
| C3 | 0.0264 (11) | 0.0292 (12) | 0.0237 (10) | −0.0060 (9) | −0.0008 (9) | −0.0020 (9) |
| C4 | 0.0197 (10) | 0.0343 (12) | 0.0220 (10) | −0.0077 (9) | −0.0028 (8) | 0.0054 (9) |
| C5 | 0.0156 (9) | 0.0323 (12) | 0.0203 (10) | −0.0024 (8) | −0.0010 (8) | 0.0050 (9) |
| C6 | 0.0176 (9) | 0.0257 (10) | 0.0147 (9) | −0.0024 (8) | 0.0010 (7) | 0.0048 (8) |
| C7 | 0.0118 (9) | 0.0304 (11) | 0.0125 (9) | −0.0024 (8) | 0.0011 (7) | 0.0023 (8) |
| C8 | 0.0333 (12) | 0.0331 (12) | 0.0220 (11) | −0.0002 (10) | 0.0106 (9) | −0.0002 (9) |
| C9 | 0.0306 (12) | 0.0528 (16) | 0.0184 (10) | −0.0029 (11) | 0.0118 (9) | −0.0069 (10) |
| C10 | 0.0229 (11) | 0.0560 (16) | 0.0153 (10) | −0.0056 (10) | 0.0026 (8) | 0.0089 (10) |
| C11 | 0.0258 (11) | 0.0387 (13) | 0.0248 (11) | −0.0037 (10) | −0.0012 (9) | 0.0135 (10) |
| C12 | 0.0229 (10) | 0.0292 (11) | 0.0189 (10) | −0.0016 (9) | 0.0023 (8) | 0.0026 (8) |
| C13 | 0.0150 (9) | 0.0239 (10) | 0.0132 (9) | −0.0031 (7) | 0.0020 (7) | 0.0022 (7) |
| C14 | 0.0221 (10) | 0.0226 (10) | 0.0194 (10) | 0.0002 (8) | 0.0040 (8) | 0.0024 (8) |
| C15 | 0.0203 (10) | 0.0272 (11) | 0.0291 (11) | 0.0034 (8) | 0.0072 (8) | 0.0082 (9) |
| C16 | 0.0175 (10) | 0.0417 (13) | 0.0277 (11) | −0.0043 (9) | −0.0008 (8) | 0.0105 (10) |
| C17 | 0.0258 (12) | 0.0440 (14) | 0.0309 (12) | −0.0098 (10) | −0.0028 (9) | −0.0102 (11) |
| C18 | 0.0212 (10) | 0.0326 (12) | 0.0277 (11) | −0.0008 (9) | 0.0026 (8) | −0.0092 (9) |
| C19 | 0.0137 (9) | 0.0223 (10) | 0.0126 (9) | −0.0004 (7) | 0.0021 (7) | 0.0018 (7) |
| C20 | 0.0272 (11) | 0.0238 (11) | 0.0197 (10) | 0.0022 (9) | 0.0074 (8) | 0.0020 (8) |
| C21 | 0.0299 (12) | 0.0307 (12) | 0.0232 (11) | 0.0008 (9) | 0.0083 (9) | 0.0099 (9) |
| C22 | 0.0259 (11) | 0.0437 (14) | 0.0155 (10) | −0.0003 (10) | 0.0081 (8) | −0.0020 (9) |
| C23 | 0.0238 (11) | 0.0371 (13) | 0.0215 (10) | −0.0016 (9) | 0.0058 (8) | −0.0104 (9) |
| C24 | 0.0190 (10) | 0.0245 (10) | 0.0202 (10) | −0.0012 (8) | 0.0029 (8) | −0.0012 (8) |
| N5 | 0.0174 (8) | 0.0244 (9) | 0.0243 (9) | −0.0029 (7) | 0.0032 (7) | 0.0026 (7) |
| C25 | 0.0150 (9) | 0.0265 (11) | 0.0210 (10) | 0.0012 (8) | −0.0010 (8) | 0.0034 (8) |
| O1 | 0.0225 (7) | 0.0285 (8) | 0.0264 (8) | −0.0028 (6) | 0.0044 (6) | 0.0041 (6) |
| N6 | 0.0197 (9) | 0.0234 (9) | 0.0250 (9) | 0.0014 (7) | 0.0033 (7) | 0.0032 (7) |
| O2 | 0.0188 (7) | 0.0254 (8) | 0.0347 (8) | −0.0006 (6) | 0.0040 (6) | −0.0012 (7) |
| O3 | 0.0188 (7) | 0.0204 (7) | 0.0371 (9) | 0.0019 (6) | 0.0033 (6) | −0.0022 (6) |
| O4 | 0.0266 (8) | 0.0276 (8) | 0.0346 (9) | 0.0043 (7) | −0.0046 (7) | −0.0007 (7) |
| O5 | 0.0346 (9) | 0.0254 (8) | 0.0317 (8) | −0.0043 (7) | −0.0073 (7) | 0.0071 (7) |
| O6 | 0.0299 (8) | 0.0273 (8) | 0.0284 (8) | −0.0018 (7) | −0.0050 (7) | 0.0039 (7) |
| C26 | 0.0216 (10) | 0.0230 (11) | 0.0297 (11) | 0.0023 (8) | 0.0012 (8) | 0.0047 (9) |
| C27 | 0.0231 (11) | 0.0208 (10) | 0.0346 (12) | 0.0016 (8) | 0.0036 (9) | 0.0025 (9) |
| C28 | 0.0200 (10) | 0.0237 (11) | 0.0320 (12) | −0.0002 (8) | 0.0058 (9) | 0.0027 (9) |
| C29 | 0.0221 (10) | 0.0264 (11) | 0.0316 (12) | 0.0065 (9) | 0.0058 (9) | 0.0029 (9) |
| C30 | 0.0249 (11) | 0.0192 (11) | 0.0472 (14) | −0.0015 (9) | 0.0089 (10) | −0.0010 (10) |
| C31 | 0.0228 (11) | 0.0211 (11) | 0.0551 (16) | −0.0006 (9) | −0.0008 (10) | −0.0061 (11) |
| C32 | 0.0300 (12) | 0.0213 (11) | 0.0386 (13) | −0.0044 (9) | −0.0127 (10) | 0.0060 (10) |
| C33 | 0.0398 (13) | 0.0223 (12) | 0.0311 (12) | −0.0064 (10) | −0.0083 (10) | 0.0032 (9) |
| C34 | 0.0335 (12) | 0.0252 (11) | 0.0289 (12) | 0.0022 (9) | −0.0028 (9) | 0.0038 (9) |
| C35 | 0.0369 (13) | 0.0270 (12) | 0.0297 (12) | 0.0003 (10) | −0.0074 (10) | 0.0049 (9) |
| C36 | 0.0318 (12) | 0.0264 (11) | 0.0278 (11) | −0.0038 (9) | 0.0024 (9) | 0.0026 (9) |
| C37 | 0.0236 (11) | 0.0283 (11) | 0.0269 (11) | 0.0020 (9) | 0.0038 (9) | −0.0001 (9) |
Geometric parameters (Å, º)
| Co1—N2 | 1.9641 (15) | C13—C14 | 1.391 (3) |
| Co1—N3 | 1.9645 (15) | C14—C15 | 1.391 (3) |
| Co1—N4 | 1.9671 (16) | C14—H14A | 0.9500 |
| Co1—N1 | 1.9751 (15) | C15—C16 | 1.383 (3) |
| Co1—O3 | 2.3380 (15) | C15—H15A | 0.9500 |
| N1—C101 | 1.380 (2) | C16—C17 | 1.371 (3) |
| N1—C102 | 1.383 (2) | C16—H16A | 0.9500 |
| N2—C104 | 1.377 (2) | C17—C18 | 1.394 (3) |
| N2—C103 | 1.383 (2) | C17—H17A | 0.9500 |
| N3—C106 | 1.380 (2) | C18—H18A | 0.9500 |
| N3—C105 | 1.384 (2) | C19—C24 | 1.387 (3) |
| N4—C108 | 1.378 (2) | C19—C20 | 1.391 (3) |
| N4—C107 | 1.378 (2) | C20—C21 | 1.389 (3) |
| C101—C301 | 1.388 (3) | C20—H20A | 0.9500 |
| C101—C201 | 1.437 (3) | C21—C22 | 1.381 (3) |
| C102—C302 | 1.387 (3) | C21—H21A | 0.9500 |
| C102—C202 | 1.438 (3) | C22—C23 | 1.369 (3) |
| C103—C302 | 1.390 (3) | C22—H22A | 0.9500 |
| C103—C203 | 1.437 (3) | C23—C24 | 1.391 (3) |
| C104—C303 | 1.395 (3) | C23—H23A | 0.9500 |
| C104—C204 | 1.437 (3) | C24—H24A | 0.9500 |
| C105—C303 | 1.390 (3) | N5—C25 | 1.385 (3) |
| C105—C205 | 1.439 (3) | N5—H5B | 0.93 (3) |
| C106—C304 | 1.394 (3) | C25—O1 | 1.232 (2) |
| C106—C206 | 1.439 (3) | C25—N6 | 1.370 (3) |
| C107—C304 | 1.391 (3) | N6—C26 | 1.464 (3) |
| C107—C207 | 1.437 (3) | N6—C37 | 1.466 (3) |
| C108—C301 | 1.388 (3) | O2—C28 | 1.421 (2) |
| C108—C208 | 1.440 (3) | O2—C27 | 1.427 (3) |
| C201—C202 | 1.346 (3) | O3—C29 | 1.424 (2) |
| C201—H(BA | 0.9500 | O3—C30 | 1.442 (3) |
| C202—H(BB | 0.9500 | O4—C32 | 1.423 (3) |
| C203—C204 | 1.349 (3) | O4—C31 | 1.433 (3) |
| C203—H(BC | 0.9500 | O5—C33 | 1.421 (3) |
| C204—H(BD | 0.9500 | O5—C34 | 1.430 (3) |
| C205—C206 | 1.348 (3) | O6—C35 | 1.419 (3) |
| C205—H(BE | 0.9500 | O6—C36 | 1.423 (3) |
| C206—H(BF | 0.9500 | C26—C27 | 1.510 (3) |
| C207—C208 | 1.349 (3) | C26—H26A | 0.9900 |
| C207—H(BG | 0.9500 | C26—H26B | 0.9900 |
| C208—H(BH | 0.9500 | C27—H27A | 0.9900 |
| C301—C1 | 1.500 (3) | C27—H27B | 0.9900 |
| C302—C7 | 1.501 (2) | C28—C29 | 1.525 (3) |
| C303—C13 | 1.497 (3) | C28—H28A | 0.9900 |
| C304—C19 | 1.492 (2) | C28—H28B | 0.9900 |
| C1—C2 | 1.389 (3) | C29—H29A | 0.9900 |
| C1—C6 | 1.402 (3) | C29—H29B | 0.9900 |
| C2—C3 | 1.392 (3) | C30—C31 | 1.494 (4) |
| C2—H2A | 0.9500 | C30—H30A | 1.01 (3) |
| C3—C4 | 1.382 (3) | C30—H30B | 1.04 (3) |
| C3—H3A | 0.9500 | C31—H31A | 1.08 (3) |
| C4—C5 | 1.380 (3) | C31—H31B | 1.04 (3) |
| C4—H4A | 0.9500 | C32—C33 | 1.502 (4) |
| C5—C6 | 1.397 (3) | C32—H32A | 1.02 (3) |
| C5—H5A | 0.9500 | C32—H32B | 0.99 (3) |
| C6—N5 | 1.424 (3) | C33—H33A | 0.98 (3) |
| C7—C12 | 1.380 (3) | C33—H33B | 1.05 (3) |
| C7—C8 | 1.394 (3) | C34—C35 | 1.511 (3) |
| C8—C9 | 1.390 (3) | C34—H34A | 0.9900 |
| C8—H8A | 0.9500 | C34—H34B | 0.9900 |
| C9—C10 | 1.378 (4) | C35—H35A | 0.9900 |
| C9—H9A | 0.9500 | C35—H35B | 0.9900 |
| C10—C11 | 1.379 (4) | C36—C37 | 1.512 (3) |
| C10—H10A | 0.9500 | C36—H36A | 0.9900 |
| C11—C12 | 1.402 (3) | C36—H36B | 0.9900 |
| C11—H11A | 0.9500 | C37—H37A | 0.9900 |
| C12—H12A | 0.9500 | C37—H37B | 0.9900 |
| C13—C18 | 1.387 (3) | ||
| N2—Co1—N3 | 89.92 (6) | C15—C14—C13 | 120.7 (2) |
| N2—Co1—N4 | 179.03 (7) | C15—C14—H14A | 119.6 |
| N3—Co1—N4 | 90.51 (6) | C13—C14—H14A | 119.6 |
| N2—Co1—N1 | 90.11 (6) | C16—C15—C14 | 120.0 (2) |
| N3—Co1—N1 | 174.16 (7) | C16—C15—H15A | 120.0 |
| N4—Co1—N1 | 89.37 (6) | C14—C15—H15A | 120.0 |
| N2—Co1—O3 | 89.05 (6) | C17—C16—C15 | 119.7 (2) |
| N3—Co1—O3 | 87.26 (6) | C17—C16—H16A | 120.2 |
| N4—Co1—O3 | 91.84 (6) | C15—C16—H16A | 120.2 |
| N1—Co1—O3 | 98.58 (6) | C16—C17—C18 | 120.7 (2) |
| C101—N1—C102 | 104.61 (15) | C16—C17—H17A | 119.7 |
| C101—N1—Co1 | 128.17 (12) | C18—C17—H17A | 119.7 |
| C102—N1—Co1 | 127.21 (13) | C13—C18—C17 | 120.3 (2) |
| C104—N2—C103 | 105.00 (15) | C13—C18—H18A | 119.9 |
| C104—N2—Co1 | 127.80 (12) | C17—C18—H18A | 119.9 |
| C103—N2—Co1 | 126.91 (12) | C24—C19—C20 | 118.83 (18) |
| C106—N3—C105 | 104.82 (15) | C24—C19—C304 | 119.40 (17) |
| C106—N3—Co1 | 127.06 (13) | C20—C19—C304 | 121.69 (18) |
| C105—N3—Co1 | 128.11 (12) | C21—C20—C19 | 120.1 (2) |
| C108—N4—C107 | 104.81 (15) | C21—C20—H20A | 120.0 |
| C108—N4—Co1 | 128.14 (12) | C19—C20—H20A | 120.0 |
| C107—N4—Co1 | 126.99 (13) | C22—C21—C20 | 120.3 (2) |
| N1—C101—C301 | 125.38 (17) | C22—C21—H21A | 119.9 |
| N1—C101—C201 | 110.72 (16) | C20—C21—H21A | 119.9 |
| C301—C101—C201 | 123.83 (18) | C23—C22—C21 | 120.14 (19) |
| N1—C102—C302 | 125.54 (17) | C23—C22—H22A | 119.9 |
| N1—C102—C202 | 110.65 (16) | C21—C22—H22A | 119.9 |
| C302—C102—C202 | 123.81 (17) | C22—C23—C24 | 119.9 (2) |
| N2—C103—C302 | 125.49 (17) | C22—C23—H23A | 120.1 |
| N2—C103—C203 | 110.29 (16) | C24—C23—H23A | 120.1 |
| C302—C103—C203 | 123.92 (17) | C19—C24—C23 | 120.8 (2) |
| N2—C104—C303 | 125.56 (17) | C19—C24—H24A | 119.6 |
| N2—C104—C204 | 110.62 (16) | C23—C24—H24A | 119.6 |
| C303—C104—C204 | 123.79 (17) | C25—N5—C6 | 121.42 (17) |
| N3—C105—C303 | 124.96 (17) | C25—N5—H5B | 116.2 (17) |
| N3—C105—C205 | 110.39 (16) | C6—N5—H5B | 118.6 (17) |
| C303—C105—C205 | 124.36 (17) | O1—C25—N6 | 122.40 (19) |
| N3—C106—C304 | 125.58 (17) | O1—C25—N5 | 122.4 (2) |
| N3—C106—C206 | 110.73 (16) | N6—C25—N5 | 115.14 (18) |
| C304—C106—C206 | 123.61 (17) | C25—N6—C26 | 122.96 (17) |
| N4—C107—C304 | 125.58 (17) | C25—N6—C37 | 116.39 (17) |
| N4—C107—C207 | 110.71 (16) | C26—N6—C37 | 118.65 (17) |
| C304—C107—C207 | 123.64 (17) | C28—O2—C27 | 113.68 (16) |
| N4—C108—C301 | 125.89 (17) | C29—O3—C30 | 115.69 (17) |
| N4—C108—C208 | 110.73 (16) | C29—O3—Co1 | 119.28 (13) |
| C301—C108—C208 | 123.38 (18) | C30—O3—Co1 | 124.87 (12) |
| C202—C201—C101 | 107.08 (17) | C32—O4—C31 | 111.13 (18) |
| C202—C201—H(BA | 126.5 | C33—O5—C34 | 113.97 (17) |
| C101—C201—H(BA | 126.5 | C35—O6—C36 | 111.54 (17) |
| C201—C202—C102 | 106.90 (17) | N6—C26—C27 | 114.33 (18) |
| C201—C202—H(BB | 126.6 | N6—C26—H26A | 108.7 |
| C102—C202—H(BB | 126.6 | C27—C26—H26A | 108.7 |
| C204—C203—C103 | 107.12 (17) | N6—C26—H26B | 108.7 |
| C204—C203—H(BC | 126.4 | C27—C26—H26B | 108.7 |
| C103—C203—H(BC | 126.4 | H26A—C26—H26B | 107.6 |
| C203—C204—C104 | 106.91 (17) | O2—C27—C26 | 107.12 (18) |
| C203—C204—H(BD | 126.5 | O2—C27—H27A | 110.3 |
| C104—C204—H(BD | 126.5 | C26—C27—H27A | 110.3 |
| C206—C205—C105 | 107.17 (17) | O2—C27—H27B | 110.3 |
| C206—C205—H(BE | 126.4 | C26—C27—H27B | 110.3 |
| C105—C205—H(BE | 126.4 | H27A—C27—H27B | 108.5 |
| C205—C206—C106 | 106.83 (16) | O2—C28—C29 | 111.49 (17) |
| C205—C206—H(BF | 126.6 | O2—C28—H28A | 109.3 |
| C106—C206—H(BF | 126.6 | C29—C28—H28A | 109.3 |
| C208—C207—C107 | 106.97 (17) | O2—C28—H28B | 109.3 |
| C208—C207—H(BG | 126.5 | C29—C28—H28B | 109.3 |
| C107—C207—H(BG | 126.5 | H28A—C28—H28B | 108.0 |
| C207—C208—C108 | 106.73 (17) | O3—C29—C28 | 109.62 (17) |
| C207—C208—H(BH | 126.6 | O3—C29—H29A | 109.7 |
| C108—C208—H(BH | 126.6 | C28—C29—H29A | 109.7 |
| C101—C301—C108 | 122.64 (18) | O3—C29—H29B | 109.7 |
| C101—C301—C1 | 119.14 (17) | C28—C29—H29B | 109.7 |
| C108—C301—C1 | 118.22 (16) | H29A—C29—H29B | 108.2 |
| C102—C302—C103 | 122.98 (17) | O3—C30—C31 | 113.2 (2) |
| C102—C302—C7 | 118.19 (17) | O3—C30—H30A | 108.8 (14) |
| C103—C302—C7 | 118.70 (16) | C31—C30—H30A | 110.9 (14) |
| C105—C303—C104 | 122.80 (17) | O3—C30—H30B | 104.9 (15) |
| C105—C303—C13 | 119.61 (16) | C31—C30—H30B | 111.6 (15) |
| C104—C303—C13 | 117.57 (16) | H30A—C30—H30B | 107 (2) |
| C107—C304—C106 | 123.00 (17) | O4—C31—C30 | 109.93 (19) |
| C107—C304—C19 | 117.78 (16) | O4—C31—H31A | 108.5 (16) |
| C106—C304—C19 | 119.21 (17) | C30—C31—H31A | 108.6 (15) |
| C2—C1—C6 | 118.86 (18) | O4—C31—H31B | 110.4 (16) |
| C2—C1—C301 | 119.05 (18) | C30—C31—H31B | 108.9 (16) |
| C6—C1—C301 | 122.02 (18) | H31A—C31—H31B | 110 (2) |
| C1—C2—C3 | 121.3 (2) | O4—C32—C33 | 110.20 (19) |
| C1—C2—H2A | 119.3 | O4—C32—H32A | 109.3 (14) |
| C3—C2—H2A | 119.3 | C33—C32—H32A | 109.7 (14) |
| C4—C3—C2 | 119.2 (2) | O4—C32—H32B | 109.1 (15) |
| C4—C3—H3A | 120.4 | C33—C32—H32B | 112.1 (15) |
| C2—C3—H3A | 120.4 | H32A—C32—H32B | 106 (2) |
| C5—C4—C3 | 120.6 (2) | O5—C33—C32 | 113.13 (19) |
| C5—C4—H4A | 119.7 | O5—C33—H33A | 109.3 (15) |
| C3—C4—H4A | 119.7 | C32—C33—H33A | 110.6 (14) |
| C4—C5—C6 | 120.4 (2) | O5—C33—H33B | 105.1 (15) |
| C4—C5—H5A | 119.8 | C32—C33—H33B | 107.4 (15) |
| C6—C5—H5A | 119.8 | H33A—C33—H33B | 111 (2) |
| C5—C6—C1 | 119.56 (19) | O5—C34—C35 | 106.57 (18) |
| C5—C6—N5 | 119.80 (18) | O5—C34—H34A | 110.4 |
| C1—C6—N5 | 120.61 (18) | C35—C34—H34A | 110.4 |
| C12—C7—C8 | 118.91 (18) | O5—C34—H34B | 110.4 |
| C12—C7—C302 | 121.05 (18) | C35—C34—H34B | 110.4 |
| C8—C7—C302 | 120.03 (19) | H34A—C34—H34B | 108.6 |
| C9—C8—C7 | 120.4 (2) | O6—C35—C34 | 108.04 (18) |
| C9—C8—H8A | 119.8 | O6—C35—H35A | 110.1 |
| C7—C8—H8A | 119.8 | C34—C35—H35A | 110.1 |
| C10—C9—C8 | 120.3 (2) | O6—C35—H35B | 110.1 |
| C10—C9—H9A | 119.9 | C34—C35—H35B | 110.1 |
| C8—C9—H9A | 119.9 | H35A—C35—H35B | 108.4 |
| C9—C10—C11 | 120.0 (2) | O6—C36—C37 | 109.12 (18) |
| C9—C10—H10A | 120.0 | O6—C36—H36A | 109.9 |
| C11—C10—H10A | 120.0 | C37—C36—H36A | 109.9 |
| C10—C11—C12 | 119.8 (2) | O6—C36—H36B | 109.9 |
| C10—C11—H11A | 120.1 | C37—C36—H36B | 109.9 |
| C12—C11—H11A | 120.1 | H36A—C36—H36B | 108.3 |
| C7—C12—C11 | 120.6 (2) | N6—C37—C36 | 113.61 (18) |
| C7—C12—H12A | 119.7 | N6—C37—H37A | 108.8 |
| C11—C12—H12A | 119.7 | C36—C37—H37A | 108.8 |
| C18—C13—C14 | 118.59 (19) | N6—C37—H37B | 108.8 |
| C18—C13—C303 | 121.95 (18) | C36—C37—H37B | 108.8 |
| C14—C13—C303 | 119.44 (18) | H37A—C37—H37B | 107.7 |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N5—H5B···O2 | 0.93 (3) | 1.99 (3) | 2.866 (2) | 156 (2) |
References
- Adler, A. D., Longo, F. R., Kampas, F. & Kim, J. (1970). J. Inorg. Nucl. Chem. 32, 2443–2445.
- Allen, F. H., Johnson, O., Shields, G. P., Smith, B. R. & Towler, M. (2004). J. Appl. Cryst. 37, 335–338.
- Bertolasi, V., Gilli, P., Ferretti, V. & Gilli, G. (1995). Acta Cryst. B51, 1004–1015.
- Bruker (2013). APEX2, SAINT, XPREP and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Collman, J. P., Wang, Z. & Straumanis, A. (1998). J. Org. Chem. 63, 2424–2425. [DOI] [PubMed]
- Comte, C. P., Gros, C., Koeller, S., Guilard, R. J., Nurco, D. & Smith, M. (1998). New J. Chem. 22, 621–626.
- Dey, S. & Rath, S. P. (2014). Dalton Trans. 43, 2301–2314. [DOI] [PubMed]
- Dürr, K., Macpherson, B. P., Warratz, R., Hampel, F., Tuczek, F., Helmreich, M., Jux, N. & Ivanović-Burmazović, I. (2007). J. Am. Chem. Soc. 129, 4217–4228. [DOI] [PubMed]
- Halime, Z., Lachkar, M., Toupet, L., Coutsolelos, A. G. & Boitrel, B. (2007). Dalton Trans. pp. 3684–3689. [DOI] [PubMed]
- Lembo, A., Tagliatesta, P., Cicero, D., Leoni, A. & Salvatori, A. (2009). Org. Biomol. Chem. 7, 1093–1096. [DOI] [PubMed]
- Michaudet, L., Richard, P. & Boitrel, B. (2000). Tetrahedron Lett. 41, 8289–8292.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Takahashi, O., Kohno, Y., Iwasaki, S., Saito, K., Iwaoka, M., Tomoda, S., Umezawa, Y., Tsuboyama, S. & Nishio, M. (2001). Bull. Chem. Soc. Jpn, 74, 2421–2430.
- Runge, S., Senge Mathias, O. & Ruhlandt-Senge, K. (1999). Z. Naturforsch. Teil B, 54, 662–666.
- Wu, X. & Starnes, S. D. (2012). Org. Lett. 14, 3652–3655. [DOI] [PubMed]
- Yamanishi, K., Miyazawa, M., Yairi, T., Sakai, S., Nishina, N., Kobori, Y., Kondo, M. & Uchida, F. (2011). Angew. Chem. 123, 6713–6716. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989017007745/qm2115sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017007745/qm2115Isup2.hkl
CCDC reference: 1552184
Additional supporting information: crystallographic information; 3D view; checkCIF report






