Abstract
Background
Gestational diabetes mellitus (GDM) is defined as a glucose tolerance disorder that arises during pregnancy. Estimates of its prevalence vary widely because of varying threshold values. Screening of all pregnant women with a two-step test has been available in Germany since 2012. This study is the first population-based, nationwide analysis of the screening coverage and the resulting one-year prevalence.
Methods
Billing data from the outpatient sector were analyzed for all persons covered by statutory health insurance in the two-year period 2014–2015. A cohort of pregnant women, constructed by using pregnancy care billing data, was studied with respect to the screening coverage. The prevalence of GDM was determined from the use of the corresponding ICD-10-GM codes.
Results
80.8% of 567 191 pregnant women were screened for GDM. Most of them (63.3%) received only the pre-test, and 12.7% received both the pre-test and the diagnostic test. 4.8% received only the diagnostic test. The overall prevalence of GDM was 13.2%. The prevalence rose with age, from 8% to 26% in women aged 45 or older. Younger women more commonly received only the pre-test; the frequency of receiving both tests rose with age.
Conclusion
Screening for GDM is comprehensively implemented. The analysis of billing data reveals a relatively high prevalence that accords with estimates in other countries, implying that earlier prevalence figures for Germany were probably underestimates.
Gestational diabetes mellitus (GDM) is defined as a glucose intolerance which is first diagnosed in pregnancy and remains below the cutoff value for manifest diabetes (1, 2). Although asymptomatic in its clinical course, GDM is associated with an increased risk of complications related to pregnancy and childbirth (3– 5). In the long term, the risk of developing manifest type 2 diabetes (T2D) is significantly increased in women with GDM in the years following initial diagnosis (6– 9). Depending on study design and methods, particularly on follow-up period and risk structure of the study sample, the rates for women developing T2D after GDM vary considerably, ranging between 3% and over 90% (7, 8). GDM during pregnancy is associated with an up to 7-fold increase in the risk of manifest T2D compared with normoglycemic pregnancies (6, 9).
The screening offer for all pregnant women was introduced within the framework of the Maternity Directive of the Federal Joint Committee (G-BA, Gemeinsamer Bundesausschuss) in 2012 (10). The G-BA used an expert report from the Institute for Quality and Efficiency in Health Care (IQWIiG, Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen) as a basis for this decision (11). According to this report, data from randomized controlled trials showed that pregnant women with GDM who were identified using a two-step screening procedure and received diabetes care/therapy experienced significantly fewer complications related to pregnancy and childbirth (risk reduction for shoulder dystocias: 60%; for preeclampsia: 36%) (3, 5, 11). The screening program defined in the Maternity Directive includes a two-step test procedure to be offered to pregnant women between 24 and 28 weeks’ gestation. This consists of a “pre-test” and a “diagnostic test”. The latter—the oral glucose tolerance test (oGTT)—is performed if the pre-test returns an abnormal result. If the oGTT is positive, the pregnant woman’s further care is provided in close collaboration with a physician qualified in diabetology. The screening procedure and the cutoff values are summarized in the Table.
Table. Screening steps and cutoff values of the two-step test for GDM.
| Testing | Cutoff value | Consequence | |||
| Dose | Timing of test | mmol/L | mg/dL | ||
| Pre-test (GCT) | |||||
| 50 g | after 1 hour | ≥ 7.5 and ≤ 11.1* | ≥ 135 and ≤ 200* | prompt application of the diagnostic test | |
| Diagnostic test (oGTT) | |||||
| directly fasting | ≥ 5.1 | ≥ 92 | upon reaching or exceeding one of the 3 cutoff values: GDM diagnosis | ||
| 75 g | after 1 hour | ≥ 10.0 | ≥ 180 | ||
| after 2 hours | ≥ 8.5 | ≥ 153 | |||
GCT, glucose challenge test; GDM, gestational diabetes mellitus; oGTT, oral glucose tolerance test
*If these cutoffs are exceeded, manifest diabetes must be ruled out or confirmed
The criteria and cutoff values for the diagnosis of GDM differ internationally (12). Depending on the test strategies used, estimated prevalence rates show considerable variation (13– 15)—with an increasing trend over the last decades (16– 18). Globally, the prevalence of hyperglycemia in pregnancy (GDM and manifest T2D in pregnancy) is estimated to be approx. 15% (15); for Europe, the prevalence is 12.6% (15). Prior to the introduction of the standardized screening program in 2012, GDM screening in Germany was undertaken on the basis of selective contracts; consequently, very different cutoffs and test strategies were used. This is reflected in the wide variation of the prevalence rates estimated during that period which vary between 2% and more than 18% (19– 21). The most comprehensive data to be used for prevalence estimation in Germany are collected in maternity hospitals within the “Quality Assurance in Obstetrics” framework (former Perinatal Survey). For this, relevant data from the Mutterpass (German maternity record) are entered into the hospital documentation system. Analysis of these data showed a steady increase in GDM during the years prior to the introduction of screening, from 2.3% in 2005 to 4.3% in 2012 and since then to 5% in 2015 (22). A recent study, evaluating the implementation of standardized screening based on outpatient service data from the North Rhine region arrived at a GDM prevalence of 6.8% for the year 2013/2014 (23). An overview of the national and international GDM prevalence studies is provided in eTable 1.
eTable 1. Summary of selected recent studies on the prevalence of gestational diabetes.
| # | Authors, year | Subject matter | Study period | Region studied | Number of evaluated pregnant women/studies *1 | GDM prevalence |
| 1 | Anna et al., 2008 (16) |
Social correlates of increase in GDM prevalence | 1995–2005 | New South Wales, Australia |
n = 956 738 | 1995: 3.0% 2005: 4.4% |
| 2 | Beyerlein et al., 2016 (19) |
Relationship between charge-free screening and GDM detection rates in deprived areas | 2008–2014 | Bavaria, Germany |
n = 587 621 | 2008: 3.4% 2014: 4.0% |
| 3 | DeSisto et al., 2014 (13) |
GDM prevalence estimate based on PRAMS data | 2007–2010 | US States: 2007–2010: 21 2010: 15 |
2007–2010: n = 123 373 2010: n = 23 479 |
2010: 9.2% 2007–2008: 8.1% 2009–2010: 8.5% |
| 4 | Djelmis et al., 2016 (14) |
GDM prevalence estimate according to diagnostic criteria (IADPSG and NICE criteria) | 2012–2014 | Zagreb, Croatia |
n = 4646 | IADPSG criteria: 17.8% NICE criteria: 23.1% |
| 5 | Donovan et al., 2016 (30) |
Prevalence and timing of screening and diagnostic testing for GDM | 2008–2012 | Alberta, Canada |
n = 86 842 | 3.4% (after two-step screening) |
| 6 | Ferrara, 2007 (17) |
Increasing prevalence of GDM over time | 1991–2003 | US States and regions in Australia | Studies: n = 6 | Increase of 1.8–3.1% Increase of 3.3–7.5% (depending on study) |
| 7 | Guariguata et al., 2014 (15) |
Global estimates of the prevalence of hyperglycemia in pregnancy | 2013 | worldwide: 34 countries |
Studies: n = 47 | Worldwide: 14.8% Europe: 12.6% |
| 8 | Huy et al., 2012 (20) |
Temporal trend and determining factors of GDM prevalence | 2006–2010 | Nationwide in Germany | n = 650 232 (German Perinatal Survey) n = 15 429 (German Health Interview and Examination Survey for Children and Adolescents (KiGGS) |
German Perinatal Survey: 2010: 3.7% 2006–2010: 1.9% KiGGS: 2006–2010: 5.3% |
| 9 | IQTIG, 2015 *2 (22) AQUA Institute, 2009–2014 BQS, 2004–2008 |
“Quality Assurance in Obstetrics,” former Perinatal Survey: standardized surveys, among others of GDM, in maternity hospitals in the context of childbirth | 2004–2015 | Germany | Varying, according to year | 2015: 50%; 2014: 4.5% 2013: 4.4%; 2012: 4.3% 2011: 4.4%; 2010: 3.7% 2009: 3.4%; 2008: 3.4% 2007: 2.7%; 2006: 2.4% 2005: 2.3%; 2004: 2.2% |
| 10 | Lavery et al., 2017 (18) |
Temporal trend in GDM prevalence rates in the US between 1979 and 2010 | 1979–2010 | USA | >125 million pregnancies | 1979–1980: 0.3% 2008–2010: 5.8% |
| 11 | Reeske et al., 2012 (21) |
Differences in GDM incidence rates between women of Turkish origin and German women | 2005–2007 | Berlin, Germany |
n = 3338 | Women of Turkish origin: 18.3% German women: 13.8% |
| 12 | Tamayo et al., 2016 (23) |
Prevalence of GDM and risk of complications before and after initiation of a general systematic two-step screening strategy in Germany | 2012–2014 | North Rhine, Germany |
2012–2013: n = 153 302 2013–2014: n = 158 839 |
2012–2013: 6.02% 2013–2014: 6.81% |
| 13 | Zhu, Zhang, 2016 (12) |
Review of global GDM prevalence rates according to country/region | 2005–2015 | Worldwide: 36 nations |
Studies: n = 77 | 2–25% |
*1 Number of studies in literature reviews or systematic reviews
*2 Data for 2015 were obtained from the IQTIG’s report “Quality Assurance in Obstetrics”; for the years 2009–2014 from the AQUA Institute’s report “Perinatal survey”; for the years 2004–2008 from the BQS’s report “Perinatal Survey”.
AQUA Institute, Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen (Institute for Applied Quality Improvement and Research in Health Care); BQS, Bundesgeschäftsstelle Qualitätssicherung (German Federal Office for Quality Assurance); GDM, gestational diabetes mellitus; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; IQTIG, Institut für Qualitätssicherung und Transparenz im Gesundheitswesen (Federal Institute for Quality Assurance and Transparency in Healthcare); NICE, National Institute for Health and Care Excellence; PRAMS, Pregnancy Risk Assessment Monitoring System
This study is the first population-based research to evaluate the nationwide implementation of the two-step screening program and the resulting 1-year prevalence for all pregnant women in Germany covered by statutory health insurance. The following research questions have been investigated:
How many pregnant women are tested for GDM during pregnancy and which method is used?
What is the 1-year prevalence of GDM?
Methods
Data source
The data source for this study is the nationwide panel doctor billing data set (referred to as “service data” in the following) of all approx. 71 million members of all German statutory health insurances (24) during the observation period from 1 January 2014 to 31 December 2015. This includes information about billed services and diagnoses. Billing of services rendered by panel doctors is based on fee schedule items (GOP) listed in the German Uniform Value Scale (Einheitlicher Bewertungsmaßstab, EBM) (25).
Formation and validation of the pregnancy cohort
For the provision of care to a pregnant woman, the flat fee-per-case ”GOP 01770” can be billed only once per quarter during pregnancy and up to 8 weeks after delivery by the treating doctor (25). For the formation of the pregnancy cohort, all women whose pregnancies started during the period from 1 July 2014 to 30 June 2015 were identified based on the billing of this flat fee in the respective quarter. This quarter of the individual start of pregnancy is here referred to as “index quarter.” To ensure that only new (incidental) pregnancies were included, it was a requirement that this flat fee-per-case had not been billed in the two quarters preceding the index quarter.
GDM screening is to be offered between 6 and 7 months’ gestation. To operationalize the existence of a pregnancy up to this point in time, women were only included in the pregnancy cohort if the flat fee was billed in the index quarter and the two consecutive quarters. Requiring that this flat fee was billed across 3 consecutive quarters was meant to ensure the exclusion of pregnancies which were not maintained to the screening period, for example due to spontaneous or induced abortion. The validation of the pregnancy cohort, based on the official statistics of live births (26, 27), is provided in eTable 2.
eTable 2. Validation of the pregnancy cohort (2010–2014).
| Year | ||||||
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
| Pregnancy cohorts *1 | ||||||
| Number of pregnant women | 520 730 | 517 582 | 524 089 | 545 775 | 563 339 | 584 006 |
| Validation cohort (DESTATIS) | ||||||
| Live births *2 | 677 947 | 662 685 | 673 544 | 682 069 | 714 927 | 737 575 |
| Women with twins *3 | 11 573 | 11 254 | 11 648 | 12 119 | 12 977 | 13 368 |
| Women with triplets *3 | 258 | 230 | 230 | 230 | 282 | 258 |
| Women with other multiple births *3 | 7 | 6 | 3 | 6 | 11 | 11 |
| Total multiple births *4 | 12 110 | 11 732 | 12 117 | 12 597 | 13 574 | 13 917 |
| Number of births *2, 3 (live births minus total multiple births) | 665 837 | 650 953 | 661 427 | 669 472 | 701 353 | 723 658 |
|
Difference between validation cohort and pregnancy cohort (number of births *2, 3 minus number of pregnant women *1) | ||||||
| Absolute frequency | 145 107 | 133 371 | 137 338 | 123 697 | 138 014 | 139 652 |
| Relative frequency | 21.8% | 20.5% | 20.8% | 18.5% | 19.7% | 19.3% |
*1 based on the methods used in this study, according to the respective calendar year
*2 German Federal Health Monitoring (GBE-Bund, Gesundheitsberichterstattung des Bundes): Live births (27)
*3 Federal Statistical Office of Germany (DESTATIS, Statistisches Bundesamt): women with multiple births (26)
*4 To prevent overestimation of the number of pregnant women due to multiple births, only one live birth was counted in all cases of multiple birth for the estimation of the number of pregnant women. Therefore, the number of women with multiple births was multiplied by the factor x-multiple births - 1 and then subtracted from the number of live births. So, the total of women with twins × 1, women with triplets × 2 and women with other multiple births × 3 was worked out
Formation of the study cohort
To form the study cohort, those women were excluded who had—according to ICD-10-GM (International Statistical Classification Of Diseases and Related Health Problems, 10th revision, German Modification)—in at least one of the two quarters preceding the index quarter a confirmed diagnosis of manifest diabetes (E10–E14 as well as O24.0–O24.3 [28]). The study cohort is the population on which the evaluation of the study questions is based.
Operationalization of screening implementation and prevalence rates
Each included pregnancy was individually assessed over three consecutive pregnancy quarters, starting from the index quarter (study period: 1 July 2014 to 31 December 2015). The two-step screening is represented by the GOP with the item numbers 01776 “Pre-test for gestational diabetes” and 01777 ”oGTT to rule out/confirm gestational diabetes” (25). For the study period it was assessed whether a GOP relevant for the respective test was billed at least once (screening implementation) and whether one of the two GDM diagnoses (O24.4 and O24.9) according to ICD-10-GM (28) was coded (prevalence estimation). In addition, it was assessed how frequently manifest diabetes (ICD-10-GM code E10–E14 as well as O24.0–O24.3) was diagnosed alone or in combination with GDM. Consistently, only confirmed diagnoses were included.
Statistical analyses
To calculate the screening implementation and prevalence rates, the percentage of persons insured with billed GOP/coded diagnosis in the study cohort was identified and described in an age-stratified way. The MicroStrategy Developer Version 10.5.0 (29) software was used for all analyses.
Results
Pregnancy cohort and study cohort
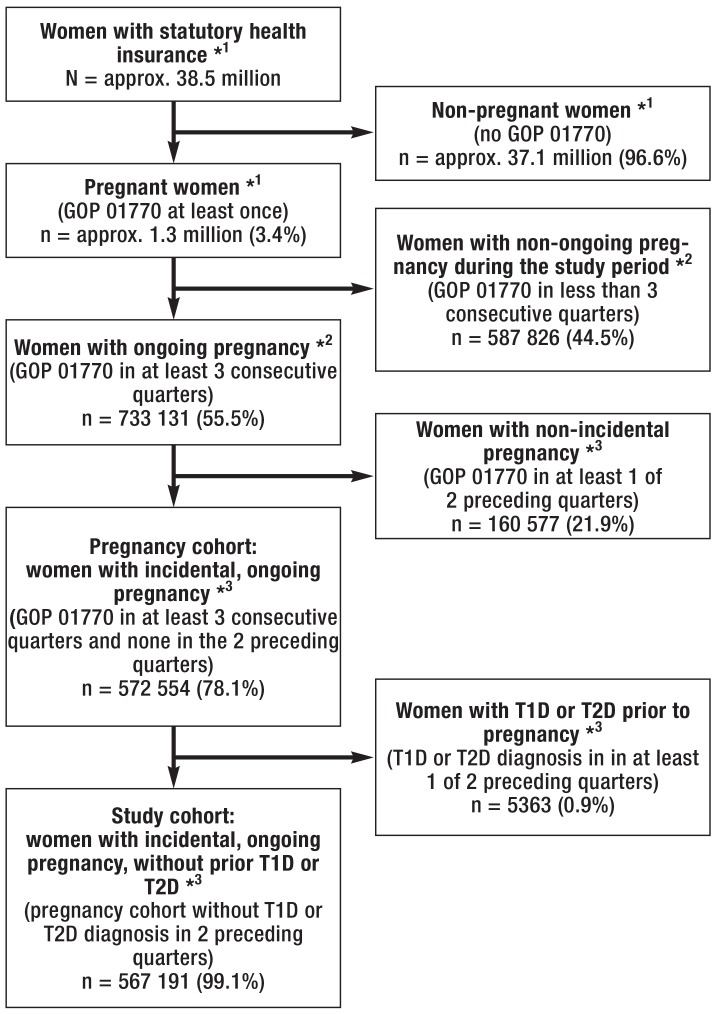
Figure 1 shows the flowchart for the formation of the pregnancy cohort and the study cohort. In 3.4% of the 38.5 million women, the pregnancy flat fee-per-case was billed at least once during the study period. For more than half of these women, pregnancies lasting at least three quarters were identified. An incidental pregnancy was found in 78.1% of these women; thus, the pregnancy cohort defined here consists of 572 554 women. This pregnancy cohort includes approximately 80% of the annual deliveries (live births) recorded in the official birth statistics (ebox).
Figure 1.
Flowchart for the formation of the pregnancy cohort and the study cohort
*1 Study period: 1 July 2014–30 June 2015
*2 Start of pregnancy in the period: 1 July 2014–30 June 2015; study period of the ongoing pregnancy: 1 July 2014–31 December 2015
*3 Start of pregnancy in the period: 1 July 2014–30 June 2015; study period of the ongoing pregnancy, with the exclusion of prior pregnancy flat fees or diagnoses of manifest diabetes: 1 January 2014–31 December 2015
GOP, fee schedule item; T1D or T2D, type 1 or type 2 diabetes mellitus
eBox. Pregnancy cohort and official birth statistics.
To validate the pregnancy cohort described, the absolute numbers of pregnant women from this cohort over the years 2010–2015 were compared with data of the Federal Statistical Office (26, 27) on the numbers of live births and multiple births for the corresponding years. The number of live births, adjusted for the number of multiple births, was calculated as an estimator for the number of pregnant women (etable 2). It was not possible to directly compare the absolute number of women of the pregnancy cohort who were used for further analyses of the study question with the official statistics, because the data for our analyses extend over a turn of the year, while the official statistics were reported on an annual basis. Across all the years studied, the validation cohort and the pregnancy cohort differed by 19 to 22%.
Altogether 0.9% (n = 5363) of the women in the pregnancy cohort were excluded from further analyses because they had already been diagnosed with manifest diabetes prior to pregnancy. Thus, the population on which the further analyses were based consisted of the 567 191 pregnant women remaining in the study cohort (figure 1).
The mean age of these women at the estimated start of pregnancy (age in the index quarter) was 30 years, with a standard deviation of 5 years. The youngest pregnant woman in our study cohort was 13 years, the oldest 58 years old. Half of these women were aged between 27 and 34 years at the start of pregnancy (median: 30 years).
Implementation rates for the two-step screening
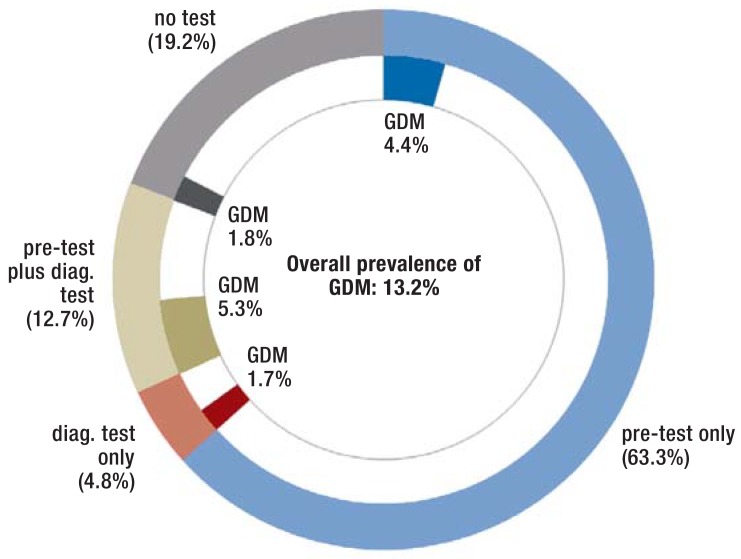
In 80.8% of all pregnant women, either the pre-test alone or the diagnostic test alone or both of the two test procedures were used (figure 2). None of the two tests was performed in 19.2% of the pregnant women. Almost all tested women underwent at least the pre-test (94%); in 83.3% of these women no further oGTT-based testing was performed. In relation to the total study cohort, 12.7% underwent oGTT in addition to the pre-test. Another 4.8% underwent only oGTT. The distribution of the test methods is depicted in Figure 2.
Figure 2.
Screening implementation and GDM prevalence according to method
outer ring: distribution of test methods or no test inner ring: pregnant women with diagnosed GDM GDM, gestational diabetes mellitus
Estimation of GDM prevalence
In our cohort, 13.2% of the pregnant women were diagnosed with GDM. Figure 2 shows the prevalence of GDM in relation to the test method used. Of the women who underwent at least one of the two tests, 11.4% were diagnosed with GDM. In the remaining 1.8% of women with GDM, the diagnosis was established, but neither of the two test methods was billed during the study period.
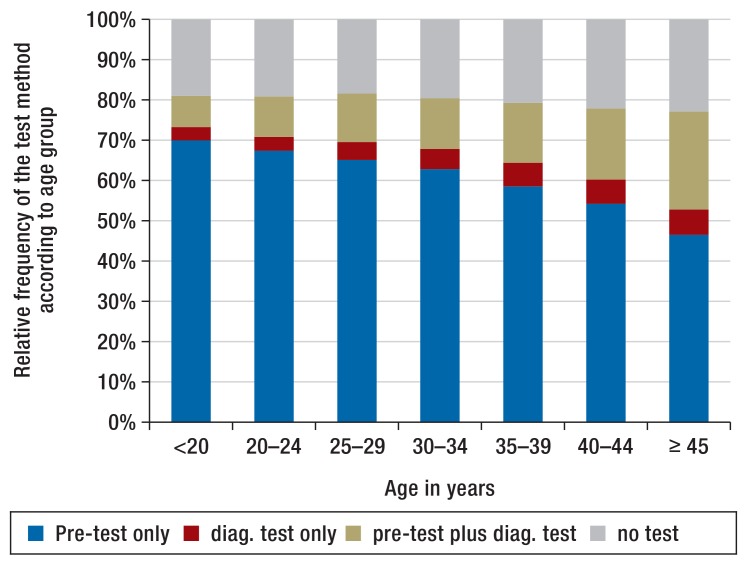
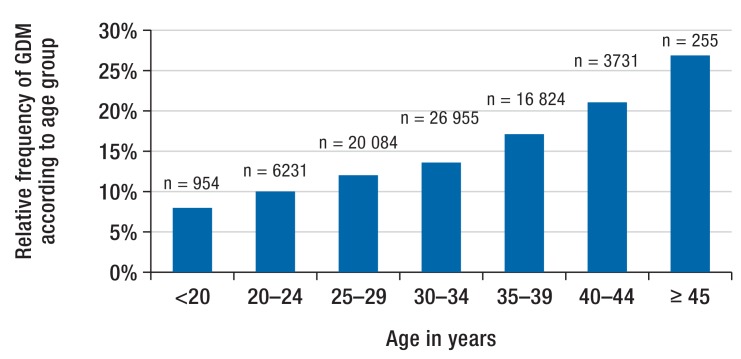
Figure 3 and Figure 4 depict the age-stratified distributions of the test methods and the GDM prevalence. The use of the pre-test alone declines with advancing age, while the use of the diagnostic test increases. With increasing age, the prevalence of GDM rises from less than 8% in the youngest age groups to over 26% among women =45 years of age. In older pregnant women, the diagnosis of GDM is more frequently based on the combination of the two test methods.
Figure 3.
Age-stratified distribution of the test methods
Figure 4.
Age-stratified distribution of GDM prevalence
GDM, gestational diabetes mellitus
Besides the diagnosis of GDM, the diagnosis of manifest diabetes was reported for the first time during the study period in 5956 women. This equals 1.0% of the study cohort. It can be assumed that these were pregnant women diagnosed for the first time with manifest diabetes (potentially as the result of GDM screening). If only women with GDM alone are included in the analysis, the overall prevalence of GDM is reduced to 12.2%.
Another 1831 pregnant women—equivalent to 0.3% of the study cohort—were first diagnosed with manifest diabetes in the pregnancy, but without additional GDM diagnosis.
If one looks at the pregnancy cohort regardless of any already diagnosed manifest diabetes or GDM, altogether 12 340 (2.2%) of 572 554 pregnant women had manifest diabetes during pregnancy (T1D: n = 4809; T2D and other manifest diabetes: n = 7531).
Discussion
This study is the first to provide outpatient care data on the nationwide implementation of GDM screening in Germany and the GDM prevalence among all statutory health insurance members. With a rate of more than 80%, GDM screening is comprehensively implemented. The remaining number of untested women reflects the voluntary nature of the screening program which is to be offered in a shared decision making process. More than two thirds of all pregnant women received the pre-test and in only 13% of women further testing based on the oral glucose tolerance test was required. This is in line with international data on the implementation of two-step test procedures for GDM (30). It can thus be assumed that the two-step screening strategy spares many women from having to undergo the more complex and demanding oGTT.
Our study found a 1-year prevalence of GDM of 13.2%. In 1% of the pregnant women, both GDM and manifest diabetes was diagnosed, indicating that it was possible to identify manifest diabetes in the process of the further evaluation of screening results. Altogether 13.5% were first diagnosed with a diabetogenic metabolic state (GDM and/or manifest diabetes) during pregnancy. With 13.2%, the GDM prevalence in our study is considerably above the 5% estimated in the Quality Report in Obstetrics for 2015 (22). The GDM data collected in maternity hospitals from the Mutterpass may underestimate the actual prevalence of GDM, because GDM testing is performed late in pregnancy and thus this diagnosis may not have been added to the Mutterpass in all cases. Furthermore, transfer errors may occur when the information from the Mutterpass is entered into the hospital documentation system after childbirth.
A recent study assessing GDM prevalence in the North Rhine region based on outpatient billing data—as we have used in our analysis—found a rate of 6.8% (23). This is considerably lower than our results presented here. This difference is explained, among other factors, by their definition of the pregnancy cohort which used the inclusion criterion of at least one billed flat fee (GOP 01770). Using this approach, the population also includes pregnant women who do not reach the screening period (6 to 7 months’ gestation), for example due to spontaneous or induced abortion; in these women a diagnosis according to the Maternity Directive cannot be established. By contrast, a study based on service data from the AOK Berlin statutory health insurance showed a considerably higher prevalence between 14% and 18%, because it used a stricter definition for the population (21). International studies estimate a prevalence of 13% for Europe (15).
Age is an important risk factor for GDM (31). Our analyses also show a considerable rise in GDM diagnoses with increasing age. This is also reflected in the implementation of screening: While younger women frequently underwent only the pre-test, which usually is sufficient to rule out GDM, a combination of the two test methods was increasingly used with rising age of the pregnant woman. This is indicative of an age-sensitive benefit of a two-step screening strategy.
Strengths and limitations
The data source of this study is the population of all pregnant women with statuary health insurance making use of outpatient care; thus, it represents the reality of care across health insurances and regions with regard to actual service provision and coding. Earlier studies evaluating GDM prevalence or screening implementation in Germany were limited to specific regions and/or health insurances; consequently, their representativeness of the total population was also limited (19, 21, 23).
Furthermore, the analyses relate to the period two years after the introduction of the screening program and one year after the introduction of the corresponding GOP. Thus, it can be assumed that there was enough time allowed for the nationwide roll-out of a new screening program to be able to capture its implementation.
A limitation of this study is the restricted validity of service data, as these are not collected for research purposes and suffer from several shortcomings. For example, diagnoses appearing without corresponding testing may have been established in a hospital setting or transferred from previous pregnancies where GDM was diagnosed. The prevalence estimates presented in our study are based on administrative data. Subject to the data source, this administrative prevalence represents a more or less accurate approximation to the actual prevalence.
Furthermore, the results depend on the definition and selection of the studied population and the operationalization of the care item. In our study, we aimed at accomplishing a content/medically-based and transparently presented cohort formation to achieve a valid estimate of the population of all pregnant women. By requiring billing of the pregnancy flat fee-per-case across three quarters, we wanted to ensure that the pregnancy had actually reached the period of the recommended screening examinations.
The comparison with official statistics on the number of live births shows a correspondence of approximately 80%. The difference of 20% is partly explained by the fact that pregnant women with private health insurance were not included in the analyzed service data. In addition, our pregnancy cohort did not account for women who changed their names during pregnancy. An “ongoing” pregnancy, as defined here, was found in 56% of all pregnant women. Pregnancy flat fees-per-case which were billed less frequently than in 3 consecutive quarters (44%) can be explained by method-related reasons (exclusion of pregnant women because of the selected cohort design, name change) and by medical reasons (for example, spontaneous or induced abortion, preterm birth). Finally, using service data it is not possible to continuously follow pregnant women who do not at all times receive services by the German outpatient care system.
Conclusion
GDM screening was widely implemented two years after its introduction by the Federal Joint Committee (G-BA). This indicates its increasing acceptance by pregnant women and their doctors. Especially the relatively low rate of pregnant women requiring oGTT is in line with the aim of the stepped screening strategy, as it reflects the ability of the pre-test to exclude a large proportion of pregnant women from further testing. The 1-year prevalence of GDM is with 13.2% in the range of current international prevalence estimates. Furthermore, the validation of the cohort we have used here and the age-based results are indicative of a reliable approximation to the actual prevalence. Prompt detection and treatment of GDM is necessary to prevent complications for both mother and child. However, based on the available data it is not possible to determine whether the high prevalence found is due to an increase in GDM cases or an increase in detection rates.
THE CLINICAL PERSPECTIVE.
According to the German Maternity Directive, gestational diabetes mellitus (GDM) screening, comprising a pre-test and a diagnostic test, shall be offered to all pregnant women not suffering from pre-existing manifest diabetes mellitus. Based on an informed decision, the pregnant women can consent to or reject GDM testing. The leaflet “I am pregnant. Why is a test for gestational diabetes offered to all pregnant women?” (annex 6 of the Maternity Directive) is provided to facilitate decision making and to explain the test procedure.
If both the pre-test and the diagnostic test are positive, the pregnant woman’s further care is provided in close collaboration with a physician qualified in diabetology. The key elements of GDM management are dietary changes and increased physical exercise; patients are educated about these in patient training session intended to promote long-term self-management. If these measures are not effective, insulin therapy may be required.
Key Messages.
More than 80% of pregnant women with statutory health insurance undergo screening for gestational diabetes mellitus (GDM).
In the majority of pregnant women, only the pre-test is performed. Thus, they are spared from having to undergo the more complex and demanding oral glucose tolerance test.
Most GDM diagnoses result from the use of the pre-test and the diagnostic test.
The overall prevalence of GDM is with 13.2% in the range of current international estimates.
With increasing age of the pregnant women, both the combined use of the two tests and the prevalence of GDM increase.
Acknowledgments
Data protection
This study complied with the provisions on data protection and conduct of analyses of the “Good Practice of Secondary Data Analysis” (GPS) guideline.
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Kleinwechter H, Schäfer-Graf U, Buhrer C, et al. Gestational diabetes mellitus (GDM) diagnosis, therapy and follow-up care: practice guideline of the German Diabetes Association (DDG) and the German Association for Gynaecology and Obstetrics (DGGG) Exp Clin Endocrinol Diabetes. 2014;122:395–405. doi: 10.1055/s-0034-1366412. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(1):S13–S22. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 3.Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 5.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 7.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 8.Löbner K, Knopff A, Baumgarten A, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. 2006;55:792–797. doi: 10.2337/diabetes.55.03.06.db05-0746. [DOI] [PubMed] [Google Scholar]
- 9.Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. 2016;59:1403–1411. doi: 10.1007/s00125-016-3927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gemeinsamer Bundesausschuss. Richtlinien über die ärztliche Betreuung während der Schwangerschaft und nach der Entbindung („Mutterschafts-Richtlinien“) www.g-ba.de/downloads/62-492-1223/Mu-RL_2016-04-21_2016-07-20pdf" (last accessed on 12 January 2017) [Google Scholar]
- 11.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG) Screening auf Gestationsdiabetes - Abschlussbericht. www.iqwig.de/download/S07-01_Abschlussbericht_Screening_auf_Gestationsdiabetes.pdf (last accessed on 12 January 2017) [Google Scholar]
- 12.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16 doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis. 2014;11 doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djelmis J, Pavic M, Mulliqi Kotori V, Pavlic Renar I, Ivanisevic M, Oreskovic S. Prevalence of gestational diabetes mellitus according to IADPSG and NICE criteria. Int J Gynaecol Obstet. 2016;135:250–254. doi: 10.1016/j.ijgo.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:176–185. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31:2288–2293. doi: 10.2337/dc08-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30:141–146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 18.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124:804–813. doi: 10.1111/1471-0528.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyerlein A, Koller D, Ziegler AG, Lack N, Maier W. Does charge-free screening improve detection of gestational diabetes in women from deprived areas: a cross-sectional study. BMC Pregnancy Childbirth. 2016;16 doi: 10.1186/s12884-016-1060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huy C, Loerbroks A, Hornemann A, Rohrig S, Schneider S. Prävalenz, Trend und Determinanten des Gestationsdiabetes in Deutschland. Geburtsh Frauenheilkd. 2012;72:311–315. doi: 10.1055/s-0031-1298390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeske A, Zeeb H, Razum O, Spallek J. Unterschiede in der Gestationsdiabetesinzidenz im Vergleich zwischen türkischstämmigen und deutschen Frauen: Eine Analyse von Abrechnungsdaten der AOK Berlin, 2005-2007. Geburtsh Frauenheilkd. 2012;72:305–310. [Google Scholar]
- 22.Institut für Qualitätssicherung und Transparenz im Gesundheitswesen (IQTIG) Beschreibung der Qualitätsindikatoren für das Erfassungsjahr 2015 - Geburtshilfe. https://iqtig.org/downloads/ergebnisse/qidb/2015/2016-05-25/QIDB_2015_INDIREKT_PDF/QIDB_2015_indirekte_Leistungsbereiche/QIDB_mit_Rechenregeln/16n1_QIDB2015_Rechenregeln.pdf (last accessed on 12 January 2017) [Google Scholar]
- 23.Tamayo T, Tamayo M, Rathmann W, Potthoff P. Prevalence of gestational diabetes and risk of complications before and after initiation of a general systematic two-step screening strategy in Germany (2012-2014) Diabetes Res Clin Pract. 2016;115:1–8. doi: 10.1016/j.diabres.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Bundesgesundheitsministerium Mitglieder und Versicherte der Gesetzlichen Krankenversicherung (GKV) www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Statistiken/GKV/Mitglieder_Versicherte/KM6_2015.xls (last accessed on 12 January 2017) [Google Scholar]
- 25.Kassenärztliche Bundesvereinigung. Einheitlicher Bewertungsmaßstab (EBM) www.kbv.de/media/sp/EBM_Gesamt___Stand_4._Quartal_2015.pdf (last accessed on 12 January 2017) [Google Scholar]
- 26.Statistische Bundesamt (DESTATIS) Frauen mit Mehrlingsgeburten. www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/Bevoelkerung/Geburten/Tabellen/GeburtenMehrlinge.html (last accessed on 12 January 2017) [Google Scholar]
- 27.Statistische Bundesamt (DESTATIS) Lebendgeborene und Gestorbene. www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/Bevoelkerung/Geburten/Tabellen/LebendgeboreneGestorbene.html (last accessed on 12 January 2017) [Google Scholar]
- 28.Deutsches Institut für Medizinische Dokumentation und Information (DIMDI) ICD-10-GM. www.dimdi.de/static/de/klassi/icd-10-gm/kodesuche/onlinefassungen/htmlgm2017/index.htm (last accessed on 12 January 2017) [Google Scholar]
- 29.MicroStrategy Inc. Advanced reporting guide. www2.microstrategy.com/producthelp/10.5/manuals/en/AdvancedReporting.pdf (last accessed on 12 January 2017) [Google Scholar]
- 30.Donovan LE, Savu A, Edwards AL, Johnson JA, Kaul P. Prevalence and timing of screening and diagnostic testing for gestational diabetes mellitus: a population-based study in Alberta, Canada. Diabetes Care. 2016;39:55–60. doi: 10.2337/dc15-1421. [DOI] [PubMed] [Google Scholar]
- 31.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286:2516–2518. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]