Abstract
Climate change is affecting both the timing of life history events and the spatial distributions of many species, including plants and pollinators. Shifts in phenology and range affect not only individual plant and pollinator species but also interactions among them, with possible negative consequences for both parties due to unfavorable abiotic conditions or mismatches caused by differences in shift magnitude or direction. Ultimately, population extinctions and reductions in pollination services could occur as a result of these climate change–induced shifts, or plants and pollinators could be buffered by plastic or genetic responses or novel interactions. Either scenario will likely involve altered selection pressures, making an understanding of plasticity and local adaptation in space and time especially important. In this review, we discuss two methods for studying plant–pollinator interactions under climate change: spatial and temporal transplants, both of which offer insight into whether plants and pollinators will be able to adapt to novel conditions. We discuss the advantages and limitations of each method and the future possibilities for this area of study. We advocate for consideration of how joint shifts in both dimensions might affect plant–pollinator interactions and point to key insights that can be gained with experimental transplants.
Keywords: climate change, phenology, plant–pollinator interactions, pollination, transplants
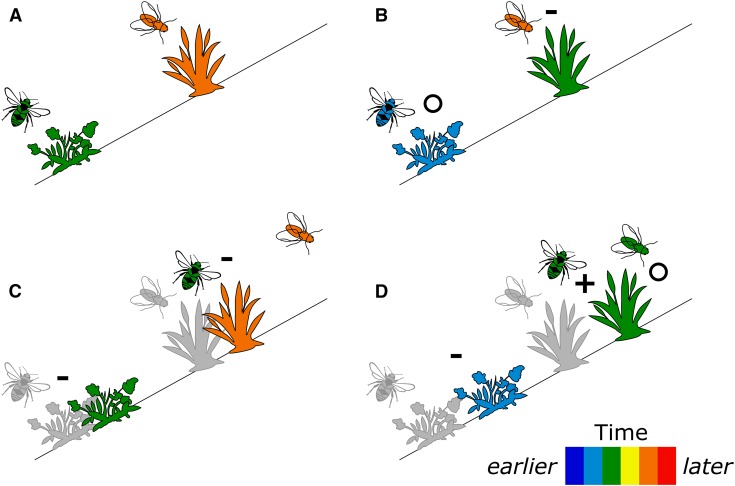
Climate change is affecting the phenology and distribution of plants and animals, potentially altering demography, species interactions, and ecosystem processes (Forrest and Miller-Rushing, 2010; Walther, 2010). Of particular interest have been the effects of climate warming upon plants, pollinators, and their interactions via changes in phenology and range (Hegland et al., 2009; Fig. 1). Many plants are flowering significantly earlier than in the past, although some first flowering dates have not changed or have shifted to later dates (Fitter and Fitter, 2002; Calinger et al., 2013; CaraDonna et al., 2014). There are fewer long-term records of pollinator phenology, but some butterfly species now appear earlier in the year than they did previously (Roy and Sparks, 2000; Forister and Shapiro, 2003) and several wild bee species have undergone a climate-associated shift, becoming active earlier in the season (Bartomeus et al., 2011), whereas the phenology of syrphid flies has not shifted significantly over two recent decades (Iler et al., 2013). At the same time, increasing evidence indicates that both plants and animals have shifted their distributions in response to climate change, by moving toward the poles and/or to higher elevations (Hughes, 2000; Parmesan and Yohe, 2003; Walther et al., 2005; Kelly and Goulden, 2008; Chen et al., 2011; Kerr et al., 2015). However, there is much variation in spatial responses to climate change, resulting in range compression for some species (Kerr et al., 2015) and range expansion for others (Doak and Morris, 2010).
Fig. 1.
Conceptual depiction of phenological and distributional shifts in populations of plants and pollinators under climate change, with populations depicted as single icons (after Alexander et al., 2016). The timing of flowering for plants and activity for pollinators is represented as shades along a color scale. Pre–climate change distributions are shown in gray. (A) Positions occupied by populations pre–climate change (historical baseline). (B) Phenological/temporal shifts alone. (C) Distributional/spatial shifts alone. (D) Joint phenological/temporal and distributional/spatial shifts. (B–D) Each panel illustrates possible outcomes of shifts: (i) maintenance of historical interactions (indicated by O), (ii) loss of historical interactions (indicated by –), and (iii) gain of novel interactions (indicated by +). For simplicity, population abundances and shapes of distributions are not shown. Although some shift types in this figure appear to be less severe than others (D changes one interaction and maintains another, while C loses both interactions), these are intended only as example outcomes. The severity of the outcome will vary depending on the pre–climate change situation, the degree of shifting of each population, and many other factors.
If phenological changes in plants and pollinators occur in parallel, the degree of synchronization between flowering and pollinator activity should be maintained, although pollination might occur earlier or later than it did prior to anthropogenic climate change (Fig. 1B). However, if one species shifts more than the other or in the opposite direction, these changes in phenology could lead to mismatches between the timing of events such as flowering and pollinator emergence (Fig. 1B). These mismatches could have significant detrimental effects upon the fitness of both the plants and pollinators, as the reduction in plant–pollinator interactions could lead to pollination depression in the plant or to pollinator starvation (Memmott et al., 2007). Using a simulation, Memmott et al. (2007) found that between 17–50% of all pollinator species experienced a reduction in floral resources due to phenological mismatch, while Burkle et al. (2013) found that phenological shifts explained 14–44% of the plant–pollinator mismatches detected by resampling sites that had been studied 120 years earlier. Two bee-pollinated plant species flowering earlier than usual in a warm spring had reduced seed set compared to cooler years, suggesting asynchrony between bee emergence and flowering phenology with warming (Kudo et al., 2004), and longer-term study of one of the two species further indicated a phenological mismatch (Kudo and Ida, 2013).
Equally, if there are mismatches between the spatial shifts of plants and their pollinators (Fig. 1C), similar consequences could arise. As climate change occurs, the conditions at previously favorable sites may change, becoming less favorable for plants growing there. If plants are unable to migrate quickly enough to track shifting environmental conditions, they may fail to adapt to rapid changes in their surroundings (Jump and Peñuelas, 2005). In addition, pollinators might shift their distributions in response to changing climatic conditions (Pyke et al., 2016) at a faster rate than plants, potentially leaving plants at trailing edges with reduced visitation (Fig. 1C). However, to date, spatial mismatches between plants and pollinators have not been observed (Hegland et al., 2009). Simulations suggest that plant–pollinator interaction network structure can be maintained under pollinator range shifts, assuming that all visitors, regardless of identity or interaction frequency, are equivalent pollinators, that no new interactions arise, and that species lacking interactions go extinct (Devoto et al., 2007). Although extinctions of both plants and pollinators occurred in the simulations, Devoto et al. (2007) attribute structural robustness to generalists and to nestedness. Of course, plant range limits may not be determined by interactions with pollinators, particularly if plants are autogamous (Hargreaves et al., 2015).
It is possible that when one plant–pollinator interaction is lost through a spatial or temporal mismatch, a new interaction will take its place. For example, Alarcón et al. (2008) found that a plant–pollinator network changed its topology year-by-year due to changes in climate, as well as other factors. Similarly, novel interactions accounted for almost half of the interactions remaining in sites resampled more than a century later by Burkle et al. (2013); however, these novel interactions can entail reduced pollinator fidelity and effectiveness (Burkle et al., 2013). Thus, data on fitness components such as pollen deposition and seed set may be needed to determine the plant reproductive consequences of novel interactions and potential mismatches.
Given that almost 88% of all plant species (Ollerton et al., 2011) and around 35% of food crops (Klein et al., 2007) depend on pollination by animals, plant–pollinator interactions form an important component of ecological communities and provide a critical ecosystem service. Thus, powerful experimental approaches are needed to study the consequences of climate change–induced shifts in phenology and distribution, both for plants themselves and for their interactions with pollinators. This review considers the utility of experimental transplants for studying the effects of climate change–induced shifts on plants and pollinators and their interactions. We discuss the key insights gained from both approaches, as well as the challenges and drawbacks of each method.
SPATIAL TRANSPLANTS
Overview
Plants or pollinators can be moved within their current ranges or beyond, and their fitness and interspecific interactions at different sites measured. To perform reciprocal transplants, individuals from sites across an elevational or latitudinal gradient are reared in common gardens at each study site. In this way, the fitness of individuals in novel environments can be determined, which will in turn demonstrate whether populations have the traits or plasticity required for persistence in novel conditions, or whether evolution would be required for persistence outside of their current range. For example, if the mean fitness of transplanted individuals is significantly lower beyond vs. within their home range, then this suggests that genetic change would be required for population persistence. Such inference could be strengthened by the use of demographic models, such as integral projection models, to determine how the performance of individuals will influence population dynamics (Merow et al., 2014; Rees et al., 2014). Plasticity and standing variation can also be measured for many different traits, allowing various aspects of within-generation plant response to climate change to be considered. In addition to reciprocal spatial transplants along existing environmental gradients, individuals can be exposed to common conditions that simulate future conditions and their fitness measured and/or interactions studied. Such an approach could use environmental chambers, greenhouses, or larger-scale manipulations of carbon dioxide, temperature, and/or soil moisture (e.g., free air carbon dioxide enrichment facilities). Species invasions and colonizations, as well as assisted migrations, could also be viewed as spatial transplants, although low replication or lack of controls present challenges.
Spatial transplants can be used to recognize: (i) locally adapted genotypes, which in their site of origin are more highly adapted to the local conditions than genotypes transplanted from other sites (Hoban et al., 2016); (ii) genotypes with “pre-adaptations,” traits that are suited to other environmental conditions such as higher temperatures; and (iii) genotypes with high levels of phenotypic plasticity, allowing them to persist in novel environments or to survive under changing conditions (Anderson and Gezon, 2015). It is important to identify these three (nonmutually exclusive) characteristics of plants and pollinators, as both plastic and evolutionary responses can influence persistence under climate change, and plasticity itself can be selected for (Franks et al., 2014).
First, local adaptation can influence persistence in changing conditions as the fitness of locally adapted genotypes may be reduced when conditions change (Franks et al., 2014). This may influence migration rates of populations, because locally adapted genotypes are likely to shift with their climate optimum. However, if migration is constrained by poor dispersal or habitat fragmentation, local adaptation may increase the risk of extinction as the climate optimum shifts away from the species’ range (Jump and Peñuelas, 2005). On the other hand, local adaptation indicates that populations possessed adequate genetic variation to enable adaptation in the past, which could signify that they will be able to adapt to changing climatic conditions (Franks et al., 2014). It is also worth noting that factors other than climate, including photoperiod, soil characteristics, and interspecific interactions, could drive local adaptation and affect population colonization of, and persistence in, novel environments (Anderson, 2016).
Second, individuals with pre-adaptations to different environmental conditions may be more likely to survive under climate change. For example, it may be that pre-adapted individuals from the warmer part of a species’ range will aid the adaptation of a population to rising temperatures associated with climate change (Jump and Peñuelas, 2005). A transplant experiment could be used to identify these pre-adapted individuals, as their fitness at sites to which they are pre-adapted will be higher than the null expectation (i.e., reduced fitness at the transplant site in comparison to the site of origin).
Third, populations that experience multiple environments with differing biotic and abiotic conditions during their life cycles may be more likely to be phenotypically plastic, as will populations in which the progeny experience environments that differ from those experienced by parents (Anderson and Gezon, 2015). A certain degree of plasticity may aid adaptation in an organism exposed to novel conditions (Nicotra et al., 2010), allowing the population to persist for long enough for adaptive evolution to occur (Forrest and Miller-Rushing, 2010). Plasticity may also allow populations to remain in their pre–climate change ranges despite novel conditions, allowing the persistence of plant–pollinator interactions, which may have otherwise been disrupted. This could be particularly important for populations with limited dispersal capacity (Anderson and Gezon, 2015).
Examples
Gómez et al. (2009) suggested that populations of pollinator-generalist plants found in “evolutionary hotspots” (where pollinator-mediated selection is strong) would have phenotypes more attractive to pollinators than plant populations in “evolutionary coldspots” (with weak pollinator-mediated selective regimes). They performed reciprocal transplants on Erysimum mediohispanicum Polatschek (Brassicaceae) plants from hotspots and coldspots and compared the attractiveness of the plants to pollinators. They found that hotspot plants were more attractive than coldspot plants, suggesting local adaptation for hotspot plants, but maladaptation in the coldspot plants. The plants from the hotspots have an advantage over the coldspot plants; they are both locally adapted to the hotspots and appear to have pre-adaptations for persistence in novel conditions, providing the pollinators remain the same in the new environment. This shows that local adaptation can occur in systems involving generalist interactions, and even on a very small spatial scale (with different sites only hundreds of meters apart). In the context of climate change, this could mean that maladapted populations from evolutionary coldspots would be unlikely to persist if plants from hotspots migrated into their environment, as the hotspot plants may outcompete them for pollinators. Similarly, if coldspot plants track climate change into areas with hotspot plants, they are less likely to persist than the locally adapted hotspot plants.
Meindl et al. (2013) reciprocally transplanted Mimulus guttatus DC. (Phrymaceae) seeds into serpentine and nonserpentine soils to determine the effects of soil type on floral morphology and display size. The results of this common garden experiment showed that plants were phenotypically plastic: regardless of which soil type they originated in, plants of both populations had smaller floral displays when grown in serpentine soil. The authors also created arrays of inflorescences grown in serpentine and nonserpentine soils. They found that pollinators visited inflorescences at the nonserpentine sites more frequently than those at serpentine sites, but that there was no significant effect of source population soil type on pollinator visitation. The pollinator assemblages differed at the serpentine and nonserpentine sites, with a higher percentage of large bees and beetles observed on the inflorescences at the nonserpentine sites compared to the serpentine sites. If plant populations move to track changing climactic conditions, they may colonize different soil types than those at their historical sites. If populations are adapted to a soil type, they may be unable to persist in novel soil conditions; however, if they are phenotypically plastic, they may be able to persist despite the differing environment. Because pollinator assemblages may vary across a landscape, the migration of a plant population to a new environment to track climate change may alter plant–pollinator interactions and, similarly, pollinators shifting across a landscape with varying soil types or another mosaic of differing abiotic environments may encounter different assemblages of plants and/or differing plant phenotypes within the same species.
Forrest and Thomson (2011) carried out reciprocal transplants of solitary bees and wasps over an elevational gradient, exchanging trap nests at high and low elevations in late summer to allow the insects to experience overwintering and springtime conditions at the new sites. This experiment revealed that local adaptation to site of origin did not affect emergence times. Instead, local conditions at the emergence sites explained differences in emergence times, with insects at the high-elevation site emerging on average 18.2 days later than those at the low-elevation site. The lack of local adaptation suggests the pollinators are plastic in their emergence phenology, which might enable them to maintain consistent overlap with floral resources under climate change. The authors combined their transplant results with observational data on flowering phenology at each site and temperature data to develop models to predict the phenologies of emergence and flowering based on degree-days. Because some pollinators were found to need higher base temperatures to trigger emergence, these results revealed the possibility of temporal mismatches between plants and pollinators occurring in the future, with an increased possibility of plants flowering before pollinators are available. Such a mismatch could reduce pollinator visitation to early flowering species, but is unlikely to result in complete loss of overlap between flowers and trap-nesting pollinators.
Advantages
By placing individuals in novel climates, spatial transplant experiments can test how plants and pollinators will respond to changing abiotic conditions and how this in turn might alter traits that influence interactions. Thus, with the appropriate controls, transplants into areas that represent likely future climate conditions can enable researchers to test for local adaptation, pre-adaptation, and plasticity. Spatial transplants can provide information that can also be used to test predictions of species distribution models that are based on observational correlational data (Alexander et al., 2016).
Another advantage of spatial transplants is that they allow researchers to create novel communities of species that are likely to co-occur under future climate conditions. Once individuals are transplanted, it is likely that the ranges of plants and pollinators will intersect in novel ways, providing an opportunity to study the ability of species to integrate into changing interaction networks, with novel pollinators or plants. Changes in interaction strength, partner identity, and effectiveness can be studied. Although the occurrence of novel interactions can be predicted based on an understanding of traits that shape plant–pollinator interactions, such as flower and tongue length matching (Nilsson, 1988), those predictions require empirical testing, which can be achieved through transplant experiments.
If siblings or individuals of otherwise known relatedness are used, this approach allows the contributions of environment and genotype to be separated, as related individuals will be exposed to differing conditions at the different sites (Anderson and Gezon, 2015). Along the same lines, individuals from different environmental backgrounds can be reciprocally transplanted to determine how the evolutionary history of populations influences performance (e.g., Gómez et al., 2009).
Limitations
Because spatial transplants often involve relatively small population sizes and study areas, issues of scale can arise. If small numbers of plants are transplanted, low density can negatively influence pollinator visitation and seed set (e.g., Dauber et al., 2010). Such effects might be a deliberate aspect of the study if the goal is to study colonization of new areas under climate change–driven range shifts, as founding or leading-edge populations might be small. Nevertheless, in studies designed to test for local adaptation, consideration of how a small population size might influence response variables that rely on mate availability and interaction frequency is important. Similarly, it is usually feasible to transplant only a small number of species, rather than entire communities (but see Alexander et al., 2015). This means that transplants can fail to accurately capture the novel communities that are likely to result from range shifts under climate change.
Spatial transplant experiments can require multiple years to establish. This is particularly true if perennial plants with slow life histories are used, multigeneration responses (e.g., recruitment, population dynamics) are of interest, or interannual environmental variation is significant. Maternal effects need to be considered, either by explicit study or by reducing their influence (e.g., Anderson and Gezon, 2015), but controlled crosses and the creation of inbred lines may be impractical for some long-lived perennials. Similarly, studies of the effects of range shifts on interactions with pollinators may require long study periods, as plants must reach maturity if they are transplanted as seeds or seedlings. An alternative would be to create experimental arrays of mature plants in pots or cut flower stems at each “transplant” site (e.g., Meindl et al., 2013; Ogilvie and Thomson, 2016), although measures of fitness or other traits could be influenced by potting, and interaction frequencies could be affected by the potting and cutting treatments.
When manipulating the distribution of plants or pollinators, the abiotic cues and environmental conditions that influence phenology are likely to be altered. Depending on the goal, the effects of spatial manipulation on phenology may not be a drawback but should be considered nonetheless (e.g., Wang et al., 2014). For instance, it may not be possible to isolate the effects of temperature on pollination success of plants transplanted from low to high elevation if the plants also flower for a much shorter time at high elevation, narrowing the window of time in which they could be pollinated.
Another difficulty with spatial transplants is that they are often more feasible for plants than for pollinators, leading to taxonomic bias (but see Forrest and Thomson, 2011). Even if mobile organisms, such as pollinators, can be transplanted, their mobility means it is challenging to maintain control of their locations, at least on smaller spatial scales. This limitation is irrelevant for studies of emergence or hatching phenology (Forrest and Thomson, 2011) but would be important for experiments that seek to study foraging, interactions with plants, or colony or nesting success. Large-scale transplants that exceed the flight ranges of the focal pollinators could be used to limit movement to within the transplant site, but the pollinators may not take to the new site, and such transplants are difficult for logistical, legal, and ethical reasons.
TEMPORAL TRANSPLANTS
Overview
Temporal transplants (Forrest, 2015) involve experimental manipulation of plants or pollinators to change their phenology. Most experiments are performed on plants as they are relatively easy to manipulate, by removing snow to simulate conditions associated with a changing climate (e.g., Dunne et al., 2003; Gezon et al., 2016), growing plants in open-top chambers that elevate temperature (e.g., Liancourt et al., 2012), or by changing the conditions in a greenhouse setting (e.g., Rafferty and Ives, 2011; Gezon et al., 2016). In the latter case, once experimental shifting has been carried out, the plants are placed into the field. Various aspects of plant performance, as well as interactions with pollinators (and other community members), can be measured and observed for experimental plants. For example, seed set of shifted plants can be compared to that of controls to see if changes in phenology confer a potential fitness advantage or are disadvantageous (Rafferty and Ives, 2012). In this way, insight into the evolutionary consequences of phenological shifts can be gained, as response traits measured for experimental plants or pollinators can indicate not only potential fitness consequences but also how phenotypically plastic genotypes are. Like spatial transplants, temporal transplants can therefore be used to test for local adaptation, phenotypic plasticity, and pre-adaptation to novel niche space in the temporal axis.
Examples
Rafferty and Ives (2011) compared current and historical dates of first bloom (DFB) for 14 plants of southern Wisconsin. They found that six of these were “historically advanced”; their DFBs had advanced 6–13 days since the historical observations were made. The others were “historically unchanged”; their modern DFBs were not significantly different from the historical data. The phenologies of these 14 species were then experimentally manipulated in a greenhouse to be advanced or delayed by increasing or decreasing (respectively) the temperature and lighting. Nectar quantity and sucrose content were measured in the plants before they were placed out into the field in arrays of potted plants. Focal observations of pollinator visitation were made, and it was found that changes in visitation differed among species. However, historically advanced species had higher visitation when flowering was advanced, perhaps suggesting pre-adaptation to earlier flowering times, while historically unchanged species had lower visitation when advanced, suggesting local adaptation to historical flowering times. Overall, these results suggest that plant–pollinator mismatches are not occurring in these species, possibly due to species tracking each other or the involvement of novel pollinators.
Snow removal was used by Gezon et al. (2016) to manipulate the flowering phenology of Claytonia lanceolata Pursh (Montiaceae), a spring-flowering forb. The number of plants in flower and the number of open flowers per plant were recorded every other day to determine phenology, and floral morphology, water potential, pollinator visitation, and seed and fruit set were also recorded. It was found that frost damage was more frequent in the plants with advanced phenology, and that this significantly reduced reproduction of the plants even with supplemental pollen. Plants that escaped frost damage had higher reproduction than control plants and also received more visits from pollinators. To control for effects of snow removal on water availability, a second experiment was carried out, with the flowering of plants being shifted using a greenhouse to create early-flowering, control, and late-flowering plants. The plants were then moved to an outdoor array and observed similarly to the plants in the initial experiment. There was no difference between pollinator visitation to the early and control plants, but visitation was much reduced in the late-phenology plants. This may have been due to a mismatch with the plants’ pollinators, suggesting local adaptation. Together, these results show that both abiotic conditions and phenological overlap with pollinators are important for plant fitness.
Parsche et al. (2011) created control and advanced-flowering wild mustard (Sinapis arvensis L.; Brassicaceae) individuals. They planted the seeds for the advanced-flowering treatment in early March and raised them under artificial long-day conditions, while the control plants were sown in mid-April and grown without supplemental heating or additional light. The advanced-flowering plants were established in the field in late April, and the control plants were established in late May, shortly before flowering. Pollinator visitation was observed in the plants over the flowering period. Visitation by wild bees and hoverflies was lower in the advanced-flowering plants. However, plants were successfully pollinated before the natural flowering time, mostly by generalist pollinators as few of the original pollinators were present, perhaps indicating pre-adaptation. There was increased reproductive output in earlier-flowering plants, possibly due to avoidance of herbivores or unfavorable weather conditions.
Advantages
A merit of some experimental manipulations of phenology is that they can isolate the effects of phenology from other cues or confounding variables in the environment that would accompany spatial transplants. This can be achieved by altering phenology in a greenhouse before placing plants out in the field. Because plant or pollinator communities may be adapted for persistence at certain times of the year, approaches that manipulate phenology independently of other factors can provide information on how timing alone might influence patterns of pollen movement among conspecifics and heterospecifics, as well as competition and facilitation among coflowering species for pollinator visitation. Studying pollinator visitation may reveal if plants flowering at their “local” time are more likely to be visited than plants flowering earlier or later than usual. Controlling for plant density, adaptation and pre-adaptation to temporal niches can be tested for in this way.
Experimental shifts can be used to create phenological “mutants,” whose timing of developmental events occurs outside the current range (e.g., Forrest, 2015; Gezon et al., 2016). These “mutants” have great utility for studying how future changes in phenology may affect plant–pollinator interactions, as plants and pollinators will likely undergo greater phenological shifts as climate change continues. Plants with experimentally advanced flowering could be used to detect pollinator availability at novel times, although as Forrest (2015) points out, it is possible to fail to observe pollinators at experimental plants even though they are active elsewhere in the environment. Nevertheless, by shifting plants or pollinators forward in time, we can gain a more predictive understanding of whether plants and pollinators will be buffered by interaction network rewiring (Burkle and Alarcón, 2011).
The reasons for current shifts in phenology in response to climate change can also be determined using temporal transplants. Changing different abiotic conditions such as precipitation and carbon dioxide levels may differentially affect phenology (e.g., Cleland et al., 2007), and this may indicate which factors are the most important drivers of phenological shifts in response to climate change. In addition, such experiments can test how plastic plants or pollinators are in their phenological responses. Mechanistic understanding can then inform the experimental methods used to manipulate phenology of focal species.
Limitations
One drawback of temporal transplants is the small size of experimental populations of plants and/or pollinators and the small spatial scale of experimental arrays (Forrest, 2015). Patches of early flowers or pollinators could experience mate limitation, as well as artificially low or high visitation or resource availability, making inference about the true effects of climate change tenuous (Forrest, 2015). For studies that aim to measure the effects of shifts in flowering onset (vs. peak flowering, for example), the small scale of temporal transplants might be less of an issue. This is because, at least for plant populations with normally distributed (or left-skewed) flowering curves, relatively few individuals flower initially. Additionally, if density is controlled for among treatment groups, results from such experiments could be interpreted in the context of the joint effects of phenological shifts and fragmentation of plant populations or declines in pollinator populations, both of which could lead to small populations in microclimates that produce aberrant phenologies.
As far as we are aware, no studies have been carried out involving experimental phenological shifts in pollinators. This means that studies are generally one-sided, with only the plants being shifted. Studies into the effects of changing pollinator phenology could be carried out by snow removal/addition on overwintering nests (Forrest, 2015) or by altering nest box temperatures. It may be more feasible to manipulate the phenologies of entire colonies of pollinators, using overwintering queen bumble bees in nest boxes, for example, and test if metrics of colony success are explained by weather variables, rather than to mark and/or track individual pollinators such that their emergence dates and fates could be documented.
Ancillary traits, such as nectar levels, plant height, and floral odor, may be affected by the treatments used to shift the phenology of plants. For example, changes in light levels affect nectar production (e.g., Boose, 1997), and increasing temperature can increase plant height and aboveground productivity (Rustad et al., 2001). Changes in these and other traits may affect pollinator visitation (e.g., Aspi et al., 2003) and may not reflect the true effects of climate change on plant traits, as the changes in these traits may be an artifact of the shifting treatment.
DISCUSSION
To understand and anticipate how climate change will affect plant–pollinator interactions, spatial and temporal transplants are valuable and underused approaches. So far, few studies have used transplants to examine how these interactions will be affected, and fewer still have focused on the effects of climate change (Table 1). Yet, experimental manipulations of phenology and distribution can provide ecological and evolutionary insight into the current and future effects of climate change on plant–pollinator interactions by generating novel contexts in which the responses of populations and individuals can be measured. These manipulations can be used to test for local adaptation and plasticity to changing conditions, which will inform researchers about persistence of populations and interactions under climate change. Moving populations outside of their current ranges in space and time provides a way to test how populations will fare in novel niche space, allowing a more predictive understanding of climate change effects. Models of range dynamics cannot anticipate how novel interactions or interactions in novel climates will shape species’ distributions; experiments are first required to determine the outcomes of such interactions (Alexander et al., 2016). The use of experiments such as spatial and temporal transplants thus provides a valuable way to further our understanding of the potential consequences of climate change for plant–pollinator interactions.
Table 1.
List of studies (published in 2000–2016) that use spatial or temporal transplants of plants or pollinators and consider plant–pollinator interactions and/or pollination success. Studies are grouped according to the type of transplant (spatial or temporal), along with synopses of study design, and whether they focus on climate change.
| Study | Transplant type | Study design | Focus on climate change? |
| Anderson et al. (2015) | Spatial | Reciprocal transplants of two subspecies of Clarkia xantiana across their ranges and contact zone, studying pollinator-mediated selection for different plant traits | No |
| Forrest and Thomson (2011) | Spatial | Reciprocal transplants of solitary bees and wasps over an elevational gradient, comparing emergence times and modeling phenological overlap with floral resources | Yes |
| Gómez et al. (2009) | Spatial | Reciprocal transplants of Erysimum mediohispanicum between sites with strong and weak pollinator-mediated selection, comparing pollinator visitation for the local and foreign plants | No |
| Kalske et al. (2012) | Spatial | Reciprocal transplants of Vincetoxicum hirundinaria, studying local adaptation to the environment, to herbivores and to pollinators, as well as local adaptation of herbivores and pollinators to sympatric plant populations | No |
| Meindl et al. (2013) | Spatial | Reciprocal transplants of Mimulus guttatus inflorescences between sites with serpentine and nonserpentine soil to measure effects on pollinator visitation | No |
| Gezon et al. (2016) | Temporal | Snow removal and greenhouse conditions used to manipulate flowering phenology of Claytonia lanceolata, with field observations of pollinator visitation | Yes |
| Hoover et al. (2012) | Temporal | Controlled-environment chambers used to test the effects of three global change treatments (elevated CO2, elevated N, and increased temperature) on plant phenology and bumble bee preferences | Yes |
| Parsche et al. (2011) | Temporal | Phenological manipulations of Sinapis arvensis carried out using early planting and artificial long-day conditions, followed by field observations of pollinator visitation | Yes |
| Rafferty and Ives (2011) | Temporal | Greenhouse manipulations of 14 plant species, prior to field observations of pollinator visitation | Yes |
| Rafferty and Ives (2012) | Temporal | Greenhouse manipulations of Tradescantia ohiensis and Asclepias incarnata, measuring pollinator effectiveness | Yes |
For both spatial and temporal transplants, the use of historical data to inform experimental manipulations can be beneficial. Historical data can provide a pre–climate change baseline of phenology or range for comparison with observed and projected shifts (e.g., Rafferty et al., 2013; Pyke et al., 2016). In combination with contemporary data, historical data can be used to determine the rate of phenological or distributional response to climate change (e.g., Fitter and Fitter, 2002; Parmesan and Yohe, 2003; Bartomeus et al., 2011). This rate can be used to project the amount of time or the number of generations required for populations to shift into novel ranges or phenologies under further climate change.
In choosing taxa to manipulate, knowledge about the mechanisms underlying shifts in space or time is beneficial, both in ensuring that study designs have relevance and realism and that manipulations are feasible. Thus, understanding of phenological cues and triggers for focal species is useful, as is a recognition that historical correlations among cues could be altered by climate change (Memmott et al., 2007). Generation time of study taxa is another important consideration, especially if evolutionary responses are of interest or controlled genetic crosses are important. Likewise, choice of study area is a critical but often constrained decision. Environmental gradients can vary from coarse to fine in the same space depending on what abiotic variables and taxonomic perspectives are of interest. Temporal responses and trends can be confounded with spatial characteristics, making it important to consider placement of spatial transplant plots carefully (de Keyzer et al., 2017).
Combining the study of spatial and temporal shifts should be especially useful, as many populations are responding across both axes (Parmesan, 2006; Fig. 1D). Furthermore, the rates and directions of response may differ on each axis. For plant–pollinator interactions, the sessile nature of one partner and the mobile nature of the other means that partners are likely to respond at different rates in both space and time, so it is important to study the effects of changes in both dimensions. In addition, spatial transplants often affect phenology due to altered abiotic conditions (e.g., Anderson and Gezon, 2015), and the timing of life history events may shape the spatial niches and distributions of species (Chuine and Beaubien, 2001). Thus, it is important to explicitly consider shifts along one axis resulting from shifts along the other.
Given that climate change often takes many years to have detectable effects upon the phenologies and distributions of populations, experimental transplants offer a relatively rapid and controlled approach to further our understanding of how plant–pollinator interactions will fare. However, experimental shifts do not allow time for adaptive evolution, as shifted populations do not have multiple generations to respond to gradually changing conditions. Experimental shifts in phenology or distribution may therefore predict more severe effects upon plants, pollinators, and their interactions than would occur under a real climatic change. Nevertheless, these manipulations can reveal how selection pressures could change under future climate conditions.
As noted, we are not aware of any studies that experimentally manipulate the phenologies of pollinators, but such work would provide important insight. Could earlier emergence times increase the risk of exposure to extreme cold and its associated impacts to survival and reproduction, in much the way that shifting plants earlier can increase their risk of frost damage? The responses of pollinators to climate warming may be more complex than a simple phenological advance, however. For example, diapause termination may be delayed by warmer winter temperatures, which may mitigate the effects of spring and summer warming on emergence time (Forrest, 2016).
Despite drawbacks, experimental transplants provide a way to empirically test how plant–pollinator interactions will be reshaped in novel environments and communities. General characteristics anticipated to influence how likely mutualisms are to develop mismatches, such as the specialization, obligacy, and seasonality of the interaction (Rafferty et al., 2015), could be tested by transplanting plants or pollinators that span the spectrum of these traits. Indeed, if several species in a community are studied, responses of transplanted plants and pollinators could be linked to functional traits, yielding more mechanistic information that can be used to predict the responses of additional species. The use of experimental transplants that explicitly consider shifts in both time and space should generate unique insight into the likelihood that plant and pollinator populations will adapt to a changing climate.
LITERATURE CITED
- Alarcón R., Waser N. M., Ollerton J. 2008. Year-to-year variation in the topology of a plant–pollinator interaction network. Oikos 117: 1796–1807. [Google Scholar]
- Alexander J. M., Diez J. M., Levine J. M. 2015. Novel competitors shape species’ responses to climate change. Nature 525: 515–518. [DOI] [PubMed] [Google Scholar]
- Alexander J. M., Diez J. M., Hart S. P., Levine J. M. 2016. When climate change reshuffles competitors: A call for experimental macroecology. Trends in Ecology & Evolution 31: 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. T. 2016. Plant fitness in a rapidly changing world. New Phytologist 210: 81–87. [DOI] [PubMed] [Google Scholar]
- Anderson J. T., Gezon Z. J. 2015. Plasticity in functional traits in the context of climate change: A case study of the subalpine forb Boechera stricta (Brassicaceae). Global Change Biology 21: 1689–1703. [DOI] [PubMed] [Google Scholar]
- Anderson J. T., Eckhart V. M., Geber M. A. 2015. Experimental studies of adaptation in Clarkia xantiana. III. Phenotypic selection across a subspecies border. Evolution 69: 2249–2261. [DOI] [PubMed] [Google Scholar]
- Aspi J., Jäkäläniemi A., Tuomi J., Siikamäki P. 2003. Multilevel phenotypic selection on morphological characters in a metapopulation of Silene tatarica. Evolution 57: 509–517. [DOI] [PubMed] [Google Scholar]
- Bartomeus I., Ascher J. S., Wagner D., Danforth B. N., Colla S., Kornbluth S., Winfree R. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proceedings of the National Academy of Sciences, USA 108: 20645–20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boose D. L. 1997. Sources of variation in floral nectar production rate in Epilobium canum (Onagraceae): Implications for natural selection. Oecologia 110: 493–500. [DOI] [PubMed] [Google Scholar]
- Burkle L. A., Alarcón R. 2011. The future of plant–pollinator diversity: Understanding interaction networks across time, space, and global change. American Journal of Botany 98: 528–538. [DOI] [PubMed] [Google Scholar]
- Burkle L. A., Marlin J. C., Knight T. M. 2013. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 339: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Calinger K. M., Queenborough S., Curtis P. S. 2013. Herbarium specimens reveal the footprint of climate change on flowering trends across north-central North America. Ecology Letters 16: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CaraDonna P. J., Iler A. M., Inouye D. W. 2014. Shifts in flowering phenology reshape a subalpine plant community. Proceedings of the National Academy of Sciences, USA 111: 4916-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.-C., Hill J. K., Ohlemüller R., Roy D. B., Thomas C. D. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333: 1024–1026. [DOI] [PubMed] [Google Scholar]
- Chuine I., Beaubien E. G. 2001. Phenology is a major determinant of tree species range. Ecology Letters 4: 500–510. [Google Scholar]
- Cleland E. E., Chuine I., Menzel A., Mooney H. A., Schwartz M. D. 2007. Shifting plant phenology in response to global change. Trends in Ecology & Evolution 22: 357–365. [DOI] [PubMed] [Google Scholar]
- Dauber J., Biesmeijer J. C., Gabriel D., Kunn W. E., Lamborn E., Meyer B., Nielsen A., et al. 2010. Effects of patch size and density on flower visitation and seed set of wild plants: A pan-European approach. Journal of Ecology 98: 188–196. [Google Scholar]
- de Keyzer C. W., Rafferty N. E., Inouye D. W., Thomson J. D. 2017. Confounding effects of spatial variation on shifts in phenology. Global Change Biology 23: 1783–1791. [DOI] [PubMed] [Google Scholar]
- Devoto M., Zimmermann M., Medan D. 2007. Robustness of plant-flower visitor webs to simulated climate change. Ecología Austral 17: 37–50. [Google Scholar]
- Doak D. F., Morris W. F. 2010. Demographic compensation and tipping points in climate-induced range shifts. Nature 467: 959–962. [DOI] [PubMed] [Google Scholar]
- Dunne J. A., Harte J., Taylor K. J. 2003. Subalpine meadow flowering phenology responses to climate change: Integrating experimental and gradient methods. Ecological Monographs 73: 69–86. [Google Scholar]
- Fitter A. H., Fitter R. S. R. 2002. Rapid changes in flowering time in British plants. Science 296: 1689–1691. [DOI] [PubMed] [Google Scholar]
- Forister M. L., Shapiro A. M. 2003. Climatic trends and advancing spring flight of butterflies in lowland California. Global Change Biology 9: 1130–1135. [Google Scholar]
- Forrest J. R. K. 2015. Plant–pollinator interactions and phenological change: What can we learn about climate impacts from experiments and observations? Oikos 124: 4–13. [Google Scholar]
- Forrest J. R. K. 2016. Complex responses of insect phenology to climate change. Current Opinion in Insect Science 17: 49–54. [DOI] [PubMed] [Google Scholar]
- Forrest J., Miller-Rushing A. J. 2010. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 365: 3101–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest J. R. K., Thomson J. D. 2011. An examination of synchrony between insect emergence and flowering in Rocky Mountain meadows. Ecological Monographs 81: 469–491. [Google Scholar]
- Franks S. J., Weber J. J., Aitken S. N. 2014. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evolutionary Applications 7: 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezon Z. J., Inouye D. W., Irwin R. E. 2016. Phenological change in a spring ephemeral: Implications for pollination and plant reproduction. Global Change Biology 22: 1779–1793. [DOI] [PubMed] [Google Scholar]
- Gómez J. M., Abdelaziz M., Camacho J. P. M., Muñoz-Pajares A. J., Perfectti F. 2009. Local adaptation and maladaptation to pollinators in a generalist geographic mosaic. Ecology Letters 12: 672–682. [DOI] [PubMed] [Google Scholar]
- Hargreaves A. L., Weiner J. L., Eckert C. G. 2015. High-elevation range limit of an annual herb is neither caused nor reinforced by declining pollinator service. Journal of Ecology 103: 572–584. [Google Scholar]
- Hegland S. J., Nielsen A., Lázaro A., Bjerknes A.-L., Totland Ø. 2009. How does climate warming affect plant-pollinator interactions? Ecology Letters 12: 184–195. [DOI] [PubMed] [Google Scholar]
- Hoban S., Kelley J. L., Lotterhos K. E., Antolin M. F., Bradburd G., Lowry D. B., Poss M. L., et al. 2016. Finding the genomic basis of local adaptation: Pitfalls, practical solutions, and future directions. American Naturalist 188: 379–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover S. E. R., Ladley J. J., Shchepetkina A. A., Tisch M., Gieseg S. P., Tylianakis J. M. 2012. Warming, CO2, and nitrogen deposition interactively affect a plant-pollinator mutualism. Ecology Letters 15: 227–234. [DOI] [PubMed] [Google Scholar]
- Hughes L. 2000. Biological consequences of global warming: Is the signal already apparent? Trends in Ecology & Evolution 15: 56–61. [DOI] [PubMed] [Google Scholar]
- Iler A. M., Inouye D. W., Høye T. T., Miller-Rushing A. J., Burkle L. A., Johnston E. B. 2013. Maintenance of temporal synchrony between syrphid flies and floral resources despite differential phenological responses to climate. Global Change Biology 19: 2348–2359. [DOI] [PubMed] [Google Scholar]
- Jump A. S., Peñuelas J. 2005. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecology Letters 8: 1010–1020. [DOI] [PubMed] [Google Scholar]
- Kalske A., Muola A., Laukkanen L., Mutikainen P., Leimu R. 2012. Variation and constraints of local adaptation of a long-lived plant, its pollinators and specialist herbivores. Journal of Ecology 100: 1359–1372. [Google Scholar]
- Kelly A. E., Goulden M. L. 2008. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences, USA 105: 11823–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. T., Pindar A., Galpern P., Packer L., Potts S. G., Roberts S. M., Rasmont P., et al. 2015. Climate change impacts on bumblebees converge across continents. Science 349: 177–180. [DOI] [PubMed] [Google Scholar]
- Klein A.-M., Vaissière B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society B. Biological Sciences 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo G., Ida T. Y. 2013. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 94: 2311–2320. [DOI] [PubMed] [Google Scholar]
- Kudo G., Nishikawa Y., Kasagi T., Kosuge S. 2004. Does seed production of spring ephemerals decrease when spring comes early? Ecological Research 19: 255–259. [Google Scholar]
- Liancourt P., Spence L. A., Boldgiv B., Lkhagva A., Helliker B. R., Casper B. B., Petraitis P. S. 2012. Vulnerability of the northern Mongolian steppe to climate change: Insights from flower production and phenology. Ecology 93: 815–824. [DOI] [PubMed] [Google Scholar]
- Meindl G. A., Bain D. J., Ashman T.-L. 2013. Edaphic factors and plant–insect interactions: Direct and indirect effects of serpentine soil on florivores and pollinators. Oecologia 173: 1355–1366. [DOI] [PubMed] [Google Scholar]
- Memmott J., Craze P. G., Waser N. M., Price M. V. 2007. Global warming and the disruption of plant–pollinator interactions. Ecology Letters 10: 710–717. [DOI] [PubMed] [Google Scholar]
- Merow C., Dahlgren J. P., Metcalf C. J. E., Childs D. Z., Evans M. E. K., Jongejans E., Record S., et al. 2014. Advancing population ecology with integral projection models: A practical guide. Methods in Ecology and Evolution 5: 99–110. [Google Scholar]
- Nicotra A. B., Atkin O. K., Bonser S. P., Davidson A. M., Finnegan E. J., Mathesius U., Poot P., et al. 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15: 684–692. [DOI] [PubMed] [Google Scholar]
- Nilsson L. A. 1988. The evolution of flowers with deep corolla tubes. Nature 334: 147–149. [Google Scholar]
- Ogilvie J. E., Thomson J. D. 2016. Site fidelity by bees drives pollination facilitation in sequentially blooming plant species. Ecology 97: 1442–1451. [DOI] [PubMed] [Google Scholar]
- Ollerton J., Winfree R., Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120: 321–326. [Google Scholar]
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics 37: 637–669. [Google Scholar]
- Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. [DOI] [PubMed] [Google Scholar]
- Parsche S., Fründ J., Tscharntke T. 2011. Experimental environmental change and mutualistic vs. antagonistic plant flower-visitor interactions. Perspectives in Plant Ecology, Evolution and Systematics 13: 27–35. [Google Scholar]
- Pyke G. H., Thompson J. D., Inouye D. W., Miller T. J. 2016. Effects of climate change on phenologies and distributions of bumble bees and the plants they visit. Ecosphere 7: e01267. [Google Scholar]
- Rafferty N. E., Ives A. R. 2011. Effects of experimental shifts in flowering phenology on plant–pollinator interactions. Ecology Letters 14: 69–74. [DOI] [PubMed] [Google Scholar]
- Rafferty N. E., Ives A. R. 2012. Pollinator effectiveness varies with experimental shifts in flowering time. Ecology 93: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty N. E., CaraDonna P. J., Burkle L. A., Iler A. M., Bronstein J. L. 2013. Phenological overlap of interacting species in a changing climate: An assessment of available approaches. Ecology and Evolution 3: 3183–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty N. E., CaraDonna P. J., Bronstein J. L. 2015. Phenological shifts and the fate of mutualisms. Oikos 124: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M., Childs D. Z., Ellner S. P. 2014. Building integral projection models: A user’s guide. Journal of Animal Ecology 83: 528–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D. B., Sparks T. H. 2000. Phenology of British butterflies and climate change. Global Change Biology 6: 407–416. [Google Scholar]
- Rustad L. E., Campbell J. L., Marion G. M., Norby R. J., Mitchell M. J., Hartley A. E., Cornelissen J. H. C., et al. 2001. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126: 543–562. [DOI] [PubMed] [Google Scholar]
- Walther G.-R. 2010. Community and ecosystem responses to recent climate change. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 365: 2019–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G.-R., Beißner S., Burga C. A. 2005. Trends in the upward shift of alpine plants. Journal of Vegetation Science 16: 541–548. [Google Scholar]
- Wang S. P., Meng F. D., Duan J. C., Wang Y. F., Cui X. Y., Piao S. L., Niu H. S., et al. 2014. Asymmetric sensitivity of first flowering date to warming and cooling in alpine plants. Ecology 95: 3387–3398. [Google Scholar]