Abstract
Mesenchymal stem cells (MSCs) have immunosuppressive and tissue repair properties, but clinical trials using MSCs to prevent or treat GVHD have shown mixed results. Macrophages (MØs) are important regulators of immunity and can promote tissue regeneration and remodeling. We have previously shown that MSCs can educate MØs toward a unique anti-inflammatory immunophenotype (MSC-educated macrophages or MEMs), however their implications for in vivo models of inflammation have not been studied yet. We now show that in comparison to MØs, MEMs have increased expression of the inhibitory molecules PD-L1, PD-L2, in addition to markers of alternatively activated macrophages: CD206 and CD163. RNA-Seq analysis of MEMs, as compared to MØs, show a distinct gene expression profile that positively correlates with multiple pathways important in tissue repair. MEMs also show increased expression of IL-6, TGF-β, Arginase-1, CD73, and decreased expression of IL-12 and TNF-α. We show that IL-6 secretion is controlled in part by the COX-2, arginase and JAK1/STAT1 pathway. When tested in vivo, we show that human MEMs significantly enhance survival from lethal GVHD, and improve survival of mice from radiation injury. We show these effects could be mediated in part through suppression of human T cell proliferation, and may have attenuated host tissue injury in part by enhancing murine fibroblast proliferation. MEMs are a unique MØ subset with therapeutic potential for the management of GVHD and/or protection from radiation-induced injury.
Keywords: Mesenchymal stem cells (MSCs), macrophages, MSC-educated macrophages (MEMs), graft-versus-host disease (GVHD), radiation injury, IL-6
Introduction
Cellular therapies such as mesenchymal stem cells (MSCs) have potent immunosuppressive properties and have demonstrated efficacy in radiation protection1, 2 and graft-versus-host-disease (GVHD) in experimental and human studies3–5. It has been shown that MSCs isolated from the bone marrow (BM) are effective in treating acute GVHD6, 7, particularly in children with gut GVHD8. However in a phase III trial to treat steroid-resistant GVHD, MSC infusions were found to be safe but did not meet the primary endpoint and were not shown to be superior to pharmacologic immunosuppression9. Thus, clinical grade MSCs are not yet approved in the U.S. for treatment of acute GVHD. While MSCs are conditionally approved in Canada and some other countries to treat acute GVHD, their ultimate role in the management of GVHD is still not clear10–12.
Recent data have shown that MSCs regulate immunity in part through regulation of monocytes13 and macrophages (MØs)14–16. MØs are recognized for their ability to polarize into subsets of classically activated (M1) MØs, which mediate tissue damage and are “pro-inflammatory”, or alternatively activated (M2) MØs, which contribute to wound healing and tissue repair and are “anti-inflammatory”17, 18. More recently, MØs are emerging as important players in mediating immunomodulation and tissue homeostasis19, 20. Previously we described in vitro a unique population of MØs, human MSC-educated MØs (MEMs), characterized by high levels of expression of the anti-inflammatory/tissue regenerative cytokines interleukin (IL)-10 and IL-6, and low levels of expression of the pro-inflammatory cytokines IL-12 and tumor necrosis factor-alpha (TNF-α)21. In contrast, M2 MØs typically express high levels of IL-10 but low levels of IL-6 in addition to low levels of IL-12, and TNF-α18, 22. Since our initial report, other investigators have published on the ability of both murine and human MSCs to recruit monocytes and MØs to sites of inflammation and switch their phenotype to M2 MØs14, 23–25. However, ex vivo generated MEMs have not been examined for their efficacy in inflammatory models in vivo.
We hypothesized that MEMs could be a potential cellular therapeutic for GVHD. In this report, we show that human MEMs have genetic and cell surface molecule expression profiles in vitro consistent with anti-inflammatory cell subsets that facilitate wound healing and tissue repair, and are characterized by high IL-6 expression that is controlled in part by redundant but non-overlapping signaling pathways. We also show that MEMs are also superior to MSCs in managing xenogeneic GVHD in vivo in part by reducing T cell proliferation. MEMs also increase survival from lethal total body irradiation in vivo in part by augmenting fibroblast expansion.
Materials and Methods
Mice
Female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and used males and females between 8–16 weeks of age. All animals were bred and housed in a pathogen-free facility throughout the study. The Animal Care and Use Committee at the University of Wisconsin approved all experimental protocols.
Human MØ & MEM cultures
Healthy donors peripheral whole blood buffy coats were purchased from Interstate Blood Bank, Inc (Memphis, Tennessee). Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coats by density-gradient separation, and monocyte isolation followed by MØ generation were performed as previously described21. MSCs were obtained from BM filters from University of Wisconsin Hospital and Clinics through an IRB approved protocol. Filters were rinsed in PBS and MSCs were isolated from erythrocytes and platelets in the filters by Ficoll density gradient separation, and then cultured in alpha MEM media (Corning CellGro, Manassas, VA) supplemented with 10% heat inactivated FBS (Hyclone, Logan, UT), 100X L-Ala-L-Glutamine (Corning GlutaGro), and 100X NEAA (Corning) and used at passage 4 or 521, 26. MSCs were verified by morphology, adherence onto 75 cm2 plastic flasks (Greiner Bio-one, Monroe, NC) and flow cytometry.
On day +7, after releasing MSCs using trypsin (TrypLe, Invitrogen, Carlsbad, CA) the cells were washed twice with PBS, and then added to MØ cultures to develop MEMs, at a ratio of 10: 1 of MØs:MSCs, as previously described21. On day +10, CD14+ cells were collected using Stem Pro Accutase cell detachment (Gibco Life Technologies) and resorted based on CD14 to eliminate the MSCs in the MEM cultures.
Measurement and inhibition of IL-6 production
On day +7 of human MØ generation from CD14+ monocytes, MØs were plated at 2x105 cells/well in a 6-well plate in media alone or stimulated with 20 ng/ml of recombinant human IL-4 (animal free) (BioLegend, San Diego, CA) or 50 ng/ml human IL-13 (animal free) (PeproTech Inc, Rocky Hill, NJ), or co-cultured with BM-MSCs (2x104 cells) in direct cell contact to generate MEMs (ratio of MØ: MSC is 10:1). Some groups of MEMs (MØ plus MSCs in direct contact) were treated with NS398, a cyclooxygenase (COX)-2 inhibitor, at 5 μM/well (Cayman Chemical, Ann Arbor, MI), NOR-NOHA, an arginase inhibitor, at 300 μM/well (Cayman Chemical), or Ruxolitinib, a JAK1/JAK2 inhibitor, at 1 μM/ml (INCB-018424, Active Biochem, Maplewood, NJ).
For transwell experiments, 0.4 μm pore size transwells (24mm insert, Corning, Kennebunk, ME) were placed in 6-well plates containing MØs (2x105 cells/well) in the lower chamber and allowed to differentiate for 7 days. Then MSCs (2x104 cells/transwell) were seeded in the upper chamber above the MØs and incubated at 37°C for 3 more days as previously described21. Supernatant from lower chamber of the transwell system were collected and frozen at −20°C for IL-6 assessment by Enzyme-Linked Immunosorbent Assay (ELISA). In other experiments, after generation of MØs or MEMs for 10 days, CD14+ cells were re-sorted from MEMs to purify the MØ population and eliminate MSCs in the MEM cultures. CD14+ MØs or CD14+ MEMs were re-plated in 96-well plates in triplicate for each donor at 1 x 105 cells/well in media or stimulated with 1 ug/ml lipopolysaccharide (LPS) from Escherichia Coli ultra pure 0111:B4 (Sigma Aldrich, St. Louis, MO). Human interleukin-6 (IL-6) levels in supernatants from human MØ, MEMs, MØ + IL-4 or MØ + IL-13 were quantified using Legend Max ELISA kit with pre-coated plates with human IL-6 according to manufacturer’s instructions (BioLegend, Inc., cat#: 430507). ELISA plates were read at 450 nm on a VERSAmax Tunable Plate Reader (Molecular Devices, Sunnyvale, CA) and data were collected using SOFTmax PRO software (Molecular Devices).
RT-PCR
Total RNA was extracted using the RNeasy mini kit (Qiagen, Germantown, MD) with on column DNAse I treatment (Qiagen) following manufacturer’s instructions. Total RNA from each sample was quantified using a Nano Drop and the resultant RNA was reverse transcribed to cDNA using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). cDNA was then used for RT-PCR using AB Step-ONE Plus (Applied Biosystems) in the presence of SYBR Green Supermix (Applied Biosystems, Warrington, UK). Human primer assays for TNF, IFN-γ, IL-6, IL-12α, IL-1α, IL-1β, IL-10, TGFβ, Arginase-1, NOS2, NOS2, CD206, CD163, CD39 and CD73 were purchased from QIAGEN. Each reaction mixture of primer, cDNA, Syber green and water was set up 3 to 5 replicates per treatment group for each of those primers. mRNA levels were calculated using the comparative threshold cycle method (Ct). Ct values for the housekeeping gene (GAPDH) and for the genes of interest were determined, and the difference between the Ct values of each gene of interest and the mean GAPDH Ct was calculated (ΔCt). Differences in ΔCt (ΔΔCt) of genes of interest in MEMs were normalized to MØ control group as shown in the following equation: ΔΔCt = ΔCt(MEMs) − ΔCt(mean of MØs). RT-PCR data are presented as fold change expression = 2−ΔΔCt of each gene in comparison to MØ group. A dissociation melt curve at the end of RT-PCR was also run to verify the homogeneity of the PCR products and absence of primer-dimers.
RNAseq library preparation
MØs or MEMs were generated for 10 days as described above. RNA was extracted from frozen cell pellets of MØ derived from blood (MØ-PBMC) or MEMs (also blood derived) or MØ derived from BM (MØ-BM) using Qiagen’s RNeasy kit. RNA was treated with DNase I (New England Biolabs, Ipswich, MA) then analyzed for quality using the RNA 6000 Pico Kit (Agilent Technologies, Santa Clara, CA) and the Agilent 2100 Bioanalyzer. 1–5ug of total RNA was treated with RiboZero rRNA Removal Reagent (Epicentre, Madison, WI) and then qualitative analysis was performed on the Bioanalyzer to ensure 18S and 28S rRNA peaks were no longer present. 0.5–50ng of rRNA-depleted total RNA was used to generate RNA sequencing libraries using the ScriptSeq v2 RNA-Seq Library Preparation Kit (Epicentre). Libraries were prepared to a mean size of 300–500 base pairs and analyzed using the High Sensitivity DNA Kit (Agilent Technologies) with the Bioanalyzer. Libraries were then diluted 1:10,000 and analyzed using the Kapa Library Quant Kit (Kapa Biosystems, Wilmington, MA) on the LightCycler96 (Roche Diagnostics, Indianapolis, IN). The samples were then normalized for accurate loading on the Illumina Hiseq2000 platform.
In vivo xenogeneic GVHD model
In the established GVHD model, on day +0, male and female NSG mice received 30x106 human PBMCs previously frozen and thawed and injected in 0.2 ml intravenously (i.v) in the absence of any conditioning. Engraftment of human CD45+ cells were assessed by flow cytometry and staining for human anti-CD45 in the spleen, peripheral blood and BM27 prior to starting treatment with PBS, MEMs or MSCs. On day +18, when mice showed clinical evidence of GVHD, mice were randomized to receive PBS, 5x105 human BM-derived MSCs (passage 4 or 5) or 5x105 MEMs in 0.2 ml of PBS i.v to treat GVHD and monitored for survival.
In vivo lethal radiation model
On day 0, NSG male and female mice received 3 Gy lethal total body irradiation followed by (3 hours later) PBS, 5x105 human MØs, 5x105 human BM-derived MSCs (passage 4 or 5) or 5x105 MEMs treatment i.v. Mice were monitored 3 times a week for survival, weight change and clinical sickness scores, similar to GVHD scoring, based on weight loss, posture, activity, fur grooming and skin texture, as previously described28.
Murine fibroblast proliferation assay
After 10 days of macrophage generation, human CD14+ sorted MØ and CD14+ sorted MEMs (to eliminate MSCs) were seeded at 2.5 x 105 cells with 1 x 104 NIH-3T3-GFP murine fibroblasts (Cell Biolabs Inc, San Diego, CA) in MØ media in T-25 flasks for 7 days at 37°C. NIH-3T3-GFP cells were also labeled with Violet Proliferation Dye 450 (VPD450) (BD Biosciences, cat #562158) when adding them with MØ or MEMs in order to check fibroblast proliferation by flow cytometry on day 7 of co-cultures. Proliferating NIH-3T3 were gated on GFP+ VPD450− cells.
Statistical analysis
Statistics were performed using GraphPad Prism version 6.0 for the Macintosh OS (GraphPad Software, San Diego, CA). Data were reported as mean ± standard error of the mean (SEM). For analysis of three or more groups, the non-parametric ANOVA test was performed with the Bonferroni or Tukey’s multiple comparisons post-test. Principal component analysis and t-tests comparing gene expression between groups were run with Matlab (version 2016a, MathWorks). Multiple hypotheses testing correction was done using the Benjamini Hochberg FDR procedure. A p-value less than 0.05 was considered statistically significant. P values: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P <0.0001.
Results
Human MEMs show increased expression of cell surface markers associated with alternatively-activated MØs
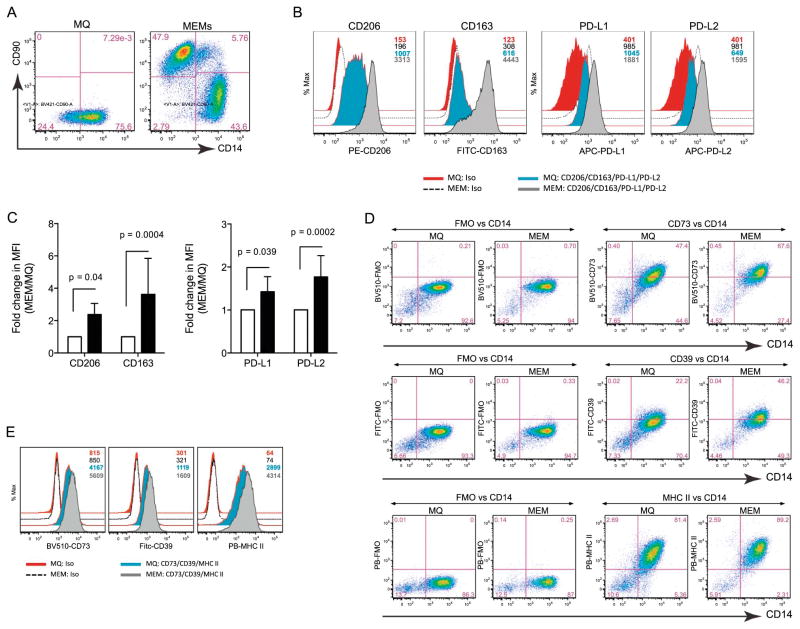
MEMs are essentially monocytes isolated from peripheral blood that are then cultured for 7 days into adherent MØs, followed by a 3 day co-culture with BM-derived MSCs at a 10:1 ratio21. On day 10, CD14+CD90− cells are re-sorted to eliminate MSCs (which are CD14−CD90+) from MEM cultures (Figure 1A). Previous work by our group has shown that MEMs show increased expression of IL-6 and IL-10, but decreased expression of IL-12 and TNF-α21. To further characterize the immunophenotype of MEMs, we examined the cell surface expression of molecules typically expressed on alternatively activated MØs, such as the mannose receptor CD20629 and the scavenger receptor CD16330. By flow cytometry, MEMs show elevated surface marker expression of both CD163 and CD206 and upregulate the inhibitory checkpoint molecules PD-L1 and PD-L231, as compared to conventional MØs (Figures 1B and 1C). MEMs also show elevated expression of the ecto-nucleotidases CD39 and CD7332, as well as MHC class II (Figures 1D and 1E). Taken together, MEMs appear to have increased protein expression of several different cell surface molecules that have been associated with cell subsets that tend to be anti-inflammatory.
Figure 1. MEMs express higher levels of CD206, CD163, PD-L1, PD-L2, CD39 and CD73.
On day +10 of ex vivo expansion, MØ or MEMs were analyzed by flow cytometry for CD90 and CD14. (A) CD14+CD90− cells are MØs. CD14−CD90+ cells are MSCs. (B) Cell surface expression of CD206, CD163, PD-L1 and PD-L2 were determined on the CD14+CD90− population in MØ and MEM cultures by MFI (Mean fluorescent intensity) of isotypes (iso), MØs and MEMs, respectively. Numbers denote the MFIs for each group with color-matched histograms. (C) CD206, CD163, PD-L1/L2 fold change in MFI in MEM over MØ from 6 donors. (D) Expression of CD73, CD39, MHC II (HLA-DR) of fluorescent minus one (FMO) vs. CD14 on MØ and MEMs. (E) MFI of iso, MØs and MEMs for CD73, CD39 or MHC II. Numbers in plots denote MFI for each group. Bar graph statistics (mean ± SEM) by Two-way ANOVA with Bonferroni multiple comparisons.
MEMs demonstrate a unique gene expression signature profile correlated with increased expression of anti-inflammatory and tissue repair genes
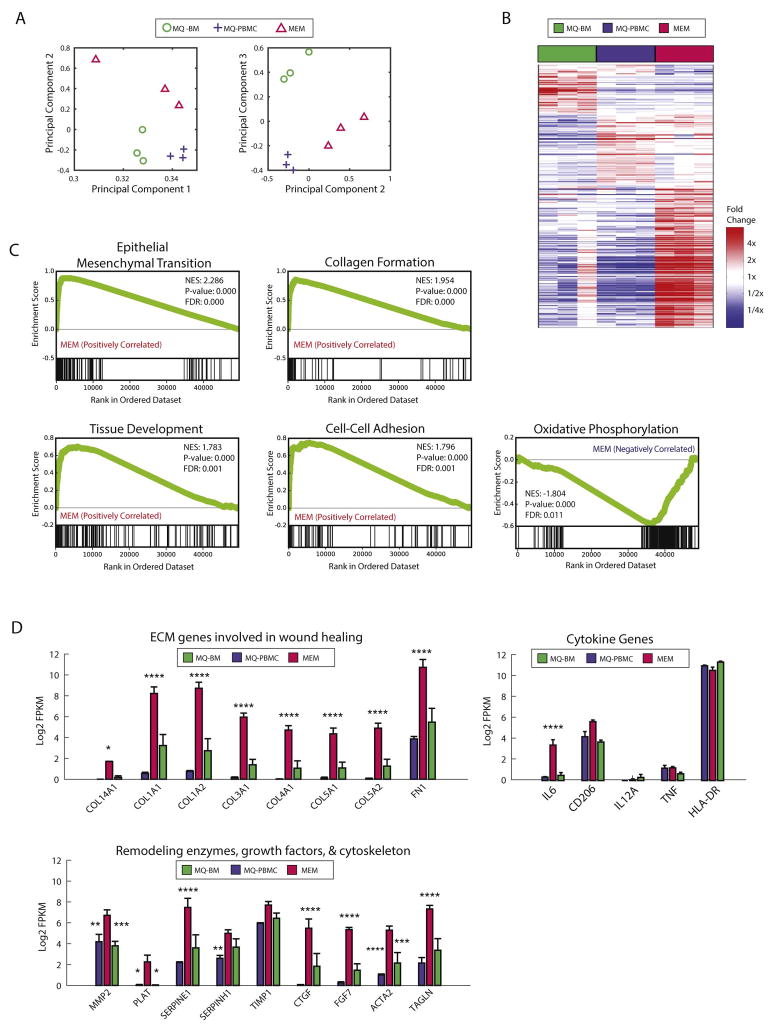
We performed RNA-Seq analysis on MØs generated from peripheral blood mononuclear cells (MØ-PBMCs), macrophages from BM (MØ-BM), and MEMs (Tables S1–S3), to compare their gene expression profiles, examine for differences in gene expression signatures and determine if the differences were associated with typical cellular pathways associated with anti-inflammatory processes and/or wound healing. The data discussed in this publication have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus and are accessible through Gene Expression Omnibus Series accession number: GSE93155. Principal component analysis showed that MEMs had a global gene expression profile that was distinct from MØ-PBMC or MØ-BM (Figure 2A). Hierarchal clustering analysis demonstrated that the replicates from the 3 sources of macrophages cluster together, and showed distinct gene expression with a subset of genes unique to each cell type (Figure 2B). In the MEM subset, genes associated with the epithelial-mesenchymal transition (EMT), collagen formation, tissue development, and cell-cell adhesion were positively correlated with MEMs, while genes associated with oxidative phosphorylation were negatively correlated with MEMs (Figure 2C). By examining select individual genes, we confirmed that MEMs had increased gene expression of IL-6 (Figure 2D), as we previously described at the protein level21. In addition, we found increased expression of 8 genes that are associated with wound healing as well as genes representing remodeling enzymes, growth factors and the cytoskeleton (Figure 2D).
Figure 2. MEMs express a unique gene expression profile that distinguishes them from macrophages cultured from peripheral blood or bone marrow.
Human MØs were generated from CD14+ monocytes from PBMCs or BM cells for 1 week. MEMs were generated by incubating PBMC-derived MØ (MØ-PBMC) with MSCs at a 10:1 ratio for 3 days. On day +10 of culture, CD14+ macrophages were re-sorted from the MØ-PBMCs, MØ-BM and MEM cultures and RNA was isolated for RNA-Seq. (A) Principal component analysis was applied to RNASeq gene expression of 9 samples, 3 each of MEM (red triangle), MØ-BM (green circle) and MØ-PBMC (blue cross) populations. Each symbol represents a unique sample. Samples are plotted based on their coefficients in principal component space. (B) Each column of the heatmap is a unique sample (red: MEM, green: MØ-BM, blue: MØ-PBMC). Genes shown are those with at least a 2-fold difference and false discovery rate<0.15 between any of the group comparisons. (C) Gene set enrichment analysis was run comparing MEMs (N=3) to other macrophages (MØ-BM and MØ-PBMC, N=6). Genes in each set are indicated by black lines along the bottom tracks. The enrichment score across the list is plotted in green. NES= Normalized enrichment score, FDR= false discovery rate. (D) Log2 transformed FPKM RNAseq gene expression, averaged across the 3 replicates for each population (red: MEM, green: MØ-BM, blue: MØ-PBMC). Error bars show SEM.
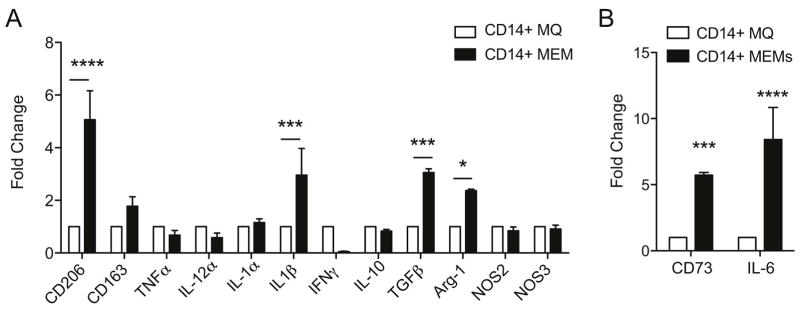
We next chose select genes that showed significantly increased gene expression in the enrichment analysis and then verified their expression by RT-PCR. Indeed, we found that MEMs demonstrate increased CD206, IL-1β, TGFβ and arginase-1 as compared to MØs (Figure 3A), as well as verified increased IL-6 and CD73 expression (Figure 3B), indicating MEMs may have increased capacity to abrogate inflammation (Arginase-1, CD73 and TGF-β) and contribute to tissue repair (IL-6 and TGF-β).
Figure 3. Decreased pro-inflammatory cytokine profile and increased expression of anti-inflammatory genes by MEMs.
On day +10 of ex vivo expansion, CD14+ sorted MØ or CD14+ sorted MEMs were collected for RT-PCR. (A–B) Fold change in mRNA expression of genes normalized to GAPDH housekeeping gene. N=3 and set up in triplicate. Mean ± SEM analyzed by Two-way ANOVA with Bonferroni’s multiple comparison. ****P<0.0001, ***P<0.001 and *P<0.05.
High IL-6 production by MEMs is stimulated by LPS exposure and is dependent on COX2, Arginase-1 and the JAK/STAT pathways
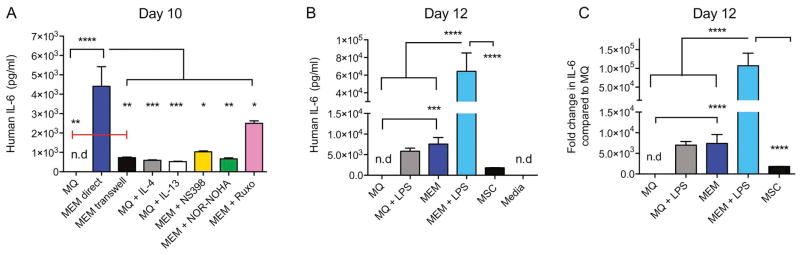
In contrast to other types of alternatively activated MØs, MEMs have increased gene expression of IL-6, and combined with other gene set enrichment analyses, appear to be a distinct subset of MØs. We next compared IL-6 protein production by MEMs versus M2 MØs by ELISA, and then examined the mechanism by which the IL-6 production is regulated. MEMs produced over 4x greater amounts of IL-6 as compared to M2 MØs (Figure 4A). To determine the mechanisms involved in MEM IL-6 production, we exposed the cells to drugs that had been previously described to attenuate IL-6 production by macrophages, including the selective COX-2 inhibitor NS39833, the arginase inhibitor NOR-NOHA34, or the JAK1/JAK2 inhibitor Ruxolitinib35. Targeting all 3 pathways led to attenuation of IL-6 production (Figure 4A), demonstrating the presence of redundant but non-overlapping mechanisms of IL-6 regulation. Because total body irradiation can lead to damage to the gastrointestinal tract, causing translocation of gut bacteria and release of lipopolysaccharide (LPS)36, we explored if LPS-stimulation could exacerbate IL-6 production. Using LPS stimulation of MEMs, IL-6 production can be increased to a greater degree than LPS-treated MØs or MSCs alone (Figure 4B).
Figure 4. MEMs secrete higher IL-6 constitutively or after LPS stimulation, and is dependent on direct contact with MSCs, via JAK1/JAK2, arginase and COX2 pathways.
(A) IL-6 production by ELISA was measured in MØs exposed to media alone (MQ), in direct contact with MSCs (MEM direct), in MØ with MSCs added to the upper chamber of a Transwell (MEM transwell), in MØ with rh-IL4 (MQ+IL-4) or rh-IL13 (MQ+IL-13), or in MEMs exposed to NS398 (MEM + NS398), NOR-NOHA (MEM + NOR-NOHA), or Ruxolitinib (MEM + Ruxo). Each group was set up in triplicate, n=3–8 donors. (B–C) IL-6 production by ELISA was measured in MØs sorted from MØs + media or MØs + MSCs (MEMs) and re-plated in fresh media with or without LPS for 2 days. N= 3 donors and each group was set up in triplicate. (B) Human IL-6 concentration and (C) fold change in human IL-6 production by each group compared to MØ in media alone is shown. Mean ± SEM analyzed by one-way ANOVA with Tukey’s multiple comparisons. Data representative of 3 experiments with reproducible results. n.d: not detected.
MEMs protect mice from xenogeneic GVHD potentially by reducing T cell proliferation
We assessed, for the first time, the ability of MEMs to protect lethally irradiated mice from established GVHD induced by human PBMCs. In this model, we obtain > 50% human CD45 engraftment in multiple murine tissues by day +12 (Figure 5A). After injecting human PBMCs on day +0, we allowed mice to develop clinical signs of GVHD28 such that by day +18, mice were randomized to infusions with vehicle (PBS), human MSCs or MEMs. Within a week, recipients of vehicle or MSCs had 100% lethality, whereas recipients of a single infusion of MEMs had 50% survival at 50 days out (Figure 5B). By examining T cells co-cultured with vehicle, MØs or MEMs ex vivo, we also found that MEMs significantly reduced human T cell proliferation (Figure 5C), explaining a potential mechanism by which MEMs could reduce GVHD-associated inflammation.
Figure 5. Treatment of xenogeneic GVHD with MEMs allow for increased survival & inhibit T cells proliferation in vitro.
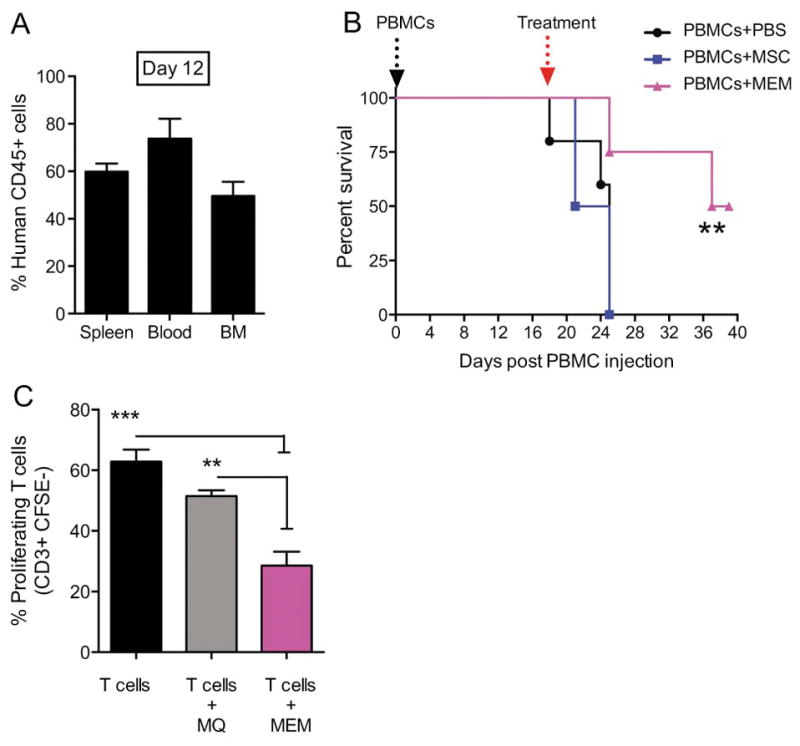
(A) Day +0, NSG mice received 30x106 PBMCs i.v to induce a xenogeneic GVHD in the absence of total body irradiation. (A) On Day +12 post transplant, human CD45+ engraftment in spleen, blood and bone marrow (BM) was assessed. (B) On day +18, when mice showed clinical evidence of GVHD, mice were randomized to receive PBS, 5x105 MSCs or MEMs i.v to treat GVHD and monitored for survival. N= 5 mice/group, one representative experiment of 3 performed. Survival curves compared by log rank analysis, **P<0.001 (C) Allogeneic CD3+ sorted CFSE-labeled T cells were cultured in triplicate with anti-CD3 and anti-CD28 antibodies either alone (T cells), with MØ (T cells + MØ), or MEM (T cells + MEM) at a 2:1 ratio for 5 days. Cells were then collected from each group and stained for CD3+ to determine the percentage of proliferating cells, which are CD3+CFSE−. Mean ± SEM calculated by one-way ANOVA.
MEMs protect mice from lethal radiation injury in part by inducing host fibroblast proliferation
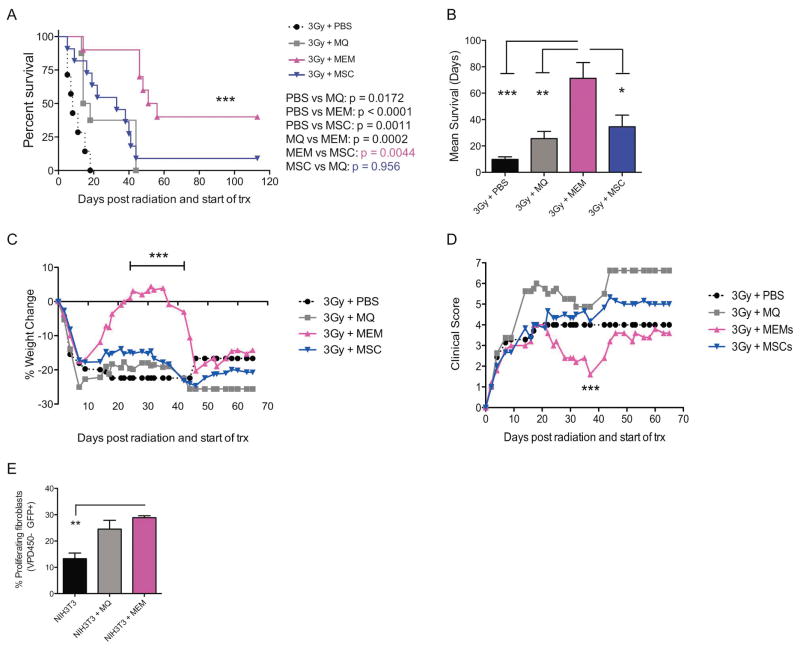
To determine if MEMs can also protect host murine tissues against radiation damage, we challenged mice with a lethal dose of radiation instead on day +0, followed by a single treatment with vehicle, MØs, MSCs or MEMs. Within 20 days, vehicle control mice had 100% lethality but the length of survival could be extended with infusion of MØs, after which all mice still died by day +42 (Figure 6A). Infusion of MSCs also led to a prolongation of survival, but lethality was 90% (Figure 6A). In contrast, infusion of MEMs prolonged the length of survival (Figure 6B), temporarily recovered weight loss (Figure 6C), and transiently reduced clinical scores (Figure 6D). Although the improvements in weight loss and clinical scores from a single MEM infusion were transient, MEM recipients had the lowest lethality (60%) as compared to all groups (Figure 6A), perhaps because of induction of other wound healing pathways contributing to radioprotective effects.
Figure 6. Treatment of lethal radiation with MEMs allows for increased survival and improved weight loss and clinical scores.
(A–D) On day 0, NSG mice received lethal total body irradiation (3Gy) followed by (3 hours later) PBS, 5x105 MØs, 5x105 MSCs or 5x105 MEMs treatment i.v. N= 8–11 mice/group. (A) Survival curve compared by log rank analysis. (B) Median survival in days for each group.*** p= 0.0003, **p= 0.0066, *p= 0.02, mean ± SEM by one-way ANOVA of analysis with Bonferroni multiple comparison post test. (C) Percent weight change compared to day 0 for each group, ***p< 0.0001, Two-way ANOVA with Tukey’s multiple comparisons post test. (D) Overall clinical score (weight loss, posture, activity, skin and fur texture). On day 37: *p=0.015 MEM vs PBS, *p= 0.011 MEM vs MSC and ***p= 0.0002 MEM vs MØ, two-way ANOVA with Tukey’s multiple comparisons post test. Data representative of one of 2 experiments with similar results. (E) MØ or MEM were co-cultured at 25:1 ratio with NIH-3T3-GFP+ cells pre-labeled with Violet proliferation dye 450. On day 7, the percentage of proliferating NIH-3T3 fibroblasts was assessed by flow cytometry by gating on GFP+ VPD450− cells and compared to NIH-3T3-GFP cells cultured alone. Mean ± SEM calculated by two-way ANOVA with Tukey’s multiple comparisons post test. Data representative of one of 2 experiments with similar results.
Because fibroblasts play an active role in normal wound healing37 and the repair of tissue injury from radiation38, 39, we examined if the potential mechanism by which human MEMs contribute to radioprotection was through effects on murine fibroblasts. We quantified the amount of murine fibroblast proliferation in the absence or presence of MEMs and found human MEMs had the ability to augment murine fibroblast proliferation comparable to murine MØs (Figure 6E).
Discussion
MØs are largely thought to be involved in the pathogenesis of GVHD as they are primed by LPS to release TNF-α, a classic M1 phenotype initiating event40. Other studies have also verified their important role in refractory acute GVHD41 and chronic GVHD42. However, these adverse effects are likely subset dependent, and as other subsets of MØs have emerged, we propose that immunomodulatory and tissue regenerative properties associated with ex vivo generated alternatively-activated MØs make them very appealing for treatment of inflammatory disorders like GVHD and radiation injury. Whereas M2 MØs typically secrete low levels of IL-6, MEMs secrete high levels of IL-6, an interleukin generally considered to be pro-inflammatory; however, recent data shows that IL-6 could have anti-inflammatory properties43, plays a critical role in promoting regeneration in many tissues44,45 and support BM hematopoiesis46, 47, and decreasing radiation-induced inflammation48. IL-6 has also been described to induce alternatively activated MØs49, 50, promote mucosal healing from colitis51 and cartilage self-repair by MSCs52. We show IL-6 production is induced in MEMs by LPS, and modulated by multiple pathways, including COX-2, arginase-1 and the JAK1/STAT1 pathway.
Using an unbiased RNA-Seq approach, we found that MEMs express genes positively correlated with several pathways that could be beneficial for anti-inflammatory effects or tissue repair (e.g. collagen formation or tissue development genes). Specifically, we verified increased expression of IL-1β, TGF-β, and Arginase-1. Classically IL-1β is described as an acute phase reactant early during inflammation, but in certain immunologic contexts plays a major role in tissue repair. IL-1β levels may contribute to early bone healing53 and prime MSCs to promote healing of airway epithelial cells54. TGF-β classically is involved in fibrosis and protects cell survival during radiation55, structural changes of tissues via EMT and inhibition of cell proliferation56, and has a protective role in acute GVHD57. Arginase-1 regulates nitric oxide production, is required for early wound healing58, and has been shown to inhibit GVHD59. Thus MEMs appear to have increased expression of several potential molecules that could have tissue protective effects in vivo. Future studies with MSCs and MEMs should compare cytokine induction profiles by each of these cell types in vivo to better understand potential overlapping mechanisms in tissue repair.
We also show that MEMs, despite some differences, share characteristics of other alternatively activated MØs, such as expression of CD274 (PD-L1) and CD273 (PD-L2), molecules that have been previously demonstrated to regulate GVHD60. Moreover, we observed increased MEM expression of molecules such as CD39 and CD73, ecto-nucleotidases that convert ADP/ATP to AMP and AMP to adenosine32. Interestingly these molecules have been shown to be upregulated by MSCs61, leading to increased adenosine and suppression of T cell proliferation62, 63. We also observed that CD39/CD73+ MEMs decrease T cell proliferation ex vivo. Since CD73 deficiency exacerbates GVHD64, 65, further studies should examine if therapies that induce CD73 expression/function would enhance MEM-mediated GVHD protection further.
Using xenogeneic models of GVHD and radiation injury, we found that MEMs were superior to MSCs in treating established GVHD, potentially due to decreased T cell proliferation. Another potential explanation could be related to the fact that in clinical GVHD, third party MSC infusions actually educate host MØs to become anti-inflammatory. Because NSG mice have been described to have dysfunctional MØs66, and MSCs have been shown to require functional CD11b+ cells to protect against colitis67, perhaps infusing MEMs overcomes this defect and thus leads to improved survival from xenogeneic GVHD. In a lethal radiation model, mice treated with MEMs had improved survival compared to MSCs or MØs. This effect may be due in part by stimulating host fibroblast proliferation, which may play an active role in normal wound healing37 and the repair of tissue injury from radiation38, 39. Future studies should examine the mechanisms of these beneficial effects and whether MEMs have to be patient-derived or could be used from a third party, “off the shelf” bank as MSCs are currently used. Alternative studies could also determine whether secreted factors present in our transwell assays, like multi-vesicular bodies such as exosomes, which have been recently shown to be produced by MSCs68, could offer an alternative, cell-free, third party product to generate radioprotective MEMs and could be banked for usage in a radiation emergency.
In summary, we have demonstrated that human MEMs represent a novel and distinct cell subset of alternatively activated MØs that express a series of cell surface molecules and cytokines at the gene and protein level that may have anti-inflammatory benefits as well as contribute to tissue repair. Using models of xenogeneic GVHD and radiation injury, we demonstrate that ex vivo generated MEMs have therapeutic potential in vivo. These studies provide the foundation for cell-based education of MØs as therapy for GVHD, radiation damage and potentially other inflammatory conditions.
Supplementary Material
Gene expression data from all expressed genes for all 9 samples. Genes have FPKM>0 in at least one sample. Data shown is log transformed FPKM (log2(FPKM+1)).
Genes shown are differentially expressed in any of the one vs. one and one vs. all comparisons between the 3 groups. Differentially expressed criteria includes at least a 2x fold change (log2(FC)>1) and FDR<0.15.
Top 20 gene sets enriched in MEMs and enriched in other MQs shown. Gene sets are ranked by normalized enrichment score. Gene set size indicates number of genes in gene set. Other GSEA related statistics are also included (gene rank at max, p-value and FDR and FWER q-values).
Highlights.
MSC-educated macrophages (MEMs) are an alternatively activated macrophage subset
MEMs secrete high levels of IL-6 controlled in part by JAK/STAT, arginase and COX
Infusion of MEMs lead to better survival than MSCs in established xenogeneic GVHD
MEMs improve survival from radiation injury in part through host fibroblasts
Acknowledgments
We would like to thank Sydney Olson for valuable assistance with this study. Thank you to the University of Wisconsin Carbone Cancer Center (UWCCC) Flow Cytometry core facility for support NIH/NCI P30 CA014520. This work was supported in part by the American Association of Immunologists Careers in Immunology Fellowship (M.N.B), the Hertz Fellowship (A.B.M.), the Don Anderson GVHD fund and Crystal Carney Fund for Leukemia Research (P.H.), NIH/NCATS UL1TR000427 to the UW ICTR and NIH/NCI P30 CA014520 to the UWCCC (P.H. and C.M.C.), and NIH/NCI K08 CA174750 (C.M.C).
Footnotes
Authorship Contributions: M.N.B. designed and performed research; collected, analyzed, and interpreted data; performed statistical analysis; and drafted the manuscript. A.M. and C.L. performed RNA-Seq; analyzed and interpreted data; and revised the manuscript. J.K., J.A.K and D.D.B. performed research and revised the manuscript. S.D. supervised RNA-Seq and revised the manuscript. P.H. designed and supervised research; analyzed and interpreted data; and revised the manuscript. C.M.C. designed and supervised research; analyzed and interpreted data; drafted and revised the manuscript.
Financial disclosure statement: The authors have declared that no relevant financial conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, et al. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One. 2011;6:e14486. doi: 10.1371/journal.pone.0014486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Balakrishnan I, Torok-Storb B, Pillai MM. Marrow Stromal Cell Infusion Rescues Hematopoiesis in Lethally Irradiated Mice despite Rapid Clearance after Infusion. Adv Hematol. 2012;2012:142530. doi: 10.1155/2012/142530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. The Journal of experimental medicine. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–644. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- 5.Sawitzki B, Brunstein C, Meisel C, et al. Prevention of graft-versus-host disease by adoptive T regulatory therapy is associated with active repression of peripheral blood Toll-like receptor 5 mRNA expression. Biol Blood Marrow Transplant. 2014;20:173–182. doi: 10.1016/j.bbmt.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 7.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Ball LM, Bernardo ME, Roelofs H, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013;163:501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- 9.Galipeau J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Hashmi S, Ahmed M, Murad MH, et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol. 2016;3:e45–52. doi: 10.1016/S2352-3026(15)00224-0. [DOI] [PubMed] [Google Scholar]
- 11.Auletta JJ, Cooke KR, Solchaga LA, Deans RJ, van’t Hof W. Regenerative stromal cell therapy in allogeneic hematopoietic stem cell transplantation: current impact and future directions. Biol Blood Marrow Transplant. 2010;16:891–906. doi: 10.1016/j.bbmt.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron F, Storb R. Mesenchymal stromal cells: a new tool against graft-versus-host disease? Biol Blood Marrow Transplant. 2012;18:822–840. doi: 10.1016/j.bbmt.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiossone L, Conte R, Spaggiari GM, et al. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells. 2016;34:1909–1921. doi: 10.1002/stem.2369. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho DI, Kim MR, Jeong HY, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 17.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews. Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 20.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggenhofer E, Hoogduijn MJ. Mesenchymal stem cell-educated macrophages. Transplant Res. 2012;1:12. doi: 10.1186/2047-1440-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PloS one. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Denu RA, Dollar BA, et al. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br J Haematol. 2012;158:336–346. doi: 10.1111/j.1365-2141.2012.09154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockridge JL, Zhou Y, Becker YA, et al. Mice engrafted with human fetal thymic tissue and hematopoietic stem cells develop pathology resembling chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:1310–1322. doi: 10.1016/j.bbmt.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 29.Deng W, Chen W, Zhang Z, et al. Mesenchymal stem cells promote CD206 expression and phagocytic activity of macrophages through IL-6 in systemic lupus erythematosus. Clin Immunol. 2015;161:209–216. doi: 10.1016/j.clim.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Moestrup SK, Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36:347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 32.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 33.Chen BC, Liao CC, Hsu MJ, et al. Peptidoglycan-induced IL-6 production in RAW 264.7 macrophages is mediated by cyclooxygenase-2, PGE2/PGE4 receptors, protein kinase A, I kappa B kinase, and NF-kappa B. J Immunol. 2006;177:681–693. doi: 10.4049/jimmunol.177.1.681. [DOI] [PubMed] [Google Scholar]
- 34.Hu H, Moon J, Chung JH, Kim OY, Yu R, Shin MJ. Arginase inhibition ameliorates adipose tissue inflammation in mice with diet-induced obesity. Biochem Biophys Res Commun. 2015;464:840–847. doi: 10.1016/j.bbrc.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 35.Maschalidi S, Sepulveda FE, Garrigue A, Fischer A, de Saint Basile G. Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice. Blood. 2016;128:60–71. doi: 10.1182/blood-2016-02-700013. [DOI] [PubMed] [Google Scholar]
- 36.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol. 1997;42:99–106. doi: 10.1016/s0167-8140(96)01880-4. [DOI] [PubMed] [Google Scholar]
- 38.Haimovitz-Friedman A, Vlodavsky I, Chaudhuri A, Witte L, Fuks Z. Autocrine effects of fibroblast growth factor in repair of radiation damage in endothelial cells. Cancer Res. 1991;51:2552–2558. [PubMed] [Google Scholar]
- 39.Fuks Z, Persaud RS, Alfieri A, et al. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994;54:2582–2590. [PubMed] [Google Scholar]
- 40.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992;175:405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiwaki S, Terakura S, Ito M, et al. Impact of macrophage infiltration of skin lesions on survival after allogeneic stem cell transplantation: a clue to refractory graft-versus-host disease. Blood. 2009;114:3113–3116. doi: 10.1182/blood-2009-03-209635. [DOI] [PubMed] [Google Scholar]
- 42.Alexander KA, Flynn R, Lineburg KE, et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124:4266–4280. doi: 10.1172/JCI75935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Pachowka M, Zegarska J, Ciecierski R, Korczak-Kowalska G. The role of IL-6 during the late phase of liver regeneration. Ann Transplant. 2008;13:15–19. [PubMed] [Google Scholar]
- 45.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73:713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 46.Keil F, Elahi F, Greinix HT, et al. Ex vivo expansion of long-term culture initiating marrow cells by IL-10, SCF, and IL-3. Transfusion. 2002;42:581–587. doi: 10.1046/j.1537-2995.2002.00102.x. [DOI] [PubMed] [Google Scholar]
- 47.Duchez P, Rodriguez L, Chevaleyre J, et al. Interleukin-6 enhances the activity of in vivo long-term reconstituting hematopoietic stem cells in “hypoxic-like” expansion cultures ex vivo. Transfusion. 2015;55:2684–2691. doi: 10.1111/trf.13175. [DOI] [PubMed] [Google Scholar]
- 48.Koukourakis MI. Radiation damage and radioprotectants: new concepts in the era of molecular medicine. Br J Radiol. 2012;85:313–330. doi: 10.1259/bjr/16386034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One. 2014;9:e94188. doi: 10.1371/journal.pone.0094188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mauer J, Chaurasia B, Goldau J, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi JS, Kim KH, Lau LF. The matricellular protein CCN1 promotes mucosal healing in murine colitis through IL-6. Mucosal Immunol. 2015;8:1285–1296. doi: 10.1038/mi.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kondo M, Yamaoka K, Sakata K, et al. Contribution of the Interleukin-6/STAT-3 Signaling Pathway to Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Arthritis Rheumatol. 2015;67:1250–1260. doi: 10.1002/art.39036. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Luo Y, Masci PP, Crawford R, Xiao Y. Influence of Interleukin-1 Beta on Platelet-Poor Plasma Clot Formation: A Potential Impact on Early Bone Healing. PLoS One. 2016;11:e0149775. doi: 10.1371/journal.pone.0149775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broekman W, Amatngalim GD, de Mooij-Eijk Y, et al. TNF-alpha and IL-1beta-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir Res. 2016;17:3. doi: 10.1186/s12931-015-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barcellos-Hoff MH, Cucinotta FA. New tricks for an old fox: impact of TGFbeta on the DNA damage response and genomic stability. Sci Signal. 2014;7:re5. doi: 10.1126/scisignal.2005474. [DOI] [PubMed] [Google Scholar]
- 56.Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 57.Asai O, Longo DL, Tian ZG, et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101:1835–1842. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol. 2013;133:2461–2470. doi: 10.1038/jid.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saha A, Aoyama K, Taylor PA, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122:3062–3073. doi: 10.1182/blood-2013-05-500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amarnath S, Foley JE, Farthing DE, et al. Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells. 2015;33:1200–1212. doi: 10.1002/stem.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saldanha-Araujo F, Ferreira FI, Palma PV, et al. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7:66–74. doi: 10.1016/j.scr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Sangiorgi B, De Freitas HT, Schiavinato JL, et al. DSP30 enhances the immunosuppressive properties of mesenchymal stromal cells and protects their suppressive potential from lipopolysaccharide effects: A potential role of adenosine. Cytotherapy. 2016;18:846–859. doi: 10.1016/j.jcyt.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Tsukamoto H, Chernogorova P, Ayata K, et al. Deficiency of CD73/ecto-5′-nucleotidase in mice enhances acute graft-versus-host disease. Blood. 2012;119:4554–4564. doi: 10.1182/blood-2011-09-375899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Fan J, Chen S, Zhang Y, Curiel TJ, Zhang B. Graft-versus-host disease is enhanced by selective CD73 blockade in mice. PLoS One. 2013;8:e58397. doi: 10.1371/journal.pone.0058397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shultz LD, Brehm MA, Bavari S, Greiner DL. Humanized mice as a preclinical tool for infectious disease and biomedical research. Ann N Y Acad Sci. 2011;1245:50–54. doi: 10.1111/j.1749-6632.2011.06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parekkadan B, Upadhyay R, Dunham J, et al. Bone marrow stromal cell transplants prevent experimental enterocolitis and require host CD11b+ splenocytes. Gastroenterology. 2011;140:966–975. doi: 10.1053/j.gastro.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen S, Dooner M, Cheng Y, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30:2221–2231. doi: 10.1038/leu.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene expression data from all expressed genes for all 9 samples. Genes have FPKM>0 in at least one sample. Data shown is log transformed FPKM (log2(FPKM+1)).
Genes shown are differentially expressed in any of the one vs. one and one vs. all comparisons between the 3 groups. Differentially expressed criteria includes at least a 2x fold change (log2(FC)>1) and FDR<0.15.
Top 20 gene sets enriched in MEMs and enriched in other MQs shown. Gene sets are ranked by normalized enrichment score. Gene set size indicates number of genes in gene set. Other GSEA related statistics are also included (gene rank at max, p-value and FDR and FWER q-values).