Abstract
The efficacy of photosensitizers in cancer phototherapy is often limited by photobleaching, low tumor selectivity, and tumor hypoxia. Assembling photosensitizers into nanostructures can improve photodynamic therapy efficacy and the safety profile of photosensitizers. Herein by employing supramolecular assembly, enhanced theranostic capability of Mn2+-assisted assembly of a photosensitizer (sinoporphyrin sodium, DVDMS) is demonstrated. A tumor environment-triggered coassembly strategy is further developed to form Mn/DVDMS nanotheranostics (nanoDVD) for cancer phototherapy. MnO2 nanosheets serve as a highly effective DVDMS carrier and in situ oxygen and nanoDVD generator. In MCF-7 cells and xenograft tumors, MnO2/DVDMS is reduced by glutathione (GSH) and H2O2 and reassembled into nanoDVD, which can be monitored by activated magnetic resonance/fluorescence/photoacoustic signals. Intriguingly, the decrease of GSH, the production of O2, and the formation of nanoDVD are shown to be synergistic with phototherapy to improve antitumor efficacy in vitro and in vivo, offering a new avenue for cancer theranostics.
Keywords: nanotheranostic generators, phototherapy, supramolecular assembly, tumor microenvironment responses
Construction of functional molecules assembled into nanostructures has been of great interest in the field of material science and technology.[1] The majority of the self-assemblies or self-organizations of nanostructures are spontaneous.[2] Recently, significant research attention has been paid to self-assembled systems that are responsive to their biological environment, and the application of such systems in gene and drug delivery and molecular imaging has already changed the landscape of nanomedicine and made a significant impact in different areas of healthcare.[3] For example, chemotherapeutics assembled into nanostructures can enter cancer cells more effectively and avoid the multidrug resistance efflux.[4] We have also explored the feasibility of in vivo formation of tumor-specific nanodrugs for cancer theranostics and our tumor microenvironment-sensitive strategies showed that tumor-specific enzyme-triggered coassembly of photosensitizers or upconversion nanocrystals could enhance the absorption for phototherapy and simultaneously improve tumor accumulation/retention.[5]
Porphyrins and porphyrin derivatives such as sinoporphyrin sodium (DVDMS) have been often applied in photodynamic therapy (PDT) due to their substantial electronic absorptions in the near-infrared (NIR) regime, ability to produce copious amounts of reactive oxygen species (ROS), and tendency to accumulate in solid tumors.[5a,6] Intriguingly, photosensitizer-based nanoscale metal-organic particles have demonstrated outstanding performance in terms of drug delivery and molecular imaging.[7] Considering the structure of DVDMS photosensitizers with unique spectroscopic and photochemical properties, it is strongly desirable to develop DVDMS-based supramolecular nanomaterials as a versatile and effective nanoplatform in cancer theranostics. Because Mn2+ features a hexacoordinate nature due to its 3d54s0 outermost shell electrons,[7b,8] we reasoned that, by using supramolecular assembly,[7b,9] Mn2+ could coordinate with the porphyrin ring and carboxylate radicals from DVDMS molecules (Figure 1A(a)) to form DVDMS nanoassemblies (Figure 1A(b)), which could potentially address the need for high-performance photosensitizers in cancer phototherapy.
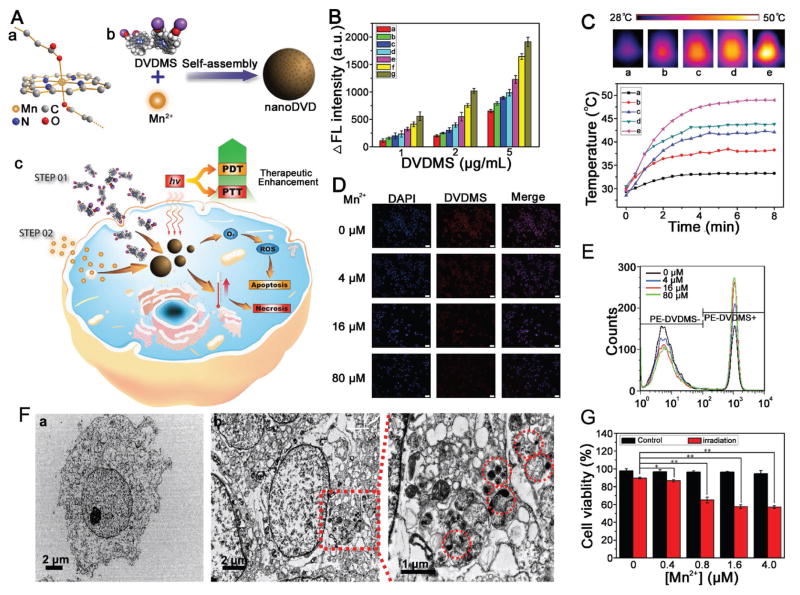
Figure 1.
A) Schematic illustration showing a molecular model of the Mn2+ ion linking porphyrin ring and two carboxylate radicals of DVDMS molecules (a), the fabrication process of Mn/DVDMS (b), and photothermal/photodynamic therapy (PTT/PDT) (c); B) the DCFH fluorescence intensity of DVDMS and Mn/DVDMS at different [Mn2+]/[DVDMS] ratios (0:1 (a), 0.5:1(b), 1:1 (c), 2:1 (d), 5:1 (e), 10:1 (f), and 20:1 (g)) after irradiation with 630 nm laser light (300 mW cm−2); C) thermal images and temperatures increase of water (a) and DVDMS (100 μg mL−1) mixed with Mn2+ at different [Mn2+]/[DVDMS] ratios (0:1 (b), 0.5:1 (c), 5:1 (d), and 10:1 (e)) after irradiation with 630 nm laser light (300 mW cm−2); D) fluorescence and E) flow cytometry data revealed the 10 μg mL−1 DVDMS reaction with different amount of Mn2+ (0 × 10−6, 4 × 10−6, 16 × 10−6, and 80 × 10−6 M) in MCF-7 cells, scale bar ≈200 μm; F) cellular TEM images of MCF-7 cells incubated with DVDMS (a) and DVDMS + Mn2+ (b); G) cell viability of MCF-7 cells treated by DVDMS (1 μg mL−1) with Mn2+ (0, 0.4 × 10−6, 0.8 × 10−6, 1.6 × 10−6, and 4.0 × 10−6 M) with or without laser irradiation 630 nm (130 mW cm−2, 5 min), *P < 0.05, **P < 0.01.
Here, we explore the use of Mn2+ to assist the nanoassembly of DVDMS. We found that simply dropping a solution of Mn2+ into the DVDMS solution resulted in the assembly of significantly different nanostructures (Figure S1, Supporting Information). Interestingly, given an increase in the [Mn2+]/[DVDMS] ratio, DVDMS could be organized to form irregular fragments, nanospheres, and space grids. These observations imply that appropriate intermolecular interactions between Mn2+ and DVDMS molecules are necessary to form well-defined nanoDVD, which might be due to the competition of the interactions of the Mn2+ and porphyrin ring/carboxylate radicals from DVDMS, and DVDMS π–π stacking.[7b,9] As expected, dynamic light scattering (DLS) data illustrated an increase in the average hydrodynamic size of nanoDVD with an increase in the [Mn2+]/[DVDMS] ratio (Figure S1H, Supporting Information), but the electronegativity of the nanoDVD was reduced as the amount of Mn2+ increased (Figure S1I, Supporting Information). Meanwhile, X-ray photoelectron spectroscopy (XPS) data further confirmed the nanoDVD supramolecular assemblies (Figure S2, Supporting Information).
We subsequently investigated the potential of nanoDVD to serve as probes for in vivo imaging. T1-weighted magnetic resonance (MR) images of Mn2+ and nanoDVD demonstrated concentration-dependent contrast enhancement under a 7T MR scanner (Figure S3A,B, Supporting Information), which is expected since Mn2+ with its five unpaired 3d electrons is an effective contrast agent in MR imaging. In addition, the fluorescence of DVDMS (Ex 630 nm) with the addition of Mn2+ was quenched (Figure S3C,D, Supporting Information) as a result of self-quenching of the nanoDVD nanostructure.[10] Therefore, the Mn2+-assisted self-assembly of DVDMS in phototherapy treatment could be monitored via fluorescence imaging.
Prior to studying the phototherapy property of nanoDVD, we investigated the UV–vis absorption of a DVDMS solution mixed with Mn2+. The absorbance value of DVDMS at 630 nm showed an obvious increase, which may have resulted from the aggregates of DVDMS (Figure S4, Supporting Information). To demonstrate the photochemical properties of nanoDVD, we compared the photoactivity of DVDMS mixed with different amounts of Mn2+ under light irradiation at 630 nm by detecting the fluorescence of 2′,7′-dichlorodihydrofluorescein (DCFH).[11] We found that Mn2+ obviously enhanced the PDT property of DVDMS (Figure 1B), regardless of fluorescence self-quenching. These observations indicate that, in the nanoDVD nanostructure, Mn2+ may enhance intersystem crossing and increase the triplet state of DVDMS, followed by efficient singlet state O2 (1O2) production when encountering triplet state O2.[12] We also examined the photothermal therapy (PTT) effect by measuring the temperature increase of various concentrations of DVDMS under laser radiation. NanoDVD exhibited a larger temperature increase than DVDMS (Figure 1C). The improved PTT effect of nanoDVD may be due to a high density of porphyrin, similar to what was reported by Zheng co-workers.[10,13] Overall, our results suggest that Mn2+ could significantly enhance the PDT and PTT properties of DVDMS and that nanoDVD nanoassemblies have the potential for imaging-guided PDT/PTT combination therapy.
In order to further study Mn2+-assisted self-assembly in cells, we sequentially incubated DVDMS and Mn2+ with MCF-7 cells (Figure 1A(c)). The fluorescence microscopy images of MCF-7 cells after incubation with DVDMS showed strong red fluorescence, which was quenched when Mn2+ was added (Figure 1D). Mn2+-induced fluorescence quenching of DVDMS was further confirmed by flow cytometry (Figure 1E). Cellular transmission electron microscope (TEM) data revealed large numbers of nanoassemblies in the cytosol (Figure 1F). The results indicate that Mn2+ could interact with DVDMS in cells. 3-(4,5-dimethyl- 2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay also demonstrated that Mn2+ could obviously enhance the NIR therapy properties of DVDMS (Figure 1G).
Notably, tumor hypoxia and oxygen consumption during PDT often hamper the efficacy of photodynamic therapy.[14] Based on the unique properties of nanoDVD, we further explored the use of tumor stimuli-responsive drug carriers that have the potential to improve cancer phototherapy by providing a rational material design approach necessary for in situ generation of both nanoDVD and O2. We reasoned that using manganese dioxide (MnO2) nanosheets[15] would offer such a “smart” DVDMS delivery system: (1) excellent efficiency for DVDMS loading, (2) targeted release of Mn2+ ions in the tumor microenvironment, (3) sustainable production of nanoDVD and O2 within the tumor tissue, (4) activatable MR/fluorescence/photoacoustic (PA) imaging, (5) enhanced photochemical properties, and (6) biocompatibility for clinical translation.
As a proof-of-concept experiment, we first assembled DVDMS molecules on the surface of MnO2 nanosheets with a high loading efficiency (≈95%, MnO2: DVDMS (wt: wt) = 1:1); their strong physisorption resulted from the large specific surface area and the Mn-N coordinate bonding (Figure 2A). The prepared MnO2 nanosheets exhibited a typical 2D sheetlike morphology (Figure 2B) a thickness of roughly 0.6 nm (Figure S5A, Supporting Information). After loading DVDMS, the MnO2/DVDMS size changed to 91.2 nm, smaller than that of pure MnO2 (128.4 nm). This change could be attributed to the additional ultrasonic treatment that was applied after MnO2 was modified with DVDMS. In addition, both UV–vis absorption and FT-IR studies implied that the nanosheet surface was coated with DVDMS (Figure S5B,C, Supporting Information). Interestingly, due to the surface modification with DVDMS, MnO2/DVDMS exhibited better stability than MnO2 in both phosphate buffer saline (PBS) and complex biological environments (i.e., dulbecco’s modified eagle medium (DMEM) and fetal bovine serum (FBS)) (Figure S6A, Supporting Information).
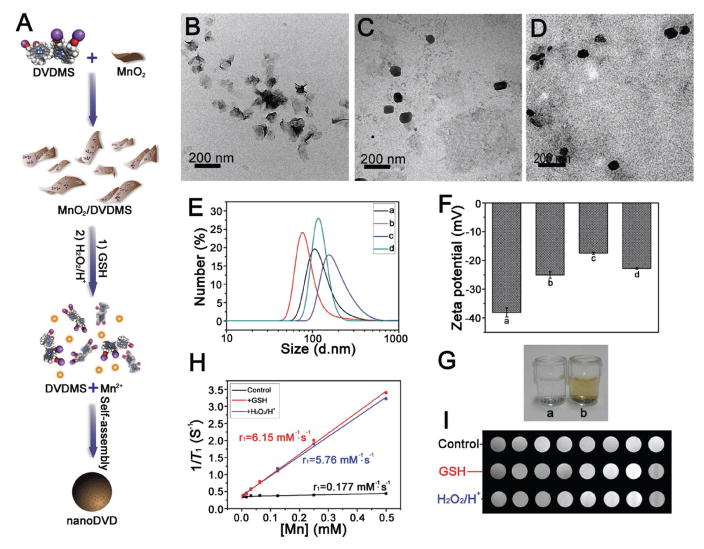
Figure 2.
A) Schematic illustration showing the fabrication process of MnO2/DVDMS and reaction in GSH solution or H2O2 in PBS (pH 5.5). TEM of B) MnO2 nanosheet, C) MnO2/DVDMS + GSH, D) and MnO2/DVDMS + H2O2/H+. E) DLS of the MnO2 nanosheet (a), MnO2/DVDMS (b), MnO2/DVDMS + GSH (c), and MnO2/DVDMS + H2O2/H+ (d). F) Zeta potential of the MnO2 nanosheet (a), MnO2/DVDMS (b), MnO2/DVDMS + GSH (c), and MnO2/DVDMS + H2O2/H+ (d). G) O2 production of MnO2 (a) and MnO2/DVDMS (b) in H2O2. H) MR imaging study of the MnO2/DVDMS: 1/T1 versus Mn concentration for the MnO2/DVDMS solution (black line), the MnO2/DVDMS + GSH solution (red line), and MnO2/DVDMS + H2O2/H+ (blue line). I) T1-weighted MR imaging phantom images obtained from MnO2/DVDMS, MnO2/DVDMS + GSH, and MnO2/DVDMS + H2O2/H+.
As expected, both MnO2 and MnO2/DVDMS could be reduced to Mn2+ ions by intracellular glutathione (GSH).[15] Alternatively, they could be reduced into Mn2+ with the production of O2 via intracellular H2O2 (Figure 2A and Figure S6B,C (Supporting Information)).[16] TEM data revealed that in GSH or H2O2 the typical 2D sheet-like nanostructure of MnO2/DVDMS (Figure 2B) changed into nanoDVD-like irregular nanoparticles (PBS, pH 5.5) (Figure 2C,D and the Supporting Information) with an average hydrodynamic size of 197.2 nm (GSH) or 122.4 nm (H2O2) (Figure 2E). Similarly, the zeta potential of MnO2/DVDMS + GSH or MnO2/DVDMS + H2O2/H+ was higher than that of MnO2 or MnO2/DVDMS (Figure 2F). Combined with the XPS study of MnO2/DVDMS + GSH and MnO2/DVDMS + H2O2/H+ (Figure S7, Supporting Information), we could conclude that after the reduction of MnO2/DVDMS in the GSH solution or H2O2/H+ solution, the released Mn2+ connected with the carboxyl and porphyrin ring of DVDMS reassembled into nanoDVD, leading to enhanced photochemical effects (Figure S8A,B, Supporting Information). Furthermore, the fluorescence intensity of the DVDMS was quenched to only 13.8% that of pure DVDMS after loading onto the surface of the MnO2 nanosheet. On the contrary, when GSH or H2O2/H+ was added, the fluorescence was partially recovered (Figure S8C,D, Supporting Information).
Because GSH and H2O2/H+ could reduce MnO2 into Mn2+, MnO2/DVDMS + GSH, and MnO2/DVDMS + H2O2/H+ in T1-weighted MR imaging exhibited much stronger enhancement than MnO2/DVDMS (Figure S2G–J, Supporting Information). The r1 value of MnO2/DVDMS was enhanced by 34.7-fold after being reduced by GSH, and by 32.5-fold in the presence of H2O2/H+, suggesting the potential of MR imaging guided drug release in the tumor area. Both the MnO2 nanosheet and MnO2/DVDMS possessed concentration-dependent PA properties (Figure S9A,B, Supporting Information). However, DVDMS and nanoDVD exhibited weaker PA signals than the MnO2 nanosheet (Figure S9C,D, Supporting Information). Taken together, such activated multimodal imaging can be readily integrated into the in situ self-assembly approach for cancer theranostic application.
Based on the promising photochemical results described above, we further explored the bioreactions that the MnO2/DVDMS nanoparticles carried out in the tumor cells (Figure 3A). Both MnO2/DVDMS and MnO2 were effectively internalized by MCF-7 cells (Figure 3B), and the cellular GSH and H2O2 levels were reduced with an increasing amount of MnO2 or MnO2/DVDMS (Figure 3B). From the thin-section cell TEM images (Figure S10, Supporting Information), we found differences in the cells incubated with MnO2 or DVDMS. The cells incubated with MnO2/DVDMS had irregular nanoparticles that could be easily distinguished from the cells themselves. In addition, the energy dispersive spectrometer (EDS) element line scanning of Mn confirmed that the nanostructure in the cells incubated with MnO2/DVDMS was Mn based. Similar findings were also observed for nanoDVD nanoassemblies, with nanoaggregates distributed in the cell cytosol (Figure 3C).
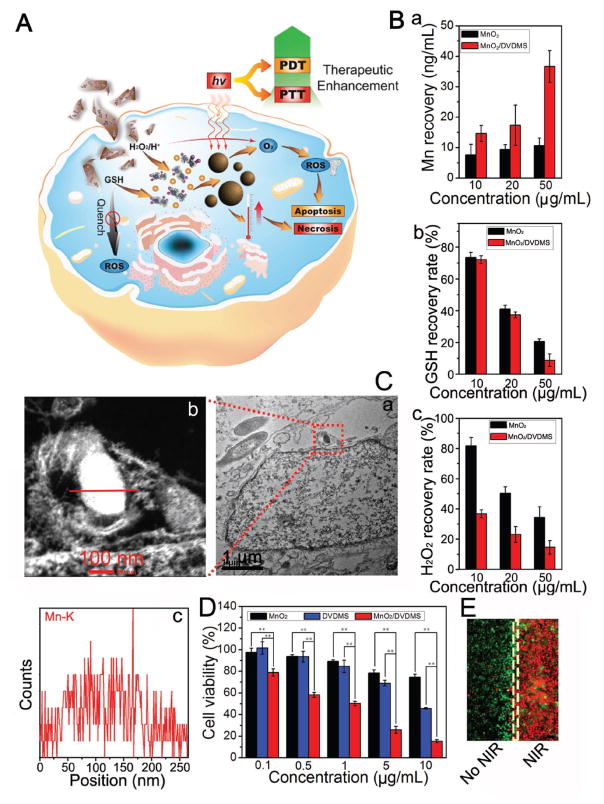
Figure 3.
A) Schematic illustration of the PDT/PTT procedure for MnO2/DVDMS. B) The amount of Mn (a), GSH (b), and H2O2 (c) recovery after the cells were cultured with MnO2 and MnO2/DVDMS (MnO2: 10, 20, and 50 μg mL−1). C) Thin-section cell TEM images of MnO2/DVDMS incubated in MCF-7 cells (a) and the element line scanning (Mn) of the reassembled nanostructure (b,c). D) Cell viability of the MCF-7 cells at different dosages of MnO2, DVDMS, and MnO2/DVDMS with laser irradiation (630 nm, 130 mW cm−2, 8 min) (**P < 0.01). E) Calcein-AM/PI staining of cells after exposure to MnO2/DVDMS with or without NIR laser irradiation, scale bar ≈200 μm.
We additionally evaluated the biocompatibility and PDT/PTT effects of MnO2/DVDMS on MCF-7 cells using the standard MTT assay and calcein acetoxymethyl ester/propidium iodide (calcein-AM/PI) experiment. As shown in Figure S10B (Supporting Information), after incubation with MCF-7 cells for 12 h, MnO2/DVDMS did not exhibit noticeable cytotoxicity. When the cells were treated with MnO2, DVDMS, and MnO2/DVDMS plus 630 mn laser irradiation, DVDMS and MnO2/DVDMS demonstrated significant cytotoxicity (Figure 3D,E and Figure S10C (Supporting Information)). MnO2/DVDMS exhibited more effective PDT/PTT killing of MCF-7 cells than DVDMS, presumably due to several advantages of the in situ self-assembly strategy: (i) MnO2/DVDMS could react with GSH in the MCF-7 cells, therefore the consumption of ROS by endogenous GSH was reduced;[16] (ii) MnO2/DVDMS could react with H2O2 in the MCF-7 cells and downregulate the expression of hypoxia-inducible factor 1α(HIF-1α) (Figure S10D, Supporting Information), ameliorating hypoxia and providing more O2 for PDT;[17] (iii) after the release of Mn2+ and DVDMS from the reduction of MnO2/DVDMS, nanoDVD was formed and both the efficacies of PDT and PTT were enhanced.
The ability to monitor anticancer treatments in real time can yield invaluable predictive information regarding drug delivery and therapeutic efficacy. Notably, the integration of fluorescence imaging, MR imaging, and PA into our MnO2/DVDMS system could offer high optical sensitivity and good spatial resolution for in vivo monitoring of various biochemical processes. As expected, after the injection of DVDMS, MnO2, and MnO2/DVDMS, the PA images and region of interest (ROI) analysis demonstrated that MnO2 in the MnO2/DVDMS reduced over time and that most of the MnO2 was gone within 24 h (Figure 4A,B and Figure S11A (Supporting Information)). On the contrary, the fluorescence signal increased quickly after MnO2/DVDMS injection (Figure 4C,D). In addition, an obvious T1 contrast enhancement could be observed in the tumor region after the injection of MnO2/DVDMS (Figure 4E,F), further evidence for the stimuli-responsiveness of MnO2/DVDMS with the released Mn2+. Consistent with the in vivo imaging results, TEM images of tumor slices confirmed that a large numbers of nanoDVD nanoassemblies were produced in the tumor area injected intratumorally (i.t.) with MnO2/DVDMS, similar to that injected with DVDMS and Mn2+ (Figure S11B, Supporting Information), but not with DVDMS or MnO2 (Figure 4G).
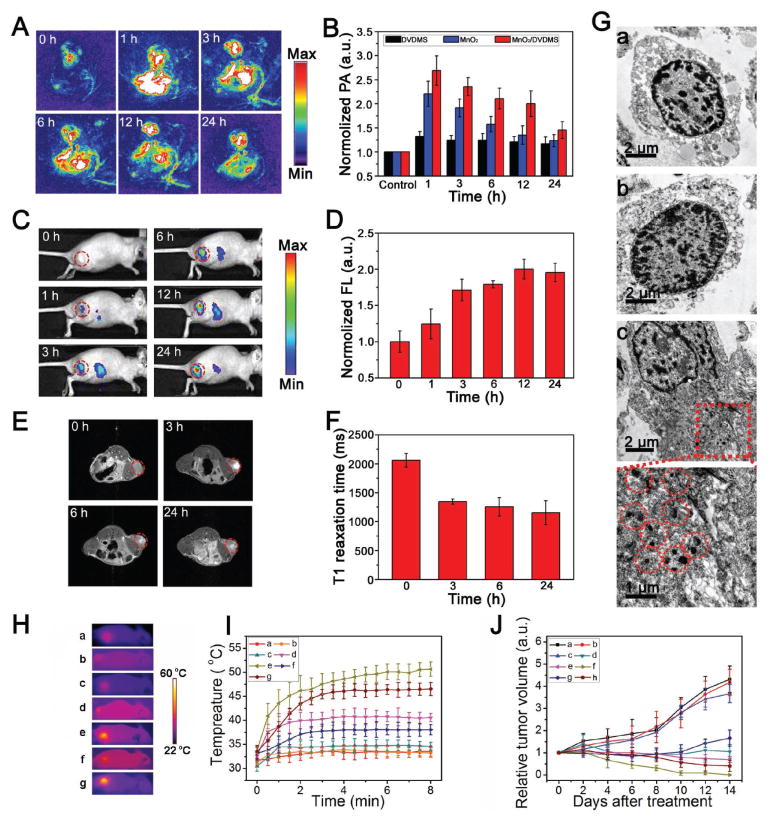
Figure 4.
A) PA images before and after an intratumoral injection of MnO2/DVDMS. B) Region of interest (ROI) analysis of the PA signal before and after injection of DVDMS, MnO2, and MnO2/DVDMS (1, 3, 6, 12, and 24 h). C) Fluorescence images and D) ROI intensity of the tumor after injection of MnO2/DVDMS. E) T1-weighted MR images and F) T1 relaxation time before and after injection of MnO2/DVDMS. G) TEM images of MCF-7 tumor thin sections at 24 h after injection of DVDMS (a), MnO2 (b), and MnO2/DVDMS (c). H) Thermal images and I) tumor temperature increase for different treatments: (a) saline control + laser; (b) MnO2 (i.t.) + laser; (c) DVDMS (i.t.) + laser; (d) DVDMS (i.t.) + Mn2+ (i.t.) + laser; (e) MnO2/DVDMS (i.t.) + laser; (f) DVDMS (i.v.) + laser; (g) MnO2/DVDMS (i.v.) + laser. J) MCF-7 tumor growth curves of mice treated with different methods: (a) saline control; (b) MnO2/DVDMS (i.t.); (c) saline control + laser; (d) DVDMS (i.t.) + laser; (e) DVDMS (i.t.) + Mn2+ (i.t.) + laser; (f) MnO2/DVDMS (i.t.) + laser; (g) DVDMS (i.v.) + laser; (h) MnO2/DVDMS (i.v.) + laser.
Encouraged by the imaging data that revealed the well-controlled tumor environment responsiveness of MnO2/DVDMS, we continued in vivo phototherapy by injecting MnO2/DVDMS into different groups of MCF-7 tumor-bearing mice, and in vivo tumor inhibition effect was evaluated upon 630 nm laser illumination. The temperature change during irradiation was monitored by an IR thermal camera (Figure 4H). The tumor temperature of mice with MnO2/DVDMS injections (DVDMS: i.v. 4.73 mg kg−1; i.t. 1.18 mg kg−1) showed rapid increase and maintained at 45 °C (i.v.) or 50 °C (i.t.) during laser irradiation. In contrast, the tumor temperature showed little change for mice injected with MnO2 or DVDMS under irradiation with the same parameters (Figure 4H,I). DVDMS (i.t.) with laser irradiation was associated with an initial delay of tumor growth, but the tumor succumbed later. However, treatment with MnO2/DVDMS (i.t.) and DVDMS (i.t.) combined with Mn2+ (i.t.) reduced tumor growth significantly, and the group treated with the MnO2/DVDMS (i.t.) experienced complete tumor regression within 14 d (Figure 4J). More importantly, tumors receiving MnO2/DVDMS (i.t.) exhibited a more significant reduction in growth compared with tumors receiving DVDMS (i.t.) combined with Mn2+ (i.t.). In addition, the tumors treated with MnO2/DVDMS (i.v.) showed greater therapeutic effect than those treated with an i.v. injection of DVDMS. These results clearly indicate that multifunctional MnO2/DVDMS considerably improves tumor therapeutic efficacy.
Besides the promising phototherapy effect in vivo, there were no changes in the relative body weights of any of the mice that we studied (Figure S12A, Supporting Information), indicating the low acute toxicity during the treatment. In addition, the biosafety assessment of the MnO2/DVDMS including the alanine aminotransferase, aspartate transaminase, and hematoxylin and eosin stained organ slices showed no obvious in vivo toxicity of MnO2/DVDMS (Figure S12B,C, Supporting Information).
In conclusion, we have reported the design and synthesis of a novel tumor environment-triggered supramolecular assembly of nanostructure. This supramolecular engineering nanoplatform integrates a variety of functions that include imaging (fluorescent imaging, MR imaging, and PA imaging) as well as synergetic combination of phototherapies (PTT and PDT). The MnO2 nanosheet served as a highly effective carrier for photosensitizer DVDMS and as an in situ oxygen and nanoDVD generator. In the tumor environment, MnO2/DVDMS could be reduced by GSH and H2O2, leading to the release of Mn2+, DVDMS, and O2. The nanoDVD assembled within the tumor cells/tissues features enhanced theranostic capability, as demonstrated by the Mn2+-assisted nanoassembly process of DVDMS. We found that the drug delivery and treatment effect could be monitored by activated fluorescence/MR/PA imaging. Furthermore, the consumption of GSH, the production of O2, and the formation of nanoDVD demonstrated overall improved phototherapy efficacy in vitro and in vivo. We believe that this report not only represents a simple approach to construct stimuli- responsive supramolecular nanomaterials but also provides a unique theranostic nanoplatform for cancer phototherapy through cancer-specific delivery and image-guided evaluation of therapy.
Supplementary Material
Acknowledgments
C.C. and H.L. contributed equally to this work. This work was supported by the Major State Basic Research Development Program of China (973 Program) (Grant Nos. 2013CB733802 and 2014CB744503), the National Natural Science Foundation of China (NSFC) (Grant Nos. 81422023, 51273165, and U1505221), the Fundamental Research Funds for the Central Universities (Grant Nos. 20720160065 and 20720150141), the Science Foundation of Fujian Province (Grant No. 2014Y2004 and 2016ZY002), and the Program for New Century Excellent Talents in University, China (NCET-13-0502). All animal experiments were approved by the Animal Management and Ethics Committee of Xiamen University.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Chengchao Chu, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
Huirong Lin, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
Heng Liu, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
Dr. Xiaoyong Wang, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China
Junqing Wang, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
Pengfei Zhang, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
Haiyan Gao, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
Chao Huang, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
Yun Zeng, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
Prof. Yuanzhi Tan, State Key Laboratory of Physical Chemistry of Solid Surfaces and The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
Prof. Gang Liu, State Key Laboratory of Molecular Vaccinology and Molecular, Diagnostics and Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China. State Key Laboratory of Physical Chemistry of Solid Surfaces and The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. State Key Laboratory of Cellular Stress Biology, Innovation Center for Cell Biology, School of Life Sciences, Xiamen University, Xiamen 361102, China
Dr. Xiaoyuan Chen, Laboratory of Molecular Imaging and Nanomedicine, National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), Bethesda, MD 20892, USA
References
- 1.a) Zhang S. Nat Biotechnol. 2003;21:1171. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]; b) Aida T, Meijer EW, Stupp SI. Science. 2012;335:813. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, de la Cruz MO, Stupp SI. Nat Mater. 2010;9:594. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitesides GM, Grzybowski B. Science. 2002;295:2418. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 3.a) Gao Y, Shi J, Yuan D, Xu B. Nat Commun. 2012;3:1033. doi: 10.1038/ncomms2040. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liang G, Ren H, Rao J. Nat Chem. 2010;2:54. doi: 10.1038/nchem.480. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Versluis F, van Esch JH, Eelkema R. Adv Mater. 2016;28:4576. doi: 10.1002/adma.201505025. [DOI] [PubMed] [Google Scholar]
- 4.Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S, Shen J, Liu H, Hu Z, Chen L, Huang Y, Koay E, Huang Y, Liu J, Ensor JE, Blanco E, Liu X, Ferrari M, Shen H. Nat Biotechnol. 2016;34:414. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Ai X, Ho CJ, Aw J, Attia AB, Mu J, Wang Y, Wang X, Wang Y, Liu X, Chen H, Gao M, Chen X, Yeow EK, Liu G, Olivo M, Xing B. Nat Commun. 2016;7:10432. doi: 10.1038/ncomms10432. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Huang P, Gao Y, Lin J, Hu H, Liao HS, Yan X, Tang Y, Jin A, Song J, Niu G, Zhang G, Horkay F, Chen X. ACS Nano. 2015;9:9517. doi: 10.1021/acsnano.5b03874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Li Y, Lin TY, Luo Y, Liu Q, Xiao W, Guo W, Lac D, Zhang H, Feng C, Wachsmann-Hogiu S, Walton JH, Cherry SR, Rowland DJ, Kukis D, Pan C, Lam KS. Nat Commun. 2014;5:4712. doi: 10.1038/ncomms5712. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lucky SS, Soo KC, Zhang Y. Chem Rev. 2015;115:1990. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]; c) Yan X, Niu G, Lin J, Jin AJ, Hu H, Tang Y, Zhang Y, Wu A, Lu J, Zhang S, Huang P, Shen B, Chen X. Biomaterials. 2015;42:94. doi: 10.1016/j.biomaterials.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Yan X, Hu H, Lin J, Al Jin J, Niu G, Zhang S, Huang P, Shen B, Chen X. Nanoscale. 2015;7:2520. doi: 10.1039/c4nr06868h. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Gu H, Xu K, Yang Z, Chang CK, Xu B. Chem Commun. 2005:4270. doi: 10.1039/b507779f. [DOI] [PubMed] [Google Scholar]; f) Liang G, Wang L, Yang Z, Koon H, Mak N, Chang CK, Xu B. Chem Commun. 2006:5021. doi: 10.1039/b611557h. [DOI] [PubMed] [Google Scholar]
- 7.a) Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C, Chang JS, Hwang YK, Marsaud V, Bories PN, Cynober L, Gil S, Ferey G, Couvreur P, Gref R. Nat Mater. 2010;9:172. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]; b) Yang Y, Liu J, Liang C, Feng L, Fu T, Dong Z, Chao Y, Li Y, Lu G, Chen M, Liu Z. ACS Nano. 2016;10:2774. doi: 10.1021/acsnano.5b07882. [DOI] [PubMed] [Google Scholar]; c) Cai W, Chu CC, Liu G, Wáng YXJ. Small. 2015;11:4806. doi: 10.1002/smll.201500802. [DOI] [PubMed] [Google Scholar]
- 8.Mi P, Kokuryo D, Cabral H, Wu H, Terada Y, Saga T, Aoki I, Nishiyama N, Kataoka K. Nat Nanotechnol. 2016;11:724. doi: 10.1038/nnano.2016.72. [DOI] [PubMed] [Google Scholar]
- 9.a) Zhang C, Chen P, Dong H, Zhen Y, Liu M, Hu W. Adv Mater. 2015;27:5379. doi: 10.1002/adma.201501273. [DOI] [PubMed] [Google Scholar]; b) Oliveras-Gonzalez C, Di Meo F, Gonzalez-Campo A, Beljonne D, Norman P, Simon-Sorbed M, Linares M, Amabilino DB. J Am Chem Soc. 2015;137:15795. doi: 10.1021/jacs.5b08081. [DOI] [PubMed] [Google Scholar]
- 10.Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WCW, Cao W, Wang LV, Zheng G. Nat Mater. 2011;10:324. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 11.Lin LS, Cong ZX, Li J, Ke KM, Guo SS, Yang HH, Chen GN. J Mater Chem B. 2014;2:1031. doi: 10.1039/c3tb21479f. [DOI] [PubMed] [Google Scholar]
- 12.a) Liu K, Xing R, Zou Q, Ma G, Möhwald H, Yan X. Angew Chem, Int Ed. 2016;55:3036. doi: 10.1002/anie.201509810. [DOI] [PubMed] [Google Scholar]; b) Xing R, Liu K, Jiao T, Zhang N, Ma K, Zhang R, Zou Q, Ma G, Yan X. Adv Mater. 2016;28:3669. doi: 10.1002/adma.201600284. [DOI] [PubMed] [Google Scholar]; c) Zhang N, Zhao F, Zou Q, Li Y, Ma G, Yan X. Small. 2016;12:5936. doi: 10.1002/smll.201602339. [DOI] [PubMed] [Google Scholar]
- 13.Huynh E, Zheng G. Nano Today. 2014;9:212. [Google Scholar]
- 14.Chen H, Tian J, He W, Guo Z. J Am Chem Soc. 2015;137:1539. doi: 10.1021/ja511420n. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Z, Fan H, Zhou G, Bai H, Liang H, Wang R, Zhang X, Tan W. J Am Chem Soc. 2014;136:11220. doi: 10.1021/ja5029364. [DOI] [PubMed] [Google Scholar]
- 16.Fan H, Yan G, Zhao Z, Hu X, Zhang W, Liu H, Fu X, Fu T, Zhang XB, Tan W. Angew Chem, Int Ed. 2016;55:5477. doi: 10.1002/anie.201510748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan W, Bu W, Shen B, He Q, Cui Z, Liu Y, Zheng X, Zhao K, Shi J. Adv Mater. 2015;27:4155. doi: 10.1002/adma.201405141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.